Abstract
Background
Ciliopathies are a class of inherited pleiotropic genetic disorders in which alterations in cilia assembly, maintenance, and/or function exhibit penetrance in the multiple organ systems. Olfactory dysfunction is one such clinical manifestation that has been shown in both patients and model organisms. Existing therapies for ciliopathies are limited to the treatment or management of symptoms. The last decade has seen an increase in potential curative therapeutic options including small molecules and biologics. Recent work in multiciliated olfactory sensory neurons has demonstrated the capacity of targeted gene therapy to restore ciliation in terminally differentiated cells and rescue olfactory function. This review will discuss the current understanding of the penetrance of ciliopathies in the olfactory system. Importantly, it will highlight both pharmacological and biological approaches, and their potential therapeutic value in the olfactory system and other ciliated tissues.
Methods
We undertook a structured and comprehensive search of peer-reviewed research literature encompassing in vitro, in vivo, model organism, and clinical studies. From these publications, we describe the olfactory system, and discuss the penetrance of ciliopathies and impact of cilia loss on olfactory function. In addition, we outlined the developing therapies for ciliopathies across different organ and cell culture systems, and discussed their potential therapeutic application to the mammalian olfactory system.
Results
One-hundred sixety-one manuscripts were included in the review, centering on the understanding of olfactory penetrance of ciliopathies, and discussing the potential therapeutic options for ciliopathies in the context of the mammalian olfactory system. Forty-four manuscripts were used to generate a table listing the known congenital causes of olfactory dysfunction, with the first ten listed are linked to ciliopathies. Twenty-three manuscripts were used to outline the potential of small molecules for the olfactory system. Emphasis was placed on HDAC6 inhibitors and lithium, both of which were shown to stabilize microtubule structures, contributing to ciliogenesis and cilia lengthening. Seventy-five manuscripts were used to describe gene therapy and gene therapeutic strategies. Included were the implementation of adenoviral, adeno-associated virus (AAV), and lentiviral vectors to treat ciliopathies across different organ systems and application toward the olfactory system. Thus far, adenoviral and AAV-meditated ciliary restoration demonstrated successful proof-of-principle preclinical studies. In addition, gene editing, ex vivo gene therapy, and transplantation could serve as alternative therapeutic and long-term approaches. But for all approaches, additional assessment of vector immunogenicity, specificity, and efficacy need further investigation. Currently, ciliopathy treatments are limited to symptomatic management with no curative options. However, the accessibility and amenability of the olfactory system to treatment would facilitate development and advancement of a viable therapy.
Conclusions
The findings of this review highlight the contribution of ciliopathies to a growing list of congenial olfactory dysfunctions. Promising results from other organ systems imply the feasibility of biologics, with results from gene therapies proving to be a viable therapeutic option for ciliopathies and olfactory dysfunction.
Keywords: olfactory dysfunction, ciliopathy, olfactory epithelium, gene therapy, small molecule
INTRODUCTION
Cilia are organelles present on the surface of cells in a wide variety of organisms from unicellular flagellates and round worms to almost all cell types in vertebrates. Cilia have a diverse set of functions, including propulsion and fluid movement for motile cilia as well as cellular signaling and detection of external stimuli such as growth factors, sound, light, and odors for non-motile cilia [1–3]. While our understanding into the diverse biological functions of cilia is continually increasing, so is our comprehension for their role in disease. Ciliopathies are a class of pleiotropic congenital human diseases with phenotypes that exhibit variable penetrance depending on the tissue, the gene and the type of mutation, as well as an individual’s genetic background [4,5]. Clinical manifestations of ciliopathies can occur during development and throughout adulthood and may result in a variety of deficits including polydactyly, obesity, hypogonadism, renal dysplasia, infertility, situs inversus, respiratory complications, liver fibrosis, retinal dystrophy, and anosmia [5].
The olfactory epithelium (OE) is a pseudo-stratified epithelium that lines the nasal turbinates and septum and is comprised of multiple cell types including immature and mature olfactory sensory neurons (OSNs), supporting sustentacular cells, and two populations of stem cells known as globose basal cells and horizontal basal cells (HBCs) (Figure 1.). Within the OE, developing neurons possess primary cilia during embryogenesis [6,7], however their function is currently unknown. In addition, recent work has shown that HBCs possess primary cilia that are involved in regulating regeneration of the OE after injury [8]. Unlike developing neurons and HBCs, mature OSNs are multiciliated bipolar neurons that project from their dendritic knob approximately 20-35 cilia, extending up to ~100 μm in length, into the olfactory lumen and cover the surface of the OE [9]. Located within these cilia are odorant receptors, which are seven-transmembrane G-protein coupled receptors (GPCRs) that bind odor molecules within the nasal cavity. GPCRs are often pharmacological targets for therapies, due in part to their involvement in various diseases [10–13]. In addition to odorant receptors, olfactory cilia also contain signal transduction machinery required for odor detection including the heterotrimeric G-proteins, adenylyl cyclase III (ACIII; EC 4.6.1.1), cyclic nucleotide gated channels, and calcium activated chloride channels. Extending from basal bodies that are organized in a ring-like fashion around the dendritic knob, OSN cilia have a transition zone, proximal segment, and a long distal segment. The proximal segment (~1-2 μm) of cilia is made up of microtubule doublets, while the distal segment (on average, 50 μm in length) is made up microtubule singlets [9]. Much like primary cilia of other cells, OSN cilia utilize anterograde and retrograde intraflagellar transport for protein movement along the ciliary axoneme [9]. Importantly, mutations in genes associated with the structural integrity or function of cilia can lead to olfactory dysfunction.
Figure 1. Olfactory epithelium cell types.
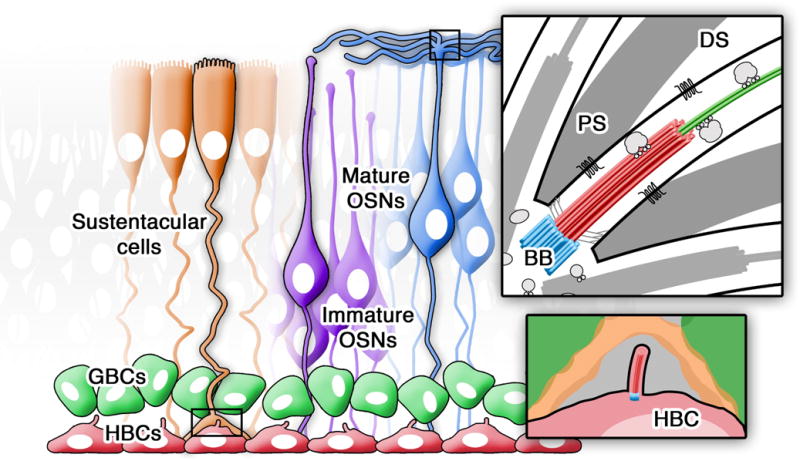
Diagram depicting the major cell types present within the mammalian olfactory epithelium. At the basal surface are the ciliated horizontal basal cells (HBCs; red) and globose basal cells (GBCs; green). At the apical surface are the sustentacular cells (orange), and immature (purple) and multiciliated mature (blue) olfactory sensory neurons (OSNs). (Inset-top) Structural organization of OSN cilia depicting basal bodies (BB), proximal segment (PS), distal segment (DS), including trafficking of ciliary proteins and membrane bound G-protein coupled odor receptors. (Inset-bottom) Magnification of the HBC primary cilia, which has a role in OE neurogenesis.
Alterations in olfactory function can range from hyposmia (reduction of olfactory function) and anosmia (loss of olfactory function), to hyperosmia (increased olfactory acuity) and dysosmia (distorted odor perception). In patients, olfactory dysfunction can impact their personal safety and reduce their quality of life [14–16]. Olfactory dysfunction is attributed to multiple causes, which include post-viral infection, head trauma, inflammation or chronic sinusitis [17]. In addition, there is a growing list of olfactory dysfunctions resulting from congenital causes, which includes ciliopathies (Table 1). The degree of penetrance of ciliopathies within the olfactory system is not well understood, nor are the associated mechanisms of action and disease phenotypes. However, over approximately the last 10 years, research in both ciliopathy patient populations and murine models has gradually started examining the occurrence and mechanisms of olfactory dysfunction in ciliopathies. Individuals with Bardet-Biedl Syndrome (BBS) as well as Leber congenital amaurosis (LCA) exhibit abnormal or anosmic scores on the B-SIT olfactory function test [18,19]. In murine ciliopathy models such as the Oak Ridge Polycystic Kidney (ORPK) mouse, as well as in knockouts of BBS1, BBS4, or BBS8, ciliation was either significantly reduced or absent from OSNs. In addition, these mice exhibit loss of olfactory function and significantly reduced olfactory bulb activity, while BBS8 knockout mice also show OSN axon mistargeting in the olfactory bulb [18,20,21]. In addition, loss of BBS1 in OSNs results in mistrafficking of core BBS complex proteins to cilia, causing a reduction in cilia length and number as well as reduced olfactory bulb activity [22]. Interestingly, in rd16 mice, which possess a genetic deletion of Cep290, cilia are still present on the surface of the OE. However, G proteins essential for olfactory signal transduction do not traffic into cilia and therefore rd16 mice are functionally anosmic [19]. These findings suggest that ciliopathies have varying degrees of penetrance in the OE and that the mechanisms underlying loss of olfactory function may vary. This may also be true for cells in the OE that elaborate primary cilia. Though primary cilia have been identified on developing OSNs [6,7], their function as well as their potential role in ciliopathy-related anosmia has yet to be determined. Recent work has shown that loss of primary cilia on HBCs, due to deletion of either Ift88 or Arl13b, two ciliopathy related genes, impairs the ability of HBCs to regenerate the OE after injury [8]. This work suggests that beyond OSNs, olfactory ciliopathies exhibit penetrance in stem cells of the OE and that impaired neurogenesis may also be a potential mechanism for congenital anosmia.
Table 1. List of congenital olfactory dysfunctions.
Known ciliopathies demonstrating olfactory dysfunction are listed #’s 1 through 10.
| # | Disease | Known Genetic Mutations | Confirmed Olfactory Dysfunction | Known Cause of Olfactory Dysfuntion | References |
|---|---|---|---|---|---|
| 1 | Bardet-Beidl Syndrome (BBS) | BBS1, BBS1(M390R), BBS2, BBS3/ARL6, BBS4, BBS5, BBS6/MKKS, BBS7, TTC8, BBS9, BBS10, TRIM32, BBS12, CEP290/NPHP6 | BBS1, BBS1(M390R), BBS2, BBS4, BBS6/MKKS, BBS8 | Olfactory epithelium (cilia loss) | 18, 20, 25, 124, 125, 126 |
| 2 | Meckel-Gruber Syndrome (MKS) | MKS1, MKS2, MKS3/TMEM67, CEP290/NPHP6, BBS1, BBS4, NPHP3 | MKS1, MKS3/TMEM67, CEP290/NPHP6 | Olfactory epithelium (signal transduction) | 19, 126, 127, 128 |
| 3 | Joubert Syndrome | CEP290/NPHP6, ARL13b, INPP5e | CEP290/NPHP6 | ??? | 19, 126, 129, 130, 131 |
| 4 | McKusick-Kaufman Syndrome | BBS6/MKKS | BBS6/MKKS | ??? | 24 |
| 5 | Refsum Disease | PEX7, PHYH | ??? | ??? | 132, 133 |
| 6 | Polycystic Kidney Disease (PKD) | PKD1, PKD2, NPHP3, IFT88 | PKD1, PKD2, IFT88 | Olfactory epithelium (cilia loss; IFT88) | 21, 128 |
| 7 | Primary Ciliary Dyskinesia (PCD) | DNAI1, DNAH5 | ??? | Olfactory epithelium (airway obstruction) | 134 |
| 8 | Leber Congenital Amaurosis (LCA) | CEP290/NPHP6, GUCY2D, RPE65, SPATA7, AIPL1, LCA5, RPGRIP1, CRX, CRB1, NMNAT1, IMPHD1, RD3, RDH12, LRAT, TULP1, KCNJ13, GDF6, PRPH2, LCA9, LCA3 | CEP290/NPHP6 | Olfactory epithelium (signal transduction; airway obstruction) | 19, 126, 135 |
| 9 | Jeune Syndrome | IFT80, DYNC2H1, WDY19, IFT40, TTC21B | ??? | ??? | 136 |
| 10 | Nephronophthisis | CEP290/NPHP6 | CEP290/NPHP6 | ??? | 126 |
| 11 | Kallmann’s Syndrome | KAL1, AKAP2, FGF8, FGFR1, KISS1R, PROK2/PROKR2, NELF, TAC3, TACR3, WDR11 | KAL1, FGFR1, PROK2/PROKR2, FGF8, CHD7, WDR11, NELF | Olfactory bulb development | 137, 138, 139, 140, 141, 142 |
| 12 | CHARGE Syndrome | CHD7 | CHD7 | ??? | 143, 144 |
| 13 | Usher Syndrome | USH1C, USH1B/MYO7A, CDH23, USH2/USH2A, PCDH15, CLRN1, SANA, USH1H, VLGF1b, DFNB31, PDZD7, USH3A | USH1 | Olfactory bulb development | 145, 146, 147, 148, 149 |
| 14 | Microcephalic osteodysplastic primordial dwarfism type II (MOPDII) | PCNT | ??? | Olfactory bulb development | 150 |
| 15 | Seckel Syndrome | ATR, PCNT, CENPJ | ??? | ??? | 21, 150, 151 |
| 16 | Bosma Arhinia Microphthalmia | PAX6 | ??? | Olfactory epithelium (airway obstruction); Olfactory bulb development | 152, 153 |
| 17 | Endocrine-Cerebro-Osteodysplasia | ICK | ??? | Olfactory bulb development | 154 |
| 18 | Mohr-Majewski Syndrome | TCTN3 | ??? | Olfactory bulb development | 155, 156, 157 |
| 19 | Isolated Congenital Anosmia | CNGA2, TENM1 | CNGA2, TENM1 | Olfactory epithelium (signal transduction) | 158, 159, 160 |
| 20 | Congenital Indifference to Pain | SCN9A | SCN9A | Olfactory epithelium (action potential propagation) | 161 |
Despite our incomplete understanding of the penetrance of cilia dysfunction within the olfactory system, both small molecule and gene therapies offer potential to treat olfactory ciliopathies. In this context, we address the current and future challenges for treating patients with olfactory dysfunction.
SMALL MOLECULE THERAPEUTIC STRATEGIES
While no small molecule therapies have been developed specifically for ciliopathy-induced olfactory dysfunction, the use of pharmaceuticals for treatment of ciliopathies in other organ systems, particularly for BBS and polycystic kidney disease (PKD), have shown progress. In BBS, cilia dysfunction leads to retinal degeneration [23–28] via protein accumulation in the inner segment of photoreceptors, stress of the endoplasmic reticulum, and the subsequent activation of the proapoptotic unfolded protein response [29]. Interestingly, in a murine model of BBS12, Mockel et al [29] showed that administration of a combination of valproic acid, guanabenz, and a specific caspase-12 inhibitor reduces apoptosis and preserves light detection in mutant animals. In PKD, extensive research and clinical trials of pharmacological therapies are ongoing. These studies frequently use pharmacological inhibitors to target cilia-associated cell proliferation signaling pathways such as mTOR (mammalian target of rapamycin), cAMP (cyclic adenosine monophosphate), and EGFR (epidermal growth factor receptor) [30–34]. Typically, reductions in kidney or liver volume were observed but kidney function was not completely restored [34–36]. More recently it has been demonstrated that cell proliferation in renal tubules was not sufficient to induce cyst formation after cilia disruption [37] and that cyst growth is regulated by the primary cilium, independent of cell proliferation signaling [38]. Other studies suggest that alterations in the length and/or stability of the primary cilium may be an important direct regulator of PKD pathogenesis [39–41].
Recent work has shown that histone deacetylase 6 (HDAC6; EC 3.5.1.98) is a major regulator of cilia stability and disassembly, and could be an important therapeutic target for ciliopathies [42]. Signaling proteins such as calmodulin and β-catenin as well as the ciliary protein nphp2/inversin interact with Aurora A, which phosphorylates and activates HDAC6, leading to deacetylation of α-tubulin and cortactin [43–46]. Deacetylation of a-tubulin leads to disassembly of primary cilia, while deacetylation of cortactin promotes interaction with filamentous actin (F-actin), ultimately leading to ciliary resorption [46]. Interestingly, calmodulin is involved in adaptation during olfactory signal transduction. It would be intriguing to examine whether calmodulin has a similar role in regulating ciliary disassembly via HDAC6 in the olfactory system and whether HDAC6 inhibitors could be used as a potential therapeutic option for olfactory dysfunction. In primary cilia, an HDAC6-specific inhibitor tubastatin A, has been shown to increase acetylated α-tubulin and restore ciliation and cilium-dependent processes [47]. Moreover, non-selective HDAC inhibitors including vorinostat, an FDA approved cancer drug, have also demonstrated increased ciliogenesis of primary cilia [42,47]. However, tubulin targeting chemotherapeutic cancer drugs such as paclitaxel (taxol) have shown negative effects on the OE, leading to severe lesions [48]. In zebrafish, characteristic ciliopathy phenotypes were observed upon reduction of BBSome-interacting protein of 10 kDa (BBIP10), a protein required for microtubule acetylation and assembly of the primary cilia [49]. In BBIP10-depleted cells, application of tubacin, an HDAC6 inhibitor, restored microtubule acetylation and ciliary assembly providing evidence that BBIP10 regulates tubulin acetylation by inhibiting HDAC6 [49]. As the BBSome is an important regulator of cilia function in the olfactory system [9], this work is suggestive that HDAC6 inhibitors may be a potential therapeutic target for olfactory ciliopathies.
Interestingly, lithium, which has been used to treat neurological disorders, also promotes tubulin acetylation and elongation of cilia [50]. Specifically, brain sections of mice chronically fed lithium carbonate demonstrate primary cilia elongation within the dorsal striatum and nucleus accumbens [51], while in cultured cells lithium chloride has also been shown to elongate cilia [50–52]. Lithium Chloride promotes acetylation of α-tubulin through the activation of α-tubulin N-acetyltransferase 1 (α-TAT1; EC 2.3.1.108) and inhibition of glycogen synthase kinase 3β (GSK3β; EC 2.7.11.26), resulting in elongation of primary cilia [50]. While lithium may increase cilia length it is currently unknown whether this is sufficient for restoring cilia function.
The use of small molecule therapy for treating olfactory dysfunction is enticing. However, extensive studies on their effects in vivo and efficacy in the context of the olfactory system remains unexplored. It also remains unclear whether restoration of cilia morphology is sufficient to reconstitute ciliary function, especially in multiciliated OSNs. In ciliopathies such as BBS, ciliary elongation and/or microtubule stabilization may be inadequate to restore the function of the missing BBS protein or the BBSome complex, which is critical for protein trafficking. Importantly, small molecule therapies are generally not curative treatments and other therapeutic approaches for restoration of olfactory function, such as gene therapy, should also be considered.
GENE THERAPEUTIC STRATEGIES
The first clinical trials of gene therapy to human patients were performed between 1989 and 2000 using retroviral and adenoviral-mediated transduction. Since then, the field has advanced dramatically with modifications of those original vectors and the development of adeno-associated viral (AAV) vectors, which demonstrated tissue specificity and lower immunogenic response, and the expansion of non-viral mediated strategies [53–55]. Clinical trial successes have been shown in multiple diseases, such as sickle cell anemia, hemophilia, cystic fibrosis, and retinal degeneration. More recently, a major breakthrough occurred in the field with the use of gene therapy for CAR-T cells and cancer immunotherapies. During the time of this review, the United States Food and Drug Association approved tisagenlecleucel (Kymriah), a cell-based gene therapy to treat acute lymphoblastic leukemia. To date there are more than 1,200 early stage and active clinical trials that uses gene therapeutic approaches to address multiple diseases (http://www.ClinicalTrials.gov; September 30, 2017; Keywords: Gene therapy). Regardless of the application, the strength of gene therapies is the potential for curative outcomes, especially monogenic diseases such as ciliopathies.
Fundamentally, the strategy of gene therapy is to deliver genetic material that would either replace or correct the disease-causing genetic mutation. The therapeutic gene is packaged in a viral vector, which then infects and is transduced in a host or target cell. The administration can be performed either in vivo, where the patient or animal is directly treated with vector, or ex vivo, where cells are collected from the host, transduced, and then transplanted back into the host. In the following section, we will focus on the various vector-mediated gene therapies, which have demonstrated successes in ciliopathy models and the capacity of treating ciliopathies affecting the olfactory system. Much like small molecule treatment, the accessibility of the olfactory system makes it conducive to gene therapeutic delivery. Several studies have demonstrated the receptiveness of the OE to transduction by multiple kinds of viral vectors, generating confidence in these approaches.
Recombinant Adenoviral-mediated Therapy
Adenoviruses are 90-100 nm non-enveloped icosahedral viruses with 57 serotypes. As a vector, adenoviral serotype 5 has been used for the majority of gene therapeutic studies due in part to its ability to efficiently infect multiple cell types, generate high titer preparations, and 3.0-8.0 kb insertional size in its ~36kb linear dsDNA genome. Early adoption of adenoviral vectors for gene therapies were hindered by the strong immunogenic responses from both animal models and human clinical trials [56], resulting in cytokine induction and activation of effector leukocytes [57]. Despite the immunogenic response, their insertion capacity and ease of production make adenovirus ideal for pre-clinical and ongoing gene therapeutic studies with efforts to reduce the immunogenic responses by capsid modifications [56,57].
Outside of the olfactory system, adenoviral-mediated strategies have been applied in ciliopathy models, particularly PKD. In vitro reintroduction of wildtype PKD2 (polycystin-2, TRPP2) restored partial function from renal cells isolated from PKD2 knockout mouse model [58]. In vivo and in vitro, postnatal PKD1 knockout mice demonstrated slower renal cyst growth 10 days following treatment with adenovirus containing the gene encoding for NGAL (neutrophil gelatinase associated lipocalin), which inhibits proliferation and increases apoptosis [59]. In an alternative strategy, in vivo administration of adenovirus encoding for a shRNA for the RAGE (receptor for advanced glycation end products) protein demonstrated slower cyst growth and improved renal function in a mouse model of autosomal dominant polycystic kidney disease (ADPKD) [60].
Within the olfactory field, several studies have demonstrated that the OE and particularly OSNs are amenable to adenoviral-mediated transduction following intranasal delivery [9,21,22,61–69]. Despite earlier reports of infiltration into the olfactory bulb [57,70], infection and transduction was highly restricted to the nasal cavity and OSNs axons [71,72]. Different studies have shown that intranasal delivery of adenovirus infects approximately 15-25% of OSNs, with viral gene expression peaking at 10 days post infection [22,61]. Longevity studies have observed the persistence of gene products to up to 21 days post infection and as long as 90 days post infection; however, there is a dramatic drop in expression levels 30 days post infection [61,72]. Together, the accessibility for non-invasive delivery and the amenability of cells in the OE to viral transduction is an important consideration for gene therapy.
As a tool, adenoviral-mediated expression of fluorescently-tagged proteins has allowed for a greater understanding of the organization and structure of OSN cilia, which includes the detailed morphology, regional divisions, and the dynamics of intraflagellar transport (IFT) protein trafficking [9]. Important as a potential therapy, the use of the adenoviral vectors is able to restore wildtype olfactory function in ciliopathy and other null mouse models [21,22,64]. First demonstrated in an OMP (olfactory marker protein) knockout mouse model, adenoviral-mediated gene replacement of the OMP gene restored normal odor response kinetics in infected OSNs [64]. The capacity of adenoviral-mediated restoration was bolstered in the ORPK ciliopathy mouse model, which possesses a hypomorphic mutation in Ift88. Intranasal delivery of wildtype Ift88 restored ciliation on OSNs and rescued olfactory function [21]. The success of adenoviral-mediated rescue was recapitulated in a patient-relevant BBS1 knockout mouse model of BBS, where ectopic expression of wildtype BBS1 was capable of restoring ciliary morphology, ciliary IFT protein trafficking, and olfactory function in vivo [22]. From the same study, adenoviral exposure and wildtype BBS1 overexpression did not exhibit changes in macrophage infiltration nor apoptosis. Suggesting the delivery and dosage did not induce an immunogenic response nor toxic effects. Together, these studies were the first to demonstrate restoration of ciliation and tissue function in a ciliopathy mouse model. More importantly, these studies provide the preclinical proof-of-concept highlighting the capacity of adenoviral-mediated gene therapy to treat olfactory dysfunctions.
Adeno-associated Viral-mediated Therapy
In the field of gene therapy, safety concerns over adenovirus has increased efforts using adeno-associated viral (AAV) vectors. This focus is due to AAV’s natural lack of pathogenicity and limited immunogenic response. Over the years, continual modification and expansion of the AAV serotype library resulted in serotype-dependent cellular specificity and distinct expression levels, onset kinetics, and viral genome copy numbers [73]. To date, there are 12 different serotypes of AAV with different AAV pseudotypes, where the genome from one serotype is packaged within the capsid of a different serotype. AAVs are 20 nm non-enveloped capsid vectors with a ~4.7 kb ssDNA genome. Unlike adenoviral vectors, the insertional capacity of AAV is relatively small and can only accommodate ~2.5 kb of genetic material. Following infection of the host cell, the single stranded AAV genome is processed into dsDNA and undergoes concatemerization, increasing episomal stabilization within the host cell’s nucleus. Although the AAV genome remains largely episomal, some AAVs demonstrate the capacity of integrating in the host’s genome. Specifically, integration occurs in the long arm of chromosome 19, on site called AAVS1 [74].
AAV-mediated gene therapies have demonstrated preclinical and clinical successes in multiple diseases including hemophilia, cystic fibrosis, and LCA. With regards to ciliopathies, the retina is one tissue that has seen significant successes. LCA is an inherited disease which can result from mutation of cilia genes. It affects retinal pigmented cells and results in severe visual loss shortly after birth. AAV-mediated therapies showed some success in RPE65-null mutations of LCA. In these studies, young and adult RPE65-null canine models exhibited functional and structural recovery of the retina [75–77]. Where 3 years following a single dosage of AAV2/2, AAV2/1, or AAV2/5 serotypes the canine models maintained rod and cone vision [78]. With the success of canine studies, clinical trials using AAV2 containing wildtype Rpe65 gene to young patients proceeded with patients also demonstrating success with visual improvement [79]. However, follow up studies show limited long-term effects with patients experiencing continual retinal degeneration despite earlier visual improvement [80,81].
In regards to other ciliopathies, similar successes were observed in the BBS4 knockout and BBS1(M390R) knock-in mouse models of BBS, where retinal degeneration is one of the symptoms [82,83]. Subretinal administration of self-complementary AAV5 containing the wildtype Bbs4 ciliary gene into BBS4-null mice restored rhodopsin localization, returned normal retinal morphology, and improved electroretinogram recordings [82]. Comparable restorations were observed in BBS1(M390R) knockin mice treated with AAV2/5 containing wildtype Bbs1, but did not prevent long-term retinal degeneration [83]. In the nasal cavity, the use of AAV-mediated transduction has been effectively observed in both olfactory and respiratory epithelia exhibiting stable expression up to 9 months in respiratory cells, while also demonstrating the capacity of reinfection [84–86]. More recently, intranasal delivery of AAV9 containing wildtype Bbs1 restored OSN cilia and olfactory function in a BBS1 knockout mouse model of BBS, recapitulating the results from the adenoviral-mediated therapy [22]. In vivo imaging of the infection showed restriction of the treatment to the nasal cavity, and the restoration of odorant detection as recorded by electro-olfactogram 3 weeks post infection. These observations, demonstrate the amenability of the olfactory system and OSNs to AAV-mediated gene therapy. However, more studies are required to examine the efficacy of the strategy for other ciliopathies.
Retroviral-mediated Therapy
Early in the development of gene therapies, retrovirus was the preferred method of gene transfer. The advantage of retrovirus is their ability to stably incorporate into the host’s genome, which would allow for long-term and stable gene expression. Retroviruses, such as gammaretroviral and lentiviral vectors, are 100 nm enveloped particles that contain two ssRNAs between 7.0-10.0 kb, with an insertion capacity of 5.0-10.0 kb. Following infection, dsDNA is generated by reverse transcription and integration into the host cell’s genome. Lentiviruses differ from other retroviruses by having the pre-integration complex transported to the nucleus, allowing for genetic integration in dividing and non-dividing cells. Use of the retroviral-mediated therapies have demonstrated successes in proof-of-principle pre-clinical studies. Recently in isolated human fibroblasts from CEP290-null LCA patients, lentiviral transduction of the wildtype Cep290 cilia gene restored primary cilia formation [87]. In studies examining DNAI1-null primary ciliary dyskinesia (PCD), successful lentiviral transduction of the Dnai1 gene restored ciliary beating in mouse and human respiratory cell cultures [88,89]. Implementation of the lentiviral-mediated therapies to treat olfactory dysfunctions has not been directly attempted. However, cells of the OE demonstrate the capacity of lentiviral transduction both in vivo and ex vivo isolated OSN cultures [90–92]. The potential use of lentivirus as a gene therapeutic vector is further supported by the repetitive administration of lentivirus without the induction of immune response [93]. In order to gain a better understanding of the capacity of retroviral-mediated treatment of olfactory dysfunctions in ciliopathies, additional studies are required.
Gene Editing Therapy
Despite the success of the gene replacement therapies across multiple diseases, the approach is limited by the longevity of therapeutic gene expression. Both adenoviral and AAV-mediated transductions are dependent on the existence and stability of the episomal genetic material. The issue of longevity is partially addressed by lentiviral transduction, where the therapeutic gene is incorporated into the host cell’s genome. However, the insertion sites are nonspecific and demonstrate a risk of insertional oncogenesis or mutagenesis [94,95]. To date, there are three prominent gene editing approaches available: zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the clustered regulatory interspaced short palindromic repeats (CRISPR)/Cas9 system [96,97]. ZFNs are artificial restriction enzymes generated by fusing a zinc-finger DNA-binding domain to cleavage domain, which can be engineered to a specific sequence. ZFNs has been shown to efficiently correct the DF508 mutation in the Ctfr gene in isolated tracheal cultures from cystic fibrosis patients [98]. More importantly, the correction of the Ctfr gene could be performed on isolated pluripotent stem cells and was retained following differentiation [99]. Similar to ZFNs, TALEN-mediated gene editing uses restriction enzymes engineered to cut specific DNA sequences by the fusion a TAL effector DNA-binding domain to a cleavage domain. Application of TALENs as a potential therapeutic agent in ciliopathies has been limited, but correction of the Dnah11 gene and restoration of the ciliary beating was demonstrated in isolated respiratory cells from PCD patients [100].
In recent years, the CRISPR-Cas9 system has become the primary method for gene editing due to its ease of use and higher specificity. Derived from the prokaryotic immune system, the CRISPR-Cas9 system utilizes a Cas9 nuclease and small guide RNAs to target and alter segments of the target gene. Applications of CRISPR-Cas9-mediated genome editing for treating ciliopathies are still premature, but encouraging results from correcting the IVS26 mutation of the Cep290 gene of LCA in vitro and demonstrating the capacity of CRISPR-Cas9-mediated genome editing in the mouse retina suggest the feasibility of future treatments [101]. Utilization of gene editing within the olfactory field has been limited to use as a tool for generating mutant models. At the time of this review, CRISPR-cas9 gene editing has found success in invertebrate animal models to examine olfactory-guided behaviors and development of the olfactory systems [102–105]. Additional studies are required to determine the feasibility and efficacy of the gene editing in the mammalian olfactory system, but could serve as an alternative long-term solution for treating olfactory dysfunctions.
Ex vivo Gene Therapy
While in vivo gene therapy remains the primary methodology for addressing many genetic diseases, the approach is not without its obstacles. Principally, there are ongoing concerns with the route of administration and viral vector specificity, both of which may result in potential systemic exposure and off-target effects of the vector. Besides off-target effects, there are also concerns of transduction efficiency, as well as, host or patient immunogenic responses. Because of these concerns, ex vivo gene therapy has become an alternative and secondary approach to in vivo gene therapy. Ex vivo therapy utilizes tissue or cells isolated from a patient or donor. These isolated cells are treated with vector containing the therapeutic gene in culture, and then transplanted back into the patient or recipient. The most advancement has been made with cancer immunotherapies and the transduction of CAR-T cells. In the recent years, successful vector transduction of isolated respiratory cells from PCD and cystic fibrosis patients are encouraging [80,89,99], opening the door for future transplantation studies. In the context of olfactory ciliopathies, ex vivo gene therapy could serve as an alternative long-term solution. In addition to the potential hurdles of in vivo therapy, there is a biological limitation to the treatment. Mammalian OSNs are constantly turning over, where the lifespan of an individual OSN lasting anywhere from 60-90 days [106,107]. Therefore, there would be a decrease in efficacy of the in vivo treatment over time as OSNs turn over. A possible solution would be isolation and treatment of the OE stem cell population, and subsequent transplantation back into the host. Proof-of-principle experiments were successful in the isolation and transplantation of globose basal cells and horizontal basal cells (HBCs), the mitotically active and inactive multipotent progenitor stem cell populations of the OE, respectively [108–110]. Furthermore, these grafted cells demonstrated the capacity of generating multiple cell types, including OSNs, as well as the supporting sustentacular cells. This ex vivo strategy may also be useful for ciliopathies that might affect the olfactory stem cell populations. Recently, the HBC population were shown to have primary cilia [8]. While HBC-specific loss of ciliation did not affect the establishment and maintenance of the OE, loss of ciliation did inhibit the ability of the OE recovery after lesion. These observations suggest a possible ciliopathy phenotype outside of the OSN cilia, and susceptibility of ciliopathy patients to OE injury. Thus, ex vivo gene therapy may be a viable option for restoring proper ciliation and ciliation function within the HBCs. However, subsequent studies will need to be performed to examine the olfactory stem cell penetrance in patient relevant ciliopathy models.
Current Therapies and Future Challenges
Currently, there is no clinically approved universal treatment to directly address ciliopathies. Due to the complexity and pleiotropic nature of many ciliopathies, the majority of the existing treatments address the symptoms that would be considered most debilitating. These immediate treatments include surgery for polydactyly, weight management to address obesity, vision aids and mobility training for vision loss, special education for learning disabilities, and kidney transplants for renal dysfunctions. In regards to ciliopathy-induced olfactory dysfunctions, management of olfactory dysfunction is possible where there is residual olfactory function [18]. Under these circumstances, patients would follow a smell training regimen that would maximize use of the remaining sensory perceptions. Smell training has demonstrated improved detection threshold and olfactory sensitivity for patients with traumatic anosmia [111,112]. Similar improvements were observed in patients following post-infection induced olfactory loss [113,114]. In addition, patients undergoing the smell training have exhibited return of neuronal activity in olfactory areas within the brain suggesting a partial restoration of central function [115]. However, smell training remains a symptomatic management of olfactory dysfunction and does not directly address the cause nor prevent disease progression. This option is also not applicable to patients who are anosmic, or complete loss of olfaction. Therefore, a targeted and long-term therapeutic solution is still necessary in severe cases.
In many ways, the peripheral olfactory system is privileged as the tissue is readily accessible and amenable to direct treatment. Because of this combination of isolation and accessibility, potential small molecule and/or gene therapeutic treatments can bypass many of the complications associated with systemic delivery, tissue specificity, dosage, and invasiveness. However, the accessibility and efficiency of the treatment could be impeded by the presence of mucus within the nasal cavity. In studies of PCD, where there is excessive mucosal accumulation, lentiviral-mediated transduction in the nasal cavity was severely reduced [88]. A similar problem was encountered in cases of cystic fibrosis, where the mucus acted as barrier for AAV and adenoviral vectors [116–118]. In such cases, there is a need to physically disrupt the mucosal accumulation or develop mucosal penetrating particles to assist in small molecule or vector penetrance.
For the most part, ciliopathies follow standard Mendelian genetic inheritance. However, some studies suggest a more complicated inheritance scheme where the disease or symptoms result from accumulated mutations across different genes [119]. This idea of the polygenic causes of ciliopathies was observed in patient populations of BBS where mutations in both Bbs6 and Bbs2 genes contribute to the disease phenotypes [120]. Mutational load was observed in other BBS patients, where Bbs4 mutations were accompanied with mutations in Bbs1 and Bbs2 [121]. With the involvement of multiple genetic mutations, the standard gene therapeutic approach of restoring a single gene may not be sufficient to restore ciliation or reverse the phenotypes. While co-infection of the same cell with different viral vectors is possible [9], the efficacy of a such a treatment is unknown. As such, alternative approaches may be required to address polygenic mutations.
At this time, the most viable treatment option for olfactory ciliopathies is through gene therapies. This is largely due to the compelling evidence of adenoviral and AAV-mediated delivery of wildtype genes to restore OSN ciliation in ORPK and BBS1 knockout mice [21,22]. However, there are still questions regarding whether this treatment is translatable to other ciliopathy models and to patients. The primary concern is the immunogenicity and toxicity of the treatment, which seem well tolerated in mouse models [22]. This is partially mitigated by the limited immunogenic response observed in cystic fibrosis studies where vector-related symptoms did not manifest in low doses of aerosolized adenoviral vectors [122]. Together, these observations suggest that the nasal cavity is one of the few areas that are immune-privileged. Another potential hurdle of gene therapy is the presence of a pre-existing immunity to adenoviral and AAV vectors. Pre-existing immunities diminishes the infection of efficiency of the several vectors, resulting in an overall reduction in the efficacy of the treatment [123]. One practical solution to this problem would be to simply change to another serotype, but the occurrence of pre-existing immunity within the olfactory system has yet to be documented.
CONCLUSION
To date, the field has made significant progress understanding the structure and function of cilia in the olfactory system while work examining the penetrance of ciliopathies in this tissue continues. The potential biological treatment options are compelling, while additional studies are required to examine the direct effect of small molecules on olfactory cilia. However, this does not detract from the most intriguing and promising finding that is the ability to restore ciliation and cilia function following gene therapy. Future work is needed to optimize and streamline the delivery, improve the infection efficiency, and increase the cell specificity. With the successes observed thus far, treating ciliopathy-induced olfactory dysfunction may provide novel insights into developing improved treatments for other ciliopathy-afflicted organ systems, as well as other congenital causes of olfactory dysfunction.
Supplementary Material
Acknowledgments
We thank the additional members of the Martens lab for their valuable discussions and input. This work was supported by NIH grant R01DC009606 to J.R.M.
LIST OF ABBREVIATIONS
- OSN
Olfactory Sensory Neuron
- OE
Olfactory Epithelium
- GPCR
G-Protein Coupled Receptor
- HBC
Horizontal Basal Cell
- IFT
Intraflagellar Transport
- BBS
Bardet Beidl Syndrome
- LCA
Leber Congenital Amaurosis
- PCD
Primary Cilia Dyskinesia
- PKD
Polycystic Kidney Disease
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- ORPK
Oak Ridge Polycystic Kidney
- AAV
Adeno-associated Virus
- ZFN
Zinc-finger Nuclease
- TALEN
Transcription Activator-like Effector Nuclease
- CRISPR
Clustered Regulatory Interspaced Short Palindromic Repeats
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The Primary Cilium as a Complex Signaling Center. Curr Biol. 2009;19(13):R526–35. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz SC, Anderson KV. The Primary Cilium: A Signalling Centre during Vertebrate Development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeda S, Narita K. Structure and Function of Vertebrate Cilia, towards a New Taxonomy. Differentiation. 2012;83(2):S4–S11. doi: 10.1016/j.diff.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Ware SM, Aygun MG-, Hildebrandt F. Spectrum of Clinical Diseases Caused By Disorders of Primary Cilia. Proc Am Thorac Soc. 2011;8(5):444–450. doi: 10.1513/pats.201103-025SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiter JF, Leroux MR. Genes and Molecular Pathways Underpinning Ciliopathies. Nat Rev Mol Cell Biol. 2017;18(9):533–547. doi: 10.1038/nrm.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menco BP, Farbman AI. Genesis of Cilia and Microvilli of Rat Nasal Epithelia during Pre-Natal Development. I. Olfactory Epithelium, Qualitative Studies. J Cell Sci. 1985;78:283–310. doi: 10.1242/jcs.78.1.283. [DOI] [PubMed] [Google Scholar]
- 7.Menco BP, Farbman AI. Genesis of Cilia and Microvilli of Rat Nasal Epithelia during Pre-Natal Development. II. Olfactory Epithelium, A Morphometric Analysis. J Cell Sci. 1985;78:311–336. doi: 10.1242/jcs.78.1.311. [DOI] [PubMed] [Google Scholar]
- 8.Joiner aM, Green WW, McIntyre JC, Allen BL, Schwob JE, Martens JR. Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium. J Neurosci. 2015;35(40):13761–13772. doi: 10.1523/JNEUROSCI.1708-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams CL, McIntyre JC, Norris SR, Jenkins PM, Zhang L, Pei Q, Verhey K, Martens JR. Direct Evidence for BBSome-Associated Intraflagellar Transport Reveals Distinct Properties of Native Mammalian Cilia. Nat Commun. 2014;5:5813. doi: 10.1038/ncomms6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulloa-Aguirre A, Conn PM. Pharmacoperones as a New Therapeutic Approach: In Vitro Identification and In Vivo Validation of Bioactive Molecules. Curr Drug Targets. 2016;17(13):1471–1481. doi: 10.2174/1389450117666160307143345. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowska A, Dev KK, Sailer AW. The Role of the Oxysterol/EBI2 Pathway in the Immune and Central Nervous Systems. Curr Drug Targets. 2016;17(16):1851–1860. doi: 10.2174/1389450117666160217123042. [DOI] [PubMed] [Google Scholar]
- 12.Liapakis G, Matsoukas M-T, Karageorgos V, Venihaki M, Mavromoustakos T. Family B G Protein-Coupled Receptors and Their Ligands: From Structure to Function. Curr Med Chem. 2017;24(31):3323–3355. doi: 10.2174/0929867324666170303162416. [DOI] [PubMed] [Google Scholar]
- 13.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat Rev Drug Discov. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temmel AFP, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T. Characteristics of Olfactory Disorders in Relation to Major Causes of Olfactory Loss. Arch Otolaryngol Head Neck Surg. 2002;128(6):635–641. doi: 10.1001/archotol.128.6.635. [DOI] [PubMed] [Google Scholar]
- 15.Gopinath B, Anstey KJ, Sue CM, Kifley A, Mitchell P. Olfactory Impairment in Older Adults Is Associated with Depressive Symptoms and Poorer Quality of Life Scores. Am J Geriatr Psychiatry. 2011;19(9):830–834. doi: 10.1097/JGP.0b013e318211c205. [DOI] [PubMed] [Google Scholar]
- 16.Philpott CM, Boak D. The Impact of Olfactory Disorders in the United Kingdom. Chem Senses. 2014;39(8):711–718. doi: 10.1093/chemse/bju043. [DOI] [PubMed] [Google Scholar]
- 17.Holbrook EH, Leopold Da. An Updated Review of Clinical Olfaction. Curr Opin Otolaryngol Head Neck Surg. 2006;14(1):23–28. doi: 10.1097/01.moo.0000193174.77321.39. [DOI] [PubMed] [Google Scholar]
- 18.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS Proteins Causes Anosmia in Humans and Defects in Olfactory Cilia Structure and Function in the Mouse. Nat Genet. 2004;36(9):994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 19.McEwen DP, Koenekoop RK, Khanna H, Jenkins PM, Lopez I, Swaroop A, Martens JR. Hypomorphic CEP290 / NPHP6 Mutations Result in Anosmia Caused by the Selective Loss of G Proteins in Cilia of Olfactory Sensory Neurons. 2007;104(40):15917–15922. doi: 10.1073/pnas.0704140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadenev ALD, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. Loss of Bardet-Biedl Syndrome Protein-8 (BBS8) Perturbs Olfactory Function, Protein Localization, and Axon Targeting. Proc Natl Acad Sci U S A. 2011;108(25):10320–10325. doi: 10.1073/pnas.1016531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mcintyre JC, Davis EE, Joiner A, Williams CL, Tsai I, Jenkins PM, Mcewen DP, Zhang L, Escobado J, Thomas S, Szymanska K, Johnson CA, Beales PL, Green ED, Mullikin JC, Comparative N, Program S, Sabo A, Muzny DM, Gibbs RA, Attié-bitach T, Yoder BK, Reed RR, Katsanis N, Martens JR. Gene Therapy Rescues Cilia Defects and Restores Olfactory Function in a Mammalian Ciliopathy Model. 2012;18(9) doi: 10.1038/nm.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams CL, Uytingco CR, Green WW, McIntyre JC, Ukhanov K, Zimmerman AD, Shively DT, Zhang L, Nishimura DY, Sheffield VC, Martens JR. Gene Therapeutic Reversal of Peripheral Olfactory Impairment in Bardet-Biedl Syndrome. Mol Ther. 2017;25(4):904–916. doi: 10.1016/j.ymthe.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired Photoreceptor Protein Transport and Synaptic Transmission in a Mouse Model of Bardet-Biedl Syndrome. Vision Res. 2007;47(27):3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-Null Mice Have a Phenotype Resembling Bardet - Biedl Syndrome. Hum Mol Genet. 2005;14(9):1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC. Bbs2-Null Mice Have Neurosensory Deficits, a Defect in Social Dominance, and Retinopathy Associated with Mislocalization of Rhodopsin. Proc Natl Acad Sci U S A. 2004;101(47):16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl Syndrome Type 4 (BBS4)-Null Mice Implicate Bbs4 in Flagella Formation but Not Global Cilia Assembly. Proc Natl Acad Sci U S A. 2004;101(23):8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A Knockin Mouse Model of the Bardet Biedl Syndrome 1 M390R Mutation Has Cilia Defects, Ventriculomegaly, Retinopathy, and Obesity. Proc Natl Acad Sci. 2007;104(49):19422–19427. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pretorius PR, Baye LM, Nishimura DY, Searby CC, Bugge K, Yang B, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform. PLoS Genet. 2010;6(3) doi: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mockel A, Obringer C, Hakvoort TBM, Seeliger M, Lamers WH, Stoetzel C, Dollfus H, Marion V. Pharmacological Modulation of the Retinal Unfolded Protein Response in Bardet-Biedl Syndrome Reduces Apoptosis and Preserves Light Detection Ability. J Biol Chem. 2012;287(44):37483–37494. doi: 10.1074/jbc.M112.386821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR Pathway Is Regulated by Polycystin-1, and Its Inhibition Reverses Renal Cystogenesis in Polycystic Kidney Disease. Proc Natl Acad Sci. 2006;103(14):5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with Sirolimus Slows Disease Progression in Han:SPRD Rats with Autosomal Dominant Polycystic Kidney Disease (ADPKD) Nephrol Dial Transplant. 2006;21(3):598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 32.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide Inhibits Hepatic Cystogenesis in a Rodent Model of Polycystic Liver Disease by Reducing Cholangiocyte Adenosine 3′,5′-Cyclic Monophosphate. Gastroenterology. 2007;132(3):1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Ibraghimov-Beskrovnaya O. Molecular Pathogenesis of ADPKD and Development of Targeted Therapeutic Options. Nephrol Dial Transplant. 2007;22(12):3367–3370. doi: 10.1093/ndt/gfm426. [DOI] [PubMed] [Google Scholar]
- 34.Chang M-Y, Ong ACM. Mechanism-Based Therapeutics for Autosomal Dominant Polycystic Kidney Disease: Recent Progress and Future Prospects. Nephron Clin Pract. 2012;120(1):c25–c35. doi: 10.1159/000334166. [DOI] [PubMed] [Google Scholar]
- 35.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2012;367(25):2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skalicka K, Kovacs L. Ciliotherapy–New Opportunity for Targeted Therapy in Autosomal Dominant Polycystic Kidney Disease. J Genet Syndr Gene Ther. 2016;7(5):5–8. [Google Scholar]
- 37.Sharma N, Malarkey EB, Berbari NF, O’Connor AK, Vanden Heuvel GB, Mrug M, Yoder BK. Proximal Tubule Proliferation Is Insufficient to Induce Rapid Cyst Formation after Cilia Disruption. J Am Soc Nephrol. 2013;24(3):456–464. doi: 10.1681/ASN.2012020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SH, Somlo S. Cyst Growth, Polycystins, and Primary Cilia in Autosomal Dominant Polycystic Kidney Disease. Kidney Res Clin Pract. 2014;33(2):73–78. doi: 10.1016/j.krcp.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal Cilia Display Length Alterations Following Tubular Injury and Are Present Early in Epithelial Repair. Nephrol Dial Transplant. 2008;23(3):834–841. doi: 10.1093/ndt/gfm743. [DOI] [PubMed] [Google Scholar]
- 40.Ong ACM. Primary Cilia and Renal Cysts: Does Length Matter? Nephrol Dial Transplant. 2013;28(11):2661–2663. doi: 10.1093/ndt/gft354. [DOI] [PubMed] [Google Scholar]
- 41.Saito S, Tampe B, Müller GA, Zeisberg M. Primary Cilia Modulate Balance of Canonical and Non-Canonical Wnt Signaling Responses in the Injured Kidney. Fibrogenesis Tissue Repair. 2015;8(1):6. doi: 10.1186/s13069-015-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu F, Ran J, Zhou J. Ciliopathies: Does HDAC6 Represent a New Therapeutic Target? Trends Pharmacol Sci. 2016;37(2):114–119. doi: 10.1016/j.tips.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, Golemis EA. Calmodulin Activation of Aurora-A Kinase (AURKA) Is Required during Ciliary Disassembly and in Mitosis. Mol Biol Cell. 2012;23(14):2658–2670. doi: 10.1091/mbc.E11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mergen M, Engel C, Müller B, Follo M, Schäfer T, Jung M, Walz G. The Nephronophthisis Gene Product NPHP2/Inversin Interacts with Aurora A and Interferes with HDAC6-Mediated Cilia Disassembly. Nephrol Dial Transplant. 2013;28(11):2744–2753. doi: 10.1093/ndt/gft316. [DOI] [PubMed] [Google Scholar]
- 45.Dere R, Perkins AL, Bawa-Khalfe T, Jonasch D, Walker CL. Beta-Catenin Links von Hippel-Lindau to Aurora Kinase A and Loss of Primary Cilia in Renal Cell Carcinoma. J Am Soc Nephrol. 2015;26(3):553–564. doi: 10.1681/ASN.2013090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ran J, Yang Y, Li D, Liu M, Zhou J. Deacetylation of α-Tubulin and Cortactin Is Required for HDAC6 to Trigger Ciliary Disassembly. Sci Rep. 2015;5:12917. doi: 10.1038/srep12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gradilone SA, Radtke BN, Bogert PS, Huang BQ, Gajdos GB, LaRusso NF. HDAC6 Inhibition Restores Ciliary Expression and Decreases Tumor Growth. Cancer Res. 2013;73(7):2259 LP–2270. doi: 10.1158/0008-5472.CAN-12-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kai K, Satoh H, Kajimura T, Kato M, Uchida K, Yamaguchi R, Tateyama S, Furuhama K. Olfactory Epithelial Lesions Induced by Various Cancer Chemotherapeutic Agents in Mice. Toxicol Pathol. 2004;32(6):701–709. doi: 10.1080/01926230490524283. [DOI] [PubMed] [Google Scholar]
- 49.Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome Subunit Links Ciliogenesis, Microtubule Stability, and Acetylation. Dev Cell. 2008;15(6):854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Nakakura T, Asano-Hoshino A, Suzuki T, Arisawa K, Tanaka H, Sekino Y, Kiuchi Y, Kawai K, Hagiwara H. The Elongation of Primary Cilia via the Acetylation of Alpha Tubulin by the Treatment with Lithium Chloride in Human Fibroblast KD Cells. Med Mol Morphol. 2015;48(1):44–53. doi: 10.1007/s00795-014-0076-x. [DOI] [PubMed] [Google Scholar]
- 51.Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Lithium Treatment Elongates Primary Cilia in the Mouse Brain and in Cultured Cells. Biochem Biophys Res Commun. 2009;388(4):757–762. doi: 10.1016/j.bbrc.2009.08.099. [DOI] [PubMed] [Google Scholar]
- 52.Thompson CL, Wiles A, Poole CA, Knight MM. Lithium Chloride Modulates Chondrocyte Primary Cilia and Inhibits Hedgehog Signaling. FASEB J. 2016;30(2):716–726. doi: 10.1096/fj.15-274944. [DOI] [PubMed] [Google Scholar]
- 53.Ramezani M, Ebrahimian M, Hashemi M. Current Strategies in the Modification of PLGA-Based Gene Delivery System. Curr Med Chem. 2017;24(7):728–739. doi: 10.2174/0929867324666161205130416. [DOI] [PubMed] [Google Scholar]
- 54.Wang K, Huang Q, Qiu F, Sui M. Non-Viral Delivery Systems for the Application in p53 Cancer Gene Therapy. Curr Med Chem. 2015;22(35):4118–4136. doi: 10.2174/0929867322666151001121601. [DOI] [PubMed] [Google Scholar]
- 55.Bhosale RR, Gangadharappa HV, Hani U, Ali M Osmani R, Vaghela R, Kulkarni PK, Koganti VS. Current Perspectives on Novel Drug Delivery Systems and Therapies for Management of Prostate Cancer: An Inclusive Review. Curr Drug Targets. 2017;18(11):1233–1249. doi: 10.2174/1389450117666160613103705. [DOI] [PubMed] [Google Scholar]
- 56.Muruve Da. The Innate Immune Response to Adenovirus Vectors. Hum Gene Ther. 2004;15(12):1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 57.Huang D, Pereboev aV, Korokhov N, He R, Larocque L, Gravel C, Jaentschke B, Tocchi M, Casley WL, Lemieux M, Curiel DT, Chen W, Li X. Significant Alterations of Biodistribution and Immune Responses in Balb/c Mice Administered with Adenovirus Targeted to CD40(+) Cells. Gene Ther. 2008;15(4):298–308. doi: 10.1038/sj.gt.3303085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 Activation by Inositol 1,4,5-Trisphosphate-Induced Ca 2+ Release Requires Its Direct Association with the Inositol 1,4,5-Trisphosphate Receptor in a Signaling Microdomain. J Biol Chem. 2010;285(24):18794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei F, Karihaloo a, Yu Z, Marlier a, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, Cantley LG. Neutrophil Gelatinase-Associated Lipocalin Suppresses Cyst Growth by Pkd1 Null Cells in Vitro and in Vivo. Kidney Int. 2008;74(10):1310–1318. doi: 10.1038/ki.2008.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park EY, Kim BH, Lee EJ, Chang E, Kim DW, Choi SY, Park JH. Targeting of Receptor for Advanced Glycation End Products Suppresses Cyst Growth in Polycystic Kidney Disease. J Biol Chem. 2014;289(13):9254–9262. doi: 10.1074/jbc.M113.514166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H, Otaki JM, Firestein S. Adenovirus-Mediated Gene Transfer in Olfactory Neurons in Vivo. J Neurobiol. 1996;30(4):521–530. doi: 10.1002/(SICI)1097-4695(199608)30:4<521::AID-NEU7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 62.Holtmaat AJGD, Hermens WTJMC, Beate Oestreicher A, Hendrik Gispen W, Kaplitt MG, Verhaagen J. Efficient Adenoviral Vector-Directed Expression of a Foreign Gene to Neurons and Sustentacular Cells in the Mouse Olfactory Neuroepithelium. Mol Brain Res. 1996;41(1-2):148–156. doi: 10.1016/0169-328x(96)00085-x. [DOI] [PubMed] [Google Scholar]
- 63.Touhara K, Sengoku S, Inaki K, Tsuboi a, Hirono J, Sato T, Sakano H, Haga T. Functional Identification and Reconstitution of an Odorant Receptor in Single Olfactory Neurons. Proc Natl Acad Sci U S A. 1999;96(7):4040–4045. doi: 10.1073/pnas.96.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivic L, Pyrski MM, Margolis JW, Richards LJ, Firestein S, Margolis FL. Adenoviral Vector-Mediated Rescue of the OMP-Null Phenotype in Vivo. Nat Neurosci. 2000;3(11):1113–1120. doi: 10.1038/80632. [DOI] [PubMed] [Google Scholar]
- 65.Arimoto Y, Nagata H, Isegawa N, Kumahara K, Isoyama K, Konno A, Shirasawa H. In Vivo Expression of Adenovirus-Mediated lacZ Gene in Murine Nasal Mucosa. Acta Otolaryngol. 2002;122(6):627–633. doi: 10.1080/000164802320396303. [DOI] [PubMed] [Google Scholar]
- 66.Ivic L, Zhang C, Zhang X, Yoon SO, Firestein S. Intracellular Trafficking of a Tagged and Functional Mammalian Olfactory Receptor. J Neurobiol. 2002;50(1):56–68. doi: 10.1002/neu.10016. [DOI] [PubMed] [Google Scholar]
- 67.Youngentob SL, Pyrski MM, Margolis FL. Adenoviral Vector-Mediated Rescue of the OMP-Null Behavioral Phenotype: Enhancement of Odorant Threshold Sensitivity. Behav Neurosci. 2004;118:636–642. doi: 10.1037/0735-7044.118.3.636. [DOI] [PubMed] [Google Scholar]
- 68.Venkatraman G, Behrens M, Pyrski M, Margolis FL. Expression of Coxsackie-Adenovirus Receptor (CAR) in the Developing Mouse Olfactory System. J Neurocytol. 2005;34(3-5):295–305. doi: 10.1007/s11068-005-8359-8. [DOI] [PubMed] [Google Scholar]
- 69.Gau P, Rodriguez S, De Leonardis C, Chen P, Lin DM. Air-Assisted Intranasal Instillation Enhances Adenoviral Delivery to the Olfactory Epithelium and Respiratory Tract. Gene Ther. 2011;18(5):432–436. doi: 10.1038/gt.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK, Eckhaus M, Nabel GJ. Enhanced Mucosal Immunoglobulin A Response of Intranasal Adenoviral Vector Human Immunodeficiency Virus Vaccine and Localization in the Central Nervous System. J Virol. 2003;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damjanovic D, Zhang X, Mu J, Medina MF, Xing Z. Organ Distribution of Transgene Expression Following Intranasal Mucosal Delivery of Recombinant Replication-Defective Adenovirus Gene Transfer Vector. Genet Vaccines Ther. 2008;6:5. doi: 10.1186/1479-0556-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doi K, Nibu K, Ishida H, Okado H, Terashima T. Adenovirus-Mediated Gene Transfer in Olfactory Epithelium and Olfactory Bulb: A Long-Term Study. Ann Otol Rhinol Laryngol. 2005;114(8):629–633. doi: 10.1177/000348940511400808. [DOI] [PubMed] [Google Scholar]
- 73.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV Serotypes 1–9 Mediated Gene Expression and Tropism in Mice After Systemic Injection. Mol Ther. 2008;16(6):1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 74.Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, Hunter LA. Targeted Integration of Adeno-Associated Virus (AAV) into Human Chromosome 19. EMBO J. 1991;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Narfström K, Katz ML, Ford M, Redmond TM, Rakoczy E, Bragadóttir R. In Vivo Gene Therapy in Young and Adult RPE65−/− Dogs Produces Long-Term Visual Improvement. Journal of Heredity. 2003;94:31–37. doi: 10.1093/jhered/esg015. [DOI] [PubMed] [Google Scholar]
- 76.Narfström K, Katz ML, Bragadottir R, Seeliger M, Boulanger A, Redmond TM, Caro L, Lai CM, Rakoczy PE. Functional and Structural Recovery of the Retina after Gene Therapy in the RPE65 Null Mutation Dog. Investig Ophthalmol Vis Sci. 2003;44(4):1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- 77.Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, Mendes-Madeira A, Provost N, Péréon Y, Cherel Y, Ali RR, Hamel C, Moullier P, Rolling F. Restoration of Vision in RPE65-Deficient Briard Dogs Using an AAV Serotype 4 Vector That Specifically Targets the Retinal Pigmented Epithelium. Gene Ther. 2007;14(4):292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- 78.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-Term Restoration of Rod and Cone Vision by Single Dose rAAV-Mediated Gene Transfer to the Retina in a Canine Model of Childhood Blindness. Mol Ther. 2005;12(6):1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bainbridge JWB, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of Gene Therapy on Visual Function in Leber’s Congenital Amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 80.Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komaromy AM, Hauswirth WW, Aguirre GD. Human Retinal Gene Therapy for Leber Congenital Amaurosis Shows Advancing Retinal Degeneration despite Enduring Visual Improvement. Proc Natl Acad Sci. 2013;110(6):E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bainbridge JWB, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJL, Casteels I, Holder GE, Tyler N, Fitzke FW, Weleber RG, Nardini M, Moore AT, Thompson DA, Petersen-Jones SM, Michaelides M, van den Born LI, Stockman A, Smith AJ, Rubin G, Ali RR. Long-Term Effect of Gene Therapy on Leber’s Congenital Amaurosis. N Engl J Med. 2015;372(20):1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simons DL, Boye SL, Hauswirth WW, Wu SM. Gene Therapy Prevents Photoreceptor Death and Preserves Retinal Function in a Bardet-Biedl Syndrome Mouse Model. Proc Natl Acad Sci U S A. 2011;108(15):6276–6281. doi: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seo S, Mullins RF, Dumitrescu AV, Bhattarai S, Gratie D, Wang K, Stone EM, Sheffield V, Drack AV. Subretinal Gene Therapy of Mice with Bardet-Biedl Syndrome Type 1. Invest Ophthalmol Vis Sci. 2013;54(9):6118–6132. doi: 10.1167/iovs.13-11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Limberis MP, Wilson JM. Adeno-Associated Virus Serotype 9 Vectors Transduce Murine Alveolar and Nasal Epithelia and Can Be Readministered. Proc Natl Acad Sci U S A. 2006;103(35):12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction Efficiencies of Novel AAV Vectors in Mouse Airway Epithelium in Vivo and Human Ciliated Airway Epithelium in Vitro. Mol Ther. 2009;17(2):294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao L, Schrank BR, Rodriguez S, Benz EG, Moulia TW, Rickenbacher GT, Gomez AC, Levites Y, Edwards SR, Golde TE, Hyman BT, Barnea G, Albers MW. Aβ Alters the Connectivity of Olfactory Neurons in the Absence of Amyloid Plaques in Vivo. Nat Commun. 2012;3:1009. doi: 10.1038/ncomms2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnight ER, Wiley LA, Drack AV, Braun TA, Anfinson KR, Kaalberg EE, Halder JA, Affatigato LM, Mullins RF, Stone EM, Tucker BA. CEP290 Gene Transfer Rescues Leber Congenital Amaurosis Cellular Phenotype. Gene Ther. 2014;21(7):662–672. doi: 10.1038/gt.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ostrowski LE, Yin W, Patel M, Sechelski J, Rogers T, Burns K, Grubb BR, Olsen JC. Restoring Ciliary Function to Differentiated Primary Ciliary Dyskinesia Cells with a Lentiviral Vector. Gene Ther. 2014;21(3):253–261. doi: 10.1038/gt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chhin B, Negre D, Merrot O, Pham J, Tourneur Y, Ressnikoff D, Jaspers M, Jorissen M, Cosset FL, Bouvagnet P. Ciliary Beating Recovery in Deficient Human Airway Epithelial Cells after Lentivirus Ex Vivo Gene Therapy. PLoS Genet. 2009;5(3) doi: 10.1371/journal.pgen.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sinn PL, Burnight ER, Hickey MA, Blissard GW, McCray PB. Persistent Gene Expression in Mouse Nasal Epithelia Following Feline Immunodeficiency Virus-Based Vector Gene Transfer. J Virol. 2005;79(20):12818–12827. doi: 10.1128/JVI.79.20.12818-12827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen H, Dadsetan S, Fomina AF, Gong Q. Expressing Exogenous Functional Odorant Receptors in Cultured Olfactory Sensory Neurons. Neural Dev. 2008;3(1):22. doi: 10.1186/1749-8104-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sadrian B, Chen H, Gong Q. Lentivirus-Mediated Genetic Manipulation and Visualization of Olfactory Sensory Neurons in Vivo. J Vis Exp. 2011;(51):5–8. doi: 10.3791/2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sinn PL, Arias AC, Brogden Ka, McCray PB. Lentivirus Vector Can Be Readministered to Nasal Epithelia without Blocking Immune Responses. J Virol. 2008;82(21):10684–10692. doi: 10.1128/JVI.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ranzani M, Cesana D, Bartholomae CC, Sanvito F, Pala M, Benedicenti F, Gallina P, Sergi LS, Merella S, Bulfone A, Doglioni C, von Kalle C, Kim YJ, Schmidt M, Tonon G, Naldini L, Montini E. Lentiviral Vector–based Insertional Mutagenesis Identifies Genes Associated with Liver Cancer. Nat Methods. 2013;10(2):155–161. doi: 10.1038/nmeth.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papayannakos C, Daniel R. Understanding Lentiviral Vector Chromatin Targeting: Working to Reduce Insertional Mutagenic Potential for Gene Therapy. Gene Ther. 2013;20(6):581–588. doi: 10.1038/gt.2012.88. [DOI] [PubMed] [Google Scholar]
- 96.Chakraborty C, Teoh SL, Das S. The Smart Programmable CRISPR Technology: A Next Generation Genome Editing Tool for Investigators. Curr Drug Targets. 2017;18(14):1653–1663. doi: 10.2174/1389450117666160527142321. [DOI] [PubMed] [Google Scholar]
- 97.LaFountaine JS, Fathe K, Smyth HDC. Delivery and Therapeutic Applications of Gene Editing Technologies ZFNs, TALENs, and CRISPR/Cas9. Int J Pharm. 2015;494(1):180–194. doi: 10.1016/j.ijpharm.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 98.Lee CM, Flynn R, Hollywood JA, Scallan MF, Harrison PT. Correction of the ΔF508 Mutation in the Cystic Fibrosis Transmembrane Conductance Regulator Gene by Zinc-Finger Nuclease Homology-Directed Repair. Biores Open Access. 2012;1(3):99–108. doi: 10.1089/biores.2012.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, Wang J, Sun HC, Paschon DE, Guschin DY, Gregory PD, Kotton DN, Holmes MC, Sorscher EJ, Davis BR. Targeted Correction and Restored Function of the CFTR Gene in Cystic Fibrosis Induced Pluripotent Stem Cells. Stem Cell Reports. 2015;4(4):569–577. doi: 10.1016/j.stemcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai M, Pifferi M, Bush A, Piras M, Michelucci A, Di Cicco M, Del Grosso A, Quaranta P, Cursi C, Tantillo E, Franceschi S, Mazzanti MC, Simi P, Saggese G, Boner A, Pistello M. Gene Editing of DNAH11 Restores Normal Cilia Motility in Primary Ciliary Dyskinesia. J Med Genet. 2016;53(4):242–249. doi: 10.1136/jmedgenet-2015-103539. [DOI] [PubMed] [Google Scholar]
- 101.Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, Scaria A. CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol Ther. 2017;25(2):331–341. doi: 10.1016/j.ymthe.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, Zhang J, Chen D, Yang P, Jiang F, Wang X, Kang L. CRISPR/Cas9 in Locusts: Successful Establishment of an Olfactory Deficiency Line by Targeting the Mutagenesis of an Odorant Receptor Co-Receptor (Orco) Insect Biochem Mol Biol. 2016;79:27–35. doi: 10.1016/j.ibmb.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 103.Koutroumpa FA, Monsempes C, François M-C, de Cian A, Royer C, Concordet J-P, Jacquin-Joly E. Heritable Genome Editing with CRISPR/Cas9 Induces Anosmia in a Crop Pest Moth. Sci Rep. 2016;6(1):29620. doi: 10.1038/srep29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trible W, Olivos-Cisneros L, McKenzie SK, Saragosti J, Chang N-C, Matthews BJ, Oxley PR, Kronauer DJC. Orco Mutagenesis Causes Loss of Antennal Lobe Glomeruli and Impaired Social Behavior in Ants. Cell. 2017;170(4):727–735.e10. doi: 10.1016/j.cell.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yan H, Opachaloemphan C, Mancini G, Yang H, Gallitto M, Mlejnek J, Leibholz A, Haight K, Ghaninia M, Huo L, Perry M, Slone J, Zhou X, Traficante M, Penick CA, Dolezal K, Gokhale K, Stevens K, Fetter-Pruneda I, Bonasio R, Zwiebel LJ, Berger SL, Liebig J, Reinberg D, Desplan C. An Engineered Orco Mutation Produces Aberrant Social Behavior and Defective Neural Development in Ants. Cell. 2017;170(4):736–747.e9. doi: 10.1016/j.cell.2017.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farbman AI. Olfactory Neurogenesis: Genetic or Environmental Controls? Trends Neurosci. 1990;13(9):362–365. doi: 10.1016/0166-2236(90)90017-5. [DOI] [PubMed] [Google Scholar]
- 107.Mackay-Sim A, Kittel PW. On the Life Span of Olfactory Receptor Neurons. Eur J Neurosci. 1991;3(3):209–215. doi: 10.1111/j.1460-9568.1991.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 108.Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of Multipotent Progenitors from the Adult Olfactory Epithelium. Neuroreport. 1998;9(7):1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- 109.Chen X, Fang H, Schwob JE. Multipotency of Purified, Transplanted Globose Basal Cells in Olfactory Epithelium. J Comp Neurol. 2004;469(4):457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- 110.Schnittke N, Herrick DB, Lin B, Peterson J, Coleman JH, Packard AI, Jang W, Schwob JE. Transcription Factor p63 Controls the Reserve Status but Not the Stemness of Horizontal Basal Cells in the Olfactory Epithelium. Proc Natl Acad Sci U S A. 2015;112(36):E5068–77. doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink K-B. Effects of Olfactory Training in Patients with Olfactory Loss. Laryngoscope. 2009;119(3):496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
- 112.Jiang RS, Twu CW, Liang KL. The Effect of Olfactory Training on the Odor Threshold in Patients with Traumatic Anosmia. Am J Rhinol Allergy. 2017;31(5):317–322. doi: 10.2500/ajra.2017.31.4466. [DOI] [PubMed] [Google Scholar]
- 113.Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, Hummel T, Hüttenbrink KB. Olfactory Training Is Helpful in Postinfectious Olfactory Loss: A Randomized, Controlled, Multicenter Study. Laryngoscope. 2014;124(4):826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 114.Konstantinidis I, Tsakiropoulou E, Constantinidis J. Long Term Effects of Olfactory Training in Patients with Post-Infectious Olfactory Loss. Rhinology. 2016;54(2):170–175. doi: 10.4193/Rhino15.264. [DOI] [PubMed] [Google Scholar]
- 115.Kollndorfer K, Kowalczyk K, Hoche E, Mueller CA, Pollak M, Trattnig S, Schöpf V. Recovery of Olfactory Function Induces Neuroplasticity Effects in Patients with Smell Loss. Neural Plast. 2014;2014:1–7. doi: 10.1155/2014/140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hida K, Lai SK, Suk JS, Won SY, Boyle MP, Hanes J. Common Gene Therapy Viral Vectors Do Not Efficiently Penetrate Sputum from Cystic Fibrosis Patients. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitson C, Angel B, Judd D, Rothery S, Severs NJ, Dewar a, Huang L, Wadsworth SC, Cheng SH, Geddes DM, Alton EW. The Extra- and Intracellular Barriers to Lipid and Adenovirus-Mediated Pulmonary Gene Transfer in Native Sheep Airway Epithelium. Gene Ther. 1999;6(4):534–546. doi: 10.1038/sj.gt.3300840. [DOI] [PubMed] [Google Scholar]
- 118.Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu PC, Leigh MW, Engelhardt JF, Edwards LJ, Jones KR. A Controlled Study of Adenoviral-Vector-Mediated Gene Transfer in the Nasal Epithelium of Patients with Cystic Fibrosis. N Engl J Med. 1995;333(13):823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 119.Novarino G, Akizu N, Gleeson JG. Modeling Human Disease in Humans: The Ciliopathies. Cell. 2011:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Katsanis N. Triallelic Inheritance in Bardet-Biedl Syndrome, a Mendelian Recessive Disorder. Science (80-) 2001;293(5538):2256–2259. doi: 10.1126/science.1063525. [DOI] [PubMed] [Google Scholar]
- 121.Katsanis N, Eichers ER, Ansley SJ, Lewis RA, Kayserili H, Hoskins BE, Scambler PJ, Beales PL, Lupski JR. BBS4 Is a Minor Contributor to Bardet-Biedl Syndrome and May Also Participate in Triallelic Inheritance. Am J Hum Genet. 2002;71(1):22–29. doi: 10.1086/341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joseph PM, O’Sullivan BP, Lapey A, Dorkin H, Oren J, Balfour R, Perricone MA, Rosenberg M, Wadsworth SC, Smith AE, St Georg JA, Meeker DP. Aerosol and Lobar Administration of a Recombinant Adenovirus to Individuals with Cystic Fibrosis. I. Methods, Safety, and Clinical Implications. Hum Gene Ther. 2001;12(11):1369–1382. doi: 10.1089/104303401750298535. [DOI] [PubMed] [Google Scholar]
- 123.Mingozzi F, High KA. Immune Responses to AAV Vectors: Overcoming Barriers to Successful Gene Therapy. Blood. 2013:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Braun JJ, Noblet V, Durand M, Scheidecker S, Zinetti-Bertschy A, Foucher J, Marion V, Muller J, Riehm S, Dollfus H, Kremer S. Olfaction Evaluation and Correlation with Brain Atrophy in Bardet-Biedl Syndrome. Clin Genet. 2014;86(6):521–529. doi: 10.1111/cge.12391. [DOI] [PubMed] [Google Scholar]
- 125.Iannaccone A, Mykytyn K, Persico AM, Searby CC, Baldi A, Jablonski MM, Sheffield VC. Clinical Evidence of Decreased Olfaction in Bardet-Biedl Syndrome Caused by a Deletion in the BBS4 Gene. Am J Med Genet. 2005;132 A(4):343–346. doi: 10.1002/ajmg.a.30512. [DOI] [PubMed] [Google Scholar]
- 126.Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a Gene with Many Faces: Mutation Overview and Presentation of CEP290base. Hum Mutat. 2010;31(10):1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 127.Ahdab-Barmada M, Claassen D. A Distinctive Triad of Malformations of the Central Nervous System in the Meckel-Gruber Syndrome. J Neuropathol Exp Neurol. 1990;49(6):610–620. doi: 10.1097/00005072-199011000-00007. [DOI] [PubMed] [Google Scholar]
- 128.Pluznick JL, Rodriguez-Gil DJ, Hull M, Mistry K, Gattone V, Johnson CA, Weatherbee S, Greer CA, Caplan MJ. Renal Cystic Disease Proteins Play Critical Roles in the Organization of the Olfactory Epithelium. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-Frame Deletion in a Novel Centrosomal/ciliary Protein CEP290/NPHP6 Perturbs Its Interaction with RPGR and Results in Early-Onset Retinal Degeneration in the rd16 Mouse. Hum Mol Genet. 2006;15(11):1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caspary T, Larkins CE, Anderson KV. The Graded Response to Sonic Hedgehog Depends on Cilia Architecture. Dev Cell. 2007;12(5):767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 131.Sun Z. A Genetic Screen in Zebrafish Identifies Cilia Genes as a Principal Cause of Cystic Kidney. Development. 2004;131(16):4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 132.Gibberd FB, Feher MD, Sidey MC, Wierzbicki aS. Smell Testing: An Additional Tool for Identification of Adult Refsum’s Disease. J Neurol Neurosurg Psychiatry. 2004;75:1334–1336. doi: 10.1136/jnnp.2003.026690. March 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wierzbicki AS, Lloyd MD, Schofield CJ, Feher MD, Gibberd FB. Refsum’s Disease: A Peroxisomal Disorder Affecting Phytanic Acid α-Oxidation. Journal of Neurochemistry. 2002:727–735. doi: 10.1046/j.0022-3042.2002.00766.x. [DOI] [PubMed] [Google Scholar]
- 134.Abdel-Hak B, Gunkel A, Kanonier G, Schrott-Fischer A, Ulmer H, Thumfart W. Ciliary Beat Frequency, Olfaction and Endoscopic Sinus Surgery. ORL J Otorhinolaryngol Relat Spec. 1998;60(4):202–205. doi: 10.1159/000027594. [DOI] [PubMed] [Google Scholar]
- 135.Papon JF, Perrault I, Coste a, Louis B, Gérard X, Hanein S, Fares-Taie L, Gerber S, Defoort-Dhellemmes S, Vojtek aM, Kaplan J, Rozet JM, Escudier E. Abnormal Respiratory Cilia in Non-Syndromic Leber Congenital Amaurosis with CEP290 Mutations. J Med Genet. 2010;47(12):829–834. doi: 10.1136/jmg.2010.077883. [DOI] [PubMed] [Google Scholar]
- 136.Lehman AM, Eydoux P, Doherty D, Glass IA, Chitayat D, Chung BYH, Langlois S, Yong SL, Lowry RB, Hildebrandt F, Trnka P. Co-Occurrence of Joubert Syndrome and Jeune Asphyxiating Thoracic Dystrophy. Am J Med Genet Part A. 2010;152(6):1411–1419. doi: 10.1002/ajmg.a.33416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maione L, Cantone E, Nettore IC, Cerbone G, De Brasi D, Maione N, Young J, Di Somma C, Sinisi AA, Iengo M, Macchia PE, Pivonello R, Colao A. Flavor Perception Test: Evaluation in Patients with Kallmann Syndrome. Endocrine. 2016;52(2):236–243. doi: 10.1007/s12020-015-0690-y. [DOI] [PubMed] [Google Scholar]
- 138.Koenigkam-Santos M, Santos AC, Versiani BR, Diniz PRB, Junior JE, De Castro M. Quantitative Magnetic Resonance Imaging Evaluation of the Olfactory System in Kallmann Syndrome: Correlation with a Clinical Smell Test. Neuroendocrinology. 2011;94(3):209–217. doi: 10.1159/000328437. [DOI] [PubMed] [Google Scholar]
- 139.Dodé C, Rondard P. PROK2/PROKR2 Signaling and Kallmann Syndrome. Frontiers in Endocrinology. 2013 doi: 10.3389/fendo.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mitchell AL, Dwyer A, Pitteloud N, Quinton R. Genetic Basis and Variable Phenotypic Expression of Kallmann Syndrome: Towards a Unifying Theory. Trends Endocrinol Metab. 2011;22(7):249–258. doi: 10.1016/j.tem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 141.Bojesen A, Juul S, Gravholt CH. Prenatal and Postnatal Prevalence of Klinefelter Syndrome: A National Registry Study. J Clin Endocrinol Metab. 2003;88(2):622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 142.Lewkowitz-Shpuntoff HM, Hughes VA, Plummer L, Au MG, Doty RL, Seminara SB, Chan YM, Pitteloud N, Crowley WF, Balasubramanian R. Olfactory Phenotypic Spectrum in Idiopathic Hypogonadotropic Hypogonadism: Pathophysiological and Genetic Implications. J Clin Endocrinol Metab. 2012;97(1) doi: 10.1210/jc.2011-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vuorela PE, Penttinen MT, Hietala MH, Laine JO, Huoponen KA, Kaariainen HA. A Familial CHARGE Syndrome with a CHD7 Nonsense Mutation and New Clinical Features. Clin Dysmorphol. 2008;17(4):249–253. doi: 10.1097/MCD.0b013e328306a704. [DOI] [PubMed] [Google Scholar]
- 144.Jongmans MCJ, van Ravenswaaij-Arts CMA, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JEH, Brunner HG, Crowley WF, Hoefsloot LH. CHD7 Mutations in Patients Initially Diagnosed with Kallmann Syndrome - The Clinical Overlap with CHARGE Syndrome. Clin Genet. 2009;75(1):65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jansen F, Kalbe B, Scholz P, Mikosz M, Wunderlich KA, Kurtenbach S, Nagel-Wolfrum K, Wolfrum U, Hatt H, Osterloh S. Impact of the Usher Syndrome on Olfaction. Hum Mol Genet. 2016;25(3):524–533. doi: 10.1093/hmg/ddv490. [DOI] [PubMed] [Google Scholar]
- 146.Seeliger M, Pfister M, Gendo K, Paasch S, Apfelstedt-Sylla E, Plinkert P, Zenner H-P, Zrenner E. Comparative Study of Visual, Auditory, and Olfactory Function in Usher Syndrome. Graefe’s Arch Clin Exp Ophthalmol. 1999;237(4):301–307. doi: 10.1007/s004170050237. [DOI] [PubMed] [Google Scholar]
- 147.Ribeiro JC, Oliveiros B, Pereira P, Antônio N, Hummel T, Paiva A, Silva ED. Accelerated Age-Related Olfactory Decline among Type 1 Usher Patients. Sci Rep. 2016;6(1):28309. doi: 10.1038/srep28309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marietta J, Walters KS, Burgess R, Moore KC, Ni L, Hejtmancik JF, Fukushima K, Smith RJH. Usher’s Syndrome Type IC: Clinical Studies and Fine-Mapping the Disease Locus. Ann Otol Rhinol Laryngol. 1997;106(2):123–128. doi: 10.1177/000348949710600206. [DOI] [PubMed] [Google Scholar]
- 149.Reiners J, Nagel-Wolfrum K, Jürgens K, Märker T, Wolfrum U. Molecular Basis of Human Usher Syndrome: Deciphering the Meshes of the Usher Protein Network Provides Insights into the Pathomechanisms of the Usher Disease. Exp Eye Res. 2006;83(1):97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 150.Endoh-Yamagami S, Karkar KM, May SR, Cobos I, Thwin MT, Long JE, Ashique AM, Zarbalis K, Rubenstein JLR, Peterson AS. A Mutation in the Pericentrin Gene Causes Abnormal Interneuron Migration to the Olfactory Bulb in Mice. Dev Biol. 2010;340(1):41–53. doi: 10.1016/j.ydbio.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 151.Hori A, Tamagawa K, Eber SW, Westmeier M, Hansmann I. Neuropathology of Seckel Syndrome in Fetal Stage with Evidence of Intrauterine Developmental Retardation. Acta Neuropathol. 1987;74(4):397–401. doi: 10.1007/BF00687219. [DOI] [PubMed] [Google Scholar]
- 152.Brasseur B, Martin CM, Cayci Z, Burmeister L, Schimmenti LA. Bosma Arhinia Microphthalmia Syndrome: Clinical Report and Review of the Literature. Am J Med Genet Part A. 2016;170(5) doi: 10.1002/ajmg.a.37572. [DOI] [PubMed] [Google Scholar]
- 153.Graham JM, Lee J. Bosma Arhinia Microphthalmia Syndrome. Am J Med Genet. 2006;140 A(2):189–193. doi: 10.1002/ajmg.a.31039. [DOI] [PubMed] [Google Scholar]
- 154.Lahiry P, Wang J, Robinson JF, Turowec JP, Litchfield DW, Lanktree MB, Gloor GB, Puffenberger EG, Strauss KA, Martens MB, Ramsay DA, Rupar CA, Siu V, Hegele RA. A Multiplex Human Syndrome Implicates a Key Role for Intestinal Cell Kinase in Development of Central Nervous, Skeletal, and Endocrine Systems. Am J Hum Genet. 2008;84(2):134–147. doi: 10.1016/j.ajhg.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moerman P, Fryns JP. Oral-Facial-Digital Syndrome Type IV (Mohr-Majewski Syndrome): A Fetopathological Study. Genet Couns. 1998;9(1):39–43. [PubMed] [Google Scholar]
- 156.Thomas S, Legendre M, Saunier S, Bessières B, Alby C, Bonnière M, Toutain A, Loeuillet L, Szymanska K, Jossic F, Gaillard D, Yacoubi MT, Mougou-Zerelli S, David A, Barthez MA, Ville Y, Bole-Feysot C, Nitschke P, Lyonnet S, Munnich A, Johnson CA, Encha-Razavi F, Cormier-Daire V, Thauvin-Robinet C, Vekemans M, Attié-Bitach T. TCTN3 Mutations Cause Mohr-Majewski Syndrome. Am J Hum Genet. 2012;91(2):372–378. doi: 10.1016/j.ajhg.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Spranger J, Grimm B, Weller M, Weißenbacher G, Herrmann J, Gilbert E, Krepler R. Short Rib-Polydactyly (SRP) Syndromes, Types Majewski and Saldino-Noonan. Z. Kinderheilkd. 1974;116(2):73–94. doi: 10.1007/BF00491508. [DOI] [PubMed] [Google Scholar]
- 158.Sailani MR, Jingga I, MirMazlomi SH, Bitarafan F, Bernstein JA, Snyder MP, Garshasbi M. Isolated Congenital Anosmia and CNGA2 Mutation. Sci Rep. 2017;7(1):2667. doi: 10.1038/s41598-017-02947-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karstensen HG, Mang Y, Fark T, Hummel T, Tommerup N. The First Mutation in CNGA2 in Two Brothers with Anosmia. Clin Genet. 2015;88(3):293–296. doi: 10.1111/cge.12491. [DOI] [PubMed] [Google Scholar]
- 160.Alkelai A, Olender T, Haffner-Krausz R, Tsoory MM, Boyko V, Tatarskyy P, Gross-Isseroff R, Milgrom R, Shushan S, Blau I, Cohn E, Beeri R, Levy-Lahad E, Pras E, Lancet D. A Role for TENM1 Mutations in Congenital General Anosmia. Clin Genet. 2016;90(3):211–219. doi: 10.1111/cge.12782. [DOI] [PubMed] [Google Scholar]
- 161.Weiss J, Pyrski M, Jacobi E, Bufe B, Willnecker V, Schick B, Zizzari P, Gossage SJ, Greer CA, Leinders-Zufall T, Woods CG, Wood JN, Zufall F. Loss-of-Function Mutations in Sodium Channel Nav1.7 Cause Anosmia. Nature. 2011;472(7342):186–190. doi: 10.1038/nature09975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


