Abstract
Persons living with HIV (PLWH) are living longer but experiencing more adverse symptoms associated with the disease and its treatment. This study aimed to examine the impact of a mHealth application (app) comprised of evidence-based self-care strategies on the symptom experience of PLWH. We conducted a 12-week feasibility study with 80 PLWH who were randomized (1:1) to a mHealth app, mobile Video Information Provider (mVIP), with self-care strategies for improving 13 commonly experienced symptoms in PLWH or to a control app. Intervention group participants showed a significantly greater improvement than the control group in 5 symptoms: anxiety (p = 0.001), depression (p = 0.001), neuropathy (p = 0.002), fever/chills/sweat (p = 0.037), and weight loss/wasting (p = 0.020). Participants in the intervention group showed greater improvement in adherence to their antiretroviral medications (p = 0.017) as compared to those in the control group. In this 12-week trial, mVIP was associated with improved symptom burden and increased medication adherence in PLWH.
Keywords: Symptom, management, Mobile, technology, mHealth, Self-care, Feasibility, trial
Introduction
HIV has evolved from a fatal diagnosis into a chronic illness largely due to the success of HIV medications [1]. In view of the change in the course of the disease, persons living HIV (PLWH) are living longer but experiencing more adverse symptoms associated with the disease and its treatment [2]. As the population of PLWH ages, there is a sharply increased risk of poorer everyday functioning and HIV-related disability supporting the need to manage adverse symptoms in this population [3]. Patients’ symptom experiences and symptom management success are strongly related to HIV disease progression and adverse clinical profiles [4, 5].
Symptom management in PLWH has been shown to decrease symptom severity [6], improve quality of life [7], reduce disability, increase medication adherence, and promote health [8]. Self-management involves helping patients set achievable goals and learn techniques of problem-solving relevant to their condition [9]. The ability to self-manage adverse symptoms of HIV illness has been shown to improve patient-centered outcomes and quality of life [10]. In response to this need, a team of researchers at the UCSF School of Nursing developed a paper-based symptom management manual with self-care strategies for 21 common HIV/AIDS symptoms. The manual was found to be efficacious in a 775-person randomized controlled trial (RCT) over 3 months at 12 sites [11].
Despite the success of the findings in the trial, uptake of these self-care strategies has been low. To facilitate dissemination of evidence-based strategies for symptom self-management, we developed a mobile application (app), mobile Video Information Provider (mVIP), which delivers these self-care strategies to PLWH based on their symptom reporting. Mobile technology is a platform that is well-suited for the implementation and dissemination of evidence-based strategies for HIV symptom management. This project is unique in that mobile health (mHealth) technology is typically developed without incorporating patient-centered outcomes research. There are currently hundreds of apps for PLWH, yet these apps have not been conceptualized using evidence-based research and/or patient-centered design [12], and as a result are expected to be off the marketplace in a few years. For instance, of the 55 apps for PLWH which were reported in Muessig’s 2013 review [13], only 18 are still available on the app marketplace. Consequently, developmental research is needed to improve understanding of how mHealth tools can be appropriately designed, functionally operated, and effectively used by PLWH to enable the dissemination of evidence-based information. In addition, incorporation of the evidence-based information has the potential to substantially improve the rigor of these technologies [14].
Use of mobile technology can improve communication, access, and information/resource delivery to racial and ethnic minority groups [15, 16]. mHealth technology has the potential to bridge a divide in healthcare delivery among these underserved groups [17]. Ownership of a mobile device is equally as common among Blacks and Whites (94%) and highest among Hispanics (98%) [18]. While mobile internet use in the US has been on the rise across all groups, Blacks and Hispanics are more likely to use a smartphone for internet use (94% for both groups) compared to Whites (85%) [19, 20]. The use of mobile technologies at nearly equal rates across racial and ethnic groups supports the use of these tools for bridging some of the current disparities in healthcare access and health outcomes.
Despite the rapid proliferation and widespread uptake of mHealth apps, there is a dearth of mHealth technology interventions focusing on PLWH’s self-management, and thus little is known about the impact of using mobile apps for managing PLWH’s symptom experience. To address these gaps, this study examined the impact of using mobile technology for the dissemination and implementation of evidence-based self-care strategies and the effect of this mHealth app on patient-reported outcomes. We hypothesized that consumers who received evidence-based self-care strategies through a mobile app would have a decrease in their symptom burden compared with patients who did not have access to self-care strategies.
Methods
We compared symptom burden in PLWH when using a mobile app with self-care strategies for symptom management versus a mobile app without self-care strategies between December 2016 and June 2017. Our study tested mVIP, which was designed to help PLWH self-manage their symptom experience. mVIP is a web-app optimized to run on a smartphone or tablet, and also capable of running on a desktop computer. It was developed through a rigorous user-centered design process described elsewhere [21, 22], consisting initially of card sorting activities that informed the architecture of the symptoms and self-care strategies, followed by a heuristic evaluation with experts, and end-user usability testing in a laboratory setting [23]. All features of the app were tested by the project team before enrolling study participants in the feasibility trial.
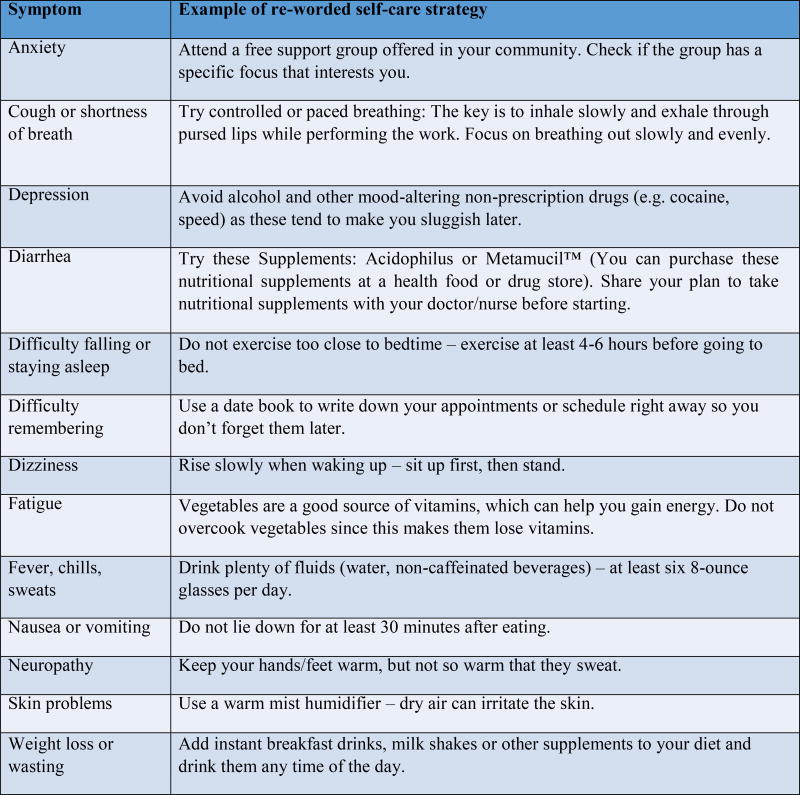
The mVIP app was comprised of 143 self-care strategies for 13 different symptoms. Symptoms included: (1) Anxiety, (2) Cough or shortness of breath, (3) Depression, (4) Diarrhea, (5) Difficulty falling or staying asleep, (6) Difficulty remembering, (7) Dizziness, (8) Fatigue, (9) Fever, chills, sweats, (10) Nausea or vomiting, (11) Neuropathy, (12) Skin problems, and (13) Weight loss or wasting. Sample self-care strategies can be found in Fig. 1.
Figure 1.
Sample self-care strategies for 13 symptoms
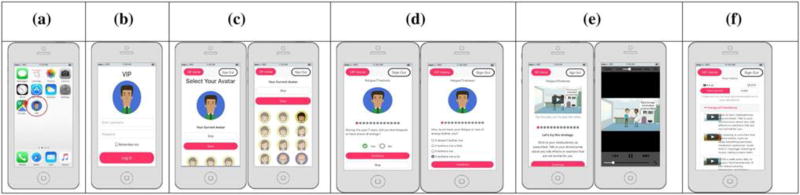
Upon enrollment, study participants installed a shortcut to the web-app on their home screen (Fig. 2a). Participants used this shortcut icon to log into mVIP (Fig. 2b), then selected an avatar (Fig. 2c) who guided them through the mVIP system. Participants were instructed to log in at least once per week and use the app to assess their symptoms and receive self-care strategies tailored to their symptom experience. Both study groups received the mVIP app but only intervention group participants received the self-care strategies. In addition to the text delivered by the self-care strategies, intervention group participants were able to view a short animated video which illustrated the self-care strategy.
Figure 2.
a mVIP shortcut, b Log-in, c Avatar selection, d Symptom assessment, e Animated video, f Summary of strategies
Participants completed survey questions each week via the app (Fig. 2d) to report if they had experienced each of the 13 symptoms in the past week and how much each symptom bothered them in the past week. The symptom questions were based on the Revised Sign and Symptom Check-List for HIV (SSC-HIVrev) [24]. Participants were first asked if they experienced the symptom in the past 7 days (Yes or No). For each symptom selected, respondents were asked how much it bothered them (a little bit, somewhat, quite a bit, or very much). If a participant did not experience the symptom in the past week, then they were not asked how much it bothered them and were not given any strategies. If a participant reported bothersome symptoms, the app would deliver 3 self-care strategies for the participant to try that week. Figure 1 illustrates sample self-care strategies for each symptom. Each strategy was accompanied by a short (3–27 s) video to illustrate the strategy (Fig. 2e). At the end of the app session, participants were able to view a summary of their strategies (Fig. 2f). The app also included a reminder system that emailed participants at 7:30 pm on 7, 14, 18, and 21 days after their last use. The reminders included a link to the mVIP app so that users could easily access the app by clicking on the link.
Study Design
This randomized, controlled study took place in New York City. Participants were recruited through flyers at a local HIV clinic and community based organizations, and through e-mail invitations. Research assistants assessed all respondents for eligibility over the phone. Eligible participants were English speaking; aged 18 years or older; diagnosed with HIV; experienced at least 2 of 13 HIV-related symptoms in the past week; had a cognitive state minimum score of 24 out of 30 as measured by the Mini-Mental State Examination (MMSE); [25] and owned a smartphone or tablet. All participants completed written informed consent prior to the start of study activities.
Following enrollment, participants were randomized to each study arm. A randomization schedule was developed prior to the start of the trial. Study participants were randomized (1:1) to mVIP with self-care strategies (intervention group) or mVIP without self-care strategies (control group). Both groups received access to the mVIP app on their smartphones. The PI created the allocation sequence through a computerized random number generator. This was a single-blinded study and the control group participants did not have access to the self-care strategies. Participation in the trial lasted 12 weeks; a follow-up survey was administered at our study site at the end of the study period.
Data Sources/Collection and Measures
Study participants completed a baseline survey comprised of demographic questions, PROMIS-29 [26], RAND 36-item health survey [27, 28], engagement with healthcare provider [29], antiretroviral therapy (ART) medication adherence using the Visual Analogue Scale (VAS) [30] and CASE Adherence Index [29], number of medical visits, and usability through the Health-ITUES [31]. All surveys were administered via Qualtrics software on either a laptop computer or iPad at our study site, the Columbia University School of Nursing. Study participants were instructed to use the app at least once per week, and symptom frequency and bothersomeness were collected via the app during each session. At the end of the 12 weeks, study participants were invited back to the study site to complete their follow-up questionnaire and receive compensation for completing the surveys each week. Participants had the opportunity to receive $155 as total compensation. Participants received $30 for attending the baseline and $40 for the follow-up visit. Participants received $5 for each week they completed a survey using the app, and they received a bonus of $25 for completion of all study components. All study activities were approved by the Columbia University Medical Center University Institutional Review Board.
Data Analysis
The study analysis followed an intention-to-treat approach. Intervention and control characteristics collected at baseline were summarized with descriptive statistics (mean ± SD or frequency). To assess the effect of the intervention on symptom burden during the follow-up period, we used a linear mixed model to analyze repeated measured data, and the models controlled for age, sex, race, education, and CD4 count.
For all secondary outcome measures, which were collected at baseline and 12-week follow-up, we used the same linear mixed model or a generalized linear mixed model. We used a linear mixed model for continuous outcomes (e.g. PROMIS score); generalized linear mixed model (Poisson or Negative binomial model) for count outcomes (e.g. number of ER visits); and the generalized linear mixed models (logistic model) for binary outcomes (e.g. CASE Adherence Index).
Results
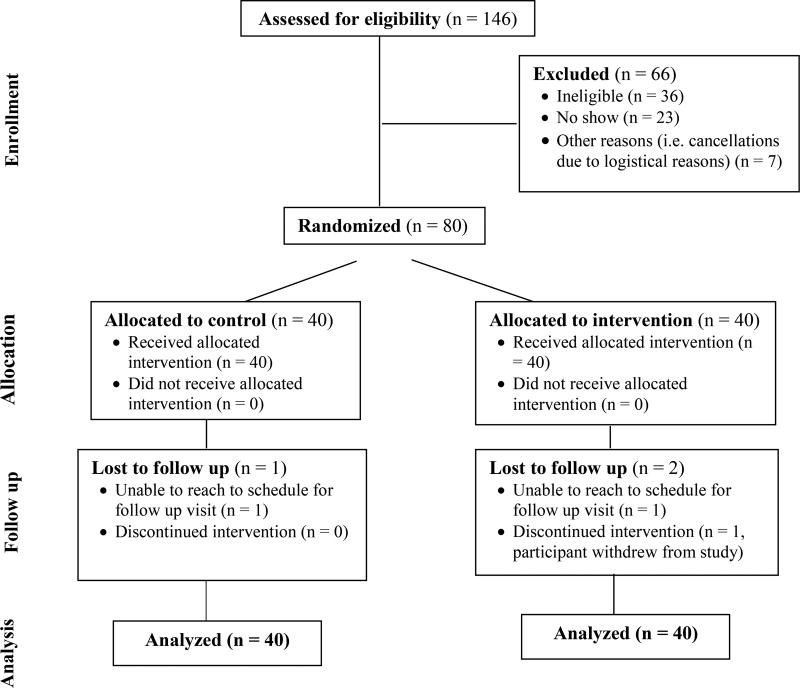
Figure 3 summarizes enrollment. A total of 80 PLWH were randomized and 76 subjects completed the study. 40 participants were randomized to the intervention group (40 allocated to intervention with one withdrawal). Table 1 summarizes demographic information for intervention and control groups. Mean age of intervention group participants was 50 years (SD 11.7) and the mean age of control group participants was 51 years (SD 9.0). Ages ranged from 23 to 72 years. Nearly half of the participants had an annual income of less than $10,000/year. 90% of our study participants belonged to a racial or ethnic minority group. There were no statistically significant differences between study groups.
Figure 3.
Enrollment Summary Diagram
Table 1.
Baseline Demographics
| Variable | Overall N = 80 |
Intervention N = 40 |
Control N = 40 |
|---|---|---|---|
| Age mean (SD) | 50.4 (10.4) | 50.0 (11.7) | 50.8 (9.0) |
| Sex, Male | 38 (47.5%) | 15 (37.5%) | 23 (57.5%) |
| Race | |||
| White, Non-Hispanic | 8 (10.0%) | 4 (10.0%) | 4 (10.0%) |
| Black, Non-Hispanic | 55 (68.8%) | 29 (72.5%) | 26 (65.0%) |
| Hispanic | 17 (21.3%) | 7 (17.5%) | 10 (25.0%) |
| Education | |||
| Less than high school | 14 (17.5%) | 9 (22.5%) | 5 (12.5%) |
| High school | 25 (31.3%) | 11 (27.5%) | 14 (35.0%) |
| Some college or associates degree | 28 (35.0%) | 15 (37.5%) | 13 (32.5%) |
| Bachelors or advanced degree | 13 (16.3%) | 5 (12.5%) | 8 (20.0%) |
| Annual Income | |||
| Less than $10,000/yr | 39 (48.8%) | 22 (55.0%) | 17 (42.5%) |
| $10,000–$19,999/yr | 19 (23.8%) | 9 (22.5%) | 10 (25.0%) |
| $20,000–$59,999/yr | 11 (13.8%) | 3 (7.5%) | 8 (20.0%) |
| Don’t know or prefer not to answer | 11 (13.8%) | 6 (15.0%) | 5 (12.5%) |
| Employment | |||
| Working (full, part, off-books) | 15 (19.7%) | 7 (18.4%) | 8 (21.1%) |
| Unemployed (looking, not looking) | 26 (34.2%) | 15 (39.5%) | 11 (29.0%) |
| Retired | 4 (5.3%) | 3 (7.9%) | 1 (2.6%) |
| Student | 4 (5.3%) | 2 (5.3%) | 2 (5.3%) |
| Disabled | 27 (35.5%) | 11 (29.0%) | 16 (42.1%) |
| ART Use | |||
| None | 4 (5.0%) | 3 (7.5%) | 1 (2.5%) |
| 2+ pills per day | 34 (42.5%) | 15 (37.5%) | 19 (47.5%) |
| 1 pill per day | 41 (51.3%) | 21 (52.5%) | 20 (50%) |
| Prefer not to answer | 1 (1.3%) | 1 (2.5%) | - |
| Virologically suppressed | 68 (85.0%) | 34 (85.0%) | 34 (85.0%) |
| Ever diagnosed with AIDS | 41 (51.3%) | 17 (42.5%) | 24 (60.0%) |
| CD4 count greater than 500 | 42 (53.2%) | 22 (55.0%) | 20 (51.3%) |
| Possible alcohol use disorder | 25 (31.3%) | 15 (37.5%) | 10 (25.0%) |
| Substance use weekly or more often | 24 (30.0%) | 15 (37.5%) | 9 (22.5%) |
Overall Use of mVIP
Of the 80 participants who completed the baseline visit, 5 (6.3%) participants (1 control, 4 intervention group) did not use the mVIP app after the baseline visit. The mean number of times participants used the app during the study period was 18.2 times (SD 15.5). 18 (45.0%) intervention group participants and 19 (47.5%) control group participants used the app greater than 14 times during the 12-week trial. 32 (80.0%) intervention group participants and 35 (87.5%) control group participants used the app at least 11 times during the 12-week trial. 14 (35.0%) participants in the intervention group and 16 (40.0%) participants in the control group used the app at least once per week (within a strict 7-day period). There was no significant difference in app use between study groups.
Impact on Symptom Burden
Table 2 presents the frequency of participants who reported experiencing the symptom at baseline. Fatigue was the most frequently reported symptom (n = 61, 76.3%), followed by difficulty falling or staying asleep (n = 59, 74.7%), neuropathy (n = 46, 59.0%), anxiety (n = 45, 57.0%), and depression (n = 43, 53.8%). There was no significant difference in symptom frequency between study groups at baseline.
Table 2.
Frequency of Symptoms at Baseline
| Variable | Overall N = 80 |
Intervention N = 40 |
Control N = 40 |
Significance (p-value) |
|---|---|---|---|---|
| Anxiety | 45 (57.0%) | 22 (56.4%) | 23 (57.5%) | 0.922 |
| Cough or shortness of breath | 37 (46.3%) | 17 (42.5%) | 20 (50.0%) | 0.501 |
| Depression | 43 (53.8%) | 25 (62.5%) | 18 (45.0%) | 0.116 |
| Diarrhea | 24 (30.4%) | 13 (33.3%) | 11 (27.5%) | 0.573 |
| Difficulty falling or staying asleep | 59 (74.7%) | 31 (77.5%) | 28 (71.8%) | 0.560 |
| Difficulty remembering | 40 (50.6%) | 22 (55.0%) | 18 (46.2%) | 0.432 |
| Dizziness | 20 (25.6%) | 12 (30.8%) | 8 (20.5%) | 0.300 |
| Fatigue | 61 (76.3%) | 31 (77.5%) | 30 (75.0%) | 0.793 |
| Fever, chills, or sweats | 20 (25.0%) | 10 (25.0%) | 10 (25.0%) | 1.000 |
| Nausea or vomiting | 15 (18.8%) | 8 (20.0%) | 7 (17.5%) | 0.775 |
| Neuropathy | 46 (59.0%) | 23 (59.0%) | 23 (59.0%) | 1.000 |
| Skin problems | 35 (44.3%) | 19 (47.5%) | 16 (41.0%) | 0.562 |
| Weight loss or wasting | 20 (25.3%) | 12 (30.0%) | 8 (20.5%) | 0.332 |
NOTE: Those who skipped a symptom question at baseline are excluded from percentages for that symptom
Table 3 provides a summary of the symptom burden results between baseline and follow-up. We conducted an intention-to-treat analysis. Compared with control group participants, intervention group participants had an improvement in 12 of 13 symptoms. Of these symptoms, intervention group participants showed a significantly greater improvement than the control group participants in 5 symptoms: anxiety (p = 0.001), depression (p = 0.001), neuropathy (p = 0.002), fever, chills, or sweats (p = 0.037), and weight loss or wasting (p = 0.020). There was a greater improvement in nausea or vomiting in the control group as compared to the intervention group but this was not significant.
Table 3.
Difference Symptom Score Between the Intervention and Control Groups
| Score change from Baseline to Week 12
|
Difference between Arm 1 & Arm 2
|
||||||
|---|---|---|---|---|---|---|---|
| Intervention
|
Control
|
||||||
| Score | Standard Error |
Score | Standard Error |
Estimate | Standard Error |
Significance (p-value) |
|
| Anxiety | −0.858 | 0.102 | −0.318 | 0.118 | −0.541 | 0.156 | 0.001 |
| Cough or shortness of breath | −0.570 | 0.105 | −0.421 | 0.122 | −0.149 | 0.161 | 0.356 |
| Depression | −0.540 | 0.106 | −0.007 | 0.123 | −0.533 | 0.163 | 0.001 |
| Diarrhea | −0.240 | 0.092 | −0.233 | 0.107 | −0.007 | 0.141 | 0.962 |
| Difficulty falling or staying asleep | −0.506 | 0.106 | −0.433 | 0.122 | −0.073 | 0.162 | 0.651 |
| Difficulty remembering | −0.343 | 0.095 | −0.169 | 0.110 | −0.174 | 0.145 | 0.230 |
| Dizziness | −0.319 | 0.083 | −0.181 | 0.095 | −0.138 | 0.126 | 0.275 |
| Fatigue | −0.566 | 0.115 | −0.563 | 0.132 | −0.003 | 0.175 | 0.987 |
| Fever, chills, sweats | −0.360 | 0.086 | −0.084 | 0.099 | −0.275 | 0.132 | 0.037 |
| Neuropathy | −0.713 | 0.103 | −0.228 | 0.119 | −0.485 | 0.157 | 0.002 |
| Skin problems | −0.219 | 0.091 | −0.089 | 0.105 | −0.130 | 0.139 | 0.349 |
| Vomiting | −0.122 | 0.066 | −0.185 | 0.077 | 0.063 | 0.101 | 0.534 |
| Weight loss or wasting | −0.254 | 0.070 | −0.004 | 0.081 | −0.250 | 0.107 | 0.020 |
Secondary Outcomes
Table 4 illustrates the findings from our secondary outcome measures. Overall, participants rated the app as highly usable. There was almost no significant difference in health-related quality of life between study groups as measured by the PROMIS-29 [32] and the RAND-36 Item Health Survey [27] instruments. Higher scores on the RAND-36 indicate more favorable health states, thus a significantly higher pain score suggests that the intervention may have had a significant effect on improving self-reported pain in the intervention group as compared to the control group. Likewise, there was no significant difference between study groups in system usability. We measured adherence to ART using two adherence measures: VAS [30] and the CASE Adherence Index [29]. Both have been shown to be reliable and valid tools and there is no gold standard measure for ART adherence. We found a significant improvement in ART adherence as measured through the CASE adherence index [29] in our intervention group as compared to our control group participants, but this difference was not detected when measuring adherence with the VAS.
Table 4.
Difference in Difference of Secondary Outcome Measures
| Variable | Estimate | Standard Error |
Significance (p-value) |
|---|---|---|---|
| PROMIS-29 | |||
| Physical Function | 0.79 | 1.25 | 0.529 |
| Anxiety | 1.71 | 1.68 | 0.312 |
| Depression | −0.36 | 1.81 | 0.841 |
| Fatigue | 0.40 | 2.07 | 0.848 |
| Sleep Disturbance | 2.58 | 2.03 | 0.208 |
| Satisfaction with Participation in Social Roles | 0.72 | 2.29 | 0.754 |
| Pain Interference | 1.25 | 1.66 | 0.454 |
| RAND-36 Item Health Survey 1.0 | |||
| Physical Functioning Scale | −3.06 | 7.27 | 0.675 |
| Role Limitations due to Physical Health Scale | 7.47 | 10.02 | 0.458 |
| Role Limitations due to Emotional Problems Scale | 3.50 | 9.91 | 0.725 |
| Energy/Fatigue Scale | −1.00 | 4.09 | 0.807 |
| Emotional Well-being Scale | 1.48 | 3.73 | 0.693 |
| Social Functioning Scale | −8.93 | 5.80 | 0.128 |
| Pain Scale | −14.33 | 5.18 | 0.007 |
| General Health Scale | −0.20 | 3.93 | 0.960 |
| Physical Health Summary Scale | −0.93 | 4.47 | 0.836 |
| Mental Health Summary Scale | −0.81 | 3.60 | 0.822 |
| Engagement With Healthcare Provider | |||
| Engagement with Healthcare Provider Scale | −2.52 | 2.19 | 0.252 |
| Medication Adherence | |||
| CASE Adherence Index | −1.51 | 0.62 | 0.017 |
| Visual Analogue Scale | −4.88 | 5.06 | 0.338 |
| Health-IT Usability Evaluation Scale (Health-ITUES) | |||
| Overall | −0.07 | 0.23 | 0.743 |
| Quality of Life | −0.28 | 0.20 | 0.166 |
| Perceived Usefulness | 0.03 | 0.26 | 0.899 |
| Perceived Ease of Use | −0.07 | 0.26 | 0.803 |
| User Control | −0.20 | 0.28 | 0.480 |
Healthcare Services Use
At the end of the trial, we asked participants to report their use of healthcare services in the past 30 days. Overall, healthcare services utilization was very low in both study groups. In summary, a total of 3 (8.1%) intervention group participants and 4 (10.3%) control group participants reported visiting the emergency room. A total of 2 (5.4%) intervention group participants and 1 (2.6%) control group participants reported being hospitalized. A total of 16 (43.2%) intervention group participants and 19 (48.7%) control group participants reported a medical office visit. Using Pearson’s Chi squared test, there was no significant difference in healthcare services use between study groups.
Discussion
Multiple studies have addressed the potential benefits of mobile phone apps for patients with chronic illnesses [33, 34]. Though this study is small, it is one of the first trials to demonstrate even a short-term impact on symptom improvement in a randomized, controlled design. In particular, this study is one of the first randomized studies of a mobile app in a sample of persons who are almost all racial/ethnic minorities and from the lowest income groups in the US.
The intervention described here provides PLWH a mobile app to self-manage their symptoms and provides evidence-based self-care strategies to help them ameliorate their symptoms. It extends the current research in several important ways. First, this short duration study demonstrated that a sub-population exists who derives value from using mHealth technology for symptom self-management. A larger, longitudinal study should be conducted to better understand how to sustain use over long periods of time in persons who can derive value from an intervention. Second, it will add to the body of literature on whether mHealth technology can be used for the dissemination of evidence-based strategies for persons living with a chronic illness. Third, it adds further support to the need for formative user-centered design during the conceptualization and development of mHealth technologies. Finally, it extends the literature on mHealth technology as a potentially effective tool for improving patient-reported outcomes in persons living with a chronic illness.
Importantly, we did detect an improvement in ART medication adherence using the CASE Adherence Index [29], although no significant association was found using the VAS. While both the CASE and the VAS are validated measures used to assess medication adherence, past research has suggested that Likert-type scales may yield more variable results in self-reports compared to global estimates of adherence [35]. ART adherence and symptom management have been strongly linked in past research, which has shown that symptom interpretation can influence adherence to treatment regimens when, for example, symptoms are assumed to be medication side-effects or when their alleviation, persistence, or worsening after treatment initiation is used to assess therapeutic efficacy.
Past research has shown that untreated HIV, as well as ART side effects, can cause more symptoms. Unlike treatments for other illnesses, ART medications are more likely to contribute to greater discomfort [36], reinforcing the need for symptom management in the treatment cascade. Interestingly, 85% of our study sample was virologically suppressed at baseline and only 5% reported not being on ART. Therefore, even patients with well-controlled HIV report symptoms that affect quality of life, which has been shown in other studies [37]. These findings further support the potential impact of the mVIP intervention for ameliorating symptoms and improving patient-reported outcomes. This is particularly relevant for PLWH who are virologically suppressed but are burdened by symptoms associated with their ART medications.
We did detect a significant improvement in the RAND-36 pain scale score in the intervention group. While this improvement is noteworthy, we acknowledge that since we examined a large number of similar outcomes measures for health-related quality of life, there is the potential for one of the scales to be significant because of random chance. Further consideration of health-related quality of life in our study demonstrates that the overall PROMIS scores at baseline in both study groups were only “mildly impaired,” making it difficult to detect a significant improvement in PROMIS scores since participants had relatively good health-related quality of life despite living with HIV. Likewise, the RAND-36 scores, another measure of health-related quality of life, were higher in our study sample than those for the general US population, making it difficult to demonstrate an intervention effect on a study sample who had generally good health-related quality of life. Future intervention studies should evaluate the effect of these self-care strategies in people who are more symptomatic and who have lower health-related quality of life at baseline.
Another important note is that our study sought to assess the effect of overall usability of the app. Usability is the measure of the quality of a user’s experience when interacting with a system, including their perceived usefulness and ease of use. In the case of our study, the Health-ITUES [30] was used as a measure of usability. The Health-ITUES is a 20-item customizable usability evaluation instrument which has been validated for use with mHealth technology [38]. This instrument is comprised of 4 subscales in addition to the overall user satisfaction: system impact, perceived usefulness, perceived ease of use, and user control. We would anticipate that there would be an improvement in overall user satisfaction, covering all of the constructs, in the intervention group at the end of the study. At the same time, we did not anticipate particularly perceived usefulness to increase in the control group, which it did. Participants in both study groups found the app to be useful in monitoring their symptom experience over time. As the mVIP app was initially developed through rigorous user-centered design processes, the overall user satisfaction scores were quite high at baseline, which reflects strong usability of mVIP. Given these findings and that both groups perceived the app as highly usable at baseline, it is not surprising that there was no significant difference in perceptions of usability between groups over time.
In regards to use of healthcare services and engagement with healthcare providers, we did not find a significant difference between groups. Given the short duration of our study and the relatively rare events of hospitalization and emergency room visits, these findings were not unexpected. Additional work evaluating mVIP’s impact on use of healthcare services over a longer study period may provide important information on healthcare use and costs to our healthcare system. Similarly, the short study duration did not allow for adequate follow up to evaluate any effect on engagement with healthcare providers; current guidelines recommend that patients on ART visit their provider every 3–4 months. For adherent patients with consistently suppressed viral load and stable immunologic status for more than 2 years, provider visits can be extended to 6-month intervals [39, 40].
Importantly, this app was designed employing earlier evidence from patient-centered outcomes research studies [41], which was a strength of the content of the app. In addition to the robustness of the content of the app, we employed rigorous user-centered design processes, which is in strong contrast to many of the extant mHealth apps on the marketplace. In particular, our design and development process adds to the rigor of current mHealth research given that our study population is comprised of racial and ethnic minority groups from the lowest income groups in the US. In short, our study sample is comprised of those persons who are most likely to suffer from disparities in healthcare yet are most likely to benefit from the mobile technology that we developed.
Our study sample is an especially important strength to our study given that past research on mobile technology has demonstrated that there are disparities in use of these technologies by African Americans [42]. In contrast to this earlier work, we found no difference in use or outcomes related to racial/ethnic or any other sociodemographic characteristic of the study sample supporting the use of mHealth technology for bridging some of the current disparities in the delivery of healthcare.
The technical capabilities of the app also created a number of limitations, which should be taken into consideration for future versions of the app. First, despite this being a smartphone app, participants wanted their reminders and login information sent via text instead of e-mail. Second, while the app provided a summary report of recommended self-care strategies for the intervention group, both study groups expressed their desire for reports of their symptoms and visualizations of their self-reported changes in symptoms over time. Finally, the self-care strategy videos did not contain sound and future versions of the app should incorporate videos that are longer and more dynamic.
There is also some limitation to the generalizability of our findings since we required individuals to possess a smartphone or tablet to be in the study. The most marginalized HIV patients likely do not have smartphones. On the other hand, an advantage to a web-based app is that individuals (assuming they own a phone/tablet) can connect using free wi-fi even if they do not have money to pay their cell phone bills, a frequent cause of service interruption.
Key elements of feasibility were successfully tested, including: acceptability, integration, demand, practicality, implementation, and limited efficacy testing [43]. Acceptability was determined through high usability scores. Participants were able to integrate use of this app into the routines of their everyday lives. Demand for the intervention was assessed by gathering data on actual use which was quite high as described above. The practicality of this app is high given that healthcare providers do not need to interact with the technology. Finally, the potential for implementation [44] of this app through its release to an app marketplace and the ability to download by targeted users is very practical. The use of mobile technology for symptom self-management holds promise, given the pervasive nature and penetration of mobile phones in our study population. Although the app was highly usable and showed preliminary efficacy, future study should consider the effect of this intervention over the long-term to demonstrate sustainability, evaluate implementation across other settings, and examine the use of this intervention in other languages.
Conclusion
The mVIP app was associated with improvement in symptoms and very strong usability. Findings from this study suggest that mobile apps have the potential to support aspects of patient-reported outcomes, including the symptom experience. Future work should use findings of this study to guide assessments of this intervention in other contexts, settings, and cultures in order to translate this intervention into the everyday lives of consumers.
Acknowledgments
The project described was supported by grant number R21HS023963 and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Compliance with Ethical Standards
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
All authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Research Involved in Animal Rights
This article does not contain any studies with animals performed by any of the authors.
Contributor Information
Rebecca Schnall, Mary Dickey Lindsay Associate Professor of Disease Prevention and Health Promotion, Columbia University School of Nursing, NY, NY 10032, rb897@columbia.edu, Phone: 212-342-6886.
Hwayoung Cho, Columbia University School of Nursing.
Alexander Mangone, Columbia University School of Nursing.
Adrienne Pichon, Columbia University School of Nursing.
Haomiao Jia, Associate Professor of Biostatistics, Columbia University School of Nursing.
References
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. The Lancet. 2013;382(9903):1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV/AIDS surveillance report 2007. Atlanta: U.S: Department of Health and Human Services, CDC; 2009. [Google Scholar]
- 3.Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61(3):341–8. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spirig R, Moody K, Battegay M, De Geest S. Symptom management in HIV/AIDS: advancing the conceptualization. ANS Adv Nurs Sci. 2005;28(4):333–44. doi: 10.1097/00012272-200510000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Leserman J, Jackson ED, Petitto JM, et al. Progression to AIDS: the effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61(3):397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Heaven CMMP. The relationship between patients’ concerns and psychological distress in a hospice setting. Psycho-Oncology. 1998;7(6):502–7. doi: 10.1002/(SICI)1099-1611(199811/12)7:6<502::AID-PON336>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Indyk DBR, Lachapelle S, Gordon G, Dewart T. A community-based approach to HIV case management: systematizing the unmanageable. Soc Work. 1993;38(4):380–7. [PubMed] [Google Scholar]
- 8.Corless IB, Bunch EH, Kemppainen JK, et al. Self-care for fatigue in patients with HIV. Oncol Nurs Forum. 2002;29(5):E60–9. doi: 10.1188/02.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- 9.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–75. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 10.Burgoyne R, Collins E, Wagner C, et al. The relationship between lipodystrophy-associated body changes and measures of quality of life and mental health for HIV-positive adults. Qual Life Res. 2005;14(4):981–90. doi: 10.1007/s11136-004-2580-2. [DOI] [PubMed] [Google Scholar]
- 11.Wantland DJ, Holzemer WL, Moezzi S, et al. A randomized controlled trial testing the efficacy of an HIV/AIDS symptom management manual. J Pain Symptom Manag. 2008;36(3):235–46. doi: 10.1016/j.jpainsymman.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Schnall R, Mosley JP, Iribarren SJ, et al. Comparison of a user-centered design, self-management app to existing mHealth apps for persons living with HIV. JMIR mHealth uHealth. 2015;3(3):e91. doi: 10.2196/mhealth.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muessig KE, Pike EC, Legrand S, Hightow-Weidman LB. Mobile phone applications for the care and prevention of HIV and other sexually transmitted diseases: a review. J Med Internet Res. 2013;15(1):e1. doi: 10.2196/jmir.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curioso WH, Mechael PN. Enhancing ‘M-health’ with south-to-south collaborations. Health Aff. 2010;29(2):264–7. doi: 10.1377/hlthaff.2009.1057. [DOI] [PubMed] [Google Scholar]
- 15.Schnall R, Cho H, Webel A. Predictors of willingness to use a smartphone for research in underserved persons living with HIV. Int J Med Inform. 2017;99:53–9. doi: 10.1016/j.ijmedinf.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schnall R, Bakken S, Rojas M, Travers J, Carballo-Dieguez A. mHealth technology as a persuasive tool for treatment, care and management of persons living with HIV. AIDS Behav. 2015;19(Suppl 2):81–9. doi: 10.1007/s10461-014-0984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenhart A, Hitlin P, Madden M. [Accessed 28 Mar 2010];Teens and Technology. 2005 http://www.pewinternet.org/Reports/2005/Teens-and-Technology/06-Communications-Tools-and-Teens/17-Cell-phone-text-messaging-emerges-as-a-formidable-force.aspx?r=1.
- 18.Pew Research Center. [Accessed 14 July 2016];Mobile Fact Sheet. 2016 http://www.pewinternet.org/fact-sheet/mobile/
- 19.Pew Research Center. [Accessed 14 July 2016];Mobile internet use on the rise among internet users. 2016 http://www.pewhispanic.org/2016/07/20/digital-divide-narrows-for-latinos-as-more-spanish-speakers-and-immigrants-go-online/ph_2016-07-21_broadband-08/
- 20.Pew Research Center. [Accessed 14 July 2016];Home Broadband 2015. 2015 http://www.pewinternet.org/2015/12/21/home-broadband-2015/
- 21.Cho H, Milian L, Schnall R. Card sorting of symptom self-management strategies to inform the development of a mHealth App in underserved persons living with HIV AMIA 2016 Annual Symposium. 2016 [Google Scholar]
- 22.Cho H, Milian L, Schnall R. Use of card sorting for incorporating symptom self-management strategies into a mHealth app for underserved persons living with HIV. IEEE Wireless Health 2016 Conference. 2016 [Google Scholar]
- 23.Cho H, Rojas H, Fulmer C, Schnall R. Usability evaluation of a prototype mHealth app for symptom self-management in underserved persons living with HIV AMIA 2017 Annual Symposium. 2017 [Google Scholar]
- 24.Holzemer WL, Hudson A, Kirksey KM, Hamilton MJ, Bakken S. The revised sign and symptom check-list for HIV (SSC-HIVrev) J Assoc Nurses AIDS Care. 2001;12(5):60–70. doi: 10.1016/s1055-3290(06)60263-x. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Schnall R, Liu J, Cho H, et al. A health-related quality-of-life measure for use in patients with HIV: a validation study. AIDS Patient Care STDS. 2017;31(2):43–8. doi: 10.1089/apc.2016.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hays RD, Sherbourne CD, Mazel RM. The rand 36-item health survey 1.0. Health Econ. 1993;2(3):217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 28.Chang C-H, Wright BD, Cella D, Hays RD. The SF-36 physical and mental health factors were confirmed in cancer and HIV/AIDS patients. J Clin Epidemiol. 2007;60(1):68–72. doi: 10.1016/j.jclinepi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Bakken S, Holzemer WL, Brown M-A, et al. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDs. 2000;14(4):189–97. doi: 10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- 30.Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5(2):74–9. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- 31.Yen P-Y, Sousa KH, Bakken S. Examining construct and predictive validity of the health-IT usability evaluation scale: confirmatory factor analysis and structural equation modeling results. J Am Med Inform Assoc JAMIA. 2014;21(e2):e241–8. doi: 10.1136/amiajnl-2013-001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. 2012;18(2):115–8. doi: 10.1258/jtt.2011.111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faridi Z, Liberti L, Shuval K, et al. Evaluating the impact of mobile telephone technology on type 2 diabetic patients’ self-management: the NICHE pilot study. J Eval Clin Pract. 2008;14(3):465–9. doi: 10.1111/j.1365-2753.2007.00881.x. [DOI] [PubMed] [Google Scholar]
- 35.Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–82. doi: 10.1007/s13142-015-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med. 2000;50(11):1599–605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- 37.Miners A, Phillips A, Kreif N, et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1(1):e32–40. doi: 10.1016/S2352-3018(14)70018-9. [DOI] [PubMed] [Google Scholar]
- 38.Schnall R, Cho H, Liu J. Validation of the health information technology usability evaluation scale (health-ITUES) for usability Assessment of Mobile Health Technology. JMIR mHealth uHealth. doi: 10.2196/mhealth.8851. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adolescents. [Accessed 17 July 2017];PoAGfAa. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016 http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 40.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1–34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 41.Schnall R, Wantland D, Velez O, Cato K, Jia H. Feasibility testing of a Web-based symptom self-management system for persons living with HIV. J Assoc Nurses AIDS Care JANAC. 2014;25(4):364–71. doi: 10.1016/j.jana.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson KB, Patterson BL, Ho Y-X, et al. The feasibility of text reminders to improve medication adherence in adolescents with asthma. J Am Med Inform Assoc JAMIA. 2016;23(3):449–55. doi: 10.1093/jamia/ocv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–7. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green LW. Public health asks of systems science: to advance our evidence-based practice, can you help us get more practice-based evidence? Am J Public Health. 2006;96(3):406–9. doi: 10.2105/AJPH.2005.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]