Abstract
Background & Aims
We aimed to evaluate the safety and effectiveness of 12 or 24 weeks treatment with ledipasvir and sofosbuvir, with or without ribavirin, in treatment-experienced patients with hepatitis C virus (HCV) genotype 1 infection and cirrhosis in routine clinical practice. Patients were followed in a multi-center, prospective, observational cohort study (HCV-TARGET).
Methods
We collected data from 667 treatment-experienced adults with chronic genotype 1 HCV infection who began treatment with ledipasvir and sofosbuvir, with or without ribavirin, from 2011 through September 15, 2016, according to the regional standards of care, at academic (n=39) and community (n=18) centers in the United States, Canada, Germany, and Israel. Information was collected from medical records and abstracted into a unique centralized data core. Independent monitors systematically reviewed data entries for completeness and accuracy. Demographic, clinical, adverse event, and virologic data were collected every 12 weeks during treatment and during the follow-up period. The primary efficacy endpoint was sustained virologic response, defined as a level of HCV RNA below the lower limit of quantification or undetectable at a minimum 64 days after the end of treatment (SVR12). The per-protocol population (n=610) was restricted to patients who completed 12 or 24 weeks of treatment (±2 weeks) and had final virologic outcomes available.
Results
The per-protocol analysis revealed that 579 patients (93.8%) achieved an SVR12, including 50/51 patients who received ledipasvir and sofosbuvir for 12 weeks (98%), 384/408 patients who received ledipasvir and sofosbuvir for 24 weeks (94.1%), 68/70 patients who received ledipasvir and sofosbuvir with ribavirin for 12 weeks (97.1%), and 57/60 patients who received ledipasvir and sofosbuvir with ribavirin for 24 weeks (95%). On multivariate analysis, neither treatment duration nor the addition of ribavirin was associated with SVR12. Compensated cirrhosis (odds ratio [OR] compared to decompensated cirrhosis, 2.41; 95% CI, 1.16–5.02), albumin ≥ 3.5 g/dL (OR, 3.15; 95% CI 1.46–6.80), or total bilirubin ≤ 1.2 mg/dL (OR 3.34; 95% CI, 1.59–7.00) were associated with SVR12.
Conclusions
In an analysis of safety and effectiveness data from the HCV-TARGET study, we found treatment with ledipasvir and sofosbuvir, with or without ribavirin, to be effective and well tolerated by treatment-experienced patients with genotype 1 HCV infection and compensated cirrhosis. There were no significant differences in rate of SVR12 among patients treated with ledipasvir and sofosbuvir for 12 or 24 weeks, with or without ribavirin. Patients with decompensated cirrhosis appear to benefit from the addition of ribavirin or extension of ledipasvir and sofosbuvir treatment to 24 weeks. ClinicalTrials.gov no: NCT10474811.
Keywords: Real world, liver disease, viral hepatitis, therapy, HCV-TARGET
Introduction
Chronic hepatitis C virus (HCV) infection is a common bloodborne infection affecting an estimated 71 million persons globally, and is associated with substantial morbidity and mortality due to complications of end-stage-liver disease and hepatocellular carcinoma (HCC).1,2 Achieving a sustained virologic response (SVR) following antiviral treatment for HCV is associated with a decrease in liver-related morbidity and mortality.3,4
Among the seven HCV genotypes (GT), HCV GT1 is the most prevalent with an estimated 83.4 million GT1-infected persons worldwide, representing 46.2% of the total.5,6 Due in part to poor tolerability and low efficacy of historic regimens such as pegylated interferon (PegIFN) plus ribavirin (RBV), few patients initiated and completed treatment, of whom only a subset achieved SVR.7 The emergence of all-oral, direct-acting antiviral drug (DAA) regimens with superior safety and efficacy in 2014 prompted the expansion of HCV treatment in real-world clinical settings.8,9 The fixed dose combination of ledipasvir (LDV), a NS5A inhibitor, and sofosbuvir (SOF), a pangenotypic NS5B nucleotide polymerase inhibitor, was approved by the U.S. FDA in 2014.10
For patients with HCV GT1, the safety and efficacy of LDV/SOF was demonstrated in a series of three phase 3 clinical trials, including ION-1, ION-2, and ION-3.11–13 Although overall SVR rates for HCV GT1 exceeded 90%, differences in SVR were observed among GT1 treatment-experienced (TE) patients with compensated cirrhosis, in whom 12 weeks of LDV/SOF or LDV/SOF/RBV resulted in SVR in 18/22 (82%) and 19/22 (86%) patients, respectively; and 24 weeks of LDV/SOF or LDV/SOF/RBV achieved SVR in 22/22 (100%) and 22/22 (100%) patients, respectively.12 A subsequent French trial of GT1 cirrhotic patients who failed telaprevir or boceprevir-based triple therapy regimens, as well as post-hoc pooled analysis of phase 2/3 trials revealed similar SVR rates among patients receiving LDV/SOF for 24 weeks or LDV/SOF/RBV for 12 weeks. 14–15 On this basis, a 12-week regimen of LDV/SOF/RBV or 24-week regimen of LDV/SOF was recommended for GT1 cirrhotic patients who failed prior PegIFN/RBV in treatment guidelines of the American Association for the Study of Liver Diseases (AASLD), Infectious Diseases Society of America (IDSA), Canadian Association for the Study of Liver (CASL), and European Association for the Study of Liver (EASL).16–18
Data confirming real-world results in this important subgroup of patients outside clinical trials are limited. The aim of this study was to investigate the safety and effectiveness of LDV/SOF for 12 or 24 weeks, with or without RBV, in the treatment of HCV GT1 TE cirrhotic patients in HCV-TARGET, an international, prospective observational study within a consortium of academic and community sites, designed to examine all-oral DAA regimens in routine clinical practice.19
Materials and Methods
Patients
HCV-TARGET is a prospective, longitudinal, observational study of patients with chronic HCV undergoing antiviral therapy within an international consortium of academic (n=39) and community (n=18) centers from the U.S., Canada, Germany, and Israel. Patients with chronic GT1 HCV infection age 18 years or older who had clinical evidence of cirrhosis, failed prior interferon-based antiviral therapy (PegIFN +/− ribavirin, boceprevir plus PegIFN/RBV, telaprevir plus PegIFN/RBV, sofosbuvir plus PegIFN/RBV, simeprevir plus PegIFN/RBV), and initiated antiviral therapy with LDV/SOF or LDV/SOF/RBV. All patients provided informed consent within 4 weeks of treatment initiation. This study analysis was restricted to patients who started LDV/SOF-based treatment prior to September 15, 2016.
Treatment regimen
The choice of treatment regimen and duration was made by the treating physician. All patients included this analysis received a fixed-dose combination of one LDV/SOF tablet (90 mg/400 mg) taken once daily. Dosing for RBV was determined at the discretion of the treating physician, although was most commonly weight-based (1000 mg daily in divided doses if <75 kg; 1200 mg daily in divided doses if ≥75 kg). Treatment duration was defined as 12 weeks (±7 days), 24 weeks (±7 days), or other duration.
Data collection
Redacted medical records including standard demographic, clinical, and virologic data, were prospectively collected by a Centralized Chart Data Abstraction Team of trained coders at the Clinical Coordinating Center at the University of Florida using a novel, standardized source data abstraction that has been described previously.20 The collected data were managed using secure, web-based Research Electronic Data Capture (REDCap) tools hosted at the University of North Carolina, Chapel Hill, and then reviewed for completeness and accuracy.21
Baseline and updated demographic, clinical, adverse event, and virologic data were collected at the time of treatment initiation, and every 12 weeks if available through end of treatment and post-treatment. Cirrhosis was defined by previously established criteria of the HCV-TARGET consortium22–24; the primary indicator of cirrhosis was a liver biopsy with METAVIR score F4. In its absence, cirrhosis was established based on transient elastography (Fibroscan) demonstrating median liver stiffness measurement ≥12.5kPa, METAVIR score F3 with at least one secondary indicator (serum fibrosis scores above thresholds for cirrhosis, signs of portal hypertension including esophagogastric varices, portal gastropathy, or platelet count <140,000/μL), or ≥2 secondary indicators. Decompensated cirrhosis was defined by the presence of prior or current ascites, hepatic encephalopathy, spontaneous bacterial peritonitis, hepatic hydrothorax, variceal hemorrhage, or the documentation of the use of medications with indication specific to these complications. Adverse events (AE) were defined as any new symptom or event captured in the medical record during the treatment period, independent of the requirement for dose reduction, treatment discontinuation, or a corrective prescription medication. All AEs were entered into the centralized database and coded in accordance with MedDRA (Medical Dictionary for Regulatory Activities) terminology. The AE of anemia was defined as either physician-reported anemia event, RBV dose reduction, administration of an erythropoietin-stimulating agent (ESA), or blood transfusion. Serious adverse events (SAEs) or suspected unexpected serious adverse reactions (SUSARs) were defined as any AE requiring hospitalization or met criteria for expedited reporting in accordance with FDA form MedWatch 3500.
Efficacy and safety endpoints
The primary efficacy endpoint was SVR, which was defined as HCV RNA below lower limit of quantification or undetectable at minimum 64 days post-end of treatment (SVR12), to account for window of evaluation surrounding clinic visits. The per protocol population (PP; N=610) for analysis was restricted to patients who completed 12 or 24 weeks of treatment (±2 weeks) and had final virologic outcomes available. Safety endpoints were evaluated in patients who completed 12 or 24 weeks of treatment (±2 weeks) and had either final virologic outcomes available or who died or were lost during post treatment follow up (counted as non-virological failures) and made up the Evaluable Population (EP; N=634).
Statistical analysis
Demographics, baseline laboratory values, treatment outcomes, and frequencies of adverse events were collected and analyzed in EP population and according to duration of treatment (12 and 24 weeks). Virological outcomes and associations between SVR and baseline covariates of interest were evaluated by Chi-square and logistic regression in PP population, focusing on patients who received either 12 or 24 week regimens (n=589). Covariates identified as significant on logistic regression without influential collinearity were then singularly evaluated in multivariate models (age and sex always included) using Firth penalized maximum likelihood estimation of predictors of SVR for LDV/SOF patients. Predictor variables of interest were selected a priori, and included: gender, age, race, MELD, albumin (g/dL), platelet count (1000/uL), total bilirubin (mg/dL), RBV dose at baseline (mg/kg), RBV dose reduction, and history of hepatic decompensation. Patients who failed to complete treatment for any reason, or were lost to follow-up during treatment, were excluded from analysis. Results are presented as odds ratios (OR) with corresponding 95% confidence interval (CI). Statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
Study oversight
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by an independent ethics committee at each participating center, or by a central institutional review board. All patients who were enrolled in this study provided written informed consent prior to participation. All authors had access to the study data and approved the final version of the manuscript.
Results
Patient population and disposition
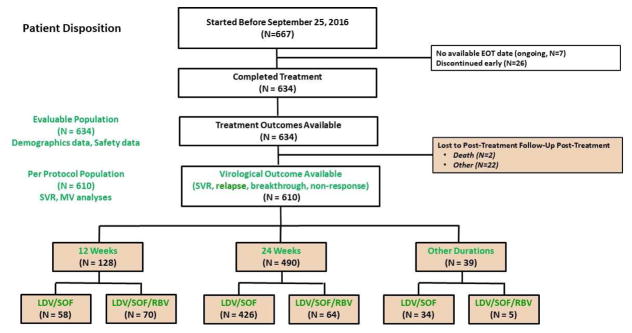
Before September 15, 2016, a total of 667 TE patients with cirrhosis started treatment with LDV/SOF with or without ribavirin. Of those, 634 completed an assigned regimen; 22 were lost to post-treatment follow-up, and 2 died prior to evaluation of virological data. The PP population with available virologic outcomes included 610 patients, including 128 patients who completed LDV/SOF for 12 weeks (58 without RBV, 70 with RBV), 490 who completed LDV/SOF for 24 weeks (426 without RBV, 64 with RBV), and 39 who completed LDV/SOF for other duration (34 without RBV, 5 with RBV). (Figure 1)
Figure 1.
Consort Diagram. Patient flow from treatment initiation, completion, and verification of virologic outcome. Pt = patient; EOT = end of treatment; Tx = treatment; SVR = sustained virologic response; MV = multivariate; LDV = ledipasvir; SOF = sofosbuvir; RBV = ribavirin.
Baseline demographic, clinical, and laboratory characteristics of participants are summarized in Table 1. All patients had GT1 HCV (66% GT1a), previously failed antiviral therapy, and had clinical evidence of cirrhosis. The study population was 66% male, 24% age 65 years or older, 75% White, 16% Black or African-American, and 66% GT1a HCV. A significant proportion of enrolled patients had prior DAA failure (27.0%), a history of hepatic decompensation (40%), or history of orthotopic liver transplantation (OLT) (14%). Most patients had elevated LFTs >2x upper limit of normal, preserved liver synthetic function, and MELD score <10 (range 6–25).
Table 1.
Baseline clinical and laboratory characteristics: Evaluable Population
| LDV/SOF | LDV/SOF/RBV | OVERALL | |||||
|---|---|---|---|---|---|---|---|
| 12 | 24 | Other | 12 | 24 | Other | Overall | |
| 53 (100%) | 424 (100%) | 18 (100%) | 70 (100%) | 64 (100%) | 5 (100%) | 634 (100%) | |
| SEX | |||||||
| Male | 39 (74%) | 273 (64%) | 11 (61%) | 49 (70%) | 43 (67%) | 4 (80%) | 419 (66%) |
| AGE | |||||||
| 65+ | 12 (23%) | 106 (25%) | 5 (28%) | 15 (21%) | 14 (22%) | 1 (20%) | 153 (24%) |
| RACE | |||||||
| White | 37 (70%) | 319 (75%) | 11 (61%) | 60 (86%) | 46 (72%) | 4 (80%) | 477 (75%) |
| Black or African American | 9 (17%) | 74 (17%) | 5 (28%) | 8 (11%) | 5 (8%) | 1 (20%) | 102 (16%) |
| Other / Not Reported | 7 (13%) | 31 (7%) | 2 (11%) | 2 (3%) | 13 (20%) | 0 (0%) | 55 (9%) |
| HCV GENOTYPE | |||||||
| 1a | 38 (72%) | 288 (68%) | 11 (61%) | 41 (59%) | 38 (59%) | 5 (100%) | 421 (66%) |
| 1b | 10 (19%) | 94 (22%) | 4 (22%) | 24 (34%) | 17 (27%) | 0 (0%) | 149 (24%) |
| 1 subtype not specified | 5 (9%) | 42 (10%) | 3 (17%) | 5 (7%) | 9 (14%) | 0 (0%) | 64 (10%) |
| Prior triple Therapy Experience | 10 (19%) | 122 (29%) | 6 (33%) | 14 (20%) | 20 (31%) | 2 (40%) | 174 (27%) |
| Prior Second Generation Experience | 0 (0%) | 2 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0%) |
| Treatment Experienced | 53 (100%) | 424 (100%) | 18 (100%) | 70 (100%) | 64 (100%) | 5 (100%) | 634 (100%) |
| Cirrhosis | 53 (100%) | 424 (100%) | 18 (100%) | 70 (100%) | 64 (100%) | 5 (100%) | 634 (100%) |
| History of Decompensation | 9 (17%) | 171 (40%) | 8 (44%) | 19 (27%) | 40 (63%) | 4 (80%) | 251 (40%) |
| History of HCC | 5 (9%) | 37 (9%) | 0 (0%) | 13 (19%) | 13 (20%) | 1 (20%) | 69 (11%) |
| HIV Co-Infection | 2 (4%) | 10 (2%) | 1 (6%) | 1 (1%) | 4 (6%) | 0 (0%) | 18 (3%) |
| Liver Transplant | 11 (21%) | 29 (7%) | 1 (6%) | 29 (41%) | 17 (27%) | 2 (40%) | 89 (14%) |
| ALT (IU/L) Median(Range) | 62 (17–228) | 64 (7–532) | 36 (21–349) | 67 (11–284) | 74 (15–235) | 51 (43–116) | 64 (7–532) |
| T. BILIRUBIN (mg/dL) Median(Range) | 0.7 (0.2–2.3) | 1 (0.2–11.3) | 0.8 (0.25–5.1) | 0.9 (0.3–2.6) | 1.1 (0.4–6.2) | 0.6 (0.5–10.3) | 0.9 (0.2–11.3) |
| ALBUMIN (g/dL) Median(Range) | 3.9 (2.1–5.3) | 3.7 (1.5–5.1) | 3.45 (2–4.3) | 4 (2–4.9) | 3.6 (1.7–4.5) | 3.9 (2.9–4) | 3.7 (1.5–5.3) |
| PLATELETS (x10/uL) Median(Range) | 134 (45–332) | 106 (23–348) | 127.5 (51–237) | 118.5 (42–279) | 84 (32–545) | 125 (26–204) | 109.5 (23–545) |
| MELD Score Median(Range) | 7 (6–11) | 8 (6–25) | 7 (6–19) | 8 (6–14) | 9 (6–21) | 8 (7–12) | 8 (6–25) |
| HCV RNA (log10 IU/mL) Median(Range) | 6.4 (4.6–7.6) | 6.1 (1.2–7.5) | 6.2 (5.0–7.1) | 6.3 (4.2–7.8) | 6.1 (4.2–7.7) | 6.3 (3.8–6.7) | 6.1 (1.2–7.8) |
The disposition of all treated patients in this cohort are summarized in Table 2. Of the 667 patients who started LDV/SOF with or without RBV, 26 (3.9%) discontinued treatment early, including 23 on LDV/SOF (4.4%) and 3 on LDV/SOF/RBV (2.1%). Ten patients (1.4%) discontinued due to adverse events or death including 7 on LDV/SOF (1.4%) and 3 on LDV/SOF/RBV (2.1%). Two patients (0.3%) discontinued early due to lack of efficacy, including 2 on LDV/SOF (0.4%) and none on LDV/SOF/RBV. Fourteen patients (2%) discontinued for administrative reasons, including 14 on LDV/SOF (2.7%) and none on LDV/SOF/RBV. In total, 634 (95.1%) completed treatment, including 495 patients on LDV/SOF (94.3%) and 139 patients on LDV/SOF/RBV (97.9%). Eight enrolled patients died during or following completion of treatment, including six on LDV/SOF (1.2%) and two on LDV/SOF/RBV (1.4%).
Table 2.
Patient disposition: Patients who started treatment with LDV/SOF +/− RBV prior to September 15, 2016
| Treatment Arm - No. Patients (%) | |||||||
|---|---|---|---|---|---|---|---|
| LDV/SOF | LDV/SOF/RBV | OVERALL | |||||
| 12 | 24 | Other | 12 | 24 | Other | Overall | |
| N=58 | N=426 | N=34 | N=70 | N=64 | N=8 | N=667 | |
| Completed Treatment | 53 (91.4%) | 424 (99.5%) | 18 (52.9%) | 70 (100%) | 64 (100%) | 5 (62.5%) | 634 (95.1%) |
| Discontinued Treatment Early | 5 (8.6%) | 2 (0.5%) | 16 (47.1%) | 0 (0.0%) | 0 (0.0%) | 3 (37.5%) | 26 (3.9%) |
| Adverse events | 2 (3.4%) | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 2 (25.0%) | 5 (0.7%) |
| Lack of efficacy | 1 (1.7%) | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| Withdrawal by subject | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Death | 1 (1.7%) | 0 (0.0%) | 3 (8.8%) | 0 (0.0%) | 0 (0.0%) | 1 (12.5%) | 5 (0.7%) |
| Non-compliance with study drug | 0 (0.0%) | 0 (0.0%) | 2 (5.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| Lost to treatment follow-up | 1 (1.7%) | 2 (0.5%) | 5 (14.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 8 (1.2%) |
| Other | 0 (0.0%) | 0 (0.0%) | 4 (11.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (0.6%) |
| On treatment | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (1.0%) |
| Treatment Arm - No. Patients (%) | |||||||
|---|---|---|---|---|---|---|---|
| LDV/SOF | LDV/SOF/RBV | OVERALL | |||||
| 12 | 24 | Other | 12 | 24 | Other | Overall | |
| N=53 | N=424 | N=18 | N=70 | N=64 | N=5 | N=634 | |
| Completed follow-up | 53 (100%) | 424 (100%) | 18 (100%) | 70 (100%) | 64 (100%) | 5 (100%) | 634 (100%) |
| Virologic outcome available | 51 (96.2%) | 408 (96.2%) | 16 (88.9%) | 70 (100%) | 60 (93.8%) | 5 (100%) | 610 (96.2%) |
| Achieved SVR | 50 (94.3%) | 384 (90.6%) | 15 (83.3%) | 68 (97.1%) | 57 (89.1%) | 5 (100%) | 579 (91.3%) |
| Non-response | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) |
| Relapse | 1 (1.9%) | 23 (5.4%) | 1 (5.6%) | 2 (2.9%) | 3 (4.7%) | 0 (0.0%) | 30 (4.7%) |
| Viral breakthrough | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Lost to post-treatment follow-up | 2 (3.8%) | 15 (3.5%) | 1 (5.6%) | 0 (0.0%) | 4 (6.3%) | 0 (0.0%) | 22 (3.5%) |
| Died in post-treatment follow-up | 0 (0.0%) | 1 (0.2%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| Died after post-treatment follow-up | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Treatment Response
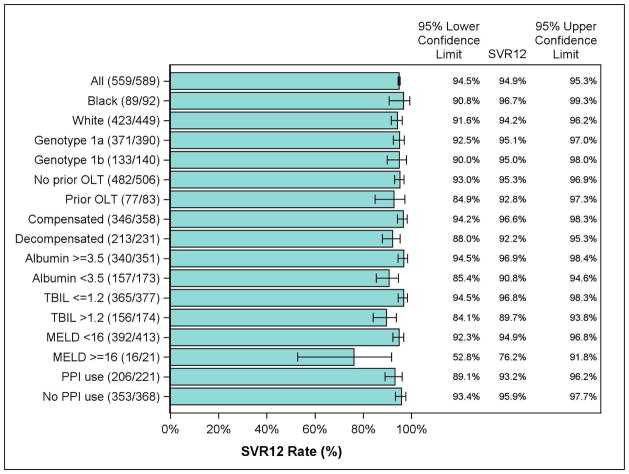
The SVR12 rates for the PP population are shown in Figure 2. Overall, 579/610 patients (93.8%) achieved SVR12, including 50/51 (98%) on LDV/SOF (12 weeks), 384/408 (94.1%) on LDV/SOF (24 weeks), 68/78 (97.1%) on LDV/SOF/RBV (12 weeks), and 57/60 (95%) on LDV/SOF/RBV (24 weeks). Patients with a history of hepatic decompensation were more likely to receive 24 weeks and/or RBV, and had an overall SVR of 92.2% (213/231), which was lower than among patients with compensated cirrhosis (346/358, 96.6%). Patients with GT1a HCV achieved similar SVR (371/390, 95.1%) as patients with GT1b HCV across regimens (133/140, 95.0%). Patients with prior OLT achieved lower SVR (77/83, 92.8%) than patients without prior OLT (482/506, 95.3%). Lower SVR was also observed among patients with markers of advanced cirrhosis including albumin <3.5 (157/173, 90.8%) and total bilirubin > 1.2 (156/174, 89.7%) compared with patients with markers of preserved liver function such as albumin ≥ 3.5 (340/351, 96.9%) and total bilirubin ≤ 1.2 (365/377, 96.8%). SVR stratified by baseline characteristics and regimen is summarized in Table 3.
Figure 2.
Rates of SVR12 for the Per Protocol population. Presented data restricted to patients receiving 12 or 24 weeks of treatment only. SVR12 = sustained virologic response; OLT = orthotopic liver transplantation; TBIL = total bilirubin; MELD = Model for End-stage Liver Disease; PPI = proton pump inhibitor.
Table 3.
Per Protocol SVR12 percentage rates with 95% CIa and sample sizes (stratified by regimen and duration)
| LDV/SOF (12 wk) N=51 | LDV/SOF/RBV (12 wk) N=70 | LDV/SOF (24 wk) N=408 | LDV/SOF/RBV (24 wk) N=60 | TOTAL N=589 | |
|---|---|---|---|---|---|
| Compensated Cirrhosis Overall | 100 (92,100) [43/43] | 96.1 (86.5,100) [49/51] | 96.7 (93.6,98.6) [234/242] | 90.9 (70.8,98.9) [20/22] | 96.6 (94.2,98.3) [346/358] |
| Incl. Albumin ≤3.5 and Tbilirubin ≥1.2 | 100b (2.5, 100) [1/1] | 100b (15.8,100) [2/2] | 96.0 (79.7, 100) [24/25] | 100b (15.8, 100) (2/2] | 96.7 (82.8,99.9) [29/30] |
| Incl. Albumin >3.5 and Tbilirubin <1.2 | 100 (88.4,100) [30/30] | 100 (89.4,100) [33/33] | 96.7 (92.4,98.9) [146/151] | 91.7 (61.5,99.8) [11/12] | 97.3 (94.3,99.0) [220/226] |
| Decompensated Cirrhosis | 87.5 (47.4,99.7) [7/8] | 100 (82.4,100) [19/19] | 90.4 (84.8, 94.4) [150/166] | 97.4 (86.2,99.9) [37/38] | 92.2 (88.0,95.3) [213/231] |
| Post-Liver Transplant | 90.9 (58.7,100) [10/11] | 96.6 (82.2,100) [28/29] | 88.9 (70.8,97.7) [24/27] | 93.8 (69.8,99.8) [15/16] | 92.8 (84.9,97.3) [77/83] |
95% confidence intervals capped at 100%.
Based on 5 or fewer observations.
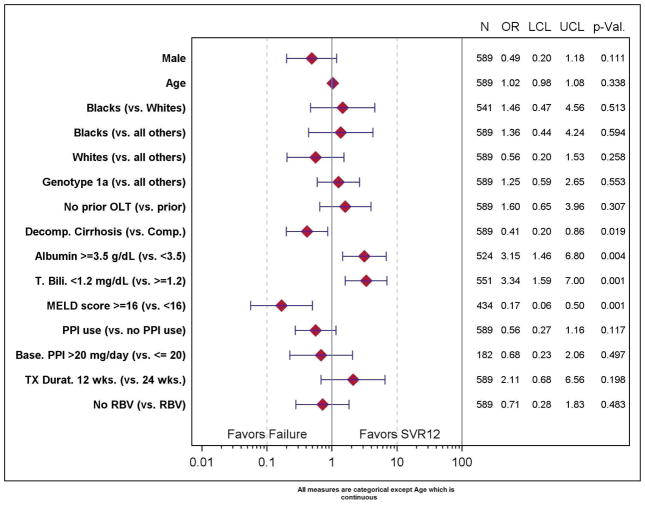
In the multivariable analysis, neither treatment duration nor the addition of RBV was predictive of SVR (p=NS). Only patients with compensated rather than decompensated cirrhosis (OR 2.41, 95% CI 1.16–5.02), albumin ≥ 3.5 (OR 3.15, 95% CI 1.46–6.80), or total bilirubin ≤ 1.2 (OR 3.34, 95% CI 1.59–7.00) were associated with higher SVR (Figure 3). A trend towards lower SVR was observed among males (OR 0.49, 95% CI 0.20–1.18) and patients with recorded use of proton pump inhibitors (OR 0.56, 95% CI 0.27–1.16) but this did not reach statistical significance.
Figure 3.
*Patients who discontinued early for any reason, did not have virological outcome or were treated with other duration were excluded. N = number observed; Odds Ratio (OR), 95% Confidence interval (LCL = lower confidence limit, UCL = upper confidence limit), and p-value. ** Estimated with logistic regression with the predictor of interest, age, and gender in the model. *** Treatment weeks 12 and 24 only.
Safety
Safety outcomes and adverse events resulting in treatment discontinuation or death are summarized in Supplementary Tables 1 and 2. Only 10/667 (1.4%) patients discontinued treatment prematurely due to an adverse event or death, with similar proportions observed among patients on LDV/SOF (7/525, 1.4%) and LDV/SOF/RBV (3/142, 2.1%). Reasons for drug discontinuation from LDV/SOF, each reported in one patient, included atrioventricular block, metastatic breast cancer, HCC, intraventricular hemorrhage, multi-organ failure, septic shock, and subdural hematoma. Reasons for drug discontinuation from LDV/SOF/RBV included hemolytic anemia, gastrointestinal perforation and road traffic accident.
Seven patients (0.1%) who started LDV/SOF or LDV/SOF/RBV died. Reported causes of death included metastatic breast cancer, HCC, intraventricular hemorrhage, multi-organ failure, septic shock, subdural hematoma, and road traffic accident. None of these events was considered treatment-related by the treating physician.
Adverse events (AEs) were reported in 484/634 (76.8%) patients of the Evaluable Population, and were lower among patients on LDV/SOF (363/495, 73.3%) compared with those on LDV/SOF/RBV (121/139, 87.1%). The most common AEs, reported in ≥ 10% of the patients, were fatigue, headache, and infections and infestations. Anemia events were reported in 49 patients (7.8%) overall, and observed more commonly in patients on LDV/SOF/RBV (42/139, 30.6%) than in patients on LDV/SOF alone (7/495, 1.4%). Management of anemia events is summarized in Supplementary Table 3.
Discussion
The results of this large, international observational real-world study of 667 HCV GT1 TE cirrhotic patients in HCV-TARGET demonstrate high efficacy and tolerability. Overall, the SVR12 rate in this traditionally difficult-to-treat population was 93.8% (579/610), including 98% (50/51) in patients on LDV/SOF (12 weeks), 94.1% (384/408) in patients on LDV/SOF (24 weeks), 97.1% (68/70) in patients on LDV/SOF/RBV (12 weeks), and 95% (57/60) in patients on LDV/SOF/RBV (24 weeks). Neither treatment duration nor the addition of RBV was associated with higher SVR12. On multivariate analysis, only decompensated cirrhosis and markers of impaired liver function (albumin < 3.5 and total bilirubin > 1.2) were associated with lower SVR12. Both LDV/SOF and LDV/SOF/RBV were well tolerated; only 10 of 667 (1.4%) patients discontinued treatment prematurely due to an adverse event or death, although adverse events (AEs) were more commonly reported among patients on LDV/SOF/RBV (121/139, 87.1%) than patients on LDV/SOF alone (363/495, 73.1%). Furthermore, anemia was observed mostly among patients on LDV/SOF/RBV (42/139, 30.6%), and 7/495 (1.4%) on LDV/SOF.
Several randomized controlled trials have evaluated LDV/SOF with or without RBV, and for 12 or 24 weeks, in TE patients with GT1 HCV infection, including the ION-1 and ION-2 protocols.12 A double-blind, placebo-controlled French trial of GT1 cirrhotic patients who failed telaprevir or boceprevir-based triple therapy regimens revealed similar SVR between LDV/SOF/RBV for 12 weeks (74/77, 96%) and LDV/SOF for 24 weeks (75/77, 97%), providing evidence for the comparability of the addition of RBV or extension of treatment to 24 weeks in this population.14 A post-hoc pooled analysis of seven phase 2 and 3 trials evaluating LDV/SOF or LDV/SOF/RBV for GT1 cirrhotic patients revealed an overall SVR of 96% (493/573 patients), and 95% in TE patients. Small differences in SVR were observed between 12 week (95%) and 24 week (98%) duration, as well as between RBV-free (95%) and RBV-containing (97%) regimens. 15
Few real-world studies have evaluated the efficacy and safety of LDV/SOF with or without RBV in GT1 TE cirrhotic patients outside clinical trials. The Spanish HEPA-C cohort study of 937 GT1 patients (46.7% cirrhotic) revealed important differences in SVR by treatment duration and the addition of RBV. SVR12 was observed in 99/108 (91.7%) patients on LDV/SOF (12 weeks), 156/162 (96.3%) patients on LDV/SOF (24 weeks), 515/541 (95.2%) patients on LDV/SOF/RBV (12 weeks), and 120/126 (95.2%) patients on LDV/SOF/RBV (24 weeks). 25 A retrospective analysis of national U.S. VA cohort data evaluating 17,487 patients undergoing oral DAA therapy also revealed important differences in SVR among GT1 TE cirrhotic patients. SVR12 was observed in 94.5% of 122 patients on LDV/SOF (12 weeks), 93.7% of 332 patients on LDV/SOF (24 weeks), 89.4% of 668 patients on LDV/SOF/RBV (12 weeks), and 94.1% of 85 patients on LDV/SOF/RBV (24 weeks). 26 In addition, two meta-analyses evaluating the efficacy and safety of LDV/SOF with or without RBV for GT1 HCV, one pooling 7 studies (n=2626 patients) and a second pooling 10 studies (n=2248 patients), revealed the absence of improved SVR with the addition of RBV or treatment extension to 24 weeks, although subgroup analyses restricted to TE patients with cirrhosis were not performed. 27–28
In this multinational real-world study in patients undergoing antiviral therapy in the U.S., Canada, Germany, and Israel, PP analysis reveals excellent rates of SVR12 with LDV/SOF in GT1 TE cirrhotic patients, with or without RBV, and regardless of 12 or 24-week treatment duration. Only patients with decompensated cirrhosis or markers of impaired liver function demonstrated lower SVR with LDV/SOF for 12 weeks; therefore these data support current guideline recommendations for LDV/SOF/RBV for 12 weeks (or LDV/SOF for 24 weeks) in this population, although with careful consideration of baseline hemoglobin and/or risk factors for anemia.
This study has several strengths. The HCV-TARGET consortium represents one of the largest reported real-world prospective observational cohorts of GT1 TE cirrhotic patients undergoing LDV/SOF treatment, with or without RBV, and for treatment duration of 12 or 24 weeks, with patient numbers exceeding those enrolled in clinical trials. This study included a large proportion of patients who were under-represented in clinical trials, including those who were DAA-experienced (27%), had a history of hepatic decompensation (40%), or cirrhosis post-OLT (14%). A significant proportion of patients were over age 65 years (24%), and a large proportion of the cohort were Black or African-American (16%), a demographic group traditionally under-represented in randomized controlled trials, including the French SIRIUS protocol (3% Black) 14 and phase 2 and 3 pooled post-hoc analysis (5% Black).15
Due to prospective observational cohort design without randomization, selection bias in choice of regimen based on characteristics predictive of treatment outcome could not be excluded. Furthermore, week 4 on-treatment HCV RNA values were not routinely collected, and data addressing pre-treatment IL28B genotype and NS5A resistance associated mutations were not available, although they may have provided valuable additional information to clarify the role of both baseline hosts and viral characteristics, as well as on-treatment viral kinetics, in DAA treatment outcomes in these patients. Finally, this analysis was restricted to patients who completed 12 or 24 weeks of LDV/SOF-based regimens per protocol, as a small number of patients who failed to complete a full treatment course or were lost to follow-up were excluded; therefore, attrition bias could not be excluded. However, sensitivity testing for SVR12 and subgroup analyses revealed no significant change in results.
In summary, in this large, multinational prospective observational cohort study, the all-oral combination of LDV/SOF was safe and effective for treatment of HCV GT1 infection in treatment-experienced patients with both compensated and decompensated cirrhosis. No significant differences in SVR were observed in patients treated with or without RBV, or treated with 12 or 24 weeks duration. Although the SVR rates in this traditionally difficult-to-treat population were high and comparable to those reported in randomized controlled trials, lower SVR12 was observed in patients with decompensated cirrhosis and markers of impaired liver function (serum albumin < 3.5, total bilirubin > 1.2). Although new oral DAA regimens such as glecaprevir/pibrentasvir and sofosbuvir/velpatasvir/voxilaprevir have been associated with encouraging efficacy and safety in phase 3 registration trials among GT1 TE cirrhotic patients, ongoing concerns regarding the safety of protease-inhibitor based regimens in cirrhotic patients with impaired hepatic function remain. Additional datasets evaluating the role of treatment duration and RBV in nucleos(t)ide polymerase inhibitor/NS5A inhibitor combination regimens in this population are needed, and continue to be of high clinical relevance.
Supplementary Material
Significance of this study.
What is already known on this subject
Due to a high efficacy in clinical trials, the all-oral combination of ledipasvir and sofosbuvir (LDV/SOF) for 12 weeks is currently considered standard of care in patients with HCV genotype 1 infection.
Based on limited data from clinical trials, treatment extension to 24 weeks of LDV/SOF or the addition of ribavirin to a 12 week regimen of LDV/SOF may augment sustained virologic response (SVR) rates in genotype 1 treatment-experienced patients with compensated cirrhosis
Information on effectiveness and safety of LDV/SOF in treatment-experienced cirrhotic patients with HCV genotype 1 infection is limited in real-world, clinical practice
What are the new findings?
SVR12 rates as observed in HCV-TARGET, a prospective, international, multicenter ‘real-word’ study that included a larger number of HCV genotype 1 treatment-experienced cirrhotic patients than any pivotal study, generally confirm the effectiveness and safety of 12 and 24 week LDV/SOF regimens in this population
While no differences in SVR were observed between 12 and 24 week regimens, with or without ribavirin in the overall treatment-experienced cirrhotic cohort, the addition of ribavirin was not associated with increased SVR in patients with decompensated cirrhosis or were post-liver transplant
How might it impact clinical practice in the foreseeable future
This large, international cohort study, confirms the effectiveness and safety of the all-oral combination of ledipasvir/sofosbuvir ± ribavirin for 12 or 24 weeks for the treatment of HCV GT1 treatment-experienced cirrhotic patients.
Whereas HCV GT1 treatment-experienced patients with compensated cirrhosis can be treated with ledipasvir/sofosbuvir for 12 weeks, those with decompensated cirrhosis may benefit from the addition of ribavirin or extension of ledipasir/sofosbuvir to 24 weeks.
Acknowledgments
Financial support. HCV-TARGET is an investigator-initiated study jointly sponsored by The University of Florida, Gainesville, FL (PI: Nelson), and The University of North Carolina at Chapel Hill, Chapel Hill, NC (PI: Fried). It was funded in part by AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, and Vertex. Funded in part by CTSA UF UL1TR000064. Dr. Fried was funded in part by NIH Mid-Career Mentoring Award K24 DK066144.
Abbreviations
- HCV
hepatitis C virus
- GT
genotype
- SOF
sofosbuvir
- RBV
ribavirin
- PegIFN
pegylated interferon
- SVR
sustained virologic response
- DAA
direct acting antiviral agent
- EP
evaluable population
- PP
per protocol
- wks
weeks
- OR
odds ratio
- LCL
lower confidence limit
- UCL
upper confidence limit
- CL
confidence limit
- MELD
model for end-stage liver disease
- Wks
weeks, mg, milligram
- kg
kilogram
- BW
bodyweight
Footnotes
Author Contributions: JKL: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision. AML, MLS, ASL, SZ, NAT, JSP, CSL, MH, JG, AK, PJP: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. MV, LA, LM: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. MWF, DRN, ZB: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision
Disclosures: JKL: Grant funding from BMS, Conatus, Genfit, Gilead, Hologic, Intercept (All to institution); consultant to AbbVie, BMS, Gilead. AML: Grant Funding from BMS, Genfit, Gilead, Hologic, Intercept, and Prometheus (All to Institution); Consultant to AbbVie, BMS, and Gilead. MS: Grant funding from AbbVie, BMS, Conatus, CymaBay, Exalenz, Galectin, Genfit, Gilead, Intercept, Immuron, Merck, NGMBio, Novartis, and Shire; Consultant to Optum Rx; sponsored lectures/honoraria from AbbVie, BMS, Gilead, Intercept, Merck; Expert Testimony/Advisory Board to AbbVie, BMS, Gilead, Intercept, Merck. ASL: Grant funding from BMS, Gilead, Target Pharma. SZ: Consultant for AbbVie, BMS, Gilead, Intercept, Janssen, and Merck; sponsored lectures/ honoraria from AbbVie, BMS, Gilead, and Merck. NAT: Grant funding from Gilead, BMS, AbbVie, Merck; consultant to Gilead and Merck; Sponsored Lectures/Honoraria to CCO Hepatitis, Practice Point Communications, Focus Medical Communications, Annenberg Center for Health Sciences, PRIME Education Inc; Other support from Research Grants from HIH. JSP: Consultant to Gilead and AbbVie; sponsored lectures/honoraria for Gilead, AbbVie, and Merck. CSL: Grant Funding from AbbVie and Gilead. MH: No disclosures. JG: Grant funding from AbbVie, BMS, Gilead, Janssen, Merck, Sangamo, Theratechnologies, ViiV/GlaxoSmithKline; expert testimony/advisory board to BMS, Gilead, Merck, Theratechonologies, ViiV Healthcare. AK: Grant funding from Gilead; Sponsored Lectures/ Honoraria from Gilead. PJP: Grant funding from Gilead, AbbVie, Merck, Intercept, Genfit; consultant to Gilead, AbbVie, Merck, Intercept and Genfit; Sponsored Lectures/Honoraria to Gilead, AbbVie, Merck, and Intercept. MV: No disclosures. LA: No disclosures. LM: No disclosures. MWF: Grant funding from AbbVie, BMS, Gilead and Merck; consultant for AbbVie, BMS, Gilead, Merck, and TARGET Pharmasolutions; Stockholder with TARGET Pharmasolutions; other research grants from NIH. DRN: Grant funding from AbbVie, Gilead, BMS, Janssen, and Merck; stockholder of TARGET Pharmasolutions. ZBA: Consultant to AbbVie, Gilead, Merck, Janssen, and BMS; Sponsored lectures/honoraria to AbbVie, Gilead, Merck, Janssen, and BMS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- 1.Blach S, Zeuzem S, Manns M, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58–68. doi: 10.1016/j.jhep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer AJ, Wedemeyer H, Feld JJ, et al. Life expectancy in patients with chronic HCV infection and cirrhosis compared with a general population. JAMA. 2014;312(18):1927–8. doi: 10.1001/jama.2014.12627. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 5.Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368(20):1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzel TM, Dultz G, Zeuzem S. Interferon-free antiviral combination therapies without nucleoside polymerase inhibitors. J Hepatol. 2014;61(1 Suppl):S98–S107. doi: 10.1016/j.jhep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Jacobson IM. Antiviral therapy with nucleotide polymerase inhibitors for chronic hepatitis C. J Hepatol. 2014;61(1 Suppl):S91–7. doi: 10.1016/j.jhep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Harvoni (ledipasvir and sofosbuvir) prescribing information. Foster City, CA: Gilead Sciences; 2014. (package insert) ( http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf) [Google Scholar]
- 11.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 12.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 13.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 14.Bourliere M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomized, double-blind phase 2 study (SIRIUS) Lancet Infect Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 15.Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015;62:79–86. doi: 10.1002/hep.27826. [DOI] [PubMed] [Google Scholar]
- 16.AASLD/IDSA HCV Guidance Panel. Hepatitis C Guidance: AASLD-IDSA Recommendations for Testing, Managing, and Treating Adults Infected With Hepatitis C Virus. Hepatology. 2015;62(3) doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 17.Myers RP, Shah H, Burak KW, et al. An update on the management of chronic hepatitis C: 2015 Consensus guidelines from the Canadian Association for the Study of the Liver. Gastroenterol Hepatol. 2015;29(1):19–34. doi: 10.1155/2015/692408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EASL Clinical Practice Guidelines: management of hepatitis C virus infection. European Association for Study of Liver. J Hepatol. 2014;60(2):392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 19. [accessed on 05/01/2017]; www.hcvtarget.org.
- 20.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection. HCV-TARGET Study Group. Gastroenterology. 2016;150:419–29. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulkowski MS, Vargas HE, Di Bisceglie AM, et al. Effectiveness of simeprevir plus sofosbuvir, with or without ribavirin, in real-world patients with HCV genotype 1 infection. Gastroenterology. 2016;150:419–29. doi: 10.1053/j.gastro.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon SC, Muir AJ, Lim JK, et al. Safety profile of boceprevir and telaprevir in chronic hepatitis C: real-world experience from HCV-TARGET. J Hepatol. 2015;62:286–93. doi: 10.1016/j.jhep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrault NA, Zeuzem S, Di Bisceglie AM, et al. Effectiveness of ledipasvir-sofosbuvir combination in patients with hepatitis C virus infection and factors associated with sustained virologic response. Gastroenterology. 2016;151:1131–1140. doi: 10.1053/j.gastro.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calleja JL, Crespo J, Rincon D, et al. Effectiveness, safety, and clinical outcomes of direct-acting antiviral therapy in HCV genotype 1 infection: results from a Spanish real-world cohort. J Hepatol. 2017;66:1138–1148. doi: 10.1016/j.jhep.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of sofosbuvir, ledipasvir/sofosbuvir, or paritaprevir/ritonavir/ombitasvir and dasabuvir regimens for treatment of patients with hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457–471. doi: 10.1053/j.gastro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao TT, Jiang XH, Chen YH, et al. Efficacy and safety of ledipasvir/sofosbuvir with and without ribavirin in patients with chronic hepatitis C virus genotype 1 infection: a meta-analysis. Int J Infect Dis. 2017;55:56–71. doi: 10.1016/j.ijid.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Rezaee-Zavareh MS, Hesamizadeh K, Behnava B, et al. Combination of ledipasvir and sofosbuvir for treatment of hepatitis C virus genotype 1 infection: systematic review and meta-analysis. Ann Hepatol. 2017;16:188–197. doi: 10.5604/16652681.1231562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.