Abstract
Addiction is a chronic relapsing disorder, in that most addicted individuals who choose to quit taking drugs fail to maintain abstinence in the long-term. Relapse is especially likely when recovering addicts encounter risk factors like small “priming” doses of drug, stress, or drug-associated cues and locations. In rodents, these same factors reinstate cocaine seeking after a period of abstinence, and extensive preclinical work has used priming, stress, or cue reinstatement models to uncover brain circuits underlying cocaine reinstatement. Here, we review common rat models of cocaine relapse, and discuss how specific features of each model influence the neural circuits recruited during reinstated drug seeking. To illustrate this point, we highlight the surprisingly specific roles played by ventral pallidum subcircuits in cocaine seeking reinstated by either cocaine-associated cues, or cocaine itself. One goal of such studies is to identify, and eventually to reverse the specific circuit activity that underlies the inability of some humans to control their drug use. Based on preclinical findings, we posit that circuit activity in humans also differs based on the triggers that precipitate craving and relapse, and that associated neural responses could help predict the triggers most likely to elicit relapse in a given person. If so, examining circuit activity could facilitate diagnosis of subgroups of addicted people, allowing individualized treatment based on the most problematic risk factors.
Keywords: neural circuits, reinstatement, self-administration, ventral pallidum, voluntary abstinence
1. Introduction
Drug addiction is characterized by a transition from controlled drug use to compulsive intake, often accompanied by extreme difficulty in quitting, and especially in maintaining abstinence. Most quit attempts result in relapse to active use within a year, and recovering addicts typically make multiple transitions between drug abuse and abstinence in the first year after deciding to quit (McLellan et al., 2000). This propensity for relapse persists long after the affective or physical symptoms of withdrawal subside, yet no effective treatments exist for reducing risk of relapse to psychostimulants, and few exist for any drug of abuse (Czoty et al., 2016; Shorter et al., 2015). Clearly, a great need exists for next-generation, neuroscience-based clinical approaches for intervening in this devastating disorder, but this will first require a better understanding of the brain substrates of relapse. Unfortunately, human research is limited by a lack of means to manipulate or observe neural activity at the cellular and circuit levels in vivo, where addiction-related changes are most likely to manifest, and therefore where future brain-based interventions are most likely to succeed.
Fortunately, behavioral features of addiction including drug intake and relapse are amenable to modeling in animals. Rats and mice readily self-administer the same psychoactive drugs as humans including cocaine, and following a period of abstinence, reinstate their drug seeking when exposed to the same factors that put humans at risk of relapse—drug-associated cues, “priming” doses of the drug, and stress. Crucially, the specific features of rodent relapse models influence which neural circuits are recruited, and this fact may also have implications for predicting which circuits are involved when humans fail to resist temptation to take drugs.
Here we provide an overview of commonly used rodent drug self-administration and reinstatement models, and describe how the neural circuits underlying renewed cocaine seeking vary based on experimental details like the type of relapse trigger that initiates cocaine seeking. Self-administration procedures and means of achieving abstinence from drug also affect circuits involved in subsequently tested reinstatement, but the details of this are poorly understood to date, so we focus here on circuit heterogeneities stemming from different relapse modalities. It is our hope that by mapping circuits engaged during a variety of relapse-related behaviors in rodents, we can make better predictions of those most likely to be involved in individuals when they encounter particular stimuli that put them at risk of returning to problematic drug use.
2. Cocaine self-administration models
Drug self-administration has become a gold standard in preclinical addiction research, owing to the overt similarity of this behavior to human voluntary drug intake (Shaham et al., 2003). Rodents are trained to perform an operant response (e.g., lever press or nose poke) for a dose of drug, typically accompanied by a concurrently-delivered, discrete cue such as a light and a tone. Like humans, individual rats vary in their propensity to acquire self-administration and in their preferred level of intoxication, and such individual variation relates to other addiction-relevant behaviors including subsequent reinstatement (Belin et al., 2009; Piazza et al., 2000; Bentzley et al., 2014).
The effort required to obtain cocaine influences the neural circuits engaged, for example those underlying high-effort, highly motivated seeking are dissociable from those underlying low effort self-administration (Floresco et al., 2008; Kurniawan et al., 2011). Most rodent relapse studies employ low effort self-administration schedules like fixed ratio 1, where one lever press yields one intravenous cocaine infusion. Under this schedule, rats typically “load up” on cocaine at the beginning of each session until preferred cocaine blood levels are reached (this level is sometimes referred to as the rats’ “hedonic set point”), then doses are self-administered regularly throughout the rest of the session in order to maintain this level of intoxication (Olmstead et al., 2000; Roberts et al., 2013; Suto and Wise, 2011). This phenomenon may also occur in humans during cocaine binging (Sughondhabirom et al., 2005), though not necessarily under all laboratory conditions (Angarita et al., 2010).
To study highly motivated, addiction-like pursuit of cocaine, more complex procedures employing higher response requirements have been developed. For example, in the progressive ratio task, effort required for reward increases incrementally throughout the session until the animal gives up on seeking (Hodos, 1961). The maximum response schedule an animal performs within a set timeframe for a fixed dose of cocaine is called its “breakpoint,” which reflects its level of motivation for cocaine.
Another factor that affects cocaine self-administration behavior is the duration, quantity, and pattern of drug use experience. Preferred daily cocaine blood levels increase with extended use, presumably due to tolerance, and motivation to pursue cocaine also increases in some rats with repeated long-access intake. For example, weeks of long access (~6 hrs/day), relative to short access (~2 hrs/day) to cocaine escalates daily intake in most animals (Ahmed and Koob, 1998), increases motivation to maintain cocaine intoxication, and decreases sensitivity of intake to punishment, e.g. shock delivered concurrently with cocaine (Ahmed et al., 2013; Edwards and Koob, 2013; Kawa et al., 2016; Koob and Kreek, 2007; Mantsch et al., 2016; Vanderschuren and Everitt, 2004). Such intake escalation is associated with alterations in the neural circuits underlying self-administration. For instance, escalation of cocaine intake under long access conditions may result from attenuated ventral striatal phasic dopamine responses when animals perform an operant response for cocaine, since observed DA responses decrease most in escalating animals, and boosting DA responses with l-dopa attenuates escalated seeking (Willuhn et al., 2014). However, we note that long access to cocaine is not necessary for development of compulsive drug seeking in rats, since susceptible individuals show compulsive seeking even after short-access continuous self-administration (Pelloux et al., 2012). In addition, intermittent access (and concomitant rapid spiking of cocaine blood levels) is as effective as extended access for promoting compulsive drug seeking, even when far less drug is taken. For example, cocaine economic demand, breakpoint, and cue-triggered reinstatement are increased to a similar extent in long- and intermittent-access animals (Allain et al., 2017a; Kawa et al., 2016; Zimmer et al., 2012). This shows that the pattern of drug intake is at least as important as total amount of drug taken for inducing an addiction-like phenotype (Allain et al., 2017b; Samaha and Robinson, 2005).
3. Modeling cessation of cocaine use
By definition, relapse follows cessation of drug use, and the means by which abstinence is achieved may affect the circuitry underlying subsequent reinstatement (Bossert et al., 2013; Fuchs et al., 2006; Venniro et al., 2017). In humans, cocaine abstinence occurs either voluntarily (e.g., choosing to cease problematic use) or involuntarily (e.g., imprisonment, non-voluntary rehabilitation treatment), and both situations have been modeled in rodent studies. The most commonly used preclinical relapse models employ either forced abstinence or extinction training to model cessation of drug use, though self-imposed abstinence models are also gaining popularity due to their face validity for modeling humans who decide to try to quit using drugs.
3.1. Forced abstinence
Forced abstinence entails removing rodents from the drug exposure context entirely by imposing a period of abstinence. Following this homecage abstinence period, animals are re-introduced to the drug context, and tested for drug seeking under extinction conditions. Relative to 1 day of abstinence, 1-4 weeks abstinence increases responding during this initial extinction test, with or without a cocaine priming injection (Neisewander et al., 2000; Tran-Nguyen et al., 1998). Grimm and colleagues (2001) went on to find that the length of this abstinence period also increases cue-induced cocaine seeking, a phenomenon they termed “incubation of craving.” This heightened cue-induced drug seeking occurred after long periods (days to months) of abstinence, relative to shorter abstinence periods (e.g., 1 day), though this effect degrades with very long abstinence periods (e.g., 180 days) (Lu et al., 2004), possibly due to memory degradation. A similar craving incubation effect is also observed in humans with several drugs of abuse (Bedi et al., 2011; Gawin and Kleber, 1986; Li et al., 2015; Parvaz et al., 2016; Wang et al., 2013). Incubation of craving may result from drug-associated memories becoming increasingly salient, and therefore liable to initiate robust drug seeking; a phenomenon that could help explain why relapse risk remains high long after quitting drugs.
3.2. Extinction-based abstinence
A common means of inducing abstinence in rodent relapse experiments is via extinction training. In extinction, operant responding no longer elicits drug delivery or cues, so animals learn to stop responding for drug (de Wit and Stewart, 1981). Extinction memories (both appetitive and aversive) do not overwrite the initial memories they suppress, but are instead mediated by anatomically distinct circuits that suppress the circuits underlying drug seeking (Bouton et al., 2006; LaLumiere et al., 2010; Peters et al., 2009; Quirk et al., 2006). Notably, drug seeking during extinction is reduced mainly by lack of drug availability despite ongoing motivation to use (and lack of reasons not to use), which may be most relevant to humans that would like to continue using drugs, but cannot. Some have speculated that strengthening these extinction memories, and/or manipulating reconsolidation of extinction or drug memories in psychotherapy may be effective at helping people maintain abstinence (Everitt, 2014; Kalivas and Volkow, 2011; Mcnally, 2014; Millan et al., 2011; Nic Dhonnchadha and Kantak, 2011; Xue et al., 2012), though evidence supporting efficacy of this strategy is mixed to date (Conklin and Tiffany, 2002; Kaplan et al., 2011; Konova et al., 2017; Kredlow et al., 2016).
3.3. Self-imposed abstinence
Forced- and extinction-based abstinence protocols, while experimentally convenient and commonly used in rodent studies, fail to capture the voluntary nature of abstinence that is characteristic of most humans who choose to quit using drugs. For this reason, the Yavin Shaham group and others have developed procedures allowing rodents to choose to quit taking drugs prior to reinstatement testing. For example, rats will cease self-administering cocaine when mutually-exclusive alternative rewards such as food are offered, or when drug is delivered in conjunction with a punishing stimulus such as a shock (Ahmed, 2010, 2012; Caprioli et al., 2015; Lenoir et al., 2013; Marchant et al., 2013a, 2013b; Pelloux et al., 2017; Venniro et al., 2016). We will discuss these models further in Section 7 below.
4. Reinstatement models
In humans, craving and subsequent relapse is often precipitated by ingestion of a small priming dose of drug, experiencing acute stress, or encountering discrete or contextual cues previously paired with drug use (O’Brien et al., 1998; Shaham et al., 2003; Sinha et al., 2011). Humans and rodents both vary individually in their sensitivity to these triggers (Bentzley et al., 2014; Homberg et al., 2004; McKay et al., 1996), and mounting evidence points to distinct neural circuits underlying relapse initiated by each. Therefore, we next describe common rodent models of cocaine reinstatement, most commonly tested following extinction training.
4.1. Cocaine-primed reinstatement
A small priming dose of drug in an abstinent individual can elicit craving, and increases risk of full-blown relapse (Donny et al., 2004; Stewart, 2000). Priming doses of cocaine also reinstate drug seeking in abstinent rats (de Wit and Stewart, 1981; Shaham et al., 2003). Importantly, cocaine selectively promotes cocaine seeking in rats, but not seeking of an alternative reward (food), demonstrating specificity of priming for enhancing motivation for cocaine in particular (Tunstall and Kearns, 2014). However, we note that cocaine dose must be carefully chosen for priming experiments in rodents, since higher doses (>15mg/kg) can induce behavioral stereotypies or other nonspecific locomotion that competes with drug seeking.
4.2. Stress reinstatement
Another significant relapse risk factor in humans is exposure to stress (Sinha et al., 2011). In rodents, acute stressors such as footshock, forced swimming, food deprivation, and administration of the aversive alpha-2 norepinephrine (NE) antagonist yohimbine (considered a ‘pharmacological stressor’) precipitate reinstatement (for reviews, see Mantsch et al., 2016; See and Waters, 2011; Shaham et al., 2000; Shalev et al., 2010). Acute delivery of intermittent footshock is perhaps the most widely used stress reinstatement model. Typically, animals are trained to self-administer drug with discrete response-contingent cues, extinguished of this behavior, then unpredictable footshocks are administered immediately prior to a cue reinstatement test. The efficacy of intermittent shock as a reinstatement trigger depends upon multiple factors including pre-shock stress levels, the amount of drug access during self-administration training, and whether the animal lives in the operant chamber or in a separate cage (Mantsch et al., 2016; Shalev et al., 2010). These sensitivities likely reflect the highly context-dependent nature of rodent stress responses, only some of which result in renewed drug seeking. The extent to which this is also true in humans is not clear, but we expect that likewise, certain types of stress are especially effective in inducing cocaine seeking in recovering addicts. To study this possibility in rats, a new test of psychosocial stress-induced relapse was developed, in which stimuli paired with social defeat robustly reinstate cocaine seeking (Manvich et al., 2016).
4.3. Cue- and context-induced reinstatement
Environments and sensory cues associated with drug-taking acquire incentive salience, or an attractive, desirable quality similar to the unconditioned rewards they predict (Berridge, 2001; Bindra, 1978). A drug-associated cue that has acquired incentive salience becomes a potent motivational trigger, and even after long-term abstinence it can capture the attention of a recovering addict, eliciting craving and temptation to relapse (Courtney et al., 2016; O’Brien et al., 1998). Cues can be either diffuse and multimodal (“context cues”) or physically localized and temporally coincident with drug (“discrete cues”), and thinking about or viewing either type of cue elicits craving in humans (Bonson et al., 2002; Courtney et al., 2016; O’Brien et al., 1998; Sinha et al., 2011; Sinha and Li, 2007).
However, context and discrete cues have importantly different features. For example, a context cue in humans might be visiting a house or neighborhood where one used to take drugs. In rodents, context reinstatement usually takes the form of “ABA” testing in which a rat is trained to self-administer cocaine in Context A, extinguished in a distinct Context B, then returned to Context A, where lever pressing is measured in the absence of drug (Bouton and Bolles, 1979; Crombag et al., 2002).
In contrast, discrete cocaine cues in humans include the feeling of cocaine-induced nasal numbing, or the sight and smell of cocaine—both stimuli experienced in close temporal association with the onset of drug effects. Such cocaine-coincident discrete cues have conditioned reinforcing properties in humans; for example smokers will smoke denicotinized cigarettes (Rose, 2006). Cocaine users also report drug-like subjective effects after experiencing stimuli associated with drug taking (Ehrman et al., 1992; O’Brien et al., 1998), which likely contributes to their conditioned reinforcing properties. To model discrete cue-induced conditioned reinforcement in rats, they can be trained to pair intravenous cocaine with a coincident temporally-discrete cue. In most studies, instrumental responses during training are rewarded with cocaine and a cue during self-administration training, but cue/cocaine pairings can alternatively be made apart from instrumental training in a separate Pavlovian training session, allowing experimental dissociation of Pavlovian and instrumental learning mechanisms (Kruzich et al., 2001; Smith et al., 2009a). Most rats show cocaine seeking when such discrete cocaine cues are delivered response-contingently during a drug-free reinstatement test, and this conditioned reinforcement-based model is commonly used since it elicits robust drug seeking behavior (Kruzich et al., 2001).
5. Cocaine reinstatement circuits—Devil in the details
Craving and relapse in humans, like renewed drug seeking after abstinence in rodents, is often assumed to be unitary in nature, and to involve specific neural circuits regardless of how reinstatement is elicited. An implication of this assumption is that if aberrant, addiction-related circuit activity could be reversed, relapse could be prevented. However, animal studies show that the brain circuits which are activated by and necessary for reinstatement under different circumstances are not completely overlapping. If this is also true in humans, it could have important implications for treating patients that vary in their susceptibility to the relapse-promoting properties of drug priming, stress, or cues (Carter and Tiffany, 1999; Flagel et al., 2009; Mahler and de Wit, 2010; Sinha et al., 2011). As mentioned above, the means by which abstinence is induced prior to reinstatement in rodent studies can also influence the neural pathways involved (Fuchs et al., 2006; Bossert et al., 2013; Venniro et al., 2017; Ma et al., 2014). Therefore, we contend that a fuller understanding of circuit/behavior relationships in rodent relapse models may inform hypotheses about the specific neural circuits that are engaged when humans encounter relapse risk factors.
Next, we briefly review the neural circuits underlying reinstatement of cocaine seeking in self-administration-based cocaine relapse models in rats. Several excellent reviews cover these relapse circuits in more detail (Bossert et al., 2013; Kalivas and McFarland, 2003; Lasseter et al., 2010a; Shalev et al., 2002; Weiss, 2005), and here we emphasize rodent findings showing that circuit involvement varies based upon experimental conditions, highlighting the fact that not all cocaine relapse is alike in the brain.
5.1. Circuitry common across post-extinction reinstatement modalities
Despite circuit heterogeneities described below, at least one neural circuit; a pathway involving ventral tegmental area (VTA) DA projections to prelimbic medial prefrontal cortex (PLC), PLC projections to nucleus accumbens core (NAcCo), and NAcCo projections to VP circuit has been described as a “final common pathway” of reinstatement (Kalivas and Volkow, 2005). This circuit is required for cocaine, stress, and cues to elicit cocaine seeking, at least following extinction training (Fig. 1). For example, temporary pharmacological inactivation of PLC or NAcCo, or excitotoxic lesions of either region block prime-, stress-, and discrete cue-induced reinstatement of cocaine seeking (Kalivas, 2008; Shaham et al., 2003), as does optogenetic inhibition or pharmacological disconnection of PLC-NAcCo projections (McGlinchey et al., 2016; Stefanik et al., 2013a). PLC-NAc projections (as well as BLA-NAc projections) also undergo plasticity during forced abstinence that results in “silent synapses,” or newly formed synaptic connections. When mature, these synapses facilitate robust cocaine seeking, unlike ILC-NAc projections, where abstinence-induced silent synapses instead suppress cocaine seeking (Ma et al., 2014; Lee et al., 2013). NAc’s major output, VP, is also required for primed, stress, and discrete cue reinstatement, which will be discussed in more detail below. VTA DA neuron projections to forebrain nuclei including PLC, orbitofrontal cortex (OFC), NAc, and basolateral amygdala (BLA) also modulate many types of cocaine reinstatement following extinction training, though DA in some of these pathways is necessary for only certain relapse modalities (Bachtell et al., 2005; Bossert et al., 2013; Capriles et al., 2003; McFarland et al., 2004; McFarland and Kalivas, 2001; See et al., 2001; Shaham et al., 2003). DA in PLC, though, is involved in all types of post-extinction cocaine reinstatement, including primed (McFarland and Kalivas, 2001), stress-induced (Capriles et al., 2003), and cue-induced (McGlinchey et al., 2016).
Figure 1. Key neural circuits underlying different types of cocaine reinstatement behavior.

Overlapping, but distinct neural networks mediate reinstatement behavior elicited by different classes of relapse triggers including A) cocaine priming, B) stress, C) discrete cues, and D) context cues. See text for references of specific findings and abbreviations. BLA: basolateral amygdala; BNST: bed nucleus of the stria terminalis; CeA: central amygdala nucleus; CRF: corticotropin-releasing factor; dHPC: dorsal hippocampus; DRN: dorsal raphe nucleus; LTN: laterodorsal tegmental nucleus; LH: lateral hypothalamus; mPFC: medial prefrontal cortex; NE: norepinephrine; NAc: nucleus accumbens; NAcCo: nucleus accumbens core; NAcSh: nucleus accumbens shell; OFC: orbitofrontal cortex; PVT: paraventricular thalamus; vHPC: ventral hippocampus; VP: ventral pallidum; VTA: ventral tegmental area
5.2. Cocaine primed reinstatement circuits
Cocaine’s main mechanism of action is to block monoaminergic transporters, and cocaine-primed reinstatement is unsurprisingly dependent upon intact monoamine neurotransmission. DA transporters in particular are crucial for cocaine’s ability to induce cocaine-primed reinstatement (Schmidt et al., 2005) by increasing synaptic DA levels by slowing its clearance from the synaptic cleft. Both self-administered and non-contingent cocaine increase the frequency of DA transients and extracellular levels in rat NAc in vivo (Stuber et al., 2005; Willuhn et al., 2012), and, consistent with this, pharmacological suppression of all VTA neuronal activity blocks cocaine-primed reinstatement (McFarland and Kalivas, 2001).
Cocaine-induced DA and other monoamines are implicated in the priming effects of cocaine in a complex manner (Fig. 1A) (Schmidt et al., 2005). Systemically, DA receptor 2 (D2; inhibitory, Gi/o-coupled) activity generally promotes reinstatement—a D2 agonist induces, and a D2 antagonist attenuates primed reinstatement (Schenk and Gittings, 2003; Self et al., 1996; Spealman et al., 1999). Paradoxically, both agonists and antagonists of DA receptor 1 (D1; stimulatory, Gs-coupled) reduce cocaine-primed reinstatement (Alleweireldt et al., 2003; Self et al., 1996; Spealman et al., 1999). These findings may be explained by distinct roles for DA receptors in individual forebrain regions in cocaine priming effects. In NAcCo, a mixed D1/2 antagonist blocks cocaine primed reinstatement (McFarland and Kalivas, 2001), and blockade of both D1 and D2 in NAc shell (NAcSh) also do so (Anderson et al., 2003, 2006), and agonists of both receptors conversely induce reinstatement (Schmidt et al., 2006). D2 but not D1 stimulation in central amygdala nucleus (CeA) reduces cocaine priming (Thiel et al., 2010), as does blockade of PLC D1 and D2 receptors (McFarland and Kalivas, 2001; Sun and Rebec, 2005, but see Capriles et al., 2003), or inhibiting PLC alpha-1 NE receptors (Schmidt et al., 2017).
In addition, 5-hydroxytryptamine (5HT/serotonin) receptors modulate cocaine-primed reinstatement, since systemic 5HT2A antagonism in monkeys, and 5HT1A blockade or 5HT2C stimulation in rat CeA suppress cocaine primed seeking (Burmeister et al., 2004; Murnane et al., 2013; Pockros-Burgess et al., 2014). Kappa opioid stimulation in VTA also suppresses cocaine-primed reinstatement (Sun et al., 2010), likely via inhibition of DA neurons there (Margolis et. al., 2003). Of note, cocaine priming effects also rely in part on conditioned reactions to cocaine’s subjective and physiological effects, including its peripheral autonomic actions (Wise and Kiyatkin, 2011). In sum, cocaine priming effects rely upon monoaminergic and other systems involved in mediating cocaine’s acute effects, and we note that the circuits of cocaine priming are likely to differ from priming effects of other drugs of abuse with actions via other neural mechanisms.
5.3. Stress-specific circuits
The brain’s major stress systems like NE, corticotropin-releasing factor (CRF), and kappa opioid receptors are heavily involved in stress-induced reinstatement of cocaine seeking, especially in extended amygdala, VTA, and lateral hypothalamus (LH) (Fig. 1B) (for comprehensive reviews, see Mantsch et al., 2016; McReynolds et al., 2014; Shalev et al., 2010; Sinha et al., 2011; Smith and Aston-Jones, 2008). Involvement of these circuits differentiate stress from other types of reinstatement, although we also point out a significant overlap in stress- and cue-related reinstatement circuits.
NE neurotransmission in stress- and aversion-related emotional circuits, including CeA and bed nucleus of the stria terminalis (BNST), are crucial for stress reinstatement of cocaine seeking. Systemic alpha-2 receptor stimulation (decreasing NE release), or intra-CeA or BNST β1/2 antagonism (blocking NE postsynaptic signaling) attenuates stress-, but not cocaine-primed reinstatement, while BNST β2 stimulation conversely induces reinstatement (Erb et al., 2000; Leri et al., 2002; Vranjkovic et al., 2014). Decreasing NE synthesis also decreases stress reinstatement (footshock and yohimbine), but also other types of reinstatement (Schroeder et al., 2013). In general, these findings are consistent with extended amygdala NE’s general role in aversive and arousal-related aspects of stress, as well as stress that induces cocaine seeking (España et al., 2016; Smith and Aston-Jones, 2011).
CRF systems (sometimes recruited by NE; Brown et al., 2009; Mantsch et al., 2016) are heavily implicated in stress-induced cocaine reinstatement, especially via CeA-BNST projections. Systemic or intracerebroventricular (i.c.v.) administration of CRF receptor antagonists attenuates footshock reinstatement, while i.c.v. CRF induces it (Erb et al., 1998; 2006). CRF in BNST is necessary and sufficient for footshock reinstatement (Erb and Stewart, 1999), and this CRF comes at least in part from CeA, since unilateral CeA inactivation, and contralateral BNST CRF antagonist blocks cocaine seeking (Erb et al., 2001a). In contrast, neither CRF signaling nor neural activity in BLA is required for footshock reinstatement, indicating specificity for stress reinstatement to extended amygdala, rather than cortical amygdala (Erb and Stewart, 1999; McFarland et al., 2004).
VTA is another site where CRF plays a role in stress reinstatement of cocaine seeking. Knockdown of VTA CRF1 receptors blocks footshock reinstatement (Chen et al., 2014), and intra-VTA CRF reinstates cocaine seeking by facilitating glutamate and dopamine release there (Wang et al., 2005). In cocaine-experienced rats, CRF together with γ-Aminobutyric acid (GABA) B receptor signaling presynaptically modulate VTA GABA and glutamate release, facilitating DA neuron excitation and cocaine relapse (Blacktop et al., 2016; Williams et al., 2014; Wise and Morales, 2010). VTA CRF may result from β2 NE receptor stimulation in BNST, as antagonism of β2 NE receptors in unilateral BNST, and concurrent CRF blockade in contralateral VTA attenuates footshock reinstatement (Vranjkovic et al., 2014). In sum, CRF plays a major role in inducing reinstatement of cocaine seeking after stress in both extended amygdala and VTA, and likely in other untested regions as well.
Kappa opioid receptors, and their endogenous ligand dynorphin, are also involved in stress-induced reinstatement of cocaine seeking, in part via actions in VTA and BNST. Systemic kappa opioid receptor antagonists abolish footshock or forced swim stress-induced, but not cocaine-primed reinstatement (Beardsley et al., 2005; Polter et al., 2014). This may result from kappa modulation of GABAergic inputs to VTA DA neurons (Graziane et al., 2013), or direct inhibition of DA neurons themselves (Margolis et al., 2003).
LH orexin neurons are involved in arousal, stress, and motivation-related processes (Alexandre et al., 2013; Berridge et al., 2010; Calipari and España, 2012; Mahler et al., 2014a), and also play a role in stress-induced cocaine reinstatement, in part via their VTA and CeA projections. Orexin projections to VTA are necessary for stress reinstatement (Boutrel et al., 2005; Tung et al., 2016), while intra-VTA orexinA conversely induces reinstatement (Wang et al., 2009). This likely involves direct orexin excitation of VTA DA neurons (España et al., 2010; Korotkova et al., 2003), as well as interactions with VTA glutamate, GABA, and endocannabinoids (Borgland et al., 2006; Mahler et al., 2013; Tung et al., 2016). Orexin in CeA is also required for yohimbine-induced reinstatement (Schmeichel et al., 2017), potentially by recruiting CRF neurons there (Erb et al., 2001b). Intriguingly, dynorphin (the main endogenous kappa opioid receptor ligand) is co-synthesized by all LH orexin neurons (Chou et al., 2001) and both dynorphin and orexin are co-released in VTA and paraventricular thalamus (PVT), where they play roles in aversion, reward, and cocaine reinstatement (Baimel and Borgland, 2017; Matzeu et al., 2017; Muschamp et al., 2014). Further research should explore how kappa and orexin signaling in VTA, PVT, and elsewhere interact to modulate stress-induced reinstatement after cocaine self-administration.
5.4. Context cue-specific circuits
In humans, revisiting places where drugs were previously used often causes craving, and potentially, relapse. In rodents, exposure to cocaine-paired places also elicits robust reinstatement (for in-depth reviews, see (Bossert et al., 2013; Crombag et al., 2008; Khoo et al., 2017; Lasseter et al., 2010a; Marchant et al., 2015). Although there is much overlap in discrete learned cue- and context-reinstatement circuitry, some differences exist between the two, particularly in the roles played by hippocampus and connected cortical regions (Fig. 1C, D).
Dorsal and ventral hippocampal circuits both play important roles in context-induced reinstatement of cocaine seeking. Inactivation of ventral hippocampus proper, but not of the hippocampal dentate gyrus, blocks the ability of contextual or response-contingent discrete cues, or cocaine prime, to cause reinstatement (Lasseter et al., 2010b; Rogers and See, 2007), suggesting a general role for ventral hippocampus in conditioned emotion/motivation. This is consistent with other evidence that ventral hippocampus mediates emotional or motivational components of spatial memory (Fanselow and Dong, 2010). In contrast, inactivation, or metabotropic glutamate receptor 1 (mGluR1) blockade in dorsal hippocampus attenuates only context reinstatement, without affecting reinstatement elicited by discrete cues or cocaine (Fuchs et al., 2005; Xie et al., 2010), in line with its general role in spatial memory (Fanselow and Dong, 2010; Moser and Moser, 1998). In other words, dorsal hippocampus (analogous to caudal hippocampus in human) is likely required for rodents to know where they are, and to use that information to trigger drug seeking, while ventral hippocampus may play a larger role in affective memory and motivation during reinstatement.
Hippocampus anchors a wider corticolimbic network that supports cocaine context-induced reinstatement (Fig. 1C). BLA, dorsomedial PFC, and lateral OFC are required, as is communication between dorsal hippocampus and these structures, and DA input (via D1 receptors) to OFC (Fuchs et al., 2005, 2007; Lasseter et al., 2010a, 2011a, 2014; Wells et al., 2011). Additionally, dorsal (but not ventral) hippocampus promotes cocaine reinstatement by indirectly disinhibiting VTA DA neurons, via innervation of lateral septum GABA neurons that project to VTA GABA interneurons (Luo et al., 2011), a pathway that is not required for discrete cue reinstatement (McGlinchey and Aston-Jones, 2017). Glutamate signaling in both NAcCo and NAcSh are also required for context reinstatement (Xie et al., 2012). In addition, systemic orexin receptor 1 (OX1R) antagonism blocks cocaine context reinstatement (Smith et al., 2010), and exposure to a cocaine-paired context (relative to an extinguished context) induces Fos expression in LH, but surprisingly, not in either orexin or melanin-concentrating hormone neurons there (Hamlin et al., 2007). In sum, the circuits that specifically subserve context reinstatement involve both spatial memory networks for identifying specific locations and environments, as well as conditioned motivation circuits.
5.5. Discrete cue-specific circuits
Cues temporally paired with cocaine elicit reinstatement via a network of cortical and subcortical regions, some of which are specific to conditioned cocaine seeking, and some of which overlap with other reinstatement circuits (Fig. 1D). BLA plays a general role in Pavlovian learning and motivation, and Fos expression there is correlated with the magnitude of cued reinstatement in male and female rats (Kufahl et al., 2009; Zhou et al., 2014). Accordingly, BLA lesion or inactivation blocks reinstatement elicited by either discrete or contextual cues (See et al., 2003). BLA’s role in discrete cue-induced cocaine seeking is dependent upon its inputs from lateral OFC, and its projections to NAc and PLC, since optogenetic inhibition of these pathways reduces cocaine seeking (Arguello et al., 2017; Stefanik and Kalivas, 2013). DA receptors play a particularly important role in BLA modulation of cue-induced cocaine seeking, since cues elicit DA release in amygdala (Weiss et al., 2000), and BLA D1 (but not D2/3) or ionotropic glutamate receptor blockade attenuates this behavior (See et al., 2001). In contrast, BLA and mPFC projections to VTA are not Fos activated during cue-induced cocaine seeking, unlike numerous other subcortical projections to VTA, including NAcCo, subregions of NAcSh, lateral septum, VP, and extended amygdala regions including BNST (Mahler and Aston-Jones, 2012). In addition to cue-induced cocaine seeking, BLA also plays a crucial role in re-consolidating Pavlovian cocaine memories, causing some to speculate that blocking drug memory re-consolidation could be helpful for treating excessively strong memories in cocaine addiction (Everitt, 2014; Lee et al., 2005; Miller and Marshall, 2005; Rich and Torregrossa, 2017). The insular cortex, which is highly interconnected with BLA and other cue-reinstatement regions, may also play a special role in cue-induced reinstatement since inhibition of its anterior aspect blocks cued but not cocaine primed reinstatement (Cosme et al., 2015), and projections from this region to CeA are required for cued methamphetamine reinstatement.
LH orexin neurons are also implicated in discrete cue-induced reinstatement, similar to context reinstatement. For example, LH’s large, mostly non-orexinergic projection to VTA is Fos activated during discrete cue reinstatement (Mahler and Aston-Jones, 2012). However, while systemic OX1R antagonism or intra-VTA OX1R blockade reduces discrete cue-induced reinstatement (James et al., 2011; Mahler et al., 2013; Smith et al., 2009a), only non-orexin LH neurons projecting to VTA are significantly Fos-activated (Mahler and Aston-Jones, 2012). It is not clear why orexin receptor signaling is required while orexin neurons are not Fos activated during cue-induced cocaine seeking, as they are during expression of conditioned place preference for cocaine or other rewards (Harris et al., 2005). This apparent disconnect could reflect intermittent orexin neuron firing during these time-locked, intermittent cue presentations that does not result in significant Fos expression (Mahler et al., 2014a). Alternatively, it could reflect orexin’s more general role in motivational activation (Mahler et al., 2014a), which is required for cue reinstatement, but also other behaviors unrelated to cocaine seeking that also occur in control groups tested in Fos expression experiments.
Regardless, in VTA orexin facilitates glutamate transmission to promote cued reinstatement of cocaine seeking. Both OX1R and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) neurotransmission are simultaneously required for discrete cues to reinstate seeking, as unilateral blockade of each receptor system in contralateral hemispheres blocks behavior, and intra-VTA OX1R antagonist-induced suppression of cue reinstatement is reversed by co-microinjection of an AMPA positive allosteric modulator (Mahler et al., 2013). These findings suggest that orexin’s facilitation of ionotropic glutamate neurotransmission upon VTA DA neurons underlies its role in cued reinstatement (Borgland et al., 2006; James et al., 2017; Mahler et al., 2012). Cocaine-primed reinstatement, in contrast, is unaffected by systemic or intra-VTA blockade of OX1Rs, or VTA AMPA/N-methyl-D-aspartate (NMDA) receptors, indicating specificity of this mechanism to conditioned (and stress-induced) cocaine seeking (Boutrel et al., 2005; Mahler et al., 2013), at least in male rats (Smith et al., 2009a; Zhou et al., 2012).
It is also noteworthy that stress facilitates the ability of cues to promote cocaine seeking. Consistent with this idea, exposing human subjects to drug cues in the absence of drug availability elicits drug craving, but also strong negative affect and cortisol response (Childress et al., 1999; Mahler and de Wit, 2005; Sinha et al., 2000; Tiffany, 1990), and yohimbine increases cue-induced cocaine craving (Moran-Santa Maria et al., 2014). Importantly, stress can also increase conditioned craving responses to cues in humans in a real-world setting, particularly for cocaine (Preston et al., 2017). In rats, cues activate the hypothalamic-pituitary-adrenal axis and induce anxiety-like behavior (DeVries et al., 1998; DeVries and Pert, 1998), and reinstatement of cocaine seeking elicited by footshock or yohimbine is observed primarily when response-contingent cues are also present (Chen et al., 2015; Feltenstein and See, 2006). Furthermore, stress-related systems like NE and CRF are required for rodent cued reinstatement, as reducing NE release with alpha-2 receptor stimulation, and antagonism or knockdown of CRF1 receptors, blocks psychostimulant seeking elicited by cues (Chen et al., 2014; Moffett and Goeders, 2007; Smith and Aston-Jones, 2011). CRF in insular cortex, a region associated with addiction-related craving in humans (Naqvi et al., 2007), is also required for cue-induced seeking (Cosme et al., 2015). Anterior insula projections to CeA are also required for cue-induced reinstatement of methamphetamine seeking (Venniro et al., 2017), though the relevance of this pathway for cocaine is yet untested. In sum, the ability of stress to facilitate conditioned and habitual response strategies may be a major means by which stress alters normal and disordered behavior (Goodman and Packard, 2016; Li and Sinha, 2008; Schwabe and Wolf, 2011; Smith and Laiks, 2017). This said, not all stress is alike, nor do all individuals respond to the same stress in the same way; for example stress-sensitive rats show suppression of cue-induced cocaine seeking after re-exposure to a stress-paired environment (Hadad et al., 2016).
6. Ventral pallidum: hidden complexities in reinstatement functions
As described above, overlapping but dissociable neural circuits underlie cocaine reinstatement elicited by different relapse triggers. We next illustrate this point by focusing on the surprisingly complex roles of a single brain region, VP, in cocaine reinstatement. We highlight this region due to our recent work showing its complex mediation of cocaine reinstatement, but point out that it is but one important node in wider reinstatement circuits. Yet we predict that like in VP, similar functional-anatomical heterogeneities may also exist in other relapse-related brain regions.
VP is the Substance P-rich region caudal of NAc, ventral of the anterior commissure and globus pallidus, and rostral of extended amygdala structures like BNST and sublenticular extended amygdala in rodents (De Olmos and Heimer, 1999; Haber and Nauta, 1983; Zahm and Heimer, 1990). VP is intimately integrated within mesocorticolimbic circuits, where it is ideally positioned to mediate both drug and natural reward-seeking behaviors. The region is best known as the pallidal output of NAc, receiving significant inputs from both D1- and D2-expressing NAc medium spiny neurons that are altered by repeated cocaine exposure (Creed et al., 2016; Kupchik et al., 2015), and are required for cue+cocaine primed reinstatement (Stefanik et al., 2013b). We and others have shown that VP projections to VTA are especially important for motivated behavior including reinstatement of cocaine seeking (Geisler et al., 2008; Grace et al., 2007; Knowland et al., 2017; Mahler et al., 2014b; Prasad and McNally, 2016; Root et al., 2015; Richard et al., 2016).
VP is characterized by spontaneously active inhibitory projection neurons, and it has long been known to gate VTA neuron activity related to reward and cognition (Fig. 2; Grace et al., 2007; Root et al., 2015; Smith et al., 2009b). Accordingly, inhibiting VP neurons activates VTA DA neurons (Floresco et al., 2003; Grace et al., 2007; Mahler et al., 2014b), while stimulating VP-VTA projections inhibits DA neurons, as well as non-DA VTA neurons (Hjelmstad et al., 2013), allowing VP to bidirectionally control VTA DA neurons via direct or indirect projections. In return, VTA sends DA and non-DA projections back to VP, which participate in memory consolidation and motivation (Stout et al., 2016; Yoo et al., 2016).
Figure 2. Ventral pallidum projection to ventral tegmental area.

Immunofluorescent visualization of Substance P (red; defining VP borders), green fluorescent protein (green; defining expression of viral-tagged neurons in VP and immediately adjacent regions, and their axons), and tyrosine hydroxylase (blue; defining dopamine neurons in VTA), in a horizontal view.
VP is crucial for reward-seeking behaviors, and its activity is modulated by salient rewards, reward cues, and punishers in rodents and primates including humans (Haber and Knutson, 2010; Root et al., 2015; Smith et al., 2009b). For example, VP and its projections to VTA and mediodorsal thalamus are essential for cue-elicited seeking of natural rewards, suggesting a role for VP in cue-triggered incentive motivation for natural as well as drug rewards (Ahrens et al., 2016; Chang et al., 2015; Leung and Balleine, 2015; Richard et al., 2016; Smith et al., 2009b). VP is also required for reinstatement of extinguished drug seeking by discrete or contextual cues (Mahler et al., 2014b; Perry and Mcnally, 2013; Prasad and McNally, 2016; Stefanik et al., 2013b; Wang et al., 2014), stress (McFarland et al., 2004), or cocaine priming injections (Mahler et al., 2014b; McFarland and Kalivas, 2001), indicating that VP is a general mediator of reinstatement regardless of trigger. As discussed above, NAcCo-VP projections are required for cocaine reinstatement, but VP projections back to NAcCo are not similarly necessary (Stefanik et al., 2013b), though these NAc projections do mediate other forms of natural and drug reward seeking (Smith and Berridge, 2007).
VP is a highly heterogeneous region, and though the implications of such heterogeneity for relapse-related behaviors are still poorly understood, they are intriguing. VP inputs from NAc are anatomically segregated, with NAcCo projecting to a dorsolateral VP subregion, and NAcSh instead projecting to ventromedial VP (Heimer et al., 1991). VP is mostly GABAergic, but glutamatergic, cholinergic, parvalbumin, and other cell types are also present (Geisler et al., 2007; Knowland et al., 2017; Root et al., 2015), the functions of which are largely unknown. Interestingly, some cell types are expressed in different rostrocaudal zones within VP borders, with glutamatergic cells projecting to VTA localized largely in medial and rostral VP, and cholinergic cells being more caudally localized (Geisler et al., 2007; Root et al., 2015), while parvalbumin-expressing neurons are scattered throughout VP (Knowland et al., 2017). These anatomical rostro-caudal differences relate to roles for VP in hedonic/disgust-related behaviors in rodents (Chan et al., 2016; Smith and Berridge, 2005) and humans (Calder et al., 2007; Royet et al., 2016), with caudal VP containing a “hedonic hotspot,” in which stimulation of μ opioid or orexin receptors increases hedonic evaluation of tastes (Ho and Berridge, 2013; Smith and Berridge, 2005). Lesions of this area produce profound anhedonia and aphagia (Cromwell and Berridge, 1993; Smith and Berridge, 2005), and chemogenetic inhibition of caudal VP blocks cocaine-primed, but not cue-induced reinstatement (Mahler et al., 2014b). The rostral half of VP instead appears to mediate cue-triggered or cue-reinforced motivation in particular, since chemogenetic inhibition of rostral VP or its projections to VTA (Fig. 2) attenuates discrete cue-induced cocaine reinstatement without affecting primed reinstatement (Mahler et al., 2014b).
To determine the circuit mechanism by which VP-VTA projections mediate reinstatement, we further examined how rostral VP efferents modulate activity of VTA DA neurons. Contralateral disconnection of rostral VP from VTA DA neurons attenuates cue-induced cocaine seeking, showing that communication between these populations is required (Mahler et al., 2014b). However, the circuit mechanisms by which VP interacts with DA neurons during cued reinstatement remains elusive. Indiscriminate chemogenetic inhibition of VP-VTA projections disinhibited DA neurons in vivo and ex vivo, confirming the previously reported predominance of GABA in this pathway. This disinhibition of DA neurons is unlikely to explain reductions in cocaine seeking after inhibition of VP afferents though, since VTA disinhibition with a GABAA antagonist facilitated, rather than attenuated cue reinstatement (Mahler et al., 2014b). We also observed that some fast-firing, short-waveform VTA neurons were inhibited upon chemogenetic suppression of VP inputs. It is therefore possible that removing VP GABA inputs disinhibited GABA interneurons, suppressing firing of the recorded VTA DA cells. Another possibility is that chemogenetic inhibition of the glutamatergic component of the VP projection to VTA (~7% of all subcortical glutamate projections to VTA; Geisler and Zahm, 2005) underlies suppression of firing in this subset of recorded VTA neurons. We attempted to test the necessity of this glutamatergic pathway for cue-induced reinstatement of cocaine seeking by disconnecting VP from VTA ionotropic glutamate neurotransmission (unilateral VP chemogenetic inhibition, plus contralateral VTA injection of AMPA/NMDA antagonists), but failed to find consistent effects of this manipulation in the small group of rats tested (Mahler et al., 2014b). Further investigation of the specific connectivity of VP glutamate versus GABA projections to VTA, and their roles in reinstatement, is therefore warranted.
Beyond revealing the complex roles for VP subregions in drug-seeking behavior, our VP manipulation experiments clearly challenge the intuitive concept that anatomical contiguity (e.g., of the Substance P-delineated VP) represents functional continuity. Recent advances in tools for manipulating genetically- and anatomically-defined pathways in vivo have led to a renewed appreciation for such functionally distinct subpopulations of neurons within defined nuclei (e.g., Lenz and Lobo, 2013; Nieh et al., 2013; Stamatakis and Stuber, 2012; Steinberg et al., 2015; Tye and Deisseroth, 2012). In our view, such functional/anatomical heterogeneity likely relates to specific roles played by brain circuits in rodent reinstatement, and potentially in human drug relapse as well.
7. Enhancing face validity of rodent relapse models
Despite the large body of evidence unraveling the neural circuits implicated in cocaine reinstatement in rats, we are far from understanding how these circuits function in human cocaine addiction and relapse. Conventional reinstatement models capture some of the overt characteristics of the addiction/relapse cycle, including voluntary drug use, cessation of drug taking, and resumption of drug use precipitated by similar stimuli to those eliciting relapse in humans. However, many details differ between the situations experienced by experimental animals in these studies and those experienced by recovering addicts, potentially hindering our ability to map circuit/behavior relationships across species (Epstein et al., 2006). Like humans and other primates, rats are sensitive to the contingencies surrounding their drug use, and most will readily cease or greatly reduce their intake when the cost of taking drugs becomes high, or alternative reinforcers are available to pursue instead (Alexander et al., 1981; Belin-Rauscent et al., 2016; Hart et al., 2000; Higgins, 1997; Nader et al., 2008). We highlight two features of addiction in humans that are absent in conventional animal relapse models: 1) presence of alternative reinforcers that may deter drug taking or relapse and 2) negative consequences associated with drug use or relapse.
People usually have many attractive options in life, some of which are mutually exclusive with excessive drug use (e.g., financial security, employment, social relationships). In humans, this idea underlies contingency management, a relatively effective type of treatment for recovering addicts in which drug-free urine can be exchanged for non-drug rewards (e.g., gift cards or money) (Prendergast et al., 2006). In contrast, laboratory rodents typically live in unstimulating environments, frequently in social isolation, and behavioral testing is by far the most engaging thing that happens to them on a regular basis. Recent rodent models have therefore incorporated alternative reinforcers (e.g., palatable food) that are delivered as an alternative to drug, and which can thereby promote voluntary abstinence from drug use. In this way, neural substrates of reinstatement following voluntary abstinence can be probed. For example, Caprioli and colleagues (2015; 2017) trained rats separately to self-administer palatable food pellets and methamphetamine, then subjected them to either forced abstinence from both rewards, or voluntary abstinence from drug due to choice of the food alternative. In this case, both forced- and voluntary-abstinence groups displayed incubation of methamphetamine craving, and both effects were attenuated by the same pharmacological enhancement of mGluR2 signaling (Caprioli et al., 2015), or activity-selective lesion of dorsomedial striatum neurons recruited during methamphetamine seeking (Caprioli et al., 2017). Venniro et. al (2017) also showed that glutamatergic projections from anterior insular cortex to central amygdala are necessary for cue-induced reinstatement of methamphetamine seeking following voluntary abstinence from “contingency management,” and involvement of this pathway in drug seeking under other conditions should be further studied.
Use of drugs in moderation is nearly ubiquitous in people under socially and legally acceptable circumstances, when the consequences of use are minimal (e.g., having a glass of wine at dinner). Yet there is a subset of individuals in which drug use becomes excessive, and use continues despite mounting consequences. Remarkably, like humans, a subset of outbred rats (usually around 10-25% depending on study conditions) are relatively insensitive to punishment—tolerating shock co-administered with cocaine (Belin et al., 2016; Belin-Rauscent et al., 2016; Vanderschuren et al., 2017), failing to suppress cocaine seeking during a shock-predictive cue (Vanderschuren and Everitt, 2004), or voluntarily crossing an electrified floor to take cocaine (Barnea-Ygael et al., 2012; Cooper et al., 2007). These punishment-resistant rats can be identified based on individual differences in the degree of pre-cocaine voluntary exploration of a novel environment (Belin et al., 2011), impulsivity in the 5-choice serial reaction time task (Economidou et al., 2009), or the pattern of cocaine intake during initial self-administration training (Belin et al., 2009). This indicates that pre-existing individual differences predict the eventual transition from regulated to uncontrolled cocaine seeking in individual animals, a finding which could have major implications for predicting vulnerability to addiction and relapse in humans. We refer readers to recent excellent reviews for more information about the characteristics and neural substrates of punished drug intake (Belin-Rauscent et al., 2016; Vanderschuren et al., 2017).
Of note, cocaine experience can reduce the ability of people (Ersche et al., 2016) or rats (Simon et al., 2009; Wied et al., 2013) to properly use negative outcomes to guide their subsequent behavior. Although punishing drug use criminally, with taxation, or even with advertising (e.g., placement of graphic, aversive photos on cigarette packages) is somewhat effective at reducing drug use (Milton and Everitt, 2012), drug-induced insensitivity to punishment may sabotage these efforts in established addicts (Ersche et al., 2016). Given these facts, some labs have begun incorporating negative outcomes of drug use into preclinical relapse models. For example, the Yavin Shaham laboratory developed a cue/context reinstatement model in which abstinence is self-imposed by rats due to co-administration of footshock with drug after initial punishment-free training (Marchant et al., 2013a, 2013b; Pelloux et al., 2017; Venniro et al., 2016). In this approach, rats are trained to lever press for drug and a discrete cue in Context A (self-administration context with distinct visual and olfactory cues), then are introduced to Context B (punishment context) where lever pressing produces not only drug and discrete cues (tone+light), but also footshock coincident with 50% of drug/cue deliveries. Initial punishment training features a weak (0.3mA) footshock, and the intensity of shock is increased across training until pressing ceases in all rats (up to 1mA). Importantly, when shocks are delivered in a non-contingent manner in Context B, drug intake is not suppressed, showing that motivation to avoid punishment, rather than shock-induced stress alone, suppresses drug (alcohol) seeking (Marchant et al., 2013a). Following self-imposed footshock-induced abstinence, rats are reintroduced to Context A in the absence of drug. As expected, drug seeking in the punishment context is low even when discrete tone/light cues are delivered upon lever pressing, yet the same cues elicit robust reinstatement when delivered in Context A where unpunished drug was delivered (Krasnova et. al., 2014).
We have recently modified Marchant and colleagues’ punishment-induced abstinence approach (Marchant et al., 2013a) for use with cocaine (similar to Pelloux et al., 2017), and characterized Long-Evans rats’ cocaine seeking during self-administration and voluntary abstinence (Fig. 3A; unpublished observations). As previously reported, rats showed marked variability in punishment resistance (Chen et al., 2013; Deroche-Gamonet et al., 2004; Marchant et al., 2014), with ~16% displaying significant resistance to punishment under this relatively mild shock intensity (Fig. 3B). When we examined the specific behaviors exhibited by rats during shock-punished training, most rats displayed “stretch and attend” hesitations following each shock delivery, a typical rodent risk assessment behavior (Blanchard et al., 2011), which here may reflect a mixed motivational state in which motivation to seek cocaine competes with motivation to avoid shock (Fig. 3C). Hesitations consisted of the rats stretching their forequarters toward the active lever and sometimes touching it (Fig. 3D), before withdrawing the forepaw rapidly without depressing the lever. Hesitations were most prevalent in the most punishment sensitive rats, and was less common in those who continued to take cocaine despite being shocked. Therefore, hesitations could be used as an objective measure of “mixed motivation” to receive cocaine in punishment sensitive rats. We also noticed that numerous rats shifted their lever pressing behavior to the unreinforced/unpunished inactive lever during punishment training (Fig. 3A), potentially representing diversion of cocaine seeking into exploration of alternate, less risky strategies to obtain cocaine. Inactive lever pressing, in addition to hesitations, could therefore be used to quantify mixed motivations in such studies.
Figure 3. Voluntary Abstinence From Cocaine Via Punishment.
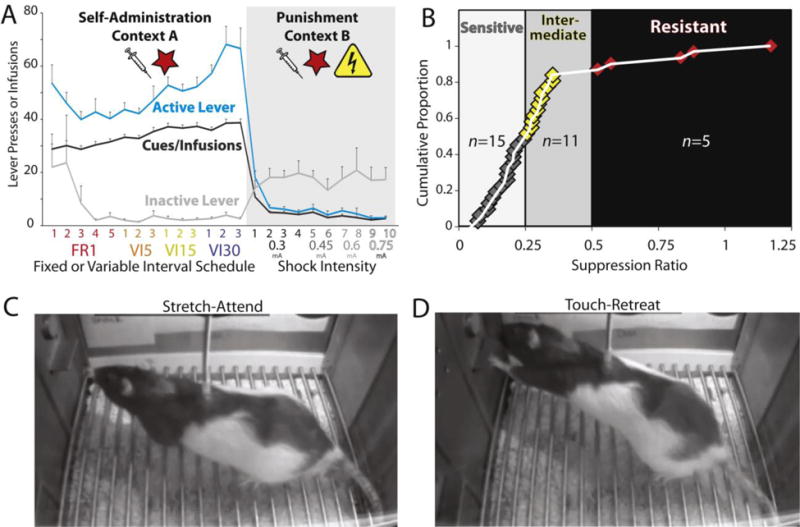
A) Cocaine self-administration behavior in Context A, followed by suppression of cocaine seeking in Context B, where lever presses yield cocaine and cues, but also shock on 50% of trials. Shock intensity was increased from 0.3-0.75mA over subsequent punishment training, until all rats voluntarily ceased pressing. B) During the punishment phase in Context B, rats varied in their sensitivity to shock for suppressing cocaine self-administration. Suppression ratio is defined as the decrease in pressing on punishment day 1, relative to infusions self-administered on the last day of unpunished self-administration [infusions on punishment day 1/infusions on last self-administration day; Y-axis indicates cumulative proportion of sampled rats, with 1 indicating 100% of tested rats (Pelloux, et al 2007)]. Most rats showed high (n=15) or intermediate (n=11) sensitivity to shock, while 16% (n=5) were resistant to punishment in this sample. During the punishment phase, rats showed putative indices of mixed motivational states, namely C) stretch-attend, and D) touch-retreat behaviors.
To date, few differences in relapse circuits following experimenter-imposed versus voluntary abstinence have been demonstrated. We predict that further study with these new self-imposed abstinence models will reveal such differences based on abstinence method and other behavioral circumstances, however. For example, we predict that fear- and aversion-related regions will be recruited during abstinence due to punishment avoidance, but not due to choice of an alternative reinforcer, experimenter-imposed abstinence, or extinction training. In sum, we believe that attention must be paid to maximizing the similarity of rodent relapse models to human behavior in addiction. This may inform our understanding of the genetic or environmental factors that cause individual differences in relapse vulnerability, and the precise neural circuits mediating decisions to take or abstain from drugs.
8. Conclusions
Cocaine relapse is a deceptively complex phenomenon, engaging a variety of brain circuits that vary due to experimental details of animal studies that are often glossed-over. In other words, the devil is in the details when it comes to identifying cocaine relapse circuits. Instead of despairing this frustrating fact, we hope that by improving preclinical models to more faithfully emulate conditions under which humans take drugs, decide to quit, and then relapse, this will facilitate our identifying the specific circuits underlying relapse risk in individual humans. This conceptual shift could facilitate development of individualized therapeutic strategies targeting aberrant activity in the circuits most relevant to an individual’s most problematic relapse risk factors (McKay et al., 1996; Moeller and Paulus, 2018). However, we must first understand how neural circuits function during clinically-relevant cocaine-seeking behaviors, and how these systems are hijacked with repeated drug use. Fortunately, modern neuroscience tools for imaging and manipulating circuit activity in vivo hold great promise for doing so, both by characterizing neural circuits underlying complex relapse-related behaviors in rodents, and perhaps for one day intervening in these circuits to selectively treat psychiatric disorders like addiction in humans.
Highlights.
Rodent reinstatement experiments may effectively model human cocaine relapse
Details of such models influence the neural circuits engaged during cocaine seeking
We summarize common rat models, and neural circuits involved
We also summarize strategies for increasing face validity of these models
Such studies could help identify relapse risk factors in individual addicted humans
Acknowledgments
Funding, and Disclosures: We thank Erik Castillo for assistance with microscopy. This paper was funded by R00 DA035251, the UCI Ayala School of Biological Sciences, Department of Neurobiology and Behavior, and Office of Research, and the Irvine Center for Addiction Neuroscience.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BLA
basolateral amygdale
- BNST
bed nucleus of the stria terminalis
- CeA
central amygdala nucleus
- CRF
corticotropin-releasing factor
- DA
dopamine
- D1
dopamine receptor 1
- D2
dopamine receptor 2
- GABA
γ-Aminobutyric acid
- ILC
infralimbic cortex
- i.c.v.
intracerebroventricular
- LTN
laterodorsal tegmental nucleus
- LH
lateral hypothalamus
- mPFC
medial prefrontal cortex
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-D-aspartate
- NE
norepinephrine
- NAc
nucleus accumbens
- NAcCo
nucleus accumbens core
- NAcSh
nucleus accumbens shell
- OFC
orbitofrontal cortex
- OX1R
orexin receptor 1
- PLC
prelimbic medial prefrontal cortex
- PVT
paraventricular thalamus
- VP
ventral pallidum
- VTA
ventral tegmental area
- 5HT
5-hydroxytryptamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial conflicts of interest exist.
References
- Ahmed SH. Validation crisis in animal models of drug addiction: Beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–25. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Ahrens AM, Meyer PJ, Ferguson LM, Robinson TE, Aldridge JW. Neural Activity in the Ventral Pallidum Encodes Variation in the Incentive Value of a Reward Cue. J Neurosci. 2016;36:7957–7970. doi: 10.1523/JNEUROSCI.0736-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB. Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav. 1981;15:571–576. doi: 10.1016/0091-3057(81)90211-2. [DOI] [PubMed] [Google Scholar]
- Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol. 2013;23:752–759. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Bouayad-Gervais K, Samaha AN. High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology (Berl) 2017a doi: 10.1007/s00213-017-4773-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Allain F, Roberts DCS, Lévesque D, Samaha AN. Intermittent intake of rapid cocaine injections promotes robust psychomotor sensitization, increased incentive motivation for the drug and mGlu2/3 receptor dysregulation. Neuropharmacology. 2017b;117:227–237. doi: 10.1016/j.neuropharm.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Alleweireldt AT, Kirschner KF, Blake CB, Neisewander JL. D1-receptor drugs and cocaine-seeking behavior: Investigation of receptor mediation and behavioral disruption in rats. Psychopharmacology (Berl) 2003;168:109–117. doi: 10.1007/s00213-002-1305-x. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Angarita GA, Pittman B, Gueorguieva R, Kalayasiri R, Lynch WJ, Sughondhabirom A, Morgan PT, Malison RT. Regulation of cocaine self-administration in humans: Lack of evidence for loading and maintenance phases. Pharmacol Biochem Behav. 2010;95:51–55. doi: 10.1016/j.pbb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Richardson BD, Hall JL, Wang R, Hodges MA, Mitchell MP, Stuber GD, Rossi DJ, Fuchs RA. Role of a Lateral Orbital Frontal Cortex-Basolateral Amygdala Circuit in Cue-Induced Cocaine-Seeking Behavior. Neuropsychopharmacology. 2017;42:727–735. doi: 10.1038/npp.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Baimel C, Borgland SL. Hypocretin/Orexin and plastic adaptations associated with drug abuse. Curr Top Behav Neurosci. 2017;33:283–304. doi: 10.1007/7854_2016_44. [DOI] [PubMed] [Google Scholar]
- Barnea-Ygael N, Yadid G, Yaka R, Ben-Shahar O, Zangen A. Cue-induced reinstatement of cocaine seeking in the rat “conflict model”: Effect of prolonged home-cage confinement. Psychopharmacology (Berl) 2012;219:875–883. doi: 10.1007/s00213-011-2416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of Intake and Drug Craving Predict the Development of Cocaine Addiction-like Behavior in Rats. Biol Psychiatry. 2009;65:863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW. In search of predictive endophenotypes in addiction: Insights from preclinical research. Genes, Brain Behav. 2016;15:74–88. doi: 10.1111/gbb.12265. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–79. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin-Rauscent A, Fouyssac M, Bonci A, Belin D. How preclinical models evolved to resemble the diagnostic criteria of drug addiction. Biol Psychiatry. 2016;79:39–46. doi: 10.1016/j.biopsych.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: A review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci. 2014;111:11822–7. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, España RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations, in: The Psychology of Learning and Motivation. 2001:223–278. doi:0079-7421/00. [Google Scholar]
- Bickel WK, Yi R, Mueller ET, Jones BA, Christensen DR. The behavioral economics of drug dependence: Towards the consilience of economics and behavioral neuroscience. Curr Top Behav Neurosci. 2010;3:319–41. doi: 10.1007/7854_2009_22. [DOI] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: a perceptual-motivational alternative to response reinforcements. Behav Brain Sci. 1978;1:41. doi: 10.1017/S0140525X00059380. [DOI] [Google Scholar]
- Blacktop JM, Vranjkovic O, Mayer M, Van Hoof M, Baker DA, Mantsch JR. Antagonism of GABA-B but not GABA-A receptors in the VTA prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats. Neuropharmacology. 2016;102:197–206. doi: 10.1016/j.neuropharm.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 2011;35(4):991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural Systems and Cue-Induced Cocaine Craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin a in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. doi: 10.1016/0023-9690(79)90057-2. [DOI] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and Temporal Modulation of Extinction: Behavioral and Biological Mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D’Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–8. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Davis MH, Van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci. 2012;6(54) doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zeric T, Li X, Adhikary S, Madangopal R, Marchant NJ, Lucantonio F, Schoenbaum G, Bossert JM, Shaham Y. Effect of the novel positive allosteric modulator of metabotropic glutamate receptor 2 AZD8529 on incubation of methamphetamine craving after prolonged voluntary abstinence in a rat model. Biol Psychiatry. 2015;78:463–473. doi: 10.1016/j.biopsych.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y. Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J Neurosci. 2017;37:1014–1027. doi: 10.1523/JNEUROSCI.3091-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME. The economic context of drug and non-drug reinforcers affects acquisition and maintenance of drug-reinforced behavior and withdrawal effects. Drug Alcohol Depend. 1993;33:201–210. doi: 10.1016/0376-8716(93)90061-T. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [DOI] [PubMed] [Google Scholar]
- Chan CL, Wheeler DS, Wheeler RA. The neural encoding of cocaine-induced devaluation in the ventral pallidum. Neurobiol Learn Mem. 2016;130:177–184. doi: 10.1016/j.nlm.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, Smith KS. Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. Eur J Neurosci. 2015;42:3105–3116. doi: 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, Chen A, Lawrence AJ. Knockdown of CRF1 Receptors in the Ventral Tegmental Area Attenuates Cue- and Acute Food Deprivation Stress-Induced Cocaine Seeking in Mice. J Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ. Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history. Addict Biol. 2015;20:690–700. doi: 10.1111/adb.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. doi:20015644 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology (Berl) 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, LaLumiere RT. The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology. 2015;40:1–35. doi: 10.1038/npp.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict Biol. 2016;21:3–22. doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron. 2016;92:214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-P. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacol Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for Addiction-like Behavior in the Rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology (Berl) 1998;137:333–340. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Taymans SE, Sundstrom JM, Pert A. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res. 1998;786:39–46. doi: 10.1016/S0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- De Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Assessing the initiation of cocaine self-administration in humans during abstinence: Effects of dose, alternative reinforcement, and priming. Psychopharmacology (Berl) 2004;172:316–323. doi: 10.1007/s00213-003-1655-z. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High Impulsivity Predicts Relapse to Cocaine-Seeking After Punishment-Induced Abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: An influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001a;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress-induced Relapse to Drug Seeking in the Rat; Role of the Bed Nucleus of the Stria Terminalis and Amygdala. Stress Int J Biol Stress. 2001b;4:289–303. doi: 10.3109/10253890109014753. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LHE, Luijten M, de Wit S, Sahakian BJ, Bullmore ET, Robbins TW. Carrots and sticks fail to change behavior in cocaine addiction. Science. 2016;352:1468–1471. doi: 10.1126/science.aaf3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Schmeichel BE, Berridge CW. Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res. 2016;1641:207–16. doi: 10.1016/j.brainres.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories - indications for novel treatments of addiction. Eur J Neurosci. 2014;40:2163–82. doi: 10.1111/ejn.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The Role of the Dorsomedial Prefrontal Cortex, Basolateral Amygdala, and Dorsal Hippocampus in Contextual Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Model. 2008;5(4):251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent activation of brainstem and pallidal afferents of the ventral tegmental area by cocaine. Neuropsychopharmacology. 2008;33:2688–700. doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Goodman J, Packard MG. Memory systems and the addicted brain. Front Psychiatry. 2016;7(24) doi: 10.3389/fpsyt.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Nauta WJH. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Hadad NA, Wu L, Hiller H, Krause EG, Schwendt M, Knackstedt LA. Conditioned stress prevents cue-primed cocaine reinstatement only in stress-responsive rats. Stress. 2016;19:406–418. doi: 10.1080/10253890.2016.1189898. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-Y. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacol Biochem Behav. 1997;57:419–427. doi: 10.1016/S0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid Modulation of Ventral Pallidal Afferents to Ventral Tegmental Area Neurons. J Neurosci. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. An Orexin Hotspot in Ventral Pallidum Amplifies Hedonic “Liking” for Sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive Ratio as a Measure of Reward Strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Raasø HS, Schoffelmeer ANM, De Vries TJ. Individual differences in sensitivity to factors provoking reinstatement of cocaine-seeking behavior. Behav Brain Res. 2004;152:157–161. doi: 10.1016/j.bbr.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 1993;33:165–172. doi: 10.1016/0376-8716(93)90058-X. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–90. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G. A Decade of Orexin/Hypocretin and addiction: Where are we now? Curr Top Behav Neurosci. 2017;33:247–281. doi: 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Addiction as a pathology in prefrontal cortical regulation of corticostriatal habit circuitry. Neurotox Res. 2008;14:185–189. doi: 10.1007/BF03033809. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168(1-2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: Neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 2016;233:3587–3602. doi: 10.1007/s00213-016-4393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo SYS, Gibson GD, Prasad AA, McNally GP. How contexts promote and prevent relapse to drug seeking. Genes, Brain Behav. 2017;16(1):185–204. doi: 10.1111/gbb.12328. [DOI] [PubMed] [Google Scholar]
- Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EHJ, Lim BK. Distinct Ventral Pallidal Neural Populations Mediate Separate Symptoms of Depression. Cell. 2017;170:284–297.e18. doi: 10.1016/j.cell.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Hall A, Winger G. Individual differences in rhesus monkeys’ demand for drugs of abuse. Addict Biol. 2012;17:887–896. doi: 10.1111/j.1369-1600.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Parvaz MA, Bernstein V, Zilverstand A, Moeller SJ, Delgado MR, Alia-Klein N, Goldstein RZ. Neural mechanisms of extinguishing drug and pleasant cue associations in human addiction: Role of the VMPFC. Addict Biol. 2017 doi: 10.1111/adb.12545. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharm. 2014;39(8):2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredlow MA, Unger LD, Otto MW. Harnessing reconsolidation to weaken fear and appetitive memories: A meta-analysis of post-retrieval extinction effects. Psychol Bull. 2016;142:314–336. doi: 10.1037/bul0000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci. 2001;115:1086–1092. doi: 10.1037/0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Zavala AR, Singh A, Thiel KJ, Dickey ED, Joyce JN, Neisewander JL. c-Fos expression associated with reinstatement of cocaine-seeking behavior by response-contingent conditioned cues. Synapse. 2009;63:823–835. doi: 10.1002/syn.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 2015;18:1230–1232. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawan IT, Guitart-Masip M, Dolan RJ. Dopamine and effort-based decision making. Front Neurosci. 2011:5. doi: 10.3389/fnins.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA. Interaction of the Basolateral Amygdala and Orbitofrontal Cortex is Critical for Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2011;36:711–720. doi: 10.1038/npp.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Arguello AA, Wells AM, Hodges MA, Fuchs RA. Contribution of a Mesocorticolimbic Subcircuit to Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacology. 2014;39:660–669. doi: 10.1038/npp.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2010a;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 2010b;171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16(11):1644–51. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Augier E, Vouillac C, Ahmed SH. A Choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci. 2013;1(SUPPL. 64) doi: 10.1002/0471142301.ns0944s64. [DOI] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. doi:20026536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. Ventral Pallidal Projections to Mediodorsal Thalamus and Ventral Tegmental Area Play Distinct Roles in Outcome-Specific Pavlovian-Instrumental Transfer. J Neurosci. 2015;35:4953–4964. doi: 10.1523/JNEUROSCI.4837-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang G Bin, Wang F, Ma MY, Xue MM, Luo YX, Yang F De, Bao YP, Shi J, Sun HQ, Lu L. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2015;20:513–522. doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: Different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking Context with Reward: A Functional Circuit from Hippocampal CA3 to Ventral Tegmental Area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones G. Fos Activation of Selective Afferents to Ventral Tegmental Area during Cue-Induced Reinstatement of Cocaine Seeking in Rats. J Neurosci. 2012;32:13309–13325. doi: 10.1124/dmd.107.016501.CYP3A4-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-Reactors: Individual Differences in Cue-Induced Craving after Food or Smoking Abstinence. PLoS One. 2010;5:e15475. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman D, Smith R, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/ hypocretin function. Nat Neurosci. 2014a;17:1298–1303. doi: 10.1038/nn.3810.Motivational. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Effects of haloperidol on reactions to smoking cues in humans. Behav Pharmacol. 2005;16:123–126. doi: 10.1097/00008877-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014b;17:577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Stowe TA, Godfrey JR, Weinshenker D. A Method for Psychosocial Stress-Induced Reinstatement of Cocaine Seeking in Rats. Biol Psychiatry. 2016;79:940–946. doi: 10.1016/j.biopsych.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–232. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biol Psychiatry. 2013a;73:256–262. doi: 10.1016/j.biopsych.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013b;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Rabei R, Kaganovsky K, Caprioli D, Bossert JM, Bonci A, Shaham Y. A Critical Role of Lateral Hypothalamus in Context-Induced Relapse to Alcohol Seeking after Punishment-Imposed Abstinence. J Neurosci. 2014;34:7447–7457. doi: 10.1523/JNEUROSCI.0256-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R. Dynorphin Counteracts Orexin in the Paraventricular Nucleus of the Thalamus: Cellular and Behavioral Evidence. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.250. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, Aston-Jones G. Dorsal Hippocampus Drives Context-Induced Cocaine Seeking via Inputs to Lateral Septum. Neuropsychopharmacology. 2017;32(3):581–597. doi: 10.1038/npp.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci. 2016;36:8700–8711. doi: 10.1523/JNEUROSCI.1291-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug Dependence, a Chronic Medical Illness. JAMA. 2000;284:1689. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Mcnally GP. Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacology. 2014;76:528–532. doi: 10.1016/j.neuropharm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Peña DF, Blacktop JM, Mantsch JR. Neurobiological mechanisms underlying relapse to cocaine use: contributions of CRF and noradrenergic systems and regulation by glucocorticoids. Stress. 2014;17:22–38. doi: 10.3109/10253890.2013.872617. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behav Brain Res. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Paulus MP. Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. 2018;80:143–154. doi: 10.1016/j.pnpbp.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007;190:171–180. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Moran-Santa Maria MM, McRae-Clark A, Baker NL, Ramakrishnan V, Brady KT. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology (Berl) 2014;231:4157–4165. doi: 10.1007/s00213-014-3555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL. Serotonin 2A Receptors Differentially Contribute to Abuse-Related Effects of Cocaine and Cocaine-Induced Nigrostriatal and Mesolimbic Dopamine Overflow in Nonhuman Primates. J Neurosci. 2013;33:13367–13374. doi: 10.1523/JNEUROSCI.1437-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3223–32. doi: 10.1098/rstb.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1038/466194a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BA, Kantak KM. Cognitive enhancers for facilitating drug cue extinction: Insights from animal models. Pharmacol Biochem Behav. 2011;99:229–244. doi: 10.1016/j.pbb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res. 2013;1511:73–92. doi: 10.1016/j.brainres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DCS. A novel IV cocaine self-administration procedure in rats: Differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl) 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DC. Behavioral Economic Assessment of Price and Cocaine Consumption Following Self-Administration Histories that Produce Escalation of Either Final Ratios or Intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: Regulation, reinforcement and activation. Psychopharmacology (Berl) 2000;152:123–131. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ. Incubation of Cue-Induced Craving in Adults Addicted to Cocaine Measured by Electroencephalography. JAMA Psychiatry. 2016;73:1127. doi: 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Dilleen R, Economidou D, Theobald D, Everitt BJ. Reduced Forebrain Serotonin Transmission is Causally Involved in the Development of Compulsive Cocaine Seeking in Rats. Neuropsychopharmacology. 2012;37:2505–2514. doi: 10.1038/npp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Hoots JK, Cifani C, Adhikary S, Martin J, Minier-Toribio A, Bossert JM, Shaham Y. Context-induced relapse to cocaine seeking after punishment-imposed abstinence is associated with activation of cortical and subcortical brain regions. Addict Biol. 2017 doi: 10.1111/adb.12527. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Mcnally GP. A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci. 2013;38:2762–2773. doi: 10.1111/ejn.12283. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockros-Burgess LA, Pentkowski NS, Der-Ghazarian T, Neisewander JL. Effects of the 5-HT2C receptor agonist CP809101 in the amygdala on reinstatement of cocaine-seeking behavior and anxiety-like behavior. Int J Neuropsychopharmacol. 2014;17:1751–1762. doi: 10.1017/S1461145714000856. [DOI] [PubMed] [Google Scholar]
- Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA. Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol Psychiatry. 2014;76:785–793. doi: 10.1016/j.biopsych.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, McNally GP. Ventral Pallidum Output Pathways in Context-Induced Reinstatement of Alcohol Seeking. J Neurosci. 2016;36:11716–11726. doi: 10.1523/JNEUROSCI.2580-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH. Exacerbated Craving in the Presence of Stress and Drug Cues in Drug-Dependent Patients. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.275. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, González-Lima F. Prefrontal Mechanisms in Extinction of Conditioned Fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rich MT, Torregrossa MM. Molecular and Synaptic Mechanisms Regulating Drug-Associated Memories: Towards a Bidirectional Treatment Strategy. Brain Res Bull. 2017 doi: 10.1016/j.brainresbull.2017.09.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, Fields HL. Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions. Neuron. 2016;90:1165–1173. doi: 10.1016/j.neuron.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Gabriele A, Zimmer BA. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: Methodological and interpretational considerations. Neurosci Biobehav Rev. 2013;37:2026–2036. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Jason L, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. 274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Royet JP, Meunier D, Torquet N, Mouly AM, Jiang T. The Neural Bases of Disgust for Cheese: An fMRI Study. Front Hum Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–7. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D. Effects of SCH 23390 and eticlopride on cocaine-seeking produced by cocaine and WIN 35,428 in rats. Psychopharmacology (Berl) 2003;168:118–123. doi: 10.1007/s00213-002-1276-y. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF. Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-administration and Stress-Induced Reinstatement in Rats. Biol Psychiatry. 2017;81:606–615. doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur J Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Schmidt KT, Schroeder JP, Foster SL, Squires K, Smith BM, Pitts EG, Epstein MP, Weinshenker D. Norepinephrine regulates cocaine-primed reinstatement via α1-adrenergic receptors in the medial prefrontal cortex. Neuropharmacology. 2017;119:134–140. doi: 10.1016/j.neuropharm.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Alisha Epps S, Grice TW, Weinshenker D. The Selective Dopamine β-Hydroxylase Inhibitor Nepicastat Attenuates Multiple Aspects of Cocaine-Seeking Behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress-induced modulation of instrumental behavior: From goal-directed to habitual control of action. Behav Brain Res. 2011;219:321–8. doi: 10.1016/j.bbr.2010.12.038. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- See RE, Parrish Waters R. Pharmacologically-induced stress: A cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2011;3:81–89. doi: 10.1016/j.neuropharm.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: A review. Brain Res Rev. 2000 doi: 10.1016/S0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shorter D, Domingo CB, Kosten TR. Emerging drugs for the treatment of cocaine use disorder: a review of neurobiological targets and pharmacotherapy. Expert Opin Emerg Drugs. 2015;20:15–29. doi: 10.1517/14728214.2015.985203. [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing Risk and Reward: A Rat Model of Risky Decision Making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sinha R, Shaham Y, Heilig M. Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 2011;218:69–82. doi: 10.1007/s00213-011-2263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid Limbic Circuit for Reward: Interaction between Hedonic Hotspots of Nucleus Accumbens and Ventral Pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The Ventral Pallidum and Hedonic Reward: Neurochemical Maps of Sucrose “Liking” and Food Intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009b;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. α 2 Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: Role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Laiks LS. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog Neuro-Psychopharmacology Biol Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.09.003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009a;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD, Barrett-Larimore RL, Rowlett JK, Platt DM, Khroyan TV. Pharmacological and environmental determinants of relapse to cocaine-seeking behavior. Pharmacol Biochem Behav. 1999;64:327–336. doi: 10.1016/S0091-3057(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Optogenetic strategies to dissect the neural circuits that underlie reward and addiction. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J Neurosci. 2013b;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013a;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Curr Opin Neurobiol. 2015;30:9–16. doi: 10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: The neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stout KA, Dunn AR, Lohr KM, Alter SP, Cliburn RA, Guillot TS, Miller GW. Selective Enhancement of Dopamine Release in the Ventral Pallidum of Methamphetamine-Sensitized Mice. ACS Chem Neurosci. 2016;7:1364–1373. doi: 10.1021/acschemneuro.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. doi:1300619 [pii]\r10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology (Berl) 2005;180:436–446. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Sun W, Xue Y, Huang Z, Steketee JD. Regulation of cocaine-reinstated drug-seeking behavior by kappa-opioid receptors in the ventral tegmental area of rats. Psychopharmacology (Berl) 2010;209:179–188. doi: 10.1007/s00213-010-1812-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Wise RA. Satiating Effects of Cocaine Are Controlled by Dopamine Actions in the Nucleus Accumbens Core. J Neurosci. 2011;31:17917–17922. doi: 10.1523/JNEUROSCI.1903-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Wenzel JM, Pentkowski NS, Hobbs RJ, Alleweireldt AT, Neisewander JL. Stimulation of dopamine D2/D3 but not D1 receptors in the central amygdala decreases cocaine-seeking behavior. Behav Brain Res. 2010;214:386–394. doi: 10.1016/j.bbr.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295X.97.2.147. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: Manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology (Berl) 2013;226:659–672. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LTL, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, Bradshaw H, Hwang LL, Hung MS, Mackie K, Zimmer A, Chiou LC. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat Commun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall BJ, Kearns DN. Reinstatement in a cocaine versus food choice situation: Reversal of preference between drug and non-drug rewards. Addict Biol. 2014;19:838–848. doi: 10.1111/adb.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Minnaard AM, Smeets JA, Lesscher HM. Punishment models of addictive behavior. Curr Opin Behav Sci. 2017;13:77–84. doi: 10.1016/j.cobeha.2016.10.007. [DOI] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug Seeking Becomes Compulsive After Prolonged Cocaine Self-Administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog Brain Res. 2016;224:25–52. doi: 10.1016/bs.pbr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D. Incubation of Methamphetamine but not Heroin Craving After Voluntary Abstinence in Female Rats. Neuropsychopharm. 2017;42(5):1126–1135. doi: 10.1038/npp.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-Induced Cocaine Seeking Requires a Beta-2 Adrenergic Receptor-Regulated Pathway from the Ventral Bed Nucleus of the Stria Terminalis That Regulates CRF Actions in the Ventral Tegmental Area. J Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine Experience Establishes Control of Midbrain Glutamate and Dopamine by Corticotropin-Releasing Factor: A Role in Stress-Induced Relapse to Drug Seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of Cocaine Seeking by Hypocretin (Orexin) in the Ventral Tegmental Area: Independence from the Local Corticotropin-Releasing Factor Network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L. Effects of Length of Abstinence on Decision-Making and Craving in Methamphetamine Abusers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shen M, Yu Y, Tao Y, Zheng P, Wang F, Ma L. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. Int J Neuropsychopharmacol. 2014;17:753–763. doi: 10.1017/S1461145713001570. [DOI] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wied HM, Jones JL, Cooch NK, Berg BA, Schoenbaum G. Disruption of model-based behavior and learning by cocaine self-administration in rats. Psychopharmacology (Berl) 2013;229:493–501. doi: 10.1007/s00213-013-3222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Buchta WC, Riegel AC. CRF-R2 and the Heterosynaptic Regulation of VTA Glutamate during Reinstatement of Cocaine Seeking. J Neurosci. 2014;34:10402–10414. doi: 10.1523/JNEUROSCI.0911-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PEM. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Kiyatkin EA. Differentiating the rapid actions of cocaine. Nat Rev Neurosci. 2011;12:479–484. doi: 10.1038/nrn3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao Y-P, Shi J, Epstein DH, Shaham Y, Lu L. A Memory Retrieval-Extinction Procedure to Prevent Drug Craving and Relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, Hnasko TS. Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 2016;7:13697. doi: 10.1038/ncomms13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 Receptor Mediation of Cocaine Seeking in Male and Female Rats. J Pharmacol Exp Ther. 2012;340:801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct. 2014;219:1831–1840. doi: 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC. The Motivation to Self-Administer is Increased After a History of Spiking Brain Levels of Cocaine. Neuropsychopharmacology. 2012;37:1901–1910. doi: 10.1038/npp.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


