Abstract
The development of humanized mice has become a prominent tool for translational animal studies of human diseases. Here we show how immune deficient mice can be “humanized” by injections of human umbilical cord stem cells. The engraftment of these cells and development into human lymphocytes has been possible because of the development of novel severely immune deficient mouse strains. Here we present proven protocols for the generation and analysis of humanized mice on the NSG mouse background.
Keywords: Humanized mice, Human Immune System (HIS), NOD scid gamma (NSG), human umbilical cord stem cells (HUSC)
INTRODUCTION
Mouse models are widely used in laboratory research and development. Mice are preferred experimental animals due to their size, ease of handling, and short reproductive cycle as well as the wealth of information generated with mouse models. However, mouse models of human disease pose limitations because of their lack of susceptibility to certain infections and a lack of a human immune system. This is true for example for the study of various human pathogens, such as retroviruses. Human immune system (HIS) mouse models are generated by engrafting human CD34+ umbilical stem cells (HUSC). The NOD.SCID Il2rγnull (NSG) mouse strain has been widely used by investigators to perform HUSC engraftment (Shultz et al., 2005). Immune deficient NSG mice lack functional mouse T cells, B cells, NK cells, and are deficient in cytokine signaling. After transfer of HUSC, phenotypically normal human immune cells develop in NSG mice. These cells circulate normally in mice, are susceptible to drugs and infections but generate only poor adaptive immune responses, if at all.
The basic protocol in this unit explains the generation of “humanized” mice through the injection of human umbilical stem cells into the liver of neonatal mice. Support protocols illustrate the techniques used to handle the sensitive human umbilical stem cells and to monitor the leukocyte populations engrafted in the humanized mice.
STRATEGIC PLANNING
Immunodeficient Mouse Strains
Two mouse strains, NOD.Cg-PrkdcscidIl2rgtm1Wjll (NSG) and BALB/c-Rag2null IL2rγnull (BRG), are used most often to produce humanized mice. Both mouse strains have either complete or partial deletion of the IL-2 receptor gamma chain (IL2rγ). In NSG mice, mutations in the Rag1 or Rag2 genes inactivate those genes whereas in BRG mice both genes are deleted. The mutations in these mouse strains result in the lack of mature T cells, B cells, or functional NK cells, and a deficiency in cytokine signaling (Coughlan et al., 2016) (Shultz et al., 2005). Although both strains are proven models for engraftment of HUSC, a study indicates that NSG mice support engraftment of HSC better than BRG mouse strains (Brehm et al., 2010).
NSG mice are easily obtainable from The Jackson Laboratory (Bar Harbor, ME) and must be bred and maintained in a specific pathogen-free and, if applicable, BSL-2 facility. All animal use protocols must be reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) before the use of laboratory animals.
Sources of human umbilical cord stem cells (HUSC)
Human umbilical cord stem cells are isolated from umbilical cord blood and purified to ≥90% CD34+ cells, a marker for HUSC. These CD34+ cells are selected using magnetic beads (Miltenyi CliniMACS system (Schumm et al., 1999)). In addition to umbilical cord blood (Ishikawa et al., 2005), HUSC can also be purified from various other human tissues, such as fetal liver, bone marrow (Holyoake, Nicolini, & Eaves, 1999), and peripheral blood (Shultz et al., 2005).
Obtaining human umbilical cord for the purification of HUSC for research requires Ethical Review approval from the Institutional Review Board (IRB) in your institution, whereas the purchase of frozen stem cells usually does not require approval. Laboratory-generated HUSC should be checked by flow cytometry for purity (CD34 expression), and especially the absence of contaminating T cells (CD3, CD4 and CD8 expression) which will lead to graft-versus-host (G-v-H) reaction in mice. Subsequently, small aliquots of purified HUSC should be tested in mice for adverse reactions (Graft-vs-Host). HUSC from commercial sources are more expensive (but less labor intensive) and usually of very good purity.
BASIC PROTOCOL 1
HUMAN UMBICIAL CORD STEM CELL ENGRAFTMENT OF NEONATAL IMMUNODEFICIENT MICE
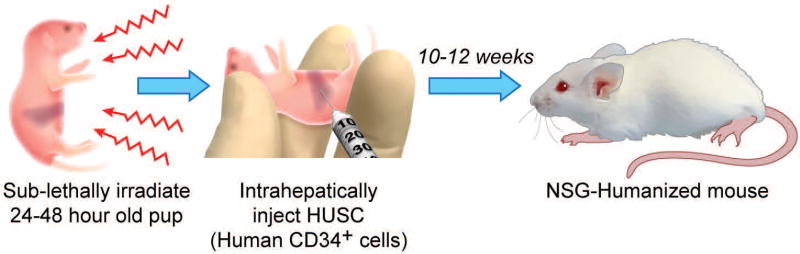
For the generation of humanized mice, neonatal pups are being injected with human umbilical stem cells. Engraftment methods include intravenous injection via the intracardiac route or facial vein, intraperitoneal or intratibial injections, or as described in this protocol, intrahepatically (Pearson, Greiner, & Shultz, 2008) figure 1.
Figure 1. HUMAN UMBICIAL CORD STEM CELL ENGRAFTMENT OF NEONATAL IMMUNODEFICIENT MICE.
Neonatal NSG pups are sub-lethally irradiated at 100cGy to help with the engraftment of the HUSC. After irradiation, intrahepatically inject HUSC and wait for 10–12 weeks for the development of Human cells.
Materials
Mouse Strain, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (The Jackson Laboratory, IMSR Cat# JAX:005557, RRID:IMSR_JAX:005557)
Wet ice
Ice Bucket
HUSC (Support Protocol 1)
26–30g insulin syringe, BD Cat#328466
Heating Pad or warming lamp
Topical petroleum based nasal decongestant; Vicks VapoRub™
Weigh boats
RS 2000 X-ray biological irradiator; RadSource, Suwanee, GA (Support Protocol 3)
Autoclaved, ventilated cage or container for housing mice in irradiator chamber
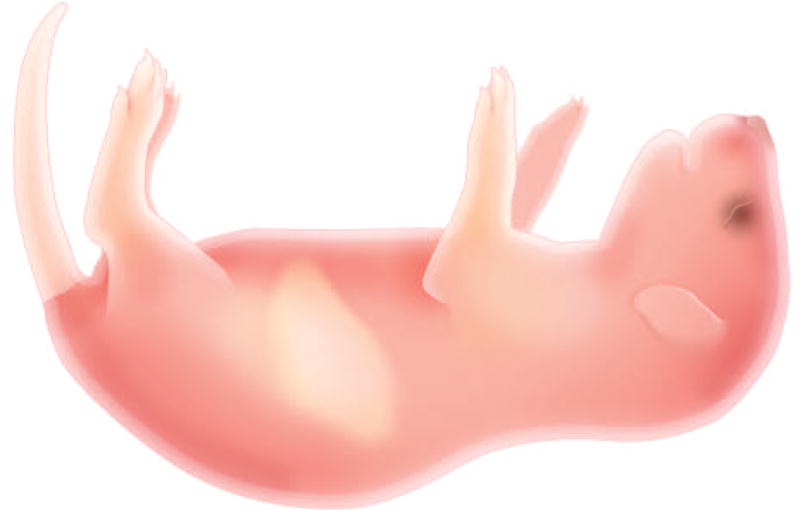
- Pair male and female NSG mice and monitor for new litters (from day 20 onwards).When pups are present watch for cannibalism, do not waste HUSC on a litter that will be cannibalized; the presence of the milk spot (stomach filled with milk) indicates the pups are nursing and are well enough to be injected. The milk spot can be seen through the skin in the abdomen (See figure 2)
Prepare Human Umbilical Stem Cells (Support Protocol 1), count, and suspend in media 3 × 104 to 1 × 105 CD34+ cell/50µl (Lonza, Allendale, NJ)
Place 24hr to 48hr old pups into the sterile, ventilated cage.
- While the pups are being irradiated and anesthetized, restrain the parents and place a small amount of Vicks VapoRub on the snouts of the parents.By placing this decongestant on the snouts of the parents it will reduce the scents transferred to the pups while being handled. This will reduce the potential of cannibalism of the pups as well as increase the acceptance by the parents.
- Irradiate the pups at 100cGy using an X-ray irradiator source.RadSource RS2000 Biological research irradiator
- Place the irradiated pups in a weigh boat on ice until anesthetized. This could take about 5 minutes or until movement subsides.Do not place pups directly on ice; use the cessation of movement as a sign of anesthesia.
- Load an insulin syringe with the HUSC suspension.Can use a 26g–30g needle
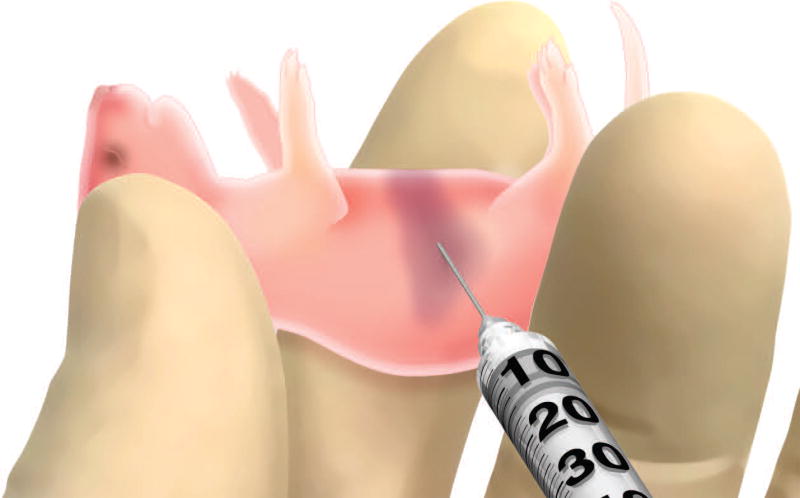
- Restrain pups for intrahepatic injection and dispense 50µl of HUSC suspension into the liver.A second person is needed to help restrain the pup to allow intrahepatic injection. For the restraint technique and location of liver see figure 3.
- Return the pups to the parent cage and place them under a warming lamp for 1–2 minutes.When the pups are returned to the parent cage, roll them in the bedding to reduce any smells associated with handling. Note: the parents will start to move the pups in various directions as they are trying to move them to “safety”.
Wean pups between 21 and 24 days post birth.
- Verify engraftment of Human Stem Cells starting at 10 weeks of age via flow cytometry. See SUPPORT PROTOCOL 2 for Flow cytometry of the analysis of the humanized mice peripheral blood.Flow cytometry will be used to analyze the peripheral blood staining for Human CD45+
Figure 2. Visualization of milk spot in Newborn NSG pup.
The milk spot can be visualized through the skin in the abdomen. The presence of the milk spot indicates the pups are nursing and are well enough to be injected.
Figure 3. Demonstrating NSG pup restraint technique and site for intrahepatic injection.
Restrain pup by having a second person gently scruff at the nape of the neck and hold the base of the tail. The liver should be visible allowing for proper injection.
SUPPORT PROTOCOL 1
PREPARATION OF HUMAN UMBILICAL CORD STEM CELLS
Human CD34+ Hematopoietic stem cells are isolated from human cord blood to reconstitute immunodeficient mice. This protocol instructs how to thaw HUSC and to prepare them for injection.
Materials
HPGM Hematopoietic Growth Media; Lonza; PT-3926
Fetal Bovine Serum
StemSpan CC100; Stem Cell Technologies; 02690
Poietics Cord Blood CD34+; Lonza; 2C-101
Add 10% FBS to HPGM Hemotopoietic Growth Media.
Pre-warm Media in 37°C Water Bath.
Wipe the exterior of the vial containing frozen cells with 70% ethanol. Quickly thaw the vial in a 37°C water bath.
Aseptically transfer the thawed cells to a 15ml tube.
- Rinse the vial (the cells were frozen in) with 1ml of warmed media and add it dropwise for about 1 minute to the cells in the 15 ml tube while swirling.This slow and gentle addition of media is important so that cell recovery is optimal.
Slowly add media dropwise to the cells, while swirling until a total volume of 5ml is reached over a 3 minute time period.
Slowly add media dropwise up to a total volume of 15ml, while gently swirling after each addition. This should take approximately 5–10 minutes.
Centrifuge the cell suspension at 200 × g for 15 minutes at room temperature.
- Remove most of the supernatant by pipette without disturbing the pellet (leave a few milliliters behind).Save all wash in secondary tubes until the procedure is complete in case you need to recover any cells if the cell recovery is low.
Gently re-suspend the pellet in the remaining media
Slowly add fresh media to the cells suspension, while gently swirling. Add media up to 15 ml.
Centrifuge the cell suspension at 200 × g for 15 minutes at room temperature.
Carefully remove the supernatant by pipette, leaving 1–2ml of supernatant.
- Gently re-suspend the cell pellet in the known volume of supernatant and count the cells.Determine how many mice will be injected and then adjust the volume to use as many of the cells as possible.
- Add 1× of StemSpan CC100 to the cells with stem cell media.StemSpan CC100 contains a combination of recombinant human cytokines to support proliferation of human hematopoietic cells. Recombinant human cytokines include: FMS-Like tyrosine kinase 3 ligand (Flt3L), interleukin 3 (IL-3), interleukin 6 (IL-6), stem cell factor (SCF)
- Place cells in incubator until ready to use in animals.Once neonatal pups have been irradiated, the HSC can be injected as described in the Basic Protocol.
SUPPORT PROTOCOL 2
SUBMANDIBULAR BLOOD COLLECTION AND ANALYSIS OF HUMANIZED MICE PERIPHERAL BLOOD VIA FLOW CYTOMETRY
Flow cytometry is an easy method for monitoring human stem cell engraftment in the peripheral blood of humanized mice. Peripheral blood from the facial vein can be collected into a microtainer tube containing EDTA and stained using antibodies specific for human markers.
Materials
BD Microtainer EDTA blood collection tubes or equivalent (BD Biosciences; Cat#365974)
BDMultitest™ CD3/CD8/CD45/CD4; (BD Biosciences, Cat# 340499, RRID:AB_400472)
Human CD3 FITC, clone SK7 (Mouse IgG1k)
Human CD8 PE, clone SK1 (Mouse IgG1k)
Human CD45 PerCP, clone SD1 (Mouse IgG1k)
Human CD4 APC, clone SK3 (Mouse IgG1k)
Rat α Mouse CD45; (BD Biosciences Cat# 553079, RRID:AB_394609)
Flow Buffer
BD lysing buffer; BD; Cat#349202
12×75mm round bottom disposable test tubes (Falcon, Cat# 352052)
AbC™ Anti-Mouse Antibody Compensation Bead Kit (Molecular Probes, Cat# A10344)
FLOW Cytometer (e.g. Attune NxT, Life Technologies)
-
Restrain mice and collect blood via a submandibular bleed.The volume of blood collected in one collection may not exceed 1% of the animal’s body weight over a 24 hour period.
See video resource for submandibular bleed procedure: https://www.youtube.com/watch?v=niTVnEAHOko
Collect 50–200µl whole blood into an EDTA collection tube from each mouse to be tested. Include a non-engrafted NSG mouse as a negative control.
- Re-suspend the blood and place 50µl of collected whole blood into a labeled 12×75mm flow tube for each animal. Pool the remaining blood to use for control staining and compensation controls.Blood cells stained with mouse CD45 specific antibodies or AbC Total compensation beads are used as compensation controls.
- Stain with the 20µl per tube of the BDMultitest™ CD3/CD8/CD45/CD4 antibody reagent.This four color direct immunofluorescence antibody reagent detects the percentage of mature human T lymphocytes (CD3+), suppressor/cytotoxic (CD3+CD8+) T-lymphocyte subsets, and helper/inducer (CD3+CD4+) T-lymphocyte subsets in erythrocyte-lysed whole blood.
Lyse red blood cells by adding 200µl of 1× BD lysing buffer to each tube.
Incubate for 15 minutes at room temperature and protected from light.
Add 2ml of flow buffer to tube and centrifuge to wash.
- Aspirate, add flow buffer to tube to measure and collect data using a flow Cytometer.Use a flow cytometer that is capable of reading 4 color cytometry. Collect at least 10,000 events.If your cytometer is equipped with greater configuration options capable of reading more colors, then you can increase your antibody panel.
SUPPORT PROTOCOL 3
IRRADIATION OF MICE
Preconditioning of immunodeficient mice with low dose sub lethal irradiation is necessary for the engraftment, proliferation and survival of human umbilical cord stem cells.
Materials
RS 2000 X-ray biological irradiator; RadSource, Suwanee, GA
Autoclaved, ventilated cage or container for housing mice in irradiator chamber
Sterile Biosafety hood
Turn on RS 2000 X-ray irradiator to allow to warm up.
Transfer mice into a sterile cage or container inside the biosafety cabinet.
- Place cage in the irradiator in RAD+ reflector block on the floor of the irradiator chamber, which is level 1 for the machine.RS 2000 is a direct replacement for cesium-137 and cobalt-60 irradiators, but note each irradiator may vary in dose rate and function.
- Irradiate mice at 100cGy (approximately 1 minute).
- Radiation Dose is measured in rad or Gray (Gy). 100 rad = 1Gy. Irradiation time corresponds with dose rates
After irradiation preconditioning, continue with the basic protocol and then transfer mice back to their parents and original cage.
REAGENTS AND SOULTIONS
HPGM Hematopoietic Growth Media 10% FBS (50ml)
Add 5ml of FBS to 45 ml of HPGM media. You can add the StemSpan CC100 at this time as well or wait till the end and add it to the final aliquot of cells prior to injection into the animal to save on cytokines.
BD lysing buffer, 1×, prepared according to manufacturer’s instructions
COMMENTARY
Background Information
Humanized mouse models have become increasingly important tools in the study of human diseases. One potential problem in the generation of humanized mice is the development of graft versus host disease when HUSC are transplanted into the mouse. Signs of graft versus host disease in mice may include a hunched posture, hair loss, weight loss and early death. Since NSG mouse strains have been reported to be the better model for engraftment of HUSC with faster expansion of human CD45+ cells, this may explain the development of graft versus host disease at a faster rate (Brehm et al., 2010) (Ali et al., 2012). It appears that the absence of T cells in the HUSC preparation is very important in avoiding graft versus host disease. In addition to the classical T cell based disease, the development of a macrophage based granulomatous disease has been described (Huey et al., in press). Granulomatosis was observed in a variety of organs with the presence of both mouse and human macrophages.
Another challenge of the humanized mouse model is the development of human lymphocytes that are not immunocompetent, following inoculation of HUSC (Villaudy et al., 2011). Part of the problem seems to be that mouse cytokines do not support fully the development of the human lymphocytes. Two approaches have been pursued to overcome these hurdles: the provision of additional fetal human tissue and the generation of human cytokines in humanized mice. In the first approach, mice are surgically transplanted with human fetal thymus and liver (BLT mouse) which allows for development of functional human leucocytes (Lan, Tonomura, Shimizu, Wang, & Yang, 2006). However, the requirements for this model are the surgical expertise and bone marrow, thymus and liver from one fetus. In addition, the use of fetal tissue for research is not legal in parts of the US. Another approach would be to produce a transgenic mouse expressing human MHC and cytokine genes in NSG or BRG mice (Shultz et al., 2010), (Billerbeck et al., 2011), (Willinger et al., 2011), (Strowig et al., 2011). An alternate approach to facilitate the development of humanized mice is to use a viral vector gene system to deliver human genes into the mouse model prior to injection of HUSC. NSG mice transduced with Adeno-associated virus (AAV) expressing human HLA-A2 and human cytokines such as IL-3, IL-15, GM-CSF have resulted in increased levels of functional human CD45+ cells (Huang, Li, Coelho-dos-Reis, Wilson, & Tsuji, 2014) (Huang et al., 2015).
Troubleshooting and Critical Parameters
For the establishment of a NSG mouse breeding colony, mice have to be kept under specific pathogen-free conditions. Mice become sexually active between 6 and 8 weeks of age. Young NSG mice usually breed better than older mice and performance usually is best if mice are paired at 5–6 weeks of age. Breeding performance and litter size may depend on various factors such as barometric pressure, temperature, humidity, noise, handling of pregnant mice, husbandry enrichments, and the overall health of the breeding pair. In our hands, 1 on 1 breeding by mating one male and one female has established stable breeding and reduction of cannibalism. In contrast, trio breeding and continuous breeding have been reported but may not be as successful. Traditional mouse diet contains roughly 6% fat (LabDiet cat#5K52 or Teklad LM-485 Cat# 7912). Breeding success can be supported by feeding a diet containing higher fat and therefore energy content (e.g. Tekland S-2335 Cat# 7904). Under optimal breeding conditions, a NSG litter should average 8 pups per litter with a range of 2 to 12 animals. The majority of NSG breeders stop breeding by 7 to 8 months of age, and should be replaced at this point. In order to keep the breeding colony active, breeder pairs which have not had a litter in 60 days or small litter size should be replaced. In addition, breeding pairs cannibalizing their pups more than once should be replaced. One way to reduce cannibalism after handling of pups during engraftment of HUSC is to place Vicks VapoRub on the parents’ snouts and to gently smear the pup with bedding from the cage. A small amount of Vicks VapoRub applied to the parent’s snouts will mask olfactory scents that have been transferred to the pups during handling (Council, 2003).
NSG mice are immune deficient and are therefore prone to infections such as Enterococcus spp., Klebsiella oxytoca, S. aureus, Pseudomonas, and C. bovis (Foreman, Kavirayani, Griffey, Reader, & Shultz, 2011), which can easily be dealt with by immunocompetent mice. In order to keep NSG mice healthy and reduce disease outbreaks, proper aseptic handling and housing practices should be performed under strict barrier conditions. Suggested strict barrier practices include the use of micro-isolator cages, autoclaving all food, water, bedding and cages prior to entering the room and manipulating cages inside a biosafety cabinet. All personnel should wear personal protective equipment (PPE) including disposable gown, hair bonnet, mask and disinfected gloves. All cages should be changed at least biweekly, however, weekly is ideal. For the monitoring of the health status of animals the testing of microorganisms typical for immune deficient animals (see above) has to be included.
Another crucial parameter for the generation of humanized mice is the state of the human umbilical cord stem cells. Human CD34+ cells are isolated from cord blood mononuclear cells via positive immunomagnetic separation (Schumm et al., 1999) and should express CD34+ to ≥90%. HUSC are very sensitive and greatly diminish in their efficacy if not thawed properly according to Support Protocol 1.
Host Mouse Strains
Many different strains of immunodeficient mice have been developed which are based on SCID mice or the understanding derived from this mouse strain that RAG 1 and 2 are crucial genes in B cell and T cell receptor development. Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice lack functional B cells, T cells, and their NK cell activity is diminished (Shultz et al., 1995). The creation of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) and BALB/c-Rag2−/−Il2rg−/− (BRG) that lack the IL2rγ gene has led to effective hosts for HUSC engraftment. The lack of the IL2rγ chain blocks NK cell development and results in further defects in innate immunity (Cao et al., 1995). The irradiation of mice before the injection of HUSC can be a problem (depending on the institutional set up). Recently a NOD.Cg-KitW-41J Tyr+PrkdcscidIl2rgtm1Wjl/ThomJ (NBSGW) mouse was derived to support engraftment of HUSC in the absence of irradiation (The Jackson Laboratory Stock #026622). This model is a cross between the NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) strain and the C57BL/6J-KitW-41J/J (C57BL/6.KitW41) strain. In our hands, the level of human lymphocytes in these mice after reconstitution with HUSC was comparable to those in NSG after radiation.
Age of Host
An alternate to neonatal engraftment of HUSC is engraftment in adult mice. When deciding the appropriate age, one has to consider time and cost advantages to manage and engraft mice. Neonatal engraftment can be optimal because pups stay with the parents until weaned, reducing per diem housing costs. Another advantage of neonatal engraftment is the fact that thymic organogenesis in young mice will better support T cell development than that in adult mice (Brehm et al., 2010). The disadvantage of using neonatal mice for engraftment is the fact that they have to be injected within 24–48 hours after birth and cannibalism is more widespread than with adults. However, it has been reported that engrafted neonatal NSG mice support human T cell development better than engrafted adults (Brehm et al., 2010).
Alternate engraftment approaches
Engraftment methods differ between neonatal and adult mice. As the basic protocol of this chapter describes intrahepatic (IH) (Traggiai et al., 2004) injections in neonates, other routes for neonates include intraperitoneal (IP) (Gimeno et al., 2004), intravenous (IV) via the facial vein (Ishikawa, Livingston, Wingard, Nishikawa, & Ogawa, 2002) and intracardiac (IC) injections (Brehm et al., 2010). Humanizing adult immunodeficient mice includes IV (Shultz et al., 2005) and intratibial (IT) injections.
Understanding Results
Results
Neonatal HIS mice
Humanized mice are generated by injecting human umbilical cord stem cell (HUSC) into the livers of sublethally irradiated neonatal NSG mice. Human peripheral blood lymphocytes (HPBL) are detectable in the blood of HUSC engrafted mice via flow cytometry. Human CD45+ cells are detectable as early as 4 weeks post inoculation of HUSC. However, the earliest time point at which a human T cell population will be detectable is 10–12 weeks post inoculation of HUSC. If engraftment was successful, the percentage of human CD45 is 10–60% of the total white blood cells at this time point. The remaining cells are mouse cells, mostly macrophages and neutrophils and can be summarily stained with antibody against mouse CD45, or antibodies against lineage-specific markers.
Time Considerations
Human stem cells engraftment of neonatal pups
The time required for the basic protocol time to inject and engraft NSG mice is dependent on litter size, stem cell preparation, irradiation, injections and the time needed for the human cells to develop in the mouse. Monitoring the breeders and waiting on a decent size litter can take time with a gestation period of about 21 days. The preparation and injection of HUSC can be accomplished in approximately 4 hours and the development of human cells takes upwards of 10–12 weeks.
Before the preparation of HUSC, it is crucial to check the pups for the presence of the milk spot (which indicates a milk filled stomach and thus that pups are viable) and for the absence of cannibalism. If the litter appears healthy, follow the stem cell preparation support protocol. The preparation of the stem cells will take approximately 2–3 hours. The mice need to be exposed to irradiation at 100cGy to aid in HUSC engraftment. The time will depend on the emission of the radiation source. Most irradiators will take less than 5 minutes for a 100cGy exposure (See Support Protocol 3). Intrahepatic injections are then performed once the pups have been irradiated and anesthetized and then returned to the parents. The development of human cells can be verified 10 weeks post inoculation of stem cells.
Acknowledgments
This work was supported by the National Cancer Institute through P01CA100730 “Retrovirus Models of Cancer” (PI Green, Director of the Animal Model Core, Stefan Niewiesk).
Footnotes
LITERATURE CITED
- Ali N, Flutter B, Rodriguez RS, Sharif-Paghaleh E, Barber LD, Lombardi G, Nestle FO. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS ONE. 2012;7(8):e44219. doi: 10.1371/journal.pone.0044219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billerbeck E, Barry WT, Mu K, Dorner M, Rice CM, Ploss A. Development of human CD4+ FoxP3+ regulatory T cells in human stem cell factor–, granulocyte-macrophage colony-stimulating factor–, and interleukin-3–expressing NOD-SCID IL2Rγ null humanized mice. Blood. 2011;117(11):3076–3086. doi: 10.1182/blood-2010-08-301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Cuthbert A, Yang C, Miller DM, DiIorio P, Laning J, Kavirayani A. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγ null mutation. Clinical immunology. 2010;135(1):84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsail BL, Russell SM, Bloom ET. Defective lymphoid development in mice lacking expression of the common cytokine receptor γ chain. Immunity. 1995;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Coughlan AM, Harmon C, Whelan S, O'Brien EC, O'Reilly VP, Crotty P, O'Farrelly C. Myeloid Engraftment in humanized mice: Impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem cells and development. 2016;25(7):530–541. doi: 10.1089/scd.2015.0289. [DOI] [PubMed] [Google Scholar]
- Council NR. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press; 2003. [PubMed] [Google Scholar]
- Foreman O, Kavirayani A, Griffey S, Reader R, Shultz L. Opportunistic bacterial infections in breeding colonies of the NSG mouse strain. Veterinary pathology. 2011;48(2):495–499. doi: 10.1177/0300985810378282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, Spits H. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/−γc−/−mice: functional inactivation of p53 in developing T cells. Blood. 2004;104(13):3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- Holyoake TL, Nicolini FE, Eaves CJ. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Experimental hematology. 1999;27(9):1418–1427. doi: 10.1016/s0301-472x(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Li X, Coelho-dos-Reis JG, Wilson JM, Tsuji M. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS ONE. 2014;9(2):e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li X, Coelho-dos-Reis JG, Zhang M, Mitchell R, Nogueira RT, Sahi V. Human immune system mice immunized with Plasmodium falciparum circumsporozoite protein induce protective human humoral immunity against malaria. Journal of immunological methods. 2015;427:42–50. doi: 10.1016/j.jim.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Huey D, Bolon B, La Perle K, Kannian P, Jacobson S, Ratner L, Green P, Niewiesk S. Wild type and recombinant human T cell leukemia viruses induce lymphoproliferative disease in humanized NSG mice. Comparative Medicine. 2017 in press. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F, Livingston AG, Wingard JR, Nishikawa S-i, Ogawa M. An assay for long-term engrafting human hematopoietic cells based on newborn NOD/SCID/β2-microglobulin null mice. Experimental hematology. 2002;30(5):488–494. doi: 10.1016/s0301-472x(02)00784-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chain null mice. Blood. 2005;106(5):1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P, Tonomura N, Shimizu A, Wang S, Yang Y-G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108(2):487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Current protocols in immunology. 2008:15.21. 11–15.21. 21. doi: 10.1002/0471142735.im1521s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm M, Lang P, Taylor G, Kuci S, Klingebiel T, Buhring H-J, Handgretinger R. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. Journal of hematotherapy. 1999;8(2):209–218. doi: 10.1089/106161299320488. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Mangada J. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. The Journal of Immunology. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Fujiwara H. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proceedings of the National Academy of Sciences. 2010;107(29):13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, Greiner DL. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. The Journal of Immunology. 1995;154(1):180–191. [PubMed] [Google Scholar]
- Strowig T, Rongvaux A, Rathinam C, Takizawa H, Borsotti C, Philbrick W, Flavell RA. Transgenic expression of human signal regulatory protein alpha in Rag2−/− γc−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proceedings of the National Academy of Sciences. 2011;108(32):13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti J-C, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Villaudy J, Wencker M, Gadot N, Gillet NA, Scoazec J-Y, Gazzolo L, Dodon MD. HTLV-1 propels thymic human T cell development in “human immune system” Rag2−/−gamma c−/−mice. PLoS pathogens. 2011;7(9):e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Manz MG. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proceedings of the National Academy of Sciences. 2011;108(6):2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]