Abstract
Frontostriatal projections have been shown to mediate impulsivity. Recent findings have demonstrated that the projection from the prefrontal cortex to the nucleus accumbens (the accumbofrontal tract) can be isolated by using probabilistic tractography on human brain MRI data, specifically, diffusion tensor images (DTI). Using DTI tractography, we isolated the tract and tested its association with the impulsivity. DTI data from 143 individuals obtained from Nathan Kline Institute-Rockland Sample was used along with the impulsivity measure assessed by the UPPS (urgency, premeditation, perseverance, and sensation seeking) impulsive behavior total score. Probabilistic tractography was first performed between the prefrontal cortex and nucleus accumbens, then, as a measure of white matter integrity in the tract, fractional anisotropy was calculated for each individual’s tract. In the multiple regression, accumbofrontal FA showed significant positive association with the impulsivity, suggesting that the accumbofrontal tract integrity may contribute to individual differences in impulsivity. This study bridges the literature in rodents, in which this glutamatergic projection has been shown to mediate impulsive behavior, and the findings in humans which allow the in-vivo isolation of the tract and comparison with behavior.
Keywords: Diffusion Tensor Imaging (DTI), MRI, frontostriatal circuit, impulsivity, accumbofrontal tract, striatum, orbitofrontal cortex, Probabilistic Tractography
Introduction
Abnormal levels of impulsive behavior are associated with a wide range of psychiatric disorders from attention deficit hyperactive disorders to substance abuse (Belin et al. 2008; Winstanley et al. 2006). The dysfunction of frontostriatal circuits has been suggested as the neuronal substrate of impulsivity (Dalley and Robbins, 2017). In particular, animal studies show that the prefrontal cortex (PFC) projections to the nucleus accumbens (NAcc) modulate dopamine release in the NAcc (Del Arco and Mora 2008) and contribute to reward processing (Jentsch and Taylor 1999), risk taking tendencies (Zalocusky et al. 2016) and impulsivity (Dalley et al. 2007). In addition, lesions and pharmacological manipulations (i.e. dopamine compounds) in the PFC and the NAcc can promote impulsive behavior (Dalley and Robbins 2017). These studies suggest that PFC-NAcc connectivity plays an important role in impulsivity.
In agreement with animal studies, neuroimaging studies show that structural changes in the PFC and the striatum are associated with impulsive behavior in humans, which suggest that impulsivity results from the dysfunction of frontostriatal circuits (Achterberg et al. 2016; Hampton et al. 2017; van den Bos et al. 2014). This idea is supported by a recent study showing that the connectivity between the ventromedial PFC and the ventral striatum is associated with discounting tendency (Hampton et al. 2017). Yet, how frontostriatal connectivity is related to impulsivity is still unclear (van den Bos et al. 2014). Given the importance of this connection, there are efforts to investigate the structural basis of PFC-NAcc projections (i.e. accumbofrontal tract). However, only recently has the underlying white matter of the accumbofrontal tract has isolated using human brain MRI data (Karlsgodt et al. 2015; Rigoard et al. 2011; Squeglia et al. 2015). Integrity of the accumbofrontal tract has been recently found to be higher in 22q11.2 deletion syndrome. Although deficits in motivational reward are found in the syndrome, tract integrity did not show association with anticipatory or consummatory pleasure (Dubourg et al. 2017). Thus, the relationship between impulsivity in humans and the integrity of the accumbofrontal tract remains unclear, while the previously described peak of accumbofrontal white matter integrity in adolescence and subsequent decline of impulsivity and accumbofrontal white matter integrity with increasing age is consistent with a positive association between the accumbofrontal white matter integrity and impulsivity (Karlsgodt et al. 2015).
Diffusion tensor imaging (DTI) can be used to estimate microstructural characteristics, or differences in the white matter, in humans (Basser et al., 1994; Alexander et al., 2007). In general, the commonly used fractional anisotropy (FA) measure is described as an index of white matter integrity that includes both organization and myelination, whereby higher FA is understood to reflect enriched and uninjured axonal structures (Song et al. 2003). In the current study, we hypothesized that white matter integrity of the accumbofrontal tract is associated with impulsivity. We used probabilistic tractography to isolate the accumbofrontal tract and then obtained the white matter integrity measure (FA) within the tract. To assess impulsivity, we used the UPPS Impulsivity Behavior Scale which measures urgency, sensation seeking, and lack of premeditation and perseverance. We then tested the relationship between accumbofrontal FA and our impulsivity measure, along with age and sex in a large community sample.
Methods
Diffusion tensor imaging data from the Nathan Kline Institute-Rockland Sample (NKI-RS) were downloaded from fcon 1000 project website, along with demographic and other behavioral and cognitive assessments (http://fcon_1000.projects.nitrc.org/indi/pro/nki.html). Samples from 185 individuals contained DTI and other demographic data, and the data from 143 individuals of age 21 years and older (44.63±17.15 years old, 57 females and 87 males) were used in the analysis to avoid developmental confounds associated with striatal changes in adolescence. The DTI series had 64 non-parallel directions (TR = 10 s, TE = 91ms, matrix = 128 × 128, FOV = 256 mm). Each volume consisted of 58 contiguous 2-mm slices with 2mm^3 isotropic resolution. UPPS (urgency, premeditation, perseverance, and sensation seeking) Impulsive Behavior Scale outcomes were also obtained as well as age and sex information.
Images were processed using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL version 5.1; Oxford, United Kingdom; http://fsl.fmrib.ox.ac.uk/fsl). Eddy-current distortions and head displacements were corrected through affine registration of the 31 diffusion volumes to the first b0 volume using FSL’s Linear Registration Tool. The b-vector table (i.e., gradient directions) for each participant was then adjusted according to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL’s Brain Extraction Tool. Fractional anisotropy (FA), an index of white matter integrity, and diffusivity measures were then calculated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least squares in FSL’s Diffusion Toolbox. FA was chosen as the primary measure for analysis because it has been the most widely used measure in relevant studies and thereby provides optimal between-study comparability. Ancillary analyses investigated axial, radial, and mean diffusivity.
The local (i.e., within-voxel) probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling in FSL's Bedpostx tool (Behrens et al., 2007). A spatial probability density function across voxels was then estimated based on these local probability density functions using FSL's Probtrackx tool (Behrens et al., 2007), in which 5000 samples were taken for each input voxel with a 0.2 curvature threshold, 0.5 mm step length, and 2000 steps per sample. To segment the accumbofrontal tracts, NAcc seed and inclusion masks were determined based on the MNI152 T1 brain provided in FSL, using FSL's FMRIB58_FA template as a DTI specific reference. The NAcc seed was determined by HarvardOxford subcortical atlas (Desikan et al., 2006). The inclusion mask (prefrontal search region) was defined MNI x<0 (left) and x>0 (right), y≤−6 and −34≤Z≤+5. The bilateral accumbofrontal tract of each subject was then thresholded at a normalized probability value of 0.06.
To assess the association between the accumbofrontal FA and impulsivity, a multiple linear regression was calculated to predict the UPPS total score based on the bilateral accumbofrontal FA, age and sex. The association between the accumbofrontal FA and age was tested by a linear regression, with sex included in the model. In order to further examine the accumbofrontal FA, axial, radial, and mean diffusivities were independently tested for the multiple regression using R 3.3.3.1.
Results
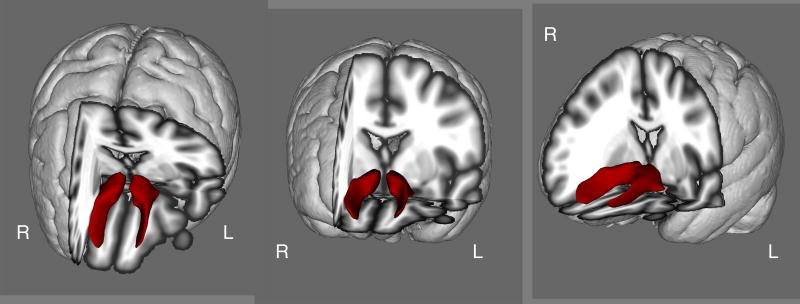
Probabilistic tractography was performed to identify the accumbofrontal tract in each subject. Tractography was verified via visual inspection of the tract in each individual subject, the tract isolated in this study (group means are shown in Figure 1) was consistent with the tract observed in previous DTI studies as well as histological findings (http://karlsgodtlab.org/HBM_accumbofrontal/) (Karlsgodt et al. 2015; Rigoard et al. 2011).
Figure 1.
The Accumbofrontal Tract (Group Mean)
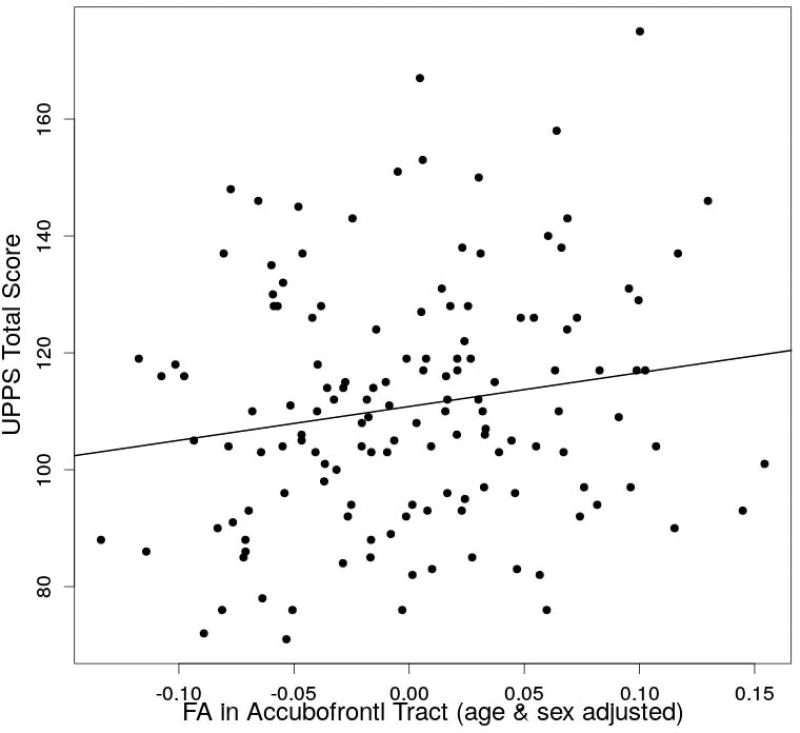
The impulsivity index (UPPS total score) ranged between 71–175 (11.01±20.53). In the statistical test of accumbofrontal FA predicting impulsivity, a significant regression equation was found (F(3,136)=7.13, p=0.00017), with an R^2 of 0.12. The predicted UPPS total score is equal to 118.40 + 57.44 (accumbofrontal FA) − 0.29 (age) – 6.77 (sex) where sex is coded as 1=male and 2=female. The accumbofrontal FA (p=0.037) as well as age (p=0.0029) and sex (p=0.046) significantly predicted the UPPS total score (Figure 2). To further investigate the relationship of impulsivity and accumbofrontal white matter integrity, axial, radial and mean diffusivities were individually tested by multiple linear regression with age and sex. None of these diffusivities showed an significant association with the impulsivity measure. There was also a significant association between accumbofrontal FA and age (F(1,140)=4.92, p=0.028), with an R^2 of 0.027. The predicted FA was equal to 0.29 – 0.00065 (age).
Figure 2.
The association between the fractional anisotoropy (FA) adjusted for age & sex and UPPS total score.
Discussion
Using probabilistic tractography on DTI data from the NKI Rockland sample, the accumbofrontal tract was isolated and a regression analysis indicated that impulsivity was predicted by the white matter integrity (FA) of the tract. These results suggest that accumbofrontal structural connectivity is associated with impulsive behavior. Importantly, this study replicates, in an independent sample, the successful isolation of the accumbofrontal tract reported in our previous study (Karlsgodt et al. 2015) which suggests that the methods used are broadly applicable across scanners, sequences, and populations.
The present study also replicates the age associated decline of accumbofrontal FA in adults shown in a recent study (Karlsgodt et al. 2015). Specifically, Karlsgodt et al. show that FA peaks in adolescence and then declines during aging. Using a unique population of individuals aged 21 years and older, our study shows a negative correlation between FA and age indicating that the input from the PFC to the NAcc is reduced during aging. The decrease in fronto-striatal white matter with age after age 21 has been previously reported (Samanez-Larkin et al. 2012). Both the previous findings of an increase in FA in adolescents and the current confirmation of a decrease of FA at later ages are in agreement with the increase of impulsive behaviors observed in adolescents and the decrease of impulsivity during aging (Roesch et al. 2012).
We find a positive correlation between the white matter integrity of the accumbofrontal tract and impulsivity which suggest that an increased accumbofrontal tract integrity is associated with impulsive behavior. Previous studies have also found a relationship between PFC and striatal structural profiles (i.e. grey matter volumes) and impulsive behaviors (Bjork et al. 2009; Tschernegg et al. 2015) and suggest that impulsivity results from the dysfunction of frontostriatal circuits in substance abusers (Jentsch and Taylor 1999) and in other psychiatric conditions (Konrad et al. 2010). However, whether increased or decreased frontostriatal connectivity is associated with increased or decreased impulsivity is still a matter of debate. In a moderately sized sample of adults with ADHD, a lower FA in the right frontostriatal fiber tract corresponded to higher measures of impulsivity (Konrad et al. 2010). Similarly, lower FA in the ventromedial PFC, caudate and putamen are associated with higher motor impulsivity in patients with schizophrenia (Hoptman et al. 2004). In contrast, in a sample of children with ADHD, another study found that increased resting state cortical striatal functional connectivity was specific to those with the hyperactive-impulsive subtype (Sanefuji et al. 2017). Another study found that heightened frontostriatal resting state functional connectivity increased in cocaine users and that this connectivity was positively correlated with amount of recent cocaine use (Hu Y et al. 2015).
Importantly, our study focused on the orbitofrontal (OFC) - NAcc tract to show higher values of white matter integrity (FA) correlated to higher impulsivity across individuals. It can be hypothesized that different cortico-striatal tracts play different roles in impulsive behavior. In fact, recent studies using discounting behavior as a behavioral paradigm to study impulsivity (Achterberg et al. 2016; Peper et al. 2012; van den Bos et al. 2014), show that different corticostriatal tracts have opposing effects on discounting (van den Bos et al. 2014). In addition, studies in experimental animals show that different parts of the OFC can play different roles in impulsivity. For instance, lesions in the lateral and medial portions of the OFC in rats increase or decrease, respectively, impulsivity (Mar et al. 2011). Although the accumbofrontal tract in this study does not distinguish between medial vs. lateral OFC, the greater part of the tract projects from the medial part of the OFC.
While none of axial, radial and mean diffusivities showed significant association with age, radial diffusivity showed marginal inverse association with the UPPS total score (p=0.086). Radial diffusivity is often used as a putative index of myelin thickness (Song et al., 2002, 2003). It is thus possible to hypothesize that myelination of the accumbofrontal tract is associated with impulsivity, although the current result failed to support this hypothesis, leaving it ambiguous which specific aspect of white matter microstructure contributed to behavior. Future studies with greater statistical power, or using diffusion modalities more directly targeted at measuring myelination, may be able to verify this potential hypothesis.
In conclusion, we first replicated the accumbofrontal (OFC-NAcc) probabilistic tractography and age associated decline in adults. The white matter integrity of the tract showed a significant positive association with impulsivity. These results emphasize the role of the accumbofrontal tract white matter integrity to predict impulsivity during the life span and future studies will determine its validity as a biomarker of impulsive disorders.
Acknowledgments
This work was supported in part by R01 MH101506 grant from the NIH to KHK. Image preprocessing was performed using the supercomputer cluster at the Mississippi Center for Supercomputing Research partly funded by the National Science Foundation (EPS-0903787).
Footnotes
Compliance with Ethical Standards
This work was partly funded by NIH (R01 MH101506). Dr. Ikuta has received speaker’s honoraria from Eli Lilly, Daiichi Sankyo, and Dainippon Sumitomo. The authors declare that they have no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Toshikazu Ikuta, Department of Communication Sciences and Disorders, School of Applied Sciences, University of Mississippi, 311 George Hall, University, MS 38677, tikuta@olemiss.edu, phone: 662-915-5121, fax: 662-915-8717.
Alberto del Arco, Department of Health, Exercise Science, and Recreation Management, School of Applied Sciences, University of Mississippi.
Katherine H. Karlsgodt, Departments of Psychology and Psychiatry and Biobehavioral Sciences, University of California, Los Angeles
References
- Achterberg M, Peper JS, van Duijvenvoorde ACK, Mandl RCW, Crone EA. Frontostriatal White Matter Integrity Predicts Development of Delay of Gratification: A Longitudinal Study. The Journal of Neuroscience. 2016;36(6):1954–1961. doi: 10.1523/JNEUROSCI.3459-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion Tensor Imaging of the Brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High Impulsivity Predicts the Switch to Compulsive Cocaine-Taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay Discounting Correlates with Proportional Lateral Frontal Cortex Volumes. Biological Psychiatry. 2009;65(8):710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, et al. Nucleus Accumbens D2/3 Receptors Predict Trait Impulsivity and Cocaine Reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Rev Neurosci. 2017;18(3):158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex–nucleus accumbens interaction: In vivo modulation by dopamine and glutamate in the prefrontal cortex. Microdialysis: recent developments. 2008;90(2):226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dubourg L, Schneider M, Padula MC, Chambaz L, Schaer M, Eliez S. Implication of reward alterations in the expression of negative symptoms in 22q11.2 deletion syndrome: a behavioural and DTI study. Psychological Medicine. 2017:1–12. doi: 10.1017/S0033291716003482. [DOI] [PubMed] [Google Scholar]
- Hampton WH, Alm KH, Venkatraman V, Nugiel T, Olson IR. Dissociable Frontostriatal White Matter Connectivity Underlies Reward and Motor Impulsivity. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron B, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72(6):584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Jentsch DJ, Taylor RJ. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146(4):373–390. doi: 10.1007/PL00005483. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, John M, Ikuta T, Rigoard P, Peters BD, Derosse P, et al. The accumbofrontal tract: Diffusion tensor imaging characterization and developmental change from childhood to adulthood. Human Brain Mapping. 2015;36(12):4954–4963. doi: 10.1002/hbm.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Dielentheis TF, El Masri D, Bayerl M, Fehr C, Gesierich T, et al. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. European Journal of Neuroscience. 2010;31(5):912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(17):6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Mandl RCW, Braams BR, de Water E, Heijboer AC, Koolschijn PCMP, Crone EA. Delay Discounting and Frontostriatal Fiber Tracts: A Combined DTI and MTR Study on Impulsive Choices in Healthy Young Adults. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoard P, Buffenoir K, Jaafari N, Giot JP, Houeto JL, Mertens P, et al. The Accumbofrontal Fasciculus in the Human BrainA Microsurgical Anatomical Study. Neurosurgery. 2011;68(4):1102–1111. doi: 10.1227/NEU.0b013e3182098e48. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G. Willingness to Wait and Altered Encoding of Time-Discounted Reward in the Orbitofrontal Cortex with Normal Aging. The Journal of Neuroscience. 2012;32(16):5525–5533. doi: 10.1523/JNEUROSCI.0586-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B. Frontostriatal White Matter Integrity Mediates Adult Age Differences in Probabilistic Reward Learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(15):5333–5337. doi: 10.1523/JNEUROSCI.5756-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanefuji M, Craig M, Parlatini V, Mehta MA, Murphy DG, Catani M, et al. Double-dissociation between the mechanism leading to impulsivity and inattention in Attention Deficit Hyperactivity Disorder: A resting-state functional connectivity study. Is a “single” brain model sufficient? 2017;86:290–302. doi: 10.1016/j.cortex.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ju W-K, Lin S-J, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Squeglia L, Sorg S, Jacobus J, Brumback T, Taylor C, Tapert S. Structural connectivity of neural reward networks in youth at risk for substance use disorders. Psychopharmacology. 2015;232(13):2217–2226. doi: 10.1007/s00213-014-3857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernegg M, Pletzer B, Schwartenbeck P, Ludersdorfer P, Hoffmann U, Kronbichler M. Impulsivity Relates To Striatal Gray Matter Volumes In Humans: Evidence From A Delay Discounting Paradigm. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Connectivity Strength of Dissociable Striatal Tracts Predict Individual Differences in Temporal Discounting. The Journal of Neuroscience. 2014;34(31):10298–10310. doi: 10.1523/JNEUROSCI.4105-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: Translation between clinical and preclinical studies. Attention Deficit Hyperactivity Disorder From A Neurosciences And Behavioral Approach. 2006;26(4):379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature, advance online publication. 2016 doi: 10.1038/nature17400. http://dx.doi.org/10.1038/nature17400 [DOI] [PMC free article] [PubMed]