Abstract
Background
Addictive-like behaviors (e.g., hoarding and shopping) may be the result of the cumulative effects of dopaminergic and other neurotransmitter genetic variants as well as elevated stress levels. We, therefore, propose that dopamine homeostasis may be the preferred goal in combating such challenging and unwanted behaviors, when simple dopaminergic activation through potent agonists may not provide any resolution.
Case presentation
C.J. is a 38-year-old, single, female, living with her mother. She has a history of substance use disorder as well as attention deficit hyperactivity disorder, inattentive type. She had been stable on buprenorphine/naloxone combination and amphetamine, dextroamphetamine mixed salts for many years when unexpectedly she lost her job for oversleeping and not calling into work. KB200z (a pro-dopamine compound) was added to her regimen for complaints of low drive and motivation. After taking this nutraceutical for 4 weeks, she noticed a marked improvement in her mental status and many behaviors. She noted that her shopping and hoarding addictions had appreciably decreased. Furthermore, her lifelong history of terrifying lucid dreams was eliminated. Finally, she felt more in control; her locus of control shifted from external to more internal.
Discussion
The hypothesis is that C.J.’s reported, behavioral, and psychological benefits resulted from the pro-dopamine-regulating effect of KB220Z across the brain reward system.
Conclusions
This effect, we surmise, could be the result of a new dopamine balance, across C.J.’s brain reward system. Dopamine homeostasis is an effect of KB220Z seen in both animal and human placebo-controlled fMRI experiments.
Keywords: reward deficiency syndrome (RDS), pro-dopamine regulation (KB220Z), hoarding and shopping behaviors, attention-deficit/hyperactivity disorder (ADHD)
Introduction
For longer than four decades, there has been an ongoing debate about the role of brain dopamine (DA) in reward and addiction. Nutt, Lingford-Hughes, Erritzoe, and Stokes (2015) proposed that DA may be central to psychostimulant dependence and possibly for alcohol dependence but not requisite for opiates, nicotine, or even cannabis dependence (Nutt et al., 2015). This view may be overly simplistic, as our group has noted in earlier publications, distinction between what constitutes “surfeit,” compared with “deficit,” regarding short-term (acute) and long-term (chronic) brain reward circuitry responsivity (Blum, Gardner, Oscar-Berman, & Gold, 2012). Others have also argued that surfeit theories of DA can explain, e.g., cocaine-seeking behavior as well as responses concerning dominant rats with elevated DA compared with subordinate rats (Jupp et al., 2016).
In an earlier study, we attempted to resolve controversy, concerning the contribution of mesolimbic DA systems to reward, by invoking three, competing, explanatory categories, namely, “liking,” “learning,” and “wanting” (Blum et al., 2012). Specifically, they are (a) the hedonic impact – “liking” reward, (b) the ability to predict rewarding effects “learning,” and (c) the incentive/salience of reward-related stimuli “wanting.”
Concerning acute effects, most of the evidence favors the “surfeit theory.” We know that because of preferential DA release in the mesolimbic–ventral tegmental area (VTA)–caudate–accumbens loci, many drugs of abuse and reward deficiency syndrome (RDS). These behaviors have been linked to heightened feelings of well-being and hyperdopaminergic states (Beeler, Faust, Turkson, Ye, & Zhuang, 2016; Beeler, Frazier, & Zhuang, 2012).
The “dopamine hypotheses” is now believed to be quite complicated, encoding the set point of hedonic tone, attention, reward expectancy, and incentive motivation (Sternat & Katzman, 2016; Willuhn, Burgeno, Groblewski, & Phillips, 2014). Willhuhn et al. (2014) have shown in a self-administration paradigm (chronic), excessive use of cocaine is associated with decreased, phasic DA signaling in the striatum. Others have shown blunted reward sites responsivity with chronic addictions, e.g., food (Stice, Spoor, Bohon, & Small, 2008), nicotine (Harrell et al., 2016), and gambling behavior (Gyollai et al., 2014; Robinson, Fischer, Ahuja, Lesser, & Maniates, 2016).
The contention is that the differences in dopaminergic function, which occur, as addictions progress can be impacted by both genetics and negative and positive environmental factors, such as epigenetics (Hamilton et al., 2017). One significant effect of dopaminergic deficiency may be linked to relapse and behavioral reinstatement (Cooper et al., 2014). In particular, addictive-like behaviors (e.g., hoarding and shopping) can result from the cumulative effects of dopaminergic and other neurotransmitter genetic variants as well as elevated stress levels. Thus, DA homeostasis may be the preferred goal in combating such challenging and unwanted behaviors, when simple dopaminergic activation through potent agonists may not provide any resolution.
DA and Motivation
DA neurons in the midbrain play a role in motivation as well as in the regulation of mood and the induction of reward-aversion (Ikemoto, Yang, & Tan, 2015; Love, 2014; Tomer, Goldstein, Wang, Wong, & Volkow, 2008). While much of this evidence points to the loci of this effect of DA being the ventral tegmental area (VTA), other evidence suggests that the motivational and other effects of DA on behavior may also derive from the substantia nigra (Ikemoto et al., 2015; Krawczyk et al., 2013).
It has been shown that, while high extracellular concentrations of DA induce euphoria, low levels of DA induce “seeking” and aversive behavioral states. For example, Ng et al. (2016) have demonstrated that neuronal calcium sensor-1, a substrate that regulates neuronal D2 expression and subsequent release at the nucleus accumbens (NAc), when deleted in the mouse’s brain, causes a reduced release of DA and subsequently decreased motivation. Other very recent work by Korte et al. (2017) evaluated the role of the 5-HT1A/1B-receptor activation and showed that the 5-HT1A/1B-receptor agonist, eltoprazine, increases both DA release in the NAc and catecholamine release in the prefrontal cortex. This agonist also decreases motivation for reward and “waiting” impulsivity but increases “stopping” impulsivity.
In addition, the D1 DA receptor-mediated, long-term potentiation, at gamma-aminobutyric acid (GABA) synapses encodes motivation to self-administer cocaine in rats (Krawczyk et al., 2013). We hypothesize that the enhanced signal to increase cocaine self-administration agrees with the report of Willuhn, Burgeno, Everitt, and Phillips (2012), which demonstrated that as DA levels go down, as cocaine use increases. This enhancement was further supported by the work of others (Bello et al., 2011; de Jong et al., 2015).
The behavior of the woman, in this case study diagnosed with attention-deficit/hyperactivity disorder (ADHD) and substance use disorder (SUD), is consistent with the findings of Volkow’s group regarding DA reward pathway dysfunction. They correlated positron emission tomography (PET) measures of DA D2/D3 receptor, as well as, DA transporter availability (measured using [(11)C]raclopride and [(11)C]cocaine, respectively) within the DA reward pathways. The DA pathways included the midbrain and NAc, with a measure of trait motivation (the achievement scale from the Multidimensional Personality Questionnaire). These authors suggested that disruption of the DA reward pathway is associated with motivation deficits in ADHD adults, which may contribute to the observed attention deficits. This finding suggests that pro-dopamine regulation, as a way of inducing “dopamine homeostasis,” may constitute a means of normalizing motivation in ADHD as well as SUD (Volkow et al., 2011).
DA and Excessive Hoarding and Shopping Behaviors
While generally, it is agreed that dopaminergic function influences motivation and that certain deficits in the brain reward pathway can impact impulsivity (hoarding and shopping excesses), a piece of the treatment puzzle may be missing.
A review of the literature (PUBMED 5/14/17) reveals that there are many studies involving food-hoarding behavior (Mogenson & Yang, 1991; Yang, Wang, Wang, & Wang, 2011), but a paucity of information on the role of DA in non-food hoarding behavior. In fact, some reports are suggesting that hoarding behavior is associated with a hyperdopaminergic trait/state and subsequent blocking of D2 receptors seems to improve the condition (Borker & Mascarenhas, 1991). Earlier work by Kelley and Stinus (1985) showed that while 6-hydroxydopamine lesions of the mesolimbic DA neurons eliminated hoarding behavior in rats, L-Dopa administration was associated with a reinstatement of this behavior. Other genetic work seems to support these earlier studies, with female hoarders. These people tend to exhibit a greater frequency of the low activity Met/Met genotype of Val158Met polymorphism, compared with females, who did not express this change, suggesting a higher concentration of DA in the synapse (Melo-Felippe et al., 2016).
Fontenelle, Oostermeijer, Harrison, Pantelis, and Yucel (2011) also suggested that obsessive–compulsive disorder, impulse control disorders, and drug addiction have common, dopaminergic features and that therapeutic intervention of these disorders should involve DA as a target. Regarding excessive shopping behavior and dopaminergic function, much less is known, except for reports regarding these behaviors and Parkinson Disease (Claassen et al., 2011; Raja & Bentivoglio, 2012; Voon et al., 2010). We contend that whether there is a hyper or hypodopaminergia, balancing the dopaminergic trait/state, especially, with a well-researched, pro-dopamine regulator, KB220, should show benefit the treatment of both excessive hoarding and shopping in a middle-aged female, diagnosed with both ADHD and SUD.
KB220
The most recent variant of KB220Z (powdered form) used in this study is composed of the following ingredients: 10 mg (500%) vitamin B6, 15 mg (1,033% of daily value) thiamine, 200 mcg (166%) chromium poly nictitate, and a fixed dose of synaptose. Synaptose is a combination of amino acids and herbs. The amino acids include L-tyrosine, DL-phenylalanine, L-glutamine, and 5-hydroxytryptophan. The herbs include passionflower extract and a complex containing astragalus, arabinogalactans, N-acetylglucosamine, aloe vera, white pine bark extract, frankincense resin Spirulina Rhodiola, as well as, thiamine hydrochloride, pyroxidal-5-phosphate, pyridoxine HCl, and N-acetylglucosamine, an amino sugar. The powder was manufactured by Cephram, Inc. (New Jersey). One other version used was the liquid nano form of the same ingredients.
Characteristics and neuropharmacology of KB220 variants
It is noteworthy that over 40 years of research over many variants and 36 published articles, many clinical benefits have been observed from double-blind placebo-controlled trials, randomized placebo-control studies, case reports, and neuroimaging studies in both humans and animals.
The following information should provide evidence for clinical benefit of evaluating KB220Z in this study, begging the question of neuromechanisms involved in producing DA homeostasis. Variations of KB220 nutraceutical complex have been studied extensively in preclinical and human trials. As reported in a detailed review article (Blum, Febo, Fried, et al., 2016), to date, KB220 variants have demonstrated the ability to enhance brain enkephalin levels in in C57/BL mice, thereby reducing alcohol-seeking behavior and pharmacogenetically convert acceptance in DBA ethanol preferring mice to non-preference as in 2J mice (Blum, Briggs, Trachtenberg, Delallo, & Wallace, 1987). In humans, KB220 has reduced alcohol and drug and withdrawal symptomatology (Blum & Gold, 2011; Blum, Trachtenberg, & Ramsay, 1988). Specifically, lowering the need for benzodiazepines, reducing the days with withdrawal tremors, no severe depression on the Minnesota Multiphasic Personality Inventory, and a lower score building up to drink score (Blum, Trachtenberg, Elliott, et al., 1988). In recovery patients, demonstrated a reduced stress response, measured by the level of skin conductance, and significantly improved physical and behavioral, emotional, social and spiritual scores (Blum, Trachtenberg, Elliott, et al., 1988). After detoxification, the KB220 variant group demonstrated a sixfold decrease in leaving against medical advice (AMA) rates in comparison with the placebo group (Blum, Allison, Trachtenberg, Williams, & Loeblich, 1988; Blum, Trachtenberg, Elliott, et al., 1988) and KB220 enhanced the focus of healthy volunteers (DeFrance et al., 1997). There is also evidence that KB220 reduced craving for alcohol (Chen et al., 2004), heroin, cocaine (Cold, 1996), food (Beitscher-Campbell et al., 2016), inappropriate sexual behavior (McLaughlin et al., 2013), and post-traumatic stress symptomatology, such as lucid nightmares (McLaughlin, Blum, Oscar-Berman, Febo, Agan, et al., 2015). Quantitative Electroencephalogram (qEEG) studies in humans have found that KB220Z modulates theta power in anterior cingulate cortex (Blum et al., 2010). Compared with placebo, a single dose of KB220Z in a pilot study of abstinent heroin addicts resulted in NAc activation and improvement in activation of the prefrontal–cerebellar–occipital neural network (Blum, Liu, et al., 2015). Known obese carriers of the DRD2 A1 allele showed enhanced compliance with KB220Z treatment, demonstrating weight loss and a significant Pearson’s correlation, relative to carriers of the DRD2 A2 allele with a usual complement DRD2 receptors (Blum et al., 2008). This result suggests the importance of balanced DA function equates to better treatment outcome.
To assist in understanding the intensive research on KB220 variants, we encourage scrutiny of some reviews on the subject (Blum, Febo, & Badgaiyan, 2016; Blum, Febo, Fried, et al., 2016). These studies, including double-blinded control studies and qEEG, have demonstrated positive effects on both craving attenuation and relapse prevention and reduction in AMA rates; see review (Blum & Gold, 2011).
Are KB220 variants putative modulators of DA homeostasis?
The development of KB220Z followed the first report concerning the enkephalinase inhibitor D-phenylalanine (Blum, Trachtenberg, Elliott, et al., 1988). The unique design of the KB220Z complex follows the brain reward cascade with the ultimate intent of facilitating DA release throughout the reward circuitry. While, as yet, the actual release of DA has not been determined we plan to study DA release using a single scan dynamic molecular imaging technique to understand the nature of DA released in the human and animal brains following administration of KB220Z (Badgaiyan, 2014). This specific technique allows detection, mapping, and measurement of DA released endogenously following a pharmacological or behavioral challenge (Badgaiyan, 2013; Badgaiyan & Wack, 2011). This molecular imaging technique was used to observe DA release in some of the same areas where enhanced connectivity was seen in the current experiment (Badgaiyan, 2010).
The hypothesis here is that these robust and selective results are due to inhibition of GABA transmission within the substantia nigra via serotonergic–opioidergic–glutaminergic interactions that reduce the inhibitory control of GABA over DA release throughout the reward network (Volkow, Fowler, Wang, Baler, & Telang, 2009). In support of these findings, similar results were found in humans showing enhanced regulation of deregulated widespread theta in the cingulate gyrus of abstinent psychostimulant abusers. Alpha and low beta waves were increased 1 hr after KB220Z administration (Blum et al., 2010). Previously, our laboratory found resting state functional abnormalities in abstinent heroin-dependent individuals affecting functional brain organization, which could negatively impact decision-making and inhibitory control (Miller et al., 2010). Moreover, compared with healthy controls, heroin addicts showed reduced activation in right amygdala in response to the affective pictures (Blum, Liu, et al., 2015), consistent with previous reports of blunted subjective experience for affective stimuli in obese carriers of the DRD2 a1 allele (Stice et al., 2008). Brain scans of these abstinent heroin addicts show evidence for “dopamine homeostasis” following 1 hr KB220Z administration: BOLD activation of caudate with reduced activation in putamen (Blum, Liu, et al., 2015). Other studies showed persistent abnormalities in the brain function following 1 month of heroin withdrawal in the orbitofrontal cortex (Zijlstra, Booij, van den Brink, & Franken, 2008). Zijlstra et al. also found lower baseline D2R availability in opiate-dependent subjects than in the left caudate nucleus of controls. After cue-exposure, opiate-dependent subjects demonstrated higher DA release in the right putamen than controls; this release positively correlated with chronic craving and anhedonia. DA 2R availability in the putamen was negatively correlated with years of opiate use (Zijlstra, Veltman, Booij, van den Brink, & Franken, 2009). Treatment strategies that increase D2Rs may be an approach that could prevent relapse not only in opiate addiction but also other behavioral addictions, such as hoarding and excessive shopping (Dahlgren et al., 2011). There is extensive evidence that indicates that current and recently abstinent cocaine abusers compared with drug-naive controls have decreased gray matter in regions, such as the anterior cingulate, lateral prefrontal, and insular cortex (Volkow et al., 2009). Following optogenetic stimulation of the rat NAc, brain regions crucial for cognitive processing including the dorsal hippocampus, anterior thalamus, and regions the central striatal reward structure were also observed to have changes in metabolic activity. Small animal PET and [18F]2-fluoro-2-deoxy-D-glucose (Thanos et al., 2013) or presentation of cues (Michaelides et al., 2013) were used to observe these changes. Wang et al. have shown a dysfunction of the frontostriatal and frontocerebellar circuits in heroin addicts. The altered function implies an altered balance between local neuronal assembly’s activities and their integrated network organizational pattern that may be involved in the process of moving from voluntary to habitual and compulsive drug use and possibly behavioral compulsion (Wang et al., 2013). In a human pilot study, KB220Z demonstrated improvement in the cerebellum, the cingulated, and other areas of the reward circuitry in abstinent heroin addicts (Blum, Liu, et al., 2015).
Importantly, the finding reported by Connolly, Bell, Foxe, and Garavan (2013), of increased areas of activation could have therapeutic value, especially considering the reduced brain gray matter volume during cocaine administration to humans. It is plausible that KB220Z due to COMT inhibition (via Rhodiola Rosea) could result in larger amounts of DA in the synapse, and for that reason enhanced DA activity (Blum, Allison, et al., 1988).
One element of the reward circuitry, the mesolimbic DA system in the brain, controls human responses to food, social interactions, and money, and is, therefore, an influential determinant of rewards and motivation. The midbrain DA neurons that project to the striatum are involved in producing the reward. In an animal study, Febo et al. (2017) illustrated the modulatory actions of a putative DA agonist (KB220Z) on the rsFC in association with a key region of the reward system, the NAc. The finding is that KB220Z, like optogenetic stimulation of the rat NAc (Thanos et al., 2013), caused an increase in the connectivity between this central striatal reward structure and the dorsal hippocampus and anterior thalamus, areas crucial for cognition. Indeed, recent work from Ferenczi et al. demonstrated that midbrain DA neuron stimulation drives both reward-seeking behavior and striatal fMRI BOLD activity. They also showed that silencing of DA neurons drives avoidance behavior (Blum et al., 1997; Ferenczi et al., 2016) and suppresses response in the striatum, as well as other brain regions like the hypothalamus. In addition, suppression of striatal responses to DA and inhibition of the behavioral drive to seek out natural rewarding stimuli through DA neuron stimulation were observed following DA neuron silencing. Most importantly, Ferenczi et al., demonstrated that medial prefrontal cortex (mPFC) excitability that is consistently elevated, synchronizes corticolimbic BOLD, and electrophysiological activity, which can, in turn, predict anhedonic behavior in individual animals (Blum, Gold, et al., 2017; Ferenczi et al., 2016). Interestingly, mPFC has glutaminergic neuronal input, and as such, there is a need to balance and optimize the fine interaction between mPFC–glutaminergic input to striatal midbrain DA, so that the resultant release of DA at the VTA–NAc is balanced (Blum, Febo, & Badgaiyan, 2016; Blum, Febo, Fried, et al., 2016). These new findings have direct implications for the notable observation that KB220Z potentially may induce BOLD activation due to a glutaminergic–dopaminergic optimization.
Case Report
Identifying information
C.J. is a 38-year-old, unmarried, female, and living with her mother. She is currently treated for ADHD, inattentive type, and SUD. Her ADHD diagnosis was based on her performance on the Connors’ Continuous Performance Test II (CPT II V.5) and her clinical history. Her SUD diagnosis was based upon her history and psychiatric evaluations over a 7-year period.
Ethics
The patient signed a consent form and the case study is part of an approved IRB from Path Foundation NY.
Past psychiatric history
Hospitalizations
-
1.
A psychiatric hospitalization at 16 years of age for a “marijuana addiction;”
-
2.
A psychiatric hospitalization at 15 years of age, when patient was diagnosed with “bipolar disorder;”
-
3.
A 6-month, psychiatric hospitalization, at the age of 14 for oppositional defiant disorder and intermittent explosive disorder signs and symptoms.
Psychotherapy
Patient engaged in individual psychotherapy regularly from 25 years of age to the present.
Abuse history
The patient reports a history of verbal/emotional abuse by her mother, who frequently called her a “bitch,” and a “whore.” She claims physical abuse by her mother from 3 to 6 years of age.
Family psychiatric history
The patient maintains her mother had “untreated bipolar disorder” (although the mother’s frequent use of coprolalia raises the question about “vocal tics” and, possibly, Tourette’s syndrome).
Family history
The patient’s mother is rageful, abusive, and hypercritical. She was unaffectionate and, otherwise, inattentive to the patient throughout her childhood.
The patient’s father is deceased. She mourned his death. He is remembered as having been “kind, attentive, and affectionate.”
Family addiction history
The patient’s father was addicted to alcohol and heroin. Her mother is addicted to food, cigarettes, and hoarding. The patient’s brother was addicted to opiates and is currently treated with methadone. The patient’s sister was addicted to heroin and is currently treated with methadone.
ADHD history
The patient states, during grade school, she was spacey, dreamy, easily distracted, and bored.
Motor tics
The patient cracks her fingers frequently. She also twists her torso, grinds her teeth, and picks her skin.
Vocal tics
The patient reports that, on a daily basis, she hums, talks to herself, clears her throat, and engages in both habitual coprolalia and palilalia.
Social history
The patient has a General Equivalency Diploma, with some community college courses. She was never married and had no children. She has had two abortions, one involving twins. She lived in foster homes from age 13 to 17, because “my mom was out of control.”
Past medical history
The patient denies any history of hypertension, heart disease, asthma, or any history of head injury.
Allergies
The patient denies any history of allergies to food or medication.
Dreamlife
The patient notes an onset of lucid dreams at about 5 years of age. These dreams, which were characterized as seeming “like… (she)… was “fully awake,” occurred nightly into adulthood. Their content was unpleasant or terrifying “100% of the time.” The themes of the dreams usually involved fears of being chased, stabbed, or murdered. These scary dreams led her to feel “paranoid” in the daytime as well. She reports awareness, during these lucid dreams that she is, in fact, dreaming. She reports an ability to control the onset or offset of these lucid dreams.
The patient was given the nutraceutical, KB220Z at four tablets a day, for 6 months, to influence her dreamlife, because of the experience by one of the authors that its use ameliorated such dreams. After following this regimen for 6 months, she reported her lucid dreams gradually remitted. She also noted increased “mental energy;” during this time, she took the KB220Z. Later, she reported that she no longer had lucid dreams, despite having stopped the KB220Z 2 years prior (McLaughlin, Blum, Oscar-Berman, Febo, Agan, et al., 2015; McLaughlin, Blum, Oscar-Berman, Febo, Demetrovics, et al., 2015; McLaughlin et al., 2016).
Patient’s addiction history
The patient reported a remote history of marijuana addiction, at 16 years of age. She also admitted to an opiate addiction for 10 years as well as a history of both shopping and hoarding addictions for this same period. The patient smokes an average of one pack of cigarettes a day and drinks 20 cups of coffee a day.
Medication regimen
Buprenorphine/naloxone combination treatment history at her current clinic.
For 5 years, the patient has been maintained on buprenorphine/naloxone combination of 8 mg/2 mg, one tablet sublingual twice a day. In addition to counseling, she attends monthly visits for her buprenorphine/naloxone combination prescriptions. During the period, since she was first seen at the clinic, she has denied any relapses, cravings, or drug use. She has been able to decrease her dose to 1½ buprenorphine/naloxone combination of 8 mg/2 mg sublingual a day.
From January 2016 to the present, the patient has also been maintained on mixed amphetamine salts [NIH National Library of Medicine] IR 30 mg every morning and 20 mg every afternoon. In addition, she is maintained on gabapentin 300 mg TID. The KB220Z compound was an addition to these medications.
Random toxicology screens
The patient has had clean, random, toxicology screens; during the 5 years, she has been treated at the clinic.
Work stressors
The patient states that she has suffered from job stress for the past 3 ½ years. This stress resulted from what she perceives to be discrimination by supervisors as well as her need to work the night shift and not getting enough sleep during the day.
Recent stressors
Three months prior, the patient reported frequent rage while driving. She stated some of this emotion had to do with her being late for work (tardiness has been an ongoing problem). During this same period, she complained of increased apathy but denied cravings, relapse, or use of substances. She continued to report stress at work.
Eventually, she lost her job, because she had overslept and failed to call into work. She was denied unemployment benefits and became severely “depressed” and unmotivated. She was given the “liquid-nano” KB200z, to augment the dopaminergic treatment (dextroamphetamine/amphetamine mixture) of her presumed, RDS (Blum, Sheridan, et al., 1996).
Phenomenological/psychological report of the effects of KB200z in a patient evidencing RDS
At this point, the patient showed cell phone pictures of her overly cluttered and hoarding-filled room, which represented its state, before taking KB200Z. After taking her KB220Z, each morning for 1 month, she had completely decluttered her apartment as seen in her post-liquid nano KB220Z photo (Figure 1) and the change in hours per week spent shopping (Figure 2).
Figure 1.
Patient’s home before use of KB220Z (left: A) and after 30 days on KB220Z (right: B). This patient admitted to a history of ADHD, opiate addiction, shopping addiction, and hoarding addiction. Traditionally, such diagnoses and behavioral addictions have been treated with pharmacotherapy (ADHD) and, more recently, with nutraceutical formulas, such as KB200z. There are, to date, no reports of the alleviation of shopping and hoarding addictions with any known pharmaceutical to our knowledge
Figure 2.
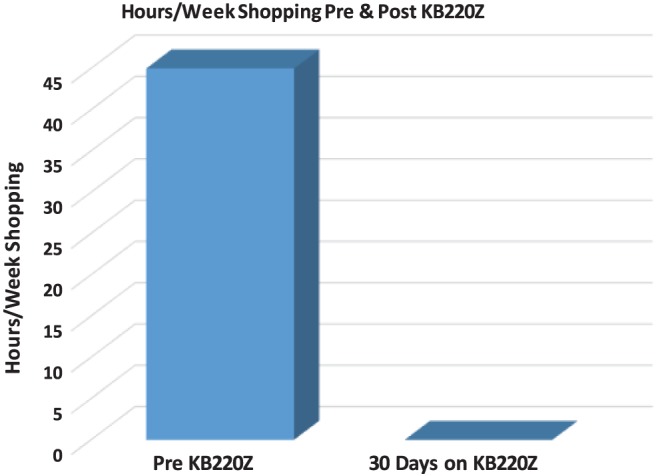
Hours per week shopping before KB220Z and after 30 days of KB220Z. The above figure depicts the change in hours spent per week shopping (42 hr/week for 2–3 years) to 0 hr/week after taking KB200z for 1 month. The change has, in fact, persisted for 2 weeks beyond the active ingestion period of 1 month on a daily basis at 1 oz a day (patient has recently reordered a new supply)
Even though there are, to date, 30 studies demonstrating the therapeutic and anti-addictive effects, there are none which demonstrate an anti-shopping or an anti-hoarding effect (Febo et al., 2017). Moreover, this is the first report to (Archer, Oscar-Berman, Blum, & Gold, 2013) describe the psychological effect of the patient’s ingestion of KB200z. These effects include her improved self-esteem, her change from a “passive victim” to a more active agent in control of her own life – in short, a change in her experience of herself and her identity. The following are the patient’s own words, presented in their unedited form:
“The month of March 2017 is when I began to drink the liquid nano KB220Z, and it worked fairly quickly for me. In fact I feel like it was almost immediate. However, realistically, it was a week or less. The first real benefit was I did not feel the need to shop. I had this sudden burst of hope or responsibility to get both my shopping and hoarding under control. Since taking KB220Z in March, my personal relationships with my family have dramatically changed. Specifically, I feel that even though I have always been kind and compassionate, I noticed that now I feel more spiritually kind towards my family. I feel my quest for spirituality (faith) has always been a struggle for me and often confused, but ironically in the month of March, I felt as though I was found, or that I found myself opening up in a raw way to GOD. I selflessly opened up. I cried for days when that happened to me. In terms of my relationship with my mother, I am so much more patient, and have not acted passionately toward her when she has maybe said something hurtful or intentionally did something to hurt my feelings. My coping skills seemed to allow me to let things slide off my back and that is a first for me. Happily I report that I am softer towards her and can do kind and loving things for her because I am thinking about her feelings now. In the pre-KB220Z period, there was so much hate and resentment. I am learning how to like her/love her. In general I feel that KB220Z and my doctor (TM) are responsible for these beautiful changes in my life.”
Discussion
Previously our laboratory provided a neuromechanism for the pathogenesis of alcohol and non-alcohol-induced depression in animals and possibly humans (Blum et al., 1987). Since we found an effect of the KB220Z on the subject’s mood (bipolar depression), it is incumbent upon us to provide a brief discussion of this notable finding. In fact, Archer et al. (2013) and our group discussed the role of epigenetics in mood disorders concluding that mood disorders manifest in in many forms, varying from anxiety to severe major clinical depression. The disorders are the complex interactive operations of environmental and genetic factors expressed in individuals through manifestations governed by the effects of genes on phenotypes like comorbidities, symptom frequency, duration, and severity.
The notion of endophenotypes, which contain markers for several underlying disorders, may help to identify and detect genetic risks for disease states and assist the unraveling of the extreme complexity of disease states. Moreover, Blum, Febo, et al. (2017) discussing SUD pointed out that relapse rate is due, in part, to untreated post-withdrawal and neurotoxicity. This impairment compromises resting state functional connectivity, by changing neurotransmitter signaling. The unwanted sequelae of these impairments include depression, lack of satisfaction and impulsivity, sleep disturbances, and sensation seeking. Neuroimaging studies reveal that neurobiological recovery can take years. SUD has a biological bidirectional (bio-directional) effect, like a “double edge sword,” on the reward circuitry of the brain. Acute psychoactive drug intake increases dopaminergic activity, while, chronic intake the opposite, hypodopaminergia occurs following with misuse (Blum, Febo, et al., 2017). Moreover, untreated hypodopaminergia is a key to relapse and may require not only 12 steps and fellowship but also epigenetic repair involving manipulation of impaired brain neurochemistry using pro-dopamine regulation directed at concomitant depressive states.
The attenuation of bipolar depression in this case study using KB220Z is in agreement with a number of other studies showing significant attenuation of depressive states following administration of this putative pro-dopamine regulator (Blum, Allison, et al., 1988; Blum et al., 2014; Chen et al., 2007; McLaughlin, Blum, Oscar-Berman, Febo, Agan, et al., 2015).
This patient provided a clinical history, consistent with the diagnosis of ADHD, inattentive type. The results of her Connors’ Continuous Performance Test supported the ADHD diagnosis. She admitted to having many vocal and motor tics and reported that vocal tics occur in her mother as well. She reported having been diagnosed with “bipolar disorder” as an adolescent. The patient’s addictions to opiates, caffeine, her prior history of marijuana addiction, and her addictions to shopping as well as to hoarding; all appear to be manifestations of RDS (Blum, Cull, & Braverman, 1996). Her reported history of lucid dreams has been reported in other patients, exhibiting RDS signs, symptoms, and diagnoses. The amelioration of these lucid dreams by KB200Z has been previously described. Finally, the complete termination of lucid dreams, even after the long-term cessation of KB200Z has also been documented, with the explanation advanced that these nutraceutical-induced neuroplasticity changes affected the reward system of the brain (McLaughlin et al., 2016).
The KB220Z compound was an addition to C.J.’s usual medications, including mixed amphetamine salts. The dextroamphetamine/amphetamine mixture is a compound combining four amphetamine isomers. The drug blocks the reuptake and increases the release of catecholamines, specifically norepinephrine and DA (van Gaalen, van Koten, Schoffelmeer, & Vanderschuren, 2006). However, long-term use of amphetamines leads to tolerance, which may be due to depletion of DA (Schechter, 1990). Therefore, adding DA precursors and stabilizing the DA system, via the adjunct therapy of KB220Z, may not only reverse this tolerance but may also augment the stimulants by reversing DA depletion (Ishikawa, Kadota, Kadota, Matsumura, & Nakamura, 2005). Furthermore, regarding RDS, creating DA homeostasis and stabilization can only be done by influencing multiple neurotransmitter pathways that converge on the DA brain reward cascade via a compound like KB220Z (Blum, Febo, et al., 2015).
What is most striking in this patient’s report is not only the cessation of her various addictive behaviors but also the activation of her psychological ego, resulting in a change from “passive victimhood” to her becoming an active agent, in control of her life, and her identity. She had changed from being a bored, hopeless, self-pitying victim to an activated agent, who had taken charge of her life. Many studies have evaluated the effect of this pro-dopaminergic regulator on psychological functions. In addition to its demonstrated anti-addiction effects, KB220Z or pro-dopamine regulation constitutes a potentially new and exciting area of brain–mind research.
Regarding both hoarding and shopping issues, the elimination of these unwanted behaviors in the patient is at the core of the mechanism of action of KB220Z. Previous work adequately showed not only selective DA activation across the brain reward circuitry (Febo et al., 2017), but also evidence for DA homeostasis in protracted heroin dependence (Blum, Febo, et al., 2017; Blum, Liu, et al., 2015).
Limitations
Currently, we do not know the actual level of “dopamine homeostasis” in the brain of this patient and although this question requires, additional, in-depth research in a much larger study her case provides an intriguing and provocative rationale for the further study.
A case study is descriptive research and, unlike true experimental placebo-control design, may help to generate experimental hypotheses rather than showing direct causality. The behavioral changes observed in this case study are consistent with a larger body of work, specifically, that of RDS and the use of the compound KB220Z. Based on this intervention, it is appropriate to extrapolate that the compound may have indeed influenced these profound behavioral changes. Finally, the mechanisms of action of KB220Z are well understood within the framework of the literature reviewed herein.
Conclusions
Based on this case study, the authors have demonstrated the elimination of many RDS-related behaviors, including lucid dreams, uncontrollable hoarding and shopping along with what appears to be enhanced motivation and activation of her psychological ego. Surprisingly, the latter reported change from “passive victimhood” to becoming an active agent, in control of her life and her identity is most intriguing.
The hypothesis that these benefits are the result of pro-dopamine regulation through KB220Z (Blum, Liu, et al., 2015), potentially leading to a needed balance of DA across the brain reward system, requires testing in further larger placebo-controlled studies.
Acknowledgements
The authors would like to thank the support of M. Hauser and a staff at Dominion Diagnostics, LLC, North Kingstown, RI, USA. The authors would also like to thank the support of T. Simpatico, MD, Department of Psychiatry, The University of Vermont, VT, USA and Margaret A. Madigan of Igene LLC. Finally, the authors would like to thank Danielle Jean Kradin for formatting the reference list.
Funding Statement
Funding sources: Drs. KB and ERB are co-recipients of a grant from The Life Extension Foundation, Ft. Lauderdale, FL, USA to Path Foundation, NY, USA. Dr. RDB is supported by the National Institutes of Health grants 1R01NS073884 and 1R21MH073624.
Authors’ contribution
The original idea to perform this trial was based on a previous clinical observation by TM and KB. The final design of the study was developed by TM, BS, and KB. The execution of the clinical data retrieval was obtained by TM and KB. The basic write up the case was developed by TM, BS, EJM, and KB. The initial writing of the manuscript was executed by KB and TM. The interpretation of the data was imputed by KB, TM, LF, RDB, EJM, and DB. The development of the tables was executed by BS, TM, and KB. The literature review was performed by KB, TM, DB, RDB, EJM, and ERB. All authors approved the final draft of the revised manuscript.
Conflict of interest
KB holds US and Foreign patents issued and pending on KB220Z and receives royalties based on its commercialization from various sources. He is also an officer and stock holder of IGENE, LLC and Geneus Health, LLC. He is a paid consultant of Dominion Diagnostics, LLC, and The Shores Treatment & Recovery Center. He is a member of the scientific advisory board of Dominion Diagnostics, LLC and Rivermend LLC, New York, NY. ERB owns Total Health Nutrients. Victory Nutrition International, Sanus Biotech, and Nupathways distribute nutritional supplements to the recovery marketplace based in-part on KB’s patents. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript part from those disclosed. All authors have approved the manuscript.
References
- Archer T., Oscar-Berman M., Blum K., Gold M. (2013). Epigenetic modulation of mood disorders. Journal of Genetic Syndrome and Gene Therapy, 4(120), 1000120. doi:10.4172/2157-7412.1000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan R. D. (2010). Dopamine is released in the striatum during human emotional processing. Neuroreport, 21(18), 1172–1176. doi:10.1097/WNR.0b013e3283410955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan R. D. (2013). A novel perspective on dopaminergic processing of human addiction. Journal of Alcoholism and Drug Dependence, 1(1), 000e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan R. D. (2014). Imaging dopamine neurotransmission in live human brain. Progress in Brain Research, 211, 165–182. doi:10.1016/b978-0-444-63425-2.00007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan R. D., Wack D. (2011). Evidence of dopaminergic processing of executive inhibition. PLoS One, 6(12), e28075. doi:10.1371/journal.pone.0028075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler J. A., Faust R. P., Turkson S., Ye H., Zhuang X. (2016). Low dopamine D2 receptor increases vulnerability to obesity via reduced physical activity, not increased appetitive motivation. Biological Psychiatry, 79(11), 887–897. doi:10.1016/j.biopsych.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler J. A., Frazier C. R., Zhuang X. (2012). Dopaminergic enhancement of local food-seeking is under global homeostatic control. The European Journal of Neuroscience, 35(1), 146–159. doi:10.1111/j.1460-9568.2011.07916.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitscher-Campbell H., Blum K., Febo M., Madigan M. A., Giordano J., Badgaiyan R. D., Braverman E. R., Dushaj K., Li M., Gold M. S. (2016). Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. Journal of Behavioral Addiction, 5(3), 533–541. doi:10.1556/2006.5.2016.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello E. P., Mateo Y., Gelman D. M., Noain D., Shin J. H., Low M. J., Alvarez V. A., Lovinger D. M., Rubinstein M. (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nature Neuroscience, 14(8), 1033–1038. doi:10.1038/nn.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Allison D., Trachtenberg M. C., Williams R. W., Loeblich L. A. (1988). Reduction of both drug hunger and withdrawal against advice rate of cocaine abusers in a 30 day inpatient treatment program by the neuronutrient tropamine. Current Therapeutic Research, 43, 1204–1214. [Google Scholar]
- Blum K., Braverman E. R., Wu S., Cull J. G., Chen T. J., Gill J., Wood R., Eisenberg A., Sherman M., Davis K. R., Matthews D., Fischer L., Schnautz N., Walsh W., Pontius A. A., Zedar M., Kaats G., Comings D. E. (1997). Association of polymorphisms of dopamine D2 receptor (DRD2), and dopamine transporter (DAT1) genes with schizoid/avoidant behaviors (SAB). Molecular Psychiatry, 2(3), 239–246. doi:10.1038/sj.mp.4000261 [DOI] [PubMed] [Google Scholar]
- Blum K., Briggs A. H., Trachtenberg M. C., Delallo L., Wallace J. E. (1987). Enkephalinase inhibition: Regulation of ethanol intake in genetically predisposed mice. Alcohol, 4(6), 449–456. doi:10.1016/0741-8329(87)90084-X [DOI] [PubMed] [Google Scholar]
- Blum K., Chen T. J. H., Chen A. L. C., Rhoades P., Prihoda T. J., Downs B. W., Bagchi D., Bagchi M., Blum S. H., William L., Braverman E. R., Kerner M., Waite R. L., Quirk B., White L., Reinking J. (2008). opamine D2 receptor Taq A1 allele predicts treatment compliance of LG839 in a subset analysis of pilot study in the Netherlands. Gene Therapy and Molecular Biology, 12(1), 129–140. [Google Scholar]
- Blum K., Chen T. J., Morse S., Giordano J., Chen A. L., Thompson J., Allen C., Smolen A., Lubar J., Stice E., Downs B. W., Waite R. L., Madigan M. A., Kerner M., Fornari F., Braverman E. R. (2010). Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D(2) agonist therapy: Part 2. Postgraduate Medicine, 122(6), 214–226. doi:10.3810/pgm.2010.11.2237 [DOI] [PubMed] [Google Scholar]
- Blum K., Cull J. G., Braverman E. R. (1996). The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. American Scientist, 84, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Febo M., Badgaiyan R. D. (2016). Fifty years in the development of a glutaminergic-dopaminergic optimization complex (KB220) to balance brain reward circuitry in reward deficiency syndrome: A pictorial. Austin Addiction Science, 1(2), 1006. [PMC free article] [PubMed] [Google Scholar]
- Blum K., Febo M., Fried L., Baron D., Braverman E. R., Dushaj K., Li M., Demetrovics Z., Badgaiyan R. D. (2017). Pro-dopamine regulator – (KB220) to balance brain reward circuitry in reward deficiency syndrome (RDS). Journal of Reward Deficiency Syndrome and Addiction Science, 3(1), 3–13. doi:10.1080/10826084.2016.1244551 [PMC free article] [PubMed] [Google Scholar]
- Blum K., Febo M., Fried L., Li M., Dushaj K., Braverman E. R., McLaughlin T., Steinberg B., Badgaiyan R. D. (2016). Hypothesizing that neuropharmacological and neuroimaging studies of glutaminergic-dopaminergic optimization complex (KB220Z) are associated with “dopamine homeostasis” in reward deficiency syndrome (RDS). Substance Use & Misuse, 52(4), 535–547. doi:10.1080/10826084.2016.1244551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Febo M., McLaughlin T., Cronje F. J., Han D., Gold S. M. (2014). Hatching the behavioral addiction egg: Reward deficiency solution system (RDSS) as a function of dopaminergic neurogenetics and brain functional connectivity linking all addictions under a common rubric. Journal of Behavioral Addiction, 3(3), 149–156. doi:10.1556/JBA.3.2014.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Febo M., Thanos P. K., Baron D., Fratantonio J., Gold M. (2015). Clinically combating reward deficiency syndrome (RDS) with dopamine agonist therapy as a paradigm shift: Dopamine for dinner? Molecular Neurobiology, 52(3), 1862–1869. doi:10.1007/s12035-015-9110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Gardner E., Oscar-Berman M., Gold M. (2012). “Liking” and “Wanting” linked to reward deficiency syndrome (RDS): Hypothesizing differential responsivity in brain reward circuitry. Current Pharmaceutical Design, 18(1), 113–118. doi:10.2174/138161212798919110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Gold M. S. (2011). Neuro-chemical activation of brain reward meso-limbic circuitry is associated with relapse prevention and drug hunger: A hypothesis. Medical Hypotheses, 76(4), 576–584. doi:10.1016/j.mehy.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Blum K., Gold M., Demetrovics Z., Archer T., Thanos P. K., Baron D., Badgaiyan R. D. (2017). Substance use disorder a bio-directional subset of reward deficiency syndrome. Frontiers in Bioscience (Landmark Edition), 22, 1534–1548. doi:10.2741/4557 [DOI] [PubMed] [Google Scholar]
- Blum K., Liu Y., Wang W., Wang Y., Zhang Y., Oscar-Berman M., Smolen A., Febo M., Han D., Simpatico T., Cronjé F. J., Demetrovics Z., Gold M. S. (2015). rsfMRI effects of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgraduate Medicine, 127(2), 232–241. doi:10.1080/00325481.2015.994879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Sheridan P. J., Wood R. C., Braverman E. R., Chen T. J., Cull J. G., Comings D. E. (1996). The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. Journal of the Royal Society of Medicine, 89(7), 396–400. doi:10.1177/014107689608900711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Trachtenberg M. C., Elliott C. l. E., Dingler M., Sexton R. L., Samuels A. I., Cataldie L. (1988). Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: Double-blind placebo-controlled study of the nutritional adjunct SAAVE™. Alcohol, 5(6), 481–493. doi:10.1016/0741-8329(88)90087-0 [DOI] [PubMed] [Google Scholar]
- Blum K., Trachtenberg M. C., Ramsay J. C. (1988). Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: A pilot study. The International Journal of the Addictions, 23(9), 991–998. doi:10.3109/10826088809058853 [DOI] [PubMed] [Google Scholar]
- Borker A. S., Mascarenhas J. F. (1991). Role of acetylcholine and dopamine in dorsal hippocampus on hoarding behavior in rats. Indian Journal of Physiology and Pharmacology, 35(1), 71–73. [PubMed] [Google Scholar]
- Chen T. J., Blum K., Payte J. T., Schoolfield J., Hopper D., Stanford M., Braverman E. R. (2004). Narcotic antagonists in drug dependence: Pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Medical Hypotheses, 63(3), 538–548. doi:10.1016/j.mehy.2004.02.051 [DOI] [PubMed] [Google Scholar]
- Chen T. J., Blum K., Waite R. L., Meshkin B., Schoolfield J., Downs B. W., Braverman E. E., Arcuri V., Varshavskiy M., Blum S. H., Mengucci J., Reuben C., Palomo T. (2007). Gene Narcotic Attenuation Program attenuates substance use disorder, a clinical subtype of reward deficiency syndrome. Advances in Therapy, 24(2), 402–414. doi:10.1007/BF02849910 [DOI] [PubMed] [Google Scholar]
- Claassen D. O., van den Wildenberg W. P., Ridderinkhof K. R., Jessup C. K., Harrison M. B., Wooten G. F., Wylie S. A. (2011). The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behavioral Neuroscience, 125(4), 492–500. doi:10.1037/a0023795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cold J. A. (1996). NeuRecover-SATM in the treatment of cocaine withdrawal and craving: A pilot study. Clinical Drug Investigation, 12(1), 1–7. doi:10.2165/00044011-199612010-00001 [Google Scholar]
- Connolly C. G., Bell R. P., Foxe J. J., Garavan H. (2013). Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS One, 8(3), e59645. doi:10.1371/journal.pone.0059645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. A., Kimmel H. L., Manvich D. F., Schmidt K. T., Weinshenker D., Howell L. L. (2014). Effects of pharmacologic dopamine beta-hydroxylase inhibition on cocaine-induced reinstatement and dopamine neurochemistry in squirrel monkeys. The Journal of Pharmacology and Experimental Therapeutics, 350(1), 144–152. doi:10.1124/jpet.113.212357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren A., Wargelius H. L., Berglund K. J., Fahlke C., Blennow K., Zetterberg H., Oreland L., Berggren U., Balldin J. (2011). Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol and Alcoholism, 46(5), 509–513. doi:10.1093/alcalc/agr045 [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Hymel C., Trachtenberg M. C., Ginsberg L. D., Schweitzer F. C., Estes S., Chen T. J., Braverman E. R., Cull J. G., Blum K. (1997). Enhancement of attention processing by Kantroll in healthy humans: A pilot study. Clinical Electroencephalography, 28(2), 68–75. [DOI] [PubMed] [Google Scholar]
- de Jong J. W., Roelofs T. J., Mol F. M., Hillen A. E., Meijboom K. E., Luijendijk M. C., van der Eerden H. A., Garner K. M., Vanderschuren L. J., Adan R. A. (2015). Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation. Neuropsychopharmacology, 40(9), 2085–2095. doi:10.1038/npp.2015.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M., Blum K., Badgaiyan R. D., Baron D., Thanos P. K., Colon-Perez L. M., Demortrovics Z., Gold M. S. (2017). Dopamine homeostasis: Brain functional connectivity in reward deficiency syndrome. Frontiers in Bioscience (Landmark Edition), 22, 669–691. doi:10.2741/4509 [DOI] [PubMed] [Google Scholar]
- Ferenczi E. A., Zalocusky K. A., Liston C., Grosenick L., Warden M. R., Amatya D., Katovich K., Mehta H., Patenaude B., Ramakrishnan C., Kalanithi P., Etkin A., Knutson B., Glover G. H., Deisseroth K. (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science, 351(6268), aac9698. doi:10.1126/science.aac9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenelle L. F., Oostermeijer S., Harrison B. J., Pantelis C., Yucel M. (2011). Obsessive-compulsive disorder, impulse control disorders and drug addiction: Common features and potential treatments. Drugs, 71(7), 827–840. doi:10.2165/11591790-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Gyollai A., Griffiths M. D., Barta C., Vereczkei A., Urban R., Kun B., Kökönyei G., Székely A., Sasvári-Székely M., Blum K., Demetrovics Z. (2014). The genetics of problem and pathological gambling: A systematic review. Current Pharmaceutical Design, 20(25), 3993–3999. doi:10.2174/13816128113199990626 [DOI] [PubMed] [Google Scholar]
- Hamilton P. J., Burek D. J., Lombroso S. I., Neve R. L., Robison A. J., Nestler E. J., Heller E. A. (2017). Cell-type-specific epigenetic editing at the Fosb gene controls susceptibility to social defeat stress. Neuropsychopharmacology. Advance online publication. doi:10.1038/npp.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell P. T., Lin H. Y., Park J. Y., Blank M. D., Drobes D. J., Evans D. E. (2016). Dopaminergic genetic variation moderates the effect of nicotine on cigarette reward. Psychopharmacology, 233(2), 351–360. doi:10.1007/s00213-015-4116-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S., Yang C., Tan A. (2015). Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behavioural Brain Research, 290, 17–31. doi:10.1016/j.bbr.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A., Kadota T., Kadota K., Matsumura H., Nakamura S. (2005). Essential role of D1 but not D2 receptors in methamphetamine-induced impairment of long-term potentiation in hippocampal-prefrontal cortex pathway. European Journal of Neuroscience, 22(7), 1713–1719. [DOI] [PubMed] [Google Scholar]
- Jupp B., Murray J. E., Jordan E. R., Xia J., Fluharty M., Shrestha S., Robbins T. W., Dalley J. W. (2016). Social dominance in rats: Effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology, 233(4), 579–589. doi:10.1007/s00213-015-4122-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley A. E., Stinus L. (1985). Disappearance of hoarding behavior after 6-hydroxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behavioral Neuroscience, 99(3), 531–545. doi:10.1037/0735-7044.99.3.531 [DOI] [PubMed] [Google Scholar]
- Korte S. M., Prins J., Van den Bergh F. S., Oosting R. S., Dupree R., Korte-Bouws G. A., Westphal K. G., Olivier B., Denys D. A., Garland A., Güntürkün O. (2017). The 5-HT1A/1B-receptor agonist eltoprazine increases both catecholamine release in the prefrontal cortex and dopamine release in the nucleus accumbens and decreases motivation for reward and “waiting” impulsivity, but increases “stopping” impulsivity. European Journal of Pharmacology, 794, 257–269. doi:10.1016/j.ejphar.2016.11.024 [DOI] [PubMed] [Google Scholar]
- Krawczyk M., Mason X., DeBacker J., Sharma R., Normandeau C. P., Hawken E. R., Di Prospero C., Chiang C., Martinez A., Jones A. A., Doudnikoff É., Caille S., Bézard E., Georges F., Dumont É. C. (2013). D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. The Journal of Neuroscience, 33(29), 11960–11971. doi:10.1523/jneurosci.1784-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love T. M. (2014). Oxytocin, motivation and the role of dopamine. Pharmacology, Biochemistry, and Behavior, 119, 49–60. doi:10.1016/j.pbb.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Blum K., Oscar-Berman M., Febo M., Agan G., Fratantonio J. L., Simpatico T., Gold M. S. (2015). Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: Role of enhanced brain reward functional connectivity and homeostasis redeeming joy. Journal of Behavioral Addictions, 4(2), 106–115. doi:10.1556/2006.4.2015.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Blum K., Oscar-Berman M., Febo M., Demetrovics Z., Agan G., Fratantonio J., Gold M. S. (2015). Using the neuroadaptagen KB200z to ameliorate terrifying, lucid nightmares in RDS patients: The role of enhanced, brain-reward, functional connectivity and dopaminergic homeostasis. Journal of Reward Deficiency Syndrome, 1(1), 24–35. doi:10.17756/jrds.2015-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Febo M., Badgaiyan R., Barh D., Dushaj K., Braverman E., Li M., Madigan M. A., Blum K. (2016). KB220Z™ a pro-dopamine regulator associated with the protracted, alleviation of terrifying lucid dreams. Can we infer neuroplasticity-induced changes in the reward circuit? Journal of Reward Deficiency Syndrome and Addiction Science, 2(1), 3–13. doi:10.17756/jrdsas.2016-022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T., Oscar-Berman M., Simpatico T., Giordano J., Jones S., Barh D., Downs W. B., Waite R. L., Madigan M., Dushaj K., Lohmann R., Braverman E. R., Han D., Blum K. (2013). Hypothesizing repetitive paraphilia behavior of a medication refractive Tourette’s syndrome patient having rapid clinical attenuation with KB220Z-nutrigenomic amino-acid therapy (NAAT). Journal of Behavioral Addictions, 2(2), 117–124. doi:10.1556/jba.2.2013.2.8 [DOI] [PubMed] [Google Scholar]
- Melo-Felippe F. B., de Salles Andrade J. B., Giori I. G., Vieira-Fonseca T., Fontenelle L. F., Kohlrausch F. B. (2016). Catechol-O-methyltransferase gene polymorphisms in specific obsessive-compulsive disorder patients’ subgroups. Journal of Molecular Neuroscience, 58(1), 129–136. doi:10.1007/s12031-015-0697-0 [DOI] [PubMed] [Google Scholar]
- Michaelides M., Anderson S. A., Ananth M., Smirnov D., Thanos P. K., Neumaier J. F., Wang G. J., Volkow N. D., Hurd Y. L. (2013). Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. The Journal of Clinical Investigation, 123(12), 5342–5350. doi:10.1172/jci72117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Bowirrat A., Manka M., Miller M., Stokes S., Manka D., Allen C., Gant C., Downs B. W., Smolen A., Stevens E., Yeldandi S., Blum K. (2010). Acute intravenous synaptamine complex variant KB220 “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: Part 1, pilot study with 2 case reports. Postgraduate Medicine, 122(6), 188–213. doi:10.3810/pgm.2010.11.2236 [DOI] [PubMed] [Google Scholar]
- Mogenson G. J., Yang C. R. (1991). The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Advances in Experimental Medicine and Biology, 295, 267–290. [DOI] [PubMed] [Google Scholar]
- Ng E., Varaschin R. K., Su P., Browne C. J., Hermainski J., Le Foll B., Pongs O., Liu F., Trudeau L. E., Roder J. C., Wong A. H. (2016). Neuronal calcium sensor-1 deletion in the mouse decreases motivation and dopamine release in the nucleus accumbens. Behavioral Brain Research, 301, 213–225. doi:10.1016/j.bbr.2015.12.037 [DOI] [PubMed] [Google Scholar]
- Nutt D. J., Lingford-Hughes A., Erritzoe D., Stokes P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews. Neuroscience, 16(5), 305–312. doi:10.1038/nrn3939 [DOI] [PubMed] [Google Scholar]
- Raja M., Bentivoglio A. R. (2012). Impulsive and compulsive behaviors during dopamine replacement treatment in Parkinson’s disease and other disorders. Current Drug Safety, 7(1), 63–75. doi:10.2174/157488612800492726 [DOI] [PubMed] [Google Scholar]
- Robinson M. J., Fischer A. M., Ahuja A., Lesser E. N., Maniates H. (2016). Roles of “wanting” and “liking” in motivating behavior: Gambling, food, and drug addictions. Current Topics of Behavioral Neuroscience, 27, 105–136. doi:10.1007/7854_2015_387 [DOI] [PubMed] [Google Scholar]
- Schechter M. D. (1990). Rats become acutely tolerant to cathine after amphetamine or cathinone administration. Psychopharmacology, 101(1), 126–131. doi:10.1007/BF02253729 [DOI] [PubMed] [Google Scholar]
- Sternat T., Katzman M. A. (2016). Neurobiology of hedonic tone: The relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatric Disease and Treatment, 12, 2149–2164. doi:10.2147/ndt.s111818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Small D. M. (2008). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322(5900), 449–452. doi:10.1126/science.1161550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos P. K., Robison L., Nestler E. J., Kim R., Michaelides M., Lobo M. K., Volkow N. D. (2013). Mapping brain metabolic connectivity in awake rats with muPET and optogenetic stimulation. The Journal of Neuroscience, 33(15), 6343–6349. doi:10.1523/jneurosci.4997-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R., Goldstein R. Z., Wang G. J., Wong C., Volkow N. D. (2008). Incentive motivation is associated with striatal dopamine asymmetry. Biological Psychology, 77(1), 98–101. doi:10.1016/j.biopsycho.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen M. M., van Koten R., Schoffelmeer A. N., Vanderschuren L. J. (2006). Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biological Psychiatry, 60(1), 66–73. doi:10.1016/j.biopsych.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Fowler J. S., Wang G. J., Baler R., Telang F. (2009). Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology, 56(Suppl 1), 3–8. doi:10.1016/j.neuropharm.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Newcorn J. H., Kollins S. H., Wigal T. L., Telang F., Fowler J. S., Goldstein R. Z., Klein N., Logan J., Wong C., Swanson J. M. (2011). Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Molecular Psychiatry, 16(11), 1147–1154. doi:10.1038/mp.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Reynolds B., Brezing C., Gallea C., Skaljic M., Ekanayake V., Fernandez H., Potenza M. N., Dolan R. J., Hallett M. (2010). Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology, 207(4), 645–659. doi:10.1007/s00213-009-1697-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhu J., Li Q., Li W., Wu N., Zheng Y., Chang H., Chen J., Wang W. (2013). Altered fronto-striatal and fronto-cerebellar circuits in heroin-dependent individuals: A resting-state FMRI study. PLoS One, 8(3), e58098. doi:10.1371/journal.pone.0058098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I., Burgeno L. M., Everitt B. J., Phillips P. E. (2012). Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20703–20708. doi:10.1073/pnas.1213460109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I., Burgeno L. M., Groblewski P. A., Phillips P. E. (2014). Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nature Neuroscience, 17(5), 704–709. doi:10.1038/nn.3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. D., Wang Q., Wang Z., Wang D. H. (2011). Food hoarding and associated neuronal activation in brain reward circuitry in Mongolian gerbils. Physiology & Behavior, 104(3), 429–436. doi:10.1016/j.physbeh.2011.04.062 [DOI] [PubMed] [Google Scholar]
- Zijlstra F., Booij J., van den Brink W., Franken I. H. (2008). Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. European Neuropsychopharmacology, 18(4), 262–270. doi:10.1016/j.euroneuro.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Zijlstra F., Veltman D. J., Booij J., van den Brink W., Franken I. H. (2009). Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence, 99(1–3), 183–192. doi:10.1016/j.drugalcdep.2008.07.012 [DOI] [PubMed] [Google Scholar]



