Introduction
Prolactin (Prl) is a 23 kDa polypeptide hormone mainly secreted by the anterior pituitary of all vertebrates1. Its functions are mediated by a non-kinase single pass transmembrane receptor, the Prl receptor (PrlR), a prototype of class 1 hematopoietic cytokine receptor superfamily that functions with various associated kinases2,3. In mammals, the best studied function of Prl is to stimulate lactation, which is mediated by the transcription factor Stat5a (Signal transducer and activator of transcription 5a). Accordingly, mice lacking one of the genes encoding Prl, PrlR or Stat5a are unable to lactate following the first pregnancy due to a failure of lobuloalveolar differentiation of the mammary epithelium4–6. In humans, the mammary gland is also considered a major target tissue of Prl, as exemplified by the fact that pharmacological blockade of pituitary Prl production using dopamine agonists stops lactation in nursing mothers, or that galactorrhea (spontaneous flow of milk from the nipple at any time other than during nursing) is one of the clinical symptoms of hyperprolactinemia.
Circulating Prl is also detected in males, though at lower levels than in females. However, its physiological role in males remains unclear. Based on the functional pleiotropy of Prl suggested from the analysis of various experimental models, it is reasonable to propose that the role of Prl in males involves some of its ‘secondary’ functions, which are mostly regulatory but not essential.. These include modulation of immune response, pancreatic beta-cell mass, lipid metabolism and hair growth, to cite only a few3,7–10. The Prl responsiveness of prostate tissue was first proposed in 1956 from studies investigating hormonal control of prostate growth in castrated rats11. During the next four decades, knowledge gained mainly from experimental models suggested that Prl participates in normal development, growth and function of the prostate gland12. The discovery that human prostate expresses the receptor for Prl13 conclusively demonstrated that this organ is a direct target of Prl. Most importantly, the detection of Prl expression by cells of the prostate suggested that this hormone may also act as a local growth factor via an autocrine/paracrine mechanism distinct from its classical endocrine route13.
This novel view has driven various investigations performed since the beginning of the 21st century regarding the potential involvement of local Prl in prostate cancer. The connections that could be made between prostate tumorigenesis and excess local Prl production contrasted with the absence of a clear link to elevated circulating Prl levels, first noticed in 197314. This paradigm shift in our understanding of Prl action on the prostate is particularly important regarding the fact that i) levels of prostate Prl production are much less accessible to clinical evaluation than circulating Prl, and ii) classical drugs which down-regulate Prl production from the pituitary are inactive in extrapituitary sites, and thus are unable to regulate production of local Prl. The past few years have been rich in studies deciphering the cellular and molecular mechanisms by which dysregulation of PrlR signaling cascade participates in prostate tumorigenesis, with the potential to impact therapeutic outcome. In addition, the recent discovery that local overexpression of Prl in mouse prostate amplifies the pool of basal/stem cells15, which have been identified as originators of prostate cancer16 and may mediate the castrate-resistant form of prostate cancer during disease progression, has provided a mechanistic basis for the oncogenic potency of Prl in prostate tissue. This review is aimed at delineating the current understanding of the role of Prl in prostate pathophysiology, with particular emphasis on cellular and molecular mechanisms linked to tumorigenesis and disease progression. The last part of this article is devoted to therapeutic perspectives for targeting PrlR signaling in prostate cancer.
Prolactin and prostate physiology
The role of Prl in prostate physiology has been almost exclusively addressed using rodent models, which involved live animals (healthy/castrated) treated with dopamine agonists or Prl. It was believed that the effects of Prl on the prostate served merely to enhance androgen action17–24 and instigate secondary endocrinological changes25. This was later disproved using organ cultures of normal and malignant prostate tissues, which demonstrated that Prl directly stimulates proliferation13,26 and inhibits apoptosis of prostate epithelial cells27. The anti-apoptotic and proliferative effects of Prl may be either direct and likely Stat5-mediated28–30, and/or indirect, implying Prl-induced expression of growth factor receptors, e.g. IGF-131 or androgen receptors24,31. In addition, earlier studies suggested a stimulatory role for Prl on epithelial secretion, function, energy metabolism and citrate production (see earlier review articles, e.g. Costello et al.12). In agreement with the growth-promoting action of Prl, mice lacking a functional Prl gene (Prl−/−) display reduction of ventral prostate weight32. Otherwise, mice harboring two non-functional PrlR alleles33 (PrlR−/−) displayed only minor histological defects (partial loss of epithelial content) and no growth or branching defects, while those lacking the Stat5a gene exhibited disorganized acini and altered secretory functions in ventral, but not other, prostate lobes34. Taken together, these various genetically-modified models failed to unanimously agree on a key role for PrlR signaling in prostate physiology, and more generally, in male reproductive functions.
The paucity of data available on the role of Prl in the normal human prostate has raised the question of the actual physiological function of Prl in prostate in humans. Indeed, although analysis of human prostate organ cultures indicated that Prl induces growth and differentiation13 of prostate epithelium, as suggested in rodents, extrapolations between species should be made very carefully as their prostate anatomy and morphology are dissimilar35,36 (Box 1). Obviously, the discovery of dysfunctional mutations of Prl or PrlR genes in humans, provided they exist, should help clarify this issue.
Box 1. Rodent versus human prostate anatomy.
Differences between rodent and human prostate have been detailed elsewhere108. In brief, the adult human prostate is a small acorn-shaped organ, with ductal–acinar histology, that lacks discernible lobular organization. Three distinct morphological regions named peripheral zone, transition zone and central zone have been identified. The peripheral zone constitutes over 70% of the gland, and is where prostate cancer most often arises. The central zone constitutes 25% of the gland. BPH occurs mainly in the transition zone. In contrast to the unique lobe anatomy of the human prostate, the mouse/rat prostate is composed of four paired lobes arranged circumferentially around the bladder and referred to as dorsal, lateral, ventral and anterior lobes (or coagulating glands). Each lobe is anatomically distinct, but the dorsal and lateral lobes are often examined together as the dorsolateral prostate due to their small size and proximity to one another. The dorsolateral prostate has been proposed to be analogous to the peripheral zone of human prostate (while the ventral lobe would have no human equivalent), therefore, rodent models that develop cancers in the dorsolateral lobes are believed to be more relevant to human prostate cancer. However, as evidence supporting human/rodent prostate analogies are primarily descriptive, this statement should be considered with caution, especially regarding the fact that in contrast to humans, mice never develop spontaneous prostate tumors. Besides anatomical differences, mouse and human prostates also differ at the histological level. Basal epithelial cells form a continuous layer in humans, while this layer is discontinuous in the mouse (4:1 ratio of luminal/basal epithelial cells). One of the hallmarks of human prostate cancer is the disappearance of basal cell markers, which is not observed in the mouse, where basal cells tend to adopt a distinct abnormal morphology and to form clusters. Neuroendocrine cells are even more rare in mouse than in human prostate. Finally, while human prostate harbors a robust fibromuscular stroma, the latter is more loose in the mouse where its density varies depending on the lobe considered and in each lobe, on pericentral vs. peripheral location.
Prolactin and prostate benign diseases
In contrast to the rather moderate consequences caused by down-regulation of PrlR signaling on mouse prostate physiology, up-regulation of PrlR signaling had a more dramatic effect. Marked enlargement of the lateral prostate was observed when chronic hyperprolactinemia was pharmacologically induced in rats using dopamine antagonists37. Similarly, systemic (using metallothionein promoter, Mt-PRL) or prostate-specific (using the probasin promoter, Pb-PRL) expression of a PRL transgene in mice resulted in dramatic prostate hypertrophy of the three lobes38,39 (Box 1). Interestingly, local over-production of Prl, rather than elevated circulating Prl levels, appeared to be the key event required to induce prostate overgrowth38. The Mt-PRL model, in which Prl overexpression occurs at neonatal stages, demonstrated that Prl is able to promote, either directly or indirectly, ductal morphogenesis in the developing prostate. After a few weeks of Prl overexpression, prostates displayed classical features of benign prostate hyperplasia (BPH), including stromal hyperplasia and focal areas of epithelial dysplasia (prostate intraepithelial neoplasia, or PIN). These effects were shown to be independent of elevated androgen levels39,40. These results underscore the significance of Prl as a mitogen and growth promoter for prostate epithelial cells. While the proliferative effect of Prl on epithelial cell primary cultures derived from BPH specimens was reported41, expression of PrlR was not found to be increased in BPH compared to normal prostate samples42. Furthermore, small case-control studies failed to correlate circulating Prl levels with BPH43. Additionally, a more recent prospective, case-control study involving 20 hyperprolactinemic young men and 20 healthy controls concluded that Prl excess had no effect on prostate volume. This was proposed to be due to the fact that hyperprolactinemia is accompanied by low testosterone and DHT levels44. At present, it is not known whether local autocrine Prl expression is increased in BPH vs. the normal secretory prostate epithelium.
There is an increasing body of evidence for the association of chronic prostate inflammation with BPH in men45,46. It has even been proposed that BPH is an inflammatory disease, based on the observation that BPH nodules are often associated with chronic inflammatory infiltrates mainly composed of chronically activated T cells and macrophages. Interestingly, mild to moderate chronic inflammation involving lymphocyte and macrophage infiltrates were also observed in prostates of Pb-PRL transgenic mice15,39. The mechanisms underlying this prostate phenotype are unknown. Prl has been suggested to mediate estrogen-induced prostate inflammation in rodents47,48. Interestingly, no inflammation was observed in estrogen-deficient aromatase knockout mice, despite the fact that they exhibited elevated levels of circulating Prl. This suggests that elevated Prl alone is not sufficient to exert pro-inflammatory effects49. Accordingly, the anti-inflammatory and anti-estrogenic actions documented for androgens in a rat model of non-bacterially-induced prostate inflammation were proposed to be partially mediated through decreased Prl-induced responses50. In humans, the involvement of Prl in prostatitis has yet to be established.
Prolactin and prostate cancer: a local affair
The much referenced epidemiological study in the field published 10 years ago by Stattin and colleagues,51 involving 144 men diagnosed with prostate cancer and 289 age-matched controls, concluded that elevated levels of circulating Prl were not related to an increase in prostate cancer risk. This result was in agreement with previous studies investigating the levels of endogenous sex hormones in prostate cancer patients52,53. Taken together, these papers suggested that endocrine Prl was not associated with the development of prostate cancer.
This finding was in apparent conflict with a wealth of data supporting a role for PrlR signaling in the promotion of prostate cancer in experimental models, specifically studies which demonstrated Prl-induced proliferation of human prostate cancer cell lines in vitro54,55 and xenograft tumors in vivo56. Additional evidence comes from genetically-modified mouse models which showed that the absence of PrlR expression (knockout model) prevented the appearance of simian virus 40 (SV40) T-induced prostate tumors33. Furthermore, systemic overexpression of Prl in Mt-PRL mice led to androgen-independent prostate hypertrophy including BPH and preneoplastic lesions (PINs)38,40. In conclusion, these data argued strongly that PrlR signaling plays a role in prostate tumorigenesis.
The lack of association between circulatory Prl levels and incidence/progression of clinical prostate cancer in patients is likely due to high local autocrine Prl production in human prostate cancer. Analysis of human prostate cancer specimens showed that i) Prl immunostaining was detected in 54% of a series of 80 prostate cancer specimens, and was positively correlated with high Gleason scores and activation of Stat5a/b, its major signaling protein29, ii) Prl was also expressed in half of recurrent prostate cancers in a series of 183 specimens30, including hormone-refractory cancer (54% of positive cases) and lymph node metastases (67%). Finally, PrlR expression, as determined by in situ hybridization and immunostaining, was homogeneously present in prostate cancer specimens, although it was more heterogeneously distributed and globally decreased in poorly differentiated high grade carcinoma42. With respect to experimental models of human prostate cancer, Prl mRNA expression was also detected in transplantable xenograft models assumed to closely mimic the clinical disease, i.e. the primary prostate tumor, CWR22P, and its castrate-resistant recurrent form, CWR22R30. Finally, the Prl gene is transcribed in a battery of human prostate cancer cell lines and their xenografts, irrespective of androgen receptor status30. Expression of the PrlR was also detected in most of these cell lines, though at a lower level compared to breast cancer cell lines42,57. Using various genetic and pharmacological approaches, the autocrine PrlR-Jak2-Stat5 cascade was demonstrated to be a critical survival pathway in CWR22Rv prostate cancer cell line30, which is in agreement with an earlier study that had demonstrated an anti-apoptotic role of Stat5 in prostate cancer cells58. In summary, these observations indicate that the autocrine/paracrine loop of Prl action is involved in prostate cancer progression. The increased expression of PrlR in pre-cancerous (dysplastic) lesions compared to normal prostate led several groups to suggest the involvement of PrlR signaling in early stages of prostate cancer development42.
Among the numerous differences between human and mouse prostate pathophysiology, mice never spontaneously develop prostate cancer59 and do not express detectable levels of Prl protein in the prostate15. Although it would be an over-interpretation to directly link these two observations, the potential importance of local Prl in human prostate cancer etiology prompted the development of animal models mimicking the human context, i.e. harboring the autocrine/paracrine Prl loop which does not intrinsically exist in mouse prostate. As already mentioned, prostate-specific expression of a Prl transgene in so-called Pb-PRL mice led to the development of BPH including preneoplastic lesions (PINs)39 similar to those reported for Mt-PRL mice. This suggests a mitogenic and anti-apoptotic role for local Prl in prostate epithelium38. In addition, we showed recently that these lesions eventually evolve to invasive carcinomas in aged animals (see below), indicating that malignancy, and not only benign disease, is the outcome of over-activation of PrlR signaling15. Interestingly, comparison of different strains of Mt-PRL mice presenting different levels of circulating Prl revealed that this had no impact on the features of their prostate tumors, stressing that locally-produced (autocrine) Prl is likely more efficient in promoting tumor growth than circulating (endocrine) Prl38,39.
Deciphering the cellular and molecular mechanisms underlying Prl promotion of prostate cancer growth
The basal/stem cell compartment as a target of autocrine/paracrine Prl
A major discovery in the field of prostate cancer etiology was reported in 2010. Owen Witte’s group demonstrated that prostate basal cells can serve as cells-of-origin for prostate cancer16. To achieve this conclusion, Witte and colleagues introduced various genetic alterations commonly found in clinical prostate cancer into non-pathological human prostate basal/stem cells sorted based on putative stem cell surface markers. These alterations included overexpression of Akt (an intracellular serine/threonine kinase with multiple signaling targets), ERG (a transcription factor whose gene is often translocated in prostate cancer), and the androgen receptor (AR). When grafted into immunodeficient mice, these genetically manipulated basal/stem cells were able to give rise to tumors displaying multifocal and heterogeneous features similar to those observed in human disease. When manipulated identically, luminal cells were unable to generate tumors. As one of the main features of human prostate cancer is the total loss of basal cell molecular markers in favor of exclusive expression of luminal markers, this was somewhat unexpected. Interestingly, similar studies performed using basal and luminal cells of mouse origin achieved exactly the same conclusions60, suggesting that rodents constitute relevant experimental models to investigate how basal/stem cells initiate prostate cancer in humans. It should be noted, however, that a small population of luminal cells in mouse prostate, referred to as castration-resistant Nkx3.1-expressing cells, or CARNs, were also able to initiate cancer upon PTEN deletion61.
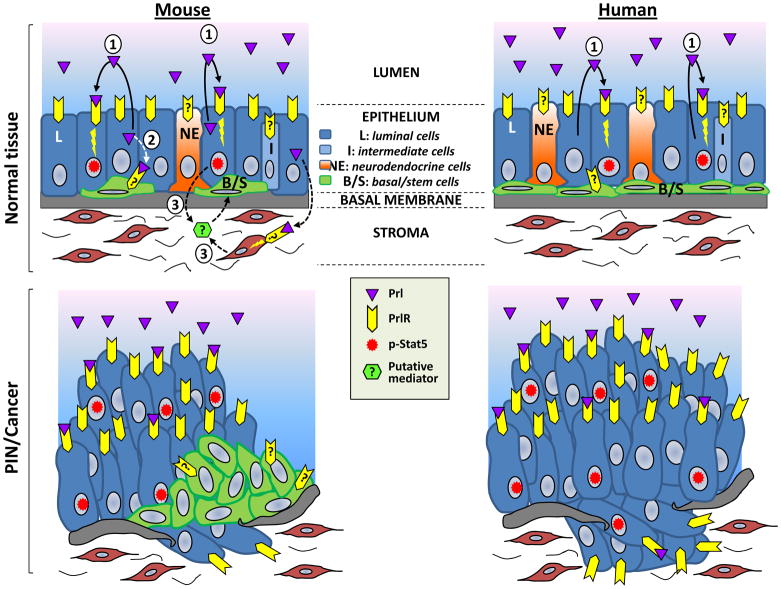
Basal/stem cells share the property of androgen-independence with late-stage castrate-resistant prostate cancer (CRPC) cells, suggesting that pathways involved in basal cell function and self-renewal may also play a role in tumor cell survival and disease recurrence after androgen ablation. Interestingly, prostate tumors harbored by Pb-PRL mice were shown to exhibit disorganized expansion of the basal/stem cell compartment throughout the entire prostate gland15. The mechanistic rationale provided by this study supports the idea that the tumorigenic properties of constitutive PrlR signaling, coupled with its potential to promote hormonal escape, potentially impacts the basal/stem cell compartment either directly or indirectly (Figure 1).
Fig 1. Potential mechanisms of autocrine Prl-induced prostate tumorigenesis.
Mouse and human prostate epithelia are schematically represented. As determined using Pb-PRL transgenic mice15, phosphorylated Stat5 (p-Stat5) colocalized with proliferating (Ki-67 positive) luminal cells (represented in blue). As the latter also produce Prl and express the PrlR, this suggests that proliferation of this compartment is mediated by direct autocrine/paracrine effects. The mechanisms underlying proliferation of the basal/stem cell compartment has yet to be elucidated. Since the basal cell marker p63 did not colocalize with p-Stat5, this either suggests that any direct effect of Prl on this compartment could be mediated by signaling pathways other than Stat5, and/or that indirect effects could involve Prl-induced production/secretion of certain growth factor(s) from other cell compartments (stroma, luminal cells). Similar mechanisms may take place in the human prostate, as increased production of local Prl and p-Stat5 have been correlated with Gleason score (see text). In contrast to the mouse, where basal cells are maintained and even amplified in Prl-induced prostate tumors, human prostate tumors lose basal cell markers (p63) in favor of tumor makers (AMACR) never shown in mouse prostate. Effects of Prl on human prostate basal cells have not been documented to our knowledge.
Prolactin signaling mechanisms in the prostate and the PrlR-Jak2-Stat5a/b signaling pathway
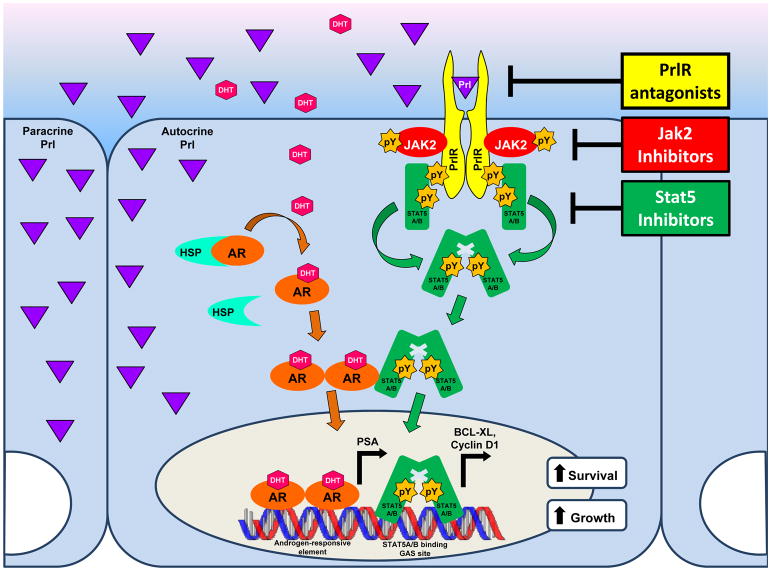
The PrlR itself lacks intrinsic enzymatic activity. In all tissues/cell types, it signals by activating various non-receptor tyrosine kinases associated with the PrlR. In the prostate, the biological effects of Prl are principally exerted by signaling through the long isoform of the PrlR and its downstream effectors, which constitute the canonical PrlR-Janus kinase 2 (Jak2)-Stat5a/b pathway (Box 2). For the purposes of this review, therefore, “PrlR” refers to those forms of the PrlR which lead to Stat5a/b phosphorylation (activation). In breast cancer, Prl is additionally capable of activating the Ras-Raf-MEK-Erk1/2 (MAPK/Erk), phosphoinositide 3-kinase (PI3K)-Akt, and protein kinase C pathways62,63. In contrast to these findings, an analysis of several human prostate cancer cell lines revealed that Prl stimulation did not activate Erk or Akt (unpublished data). While the contribution of MAPK/Erk, PI3K-Akt, and various other signaling pathways has yet to be conclusively ruled out, thus far only Stat5a and Stat5b have been established as key mediators of Prl in normal and malignant prostate28–30. Accordingly, Stat5 was the only classical component of the PrlR signaling pathway that was detectably activated in prostate tumors of Pb-PRL mice15 (Figure 2).
Box 2. The Prolactin Receptor.
The human PrlR is a transmembrane, single-pass, class I cytokine receptor109,110, encoded by the PrlR gene, which is located on chromosome 5p13-14 and contains 11 exons111,112. In both rodents and humans, the PrlR gene can give rise to various PrlR isoforms, referred to simply as long, intermediate, and short112,113. Synthesized as precursors with a signal peptide of ~19 to 24 amino acids, PrlR isoforms contain identical extracellular domains, which bind Prl, and differ only in the length of their cytoplasmic tails114. The PrlR is N-glycosylated at three sites on its extracellular ligand-binding domain, Asn35, Asn80 and Asn108115,116, which are crucial for proper trafficking of the receptor to the cell membrane117.
The PrlR lacks intrinsic enzymatic activity. In 1994, Jak2 was identified as the non-receptor tyrosine kinase associated with PrlR118. Upon Prl binding, preformed PrlR homodimers are assumed to undergo conformational changes which allow Jak2 molecules associated with the receptor’s cytosolic domain to move closer in proximity to each other, facilitating their activation by transphosphorylation. Subsequently, activated Jak2 is able to phosphorylate key tyrosine residues of the PrlR cytoplasmic domain of long and intermediate isoforms3. These phosphotyrosine motifs then act as docking sites for various signaling effectors, including Stat5a/b, typically via their SH2 domain (note that short PrlR isoforms are not tyrosine-phosphorylated, therefore they are believed to act as a dominant-negative on Stat5 activation). Following docking, Stat5a/b is rapidly phosphorylated by Jak2 at a conserved tyrosine residue in their C-terminus. Phosphorylation at Tyr694 and Tyr699 for Stat5a and Stat5b isoforms, respectively, leads to homo- or heterodimerization through phosphotyrosine-SH2 domain interactions between Stat monomers119,120. There is evidence that several protein kinases are capable of phosphorylating Stat proteins on serine residues, allowing for further potentiation of the initial Prl-mediated signal121. Interestingly, phosphorylation of Ser726 on Stat5a and Ser731 on Stat5b appears to have an inhibitory effect on transcriptional activity of these proteins76,122,123. Another level of regulation concerns crosstalk which is known to occur between the PrlR and AR signaling pathways, whereby Stat5a/b and AR exhibit a reciprocal interaction leading to the increased nuclear localization and transcriptional activity of both proteins. The PrlR may thus signal through the canonical Jak2-Stat5a/b pathway, as well as a non-canonical pathway involving AR.
Fig 2. PrlR-signaling in prostate epithelium and current drug development strategies.
The Stat5a/b pathway (see Box 2 for explanations) appears to be the major, if not the only, signaling pathway triggered by the PrlR in mouse and human prostate epithelial cells. Physical interactions between Stat5 and AR have been documented, providing a mechanistic basis for crosstalk between these two pathways resulting in reciprocal synergistic effects. Various levels of inhibition of this signaling cascade are in development (represented in yellow), which involve targeting of PrlR activation (competitive PrlR antagonist), Jak2 activity/stability (Jak2 inhibitors), and Stat5 DNA binding/expression (Stat5 inhibitors).
Stat proteins are a family of transcription factors that mediate cytokine and growth factor responses64–66, and the repertoire of genes activated by Prl depends largely on Stat5a/b binding to DNA. While highly homologous, the 94 kDa Stat5a and 92kDa Stat5b are distinct isoforms67–69. Once phosphorylated by Jak2 following PrlR activation (Box 2), Stat5a/b dimerizes and rapidly translocates to the nucleus. The available evidence has characterized the sequence of events leading to nuclear translocation as an energy-dependent process70,71 requiring interaction with Ran-dependent import machinery72,73: Rac 1, a small G protein, MgcRacGAP, a chaperone protein, and β1-karyopherin/β1-importin, a carrier protein, cooperate to form a nuclear shuttling complex with the Stat5a/b dimer74. In contrast, unphosphorylated Stat proteins, which exist as monomers, are believed to shuttle freely at a high exchange rate between distinct nuclear and cytoplasmic pools even in the absence of upstream cytokine signals70,75.
Nuclear-localized Stat5a/b binds to an 8–10 bp inverted repeat consensus sequence, TTC(C/T)N(G/A)GAA, known as the GAS (gamma-interferon activation sequence) element. Stat5a and Stat5b homodimers possess equal binding affinity for half palindromes spaced 3 bp apart. Tetrameric binding of Stat proteins can occur in the presence of tandemly linked consensus (GAS) or non-consensus motifs optimally spaced at 6 bp apart76. Stat5a/b target genes include the anti-apoptosis protein Bcl-XL and the cell cycle protein cyclin D1, among other pro-survival and growth-promoting factors77.
Stat5a/b in growth promotion and clinical progression of prostate cancer
The PrlR-Jak2-Stat5a/b pathway has been validated as a molecular target for the development of novel pharmaceutical therapies for prostate cancer. The relevance of active Stat5a/b to clinical prostate cancer progression is supported by evidence of its association with high histological grade29 and ability to predict early disease recurrence78. To emphasize the predictive value of active Stat5a/b, it should be noted that sub-analysis of intermediate Gleason grade prostate cancer samples still revealed Stat5a/b as an independent prognostic marker for early recurrent disease78. Active Stat5a/b critically regulates prostate cancer cell viability. Introduction of an adenovirally expressed dominant negative Stat5a/b mutant into cells in culture led to massive apoptosis detectable by changes in cell morphology, metabolic cell viability assays, DNA fragmentation, and caspase-3 and caspase-9 activation58. Later work confirmed these initial findings by using inducible expression of a carboxy terminal-truncated Stat5b mutant, which inhibits both Stat5a and Stat5b, in cell lines derived from the TRAMP mouse prostate cancer model. Expression of a mutant Stat5b reduced the ability of cells to grow in soft agar or form tumors in nude mice79. Dagvadorj et al. (2008)77 offered further support for the cruciality of active Stat5a/b in maintaining cell viability by triggering immense cell death across numerous Stat5-positive prostate cancer cell lines, using a variety of methodological approaches to inhibit Stat5a/b. This same study extended the results to the in vivo setting by showing decreased incidence and growth of subcutaneous human prostate xenograft tumors in nude mice77. Intriguingly, it was recently found that Stat5a/b inhibition also induced rapid death of DU145 prostate cancer cells, which are AR-negative, indicating AR-independent effects on prostate cancer cell viability80. This result highlights the importance of evaluating the role of Stat5a/b in progression to castrate-resistant stage from multiple perspectives. One conceivable approach would be to investigate active Stat5a/b as a survival factor acting independently of AR under castrate conditions, while another would be to look for possible enhancement of AR activity by Stat5a/b in the same setting.
Stat5a/b has emerged as a promoter of metastatic behavior of prostate cancer cells in vitro and in vivo81. Introduction of active Stat5a/b into DU145 prostate cancer cells increased lung metastases formation by 11-fold in nude mice following tail-vein injection80,81. Moreover, active Stat5a/b is associated with increased cell migration, decreased cell-surface E-cadherin levels, and increased heterotypic adhesion of prostate cancer cells to endothelial cells, all hallmarks of the epithelial to mesenchymal transition (EMT) which presages metastasis81. As further confirmation, distant prostate cancer metastases and CRPC were found to display increased nuclear-localized Stat5a/b and Prl expression levels30,82. In fact, in over 60% of metastatic prostate cancer clinical samples, Stat5a/b was found to be constantly active81. The mechanisms by which active Stat5a/b stimulates mobility, migration, and invasiveness of prostate cancer cells remain an area of active investigation. The implications of Prl-PrlR-Jak2-Stat5a/b pathway involvement in prostate cancer development and progression, when coupled with the paucity of treatment options for patients with CRPC or metastatic disease, provides a strong rationale for targeting the Prl signaling axis, either directly or through one of its downstream effectors.
Crosstalk between Prl and steroid hormone signaling pathways in prostate cancer
The Prl signaling pathway displays significant crosstalk with the AR signaling pathway in prostate cancer. While CRPC is known to continually express AR, implying sustained activation of AR despite low levels of circulating androgens following androgen ablation therapy83, the presence of active Stat5a/b in 95% of castrate-resistant clinical human prostate cancers was recently highlighted82. Furthermore, it was demonstrated that activated Stat5a/b increased the transcriptional activity of AR, while ligand-bound AR, in turn, increased the transcriptional activity of Stat5a/b (Figure 2). This synergy between Stat5a/b and AR extended to a physical interaction between the two proteins, as detected by co-immunoprecipitation and double immunofluorescent staining, with both Stat5a/b and AR enhancing each other’s nuclear localization in prostate cancer cells82,84. Interestingly, software analysis predicted the presence of GAS elements in the AR promoter region, suggesting recruitment of Stat5a/b to the promoter and a direct role for Stat5a/b in regulation of AR transcript levels (unpublished data). Given that both Stat5a/b and AR act as growth-promoting transcription factors, it is highly plausible that their co-action may permit continued proliferation despite low levels of androgens and support viability in conditions otherwise conducive to apoptosis. Further investigation will help dissect the molecular mechanisms of Stat5a/b-AR synergy, as well as extend these findings to in vivo studies.
Therapeutic targeting of the PrlR signaling axis in prostate cancer: perspectives
Organ-confined prostate cancer is amenable to treatment by surgery or local radiation, while the treatment options for disseminated malignancy include radiation, hormone therapy, and chemotherapy85. Unfortunately, response to hormone therapy in the form of androgen ablation is of limited duration, and late-stage disease inevitably recurs as CRPC, for which there is no curative treatment.
Pharmacological inhibitors of PrlR
Direct anti-Prl therapy has yet to be employed in the clinic, despite the existence of PrlR antagonists for some time now. Historically, the primary anti-Prl drugs have been dopamine agonists, and they continue to be used in this capacity today to block pituitary Prl secretion86. Unfortunately, this class of drugs is inactive in regulating Prl produced by extrapituitary sources such as the prostate since the mechanism of action of dopamine is based on inhibition of Prl secretion by anterior pituitary cells. Absent the development of potent neutralizing anti-PrlR monoclonal antibodies, PrlR antagonists are a logical therapeutic choice, given their ability to block local autocrine Prl action in various organs.
The competitive PrlR antagonist G129R-Prl87 and its second-generation analog, Δ1-9-G129R-hPrl30 (a pure PrlR antagonist lacking the residual agonism which characterizes G129R-Prl), provide a promising means of pharmacologically inhibiting PrlR signaling. Notably, work by Rouet and colleagues15 recently established proof-of-concept for the use of a pure PrlR antagonist to prevent early prostate tumorigenesis initiated by local Prl. Indeed, the hallmarks of prostate tumorigenesis observed in Pb-PRL transgenic mice (hypertrophy, expansion of a putative tumor-initiating/stem-like cell population, constitutive Stat5 activation, etc) were all prevented upon crossing these mice with another transgenic strain ubiquitously expressing Δ1-9-G129R-hPrl15. Future work will be geared towards improving the pharmacokinetics of the recombinant Δ1-9-G129R-hPrl compound, which should be possible by conjugation to polyethylene glycol moieties or carrier proteins88.
While competitive receptor antagonists are designed to block functional PrlR dimerization, another means of achieving PrlR blockade is via the use of pseudo-phosphorylated Prl (S179D-hPrl). Rather than exerting an antagonistic effect at the receptor, the S179D-hPRL variant stimulates production of the short PrlR isoform (SF1b), which was hypothesized to act in a dominant negative capacity to decrease PrlR-Stat5a/b signaling89. However, the molecular mechanisms underlying the induction of alternative splicing of the PrlR are not known.
PrlR antagonists are predicted to exert their effects primarily by ablation of signaling through the canonical Jak2-Stat5a/b pathway. Due to known PrlR promiscuity, however, it is possible that signaling through alternative pathways in non-prostate tissues may be disrupted. Of particular note, numerous studies reported activation of the MAPK pathway in response to Prl stimulation in rat Nb2 lymphoma cells90–92. The Src family kinase Fyn93, as well as Src itself94, have been shown to be associated with PrlR and capable of activation in a Prl-dependent manner. Complexing of PrlR and G-proteins was demonstrated by cross-linking experiments95. Finally, there is evidence that Prl induces association of IRS-1 and PI3K with PrlR, leading to rapid phosphorylation of IRS-1 and the 85-kDa subunit of PI3K96,97.
Pharmacological inhibitors of Jak2
Constitutive activation of Jak2 is conferred by an activating somatic mutation involving the JH2 pseudokinase domain, V617F, which can lead to the development of a number of myeloproliferative disorders, including polycythemia vera, essential thrombocythemia, and myelofibrosis with myeloid metaplasia98. The V617F mutation is also found in acute myeloid leukemia (AML), which may develop from the aforementioned disorders and in these patients is associated with a poor outcome. Indeed, persistent Stat3 activation associated with hyperactive Jak2 V617F has been found to be oncogenic65. Consequently, Jak2 inhibitors were first developed in the context of hematopoietic malignancies. Although V617F was recently determined to be absent in both locally-confined prostate cancer and CRPC99, the concept of Jak2 inhibition still holds promise in disrupting aberrant Prl signaling (Figure 2).
The compound WP1066 (also known as (E)-3(6-bromopyridin-2-yl)-2-cyano-N-(S0-1-phenylethyl) acrylamide), originally synthesized at M.D. Anderson Cancer Center in Houston, Texas, was one of the first compounds to demonstrate effective inhibition of Jak2 phosphorylation, and accordingly, inhibition of downstream phosphorylation of Stat3, Stat5, and Akt. Interestingly, WP1066 also led to degradation of Jak2, providing yet another means for attenuating the kinase’s effects. Incubation of several AML cell lines with the compound ultimately resulted in caspase-dependent cell death100.
AZD1480 is a potent pyrazolyl pyrimidine developed by AstraZeneca to function as a small-molecule, competitive ATP inhibitor of Jak2 (Ki = 0.26nM). In addition to inhibiting the growth of a panel of cancer cell lines in vitro, it was shown that nude mice bearing DU145 (prostate), MB-MDA-468 (breast), and MDAH2774 (ovarian) human xenografts demonstrated reduced tumor volume and abrogated Stat3 activity when treated with AZD1480, compared to control101. The demonstration that AZD1480 blocks oncogenesis in solid tumors validates Jak2 inhibition potentially as a rational pharmacological approach in treatment of prostate cancer.
As with many kinase inhibitors, selectivity is a concern; for example, evaluation of AZD1480 against a panel of 82 kinases, selected to represent the diversity of the kinome, demonstrated that 11 kinases were inhibited by greater than 50% at 0.10 μM101. Given the potential for non-specific inhibition, combined with the fact that other cytokines signal through Jak family kinases (i.e. IL-6 via Stat3) in various tissues, it may be necessary to optimize dose and/or schedule in order to avoid off-target toxicity101.
Pharmacological inhibitors of Stat5a/b
Stat5a/b has emerged as an attractive target for rational drug design in prostate cancer. Theoretically, dimerization of Stats can be inhibited by targeting the SH2 domain, transactivation can be inhibited by targeting the C-terminal transactivation domain, and DNA binding can be blocked by targeting the DNA-binding domain.
Early work in this area highlighted the feasibility of disrupting Stat5 transcriptional activity by using decoy oligonucleotides, which mimic Stat5a/b binding sites, to sequester Stat5a/b from its target genes. Sequestration of Stat5a/b successfully inhibited cyclin D1 expression in NRK-49F kidney cells102. Building on this approach, it was later shown that peptide aptamers selected from combinatorial libraries were capable of blocking Stat5a/b binding to DNA, as well as Stat5a/b dimerization103. A variety of methods to downregulate Stat5a/b have now been characterized, including a dominant negative mutant lacking the transactivation domain58,77, antisense oligonucleotides, and RNA interference77. Finally, phosphotyrosyl peptides or peptidomimetics which bind the SH2 domain effectively inhibited Stat3 dimerization104,105, but these studies did not extend the strategy to Stat5. As of yet, all strategies to inhibit Stat5a/b require further development before clinical application. Similar to Jak2 inhibitors, there is a question of off-target effects due to Stat5 inhibition, particularly in regard to hematopoietic106 and immune cell development107. Additionally, Stat5a/b (and Jak2) inhibitors are at a disadvantage when compared with PrlR antagonists acting at the cell surface, in that they require a mode of intracellular delivery to achieve efficacy. Overcoming this obstacle will be of prime importance in bringing Stat5a/b-targeted therapeutics to the clinic. Nonetheless, the continued development of lead compounds to produce small molecule inhibitors of Stat5a/b promises to expand the available armamentarium for treating prostate cancer in the near future.
Conclusions
Prl has recently entered the still limited panel of non-androgenic signaling pathways that are emerging as potential candidates for targeted therapy in a subset of prostate cancer patients. Indeed, evidence has accumulated within the past few years to suggest that activation of Stat5, the downstream effector of the canonical PrlR signaling pathway, plays a key role in prostate cancer progression. Interestingly, locally produced Prl of prostate origin, rather than circulating Prl secreted by the pituitary, seems to be the biochemical source for activation of the pathway, and may therefore represent another relevant target in PrlR-Jak2-Stat5a/b signaling pathway. This raises several important questions for translating these findings from bench-to-bedside: What additional evidence is required to advance the development of targeted therapies for Prl and its signaling cascade? Will the analysis of PrlR-Jak2-Stat5a/b activation status provide a routinely employed diagnostic tool to guide the clinical management of prostate cancer? If so, which readout of PrlR signaling will be most relevant to disease status (Prl expression levels, Stat5 phosphosylation, etc.)? And perhaps most importantly, which therapeutic approach will ultimately prove most effective in downregulating over-activated PrlR signaling in patients selected for targeted therapy? These are the key challenges to be addressed in future research.
Key points.
While mouse models with germline knock-out of Prl or PrlR alleles have suggested that Prl signaling contributes to prostate development and normal physiology, corresponding data for humans is currently lacking and awaits further investigation.
Involvement of Prl in benign diseases of the prostate (inflammation, BPH) has been suggested based on the identification of such phenotypes in Prl-overexpressing rodent models. However, there are no data so far indicating a direct role for Prl in inflammation or BPH in humans.
Circulating Prl levels do not correlate with prostate cancer risk in humans. However, several experimental and clinical studies indicate that over-activation of PrlR signaling by increased expression of prostate autocrine Prl may contribute to prostate cancer progression.
The basal/stem cell compartment has been proposed as a potential location for putative tumor-initiating cells in the prostate. Strikingly, the primary histological phenotype of prostate tumors developed by transgenic mice over-expressing Prl in the prostate involves disorganized amplification of the basal/stem cell compartment. This finding provides new insight into potential targets of autocrine/paracrine Prl which could drive prostate carcinogenesis and contribute to disease progression.
Stat5a/b is currently identified as a primary mediator of Prl effects in prostate epithelium. It has been known for some time that Prl directly regulates production of citrate in prostate epithelial cells124. Evidence is now emerging for the critical role of Stat5a/b in prostate cancer progression, documented by its role in promoting the growth and metastatic behavior of human prostate cancer cells, as well as its association with high histological grade and early recurrence. The dependence of prostate cancer cells on Stat5a/b for viability is amply illustrated by inhibition of Stat5a/b leading to widespread apoptosis. Finally, Stat5a/b-AR functional interactions represent a possible means of sustaining prostate cancer cell survival, especially under castrate conditions.
Current available therapies for advanced castrate-resistant prostate cancer are not curative. In recent years, targeted cancer therapies aimed at inhibiting particular signaling pathways have emerged as potential alternatives to traditional cytotoxic chemotherapy. In this context, PrlR-Jak2-Stat5a/b pathway offers several novel molecular candidates for pharmaceutical intervention, based on evidence supporting its involvement in human prostate cancer growth. This has led to the development of a number of antagonists and small-molecule inhibitors capable of either inactivating the PrlR itself, the receptor-associated Jak2 kinase, or the transcription factor Stat5a/b. Although none of these compounds are available yet for routine clinical use, their promise is highlighted by proof-of-concept obtained in cellular and/or animal models. In the future, judicious use of pharmacological inhibitors of the PrlR-Jak2-Stat5a/b pathway may have the potential to improve clinical outcomes for prostate cancer patients.
REVIEW CRITERIA.
In addition to the combined reference databases of the Paris and Philadelphia laboratories (~6,000 articles), we searched PubMed for original and review publications containing in the title or abstract the terms “prolactin” and/or “prostate” and/or “cancer” and/or “hyperplasia” and/or “prostatitis” and/or “dopamine agonist” and/or “hyperprolactinemia”.
Acknowledgments
The authors are grateful to Dr Lucila Sackmann-Sala for critical reading of this manuscript. They acknowledge grant support from Association for International Cancer Research (AICR grant 05-0603) and NIH National Cancer Institute grant 1RO1CA113580-01A1.
Abbreviations
- Prl
prolactin
- PrlR
Prl receptor
- Stat
signal transducer and activator of transcription
- Mt-PRL
Metallothionein promoter-Prl transgene
- Pb-PRL
probasin promoter-Prl transgene
- PIN
Prostate intraepithelial neoplasia
- CRPC
Castrate-resistant prostate cancer
- AR
Androgen receptor
- TRAMP
Transgenic Adenocarcinoma of Mouse Prostate
References
- 1.Riddle O, Bates RW, Dykshorn SW. The preparation, identification and assay of prolactin - a hormone of the anterior pituitary. Am J Physiol. 1933;105:191–216. [Google Scholar]
- 2.Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991;12:235–51. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- 3.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–68. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 4.Ormandy CJ, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–78. doi: 10.1101/gad.11.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Horseman ND, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. Embo J. 1997;16:6926–35. doi: 10.1093/emboj/16.23.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–86. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langan EA, et al. Mind the (gender) gap: does prolactin exert gender and/or site-specific effects on the human hair follicle? J Invest Dermatol. 130:886–91. doi: 10.1038/jid.2009.340. [DOI] [PubMed] [Google Scholar]
- 9.LaPensee CR, et al. The prolactin-deficient mouse has an unaltered metabolic phenotype. Endocrinology. 2006;147:4638–45. doi: 10.1210/en.2006-0487. [DOI] [PubMed] [Google Scholar]
- 10.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- 11.Flores A, Segaloff A, Steelman SL. Prolactin as a factor in the ventral prostate assay for luteinizing hormone. Endocrinology. 1956;59:233–40. doi: 10.1210/endo-59-2-233. [DOI] [PubMed] [Google Scholar]
- 12.Costello LC, Franklin RB. Effect of prolactin on the prostate. Prostate. 1994;24:162–6. doi: 10.1002/pros.2990240311. [DOI] [PubMed] [Google Scholar]
- 13.Nevalainen MT, et al. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–27. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newball HH, Byar DP. Does reserpine increase prolactin and exacerbate cancer of prostate? Case control study. Urology. 1973;2:525–9. doi: 10.1016/0090-4295(73)90560-8. [DOI] [PubMed] [Google Scholar]
- 15.Rouet V, et al. Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc Natl Acad Sci U S A. 2010;107:15199–204. doi: 10.1073/pnas.0911651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walvoord DJ, Resnick MI, Grayhack JT. Effect of testosterone, dihydrotestosterone, estradiol, and prolactin on the weight and citric acid content of the lateral lobe of the rat prostate. Invest Urol. 1976;14:60–5. [PubMed] [Google Scholar]
- 18.Coert A, Nievelstein H, Kloosterboer HJ, Loonen P, van deer Vies J. Effects of hyperprolactinemia on the accessory sexual organs of the male rat. Prostate. 1985;6:269–276. doi: 10.1002/pros.2990060306. [DOI] [PubMed] [Google Scholar]
- 19.Jones R, Riding PR, Parker MG. Effects of prolactin on testosterone-induced growth and protein synthesis in rat accessory sex glands. J Endocrinol. 1983;96:407–16. doi: 10.1677/joe.0.0960407. [DOI] [PubMed] [Google Scholar]
- 20.Holland JM, Lee C. Effects of pituitary grafts on testosterone stimulated growth of rat prostate. Biol Reprod. 1980;22:351–5. doi: 10.1093/biolreprod/22.2.351. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JA, Manandhar MS. Effects of prolactin on the dorsolateral lobe of the rat prostate gland. Invest Urol. 1977;14:398–9. [PubMed] [Google Scholar]
- 22.Schacht MJ, et al. A local direct effect of pituitary graft on growth of the lateral prostate in rats. Prostate. 1992;20:51–8. doi: 10.1002/pros.2990200107. [DOI] [PubMed] [Google Scholar]
- 23.Keenan EJ, Klase PA, Thomas JA. Effects of prolactin on DNA synthesis and growth of the accessory sex organs in male mice. Endocrinology. 1981;109:170–5. doi: 10.1210/endo-109-1-170. [DOI] [PubMed] [Google Scholar]
- 24.Prins GS. Prolactin influence on cytosol and nuclear androgen receptors in the ventral, dorsal, and lateral lobes of the rat prostate. Endocrinology. 1987;120:1457–64. doi: 10.1210/endo-120-4-1457. [DOI] [PubMed] [Google Scholar]
- 25.Adams JB. Control of secretion and the function of C19-delta 5-steroids of the human adrenal gland. Mol Cell Endocrinol. 1985;41:1–17. doi: 10.1016/0303-7207(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 26.Nevalainen MT, et al. Estrogen and prolactin regulation of rat dorsal and lateral prostate in organ culture. Endocrinology. 1991;129:612–22. doi: 10.1210/endo-129-2-612. [DOI] [PubMed] [Google Scholar]
- 27.Ahonen TJ, et al. Prolactin is a survival factor for androgen-deprived rat dorsal and lateral prostate epithelium in organ culture. Endocrinology. 1999;140:5412–21. doi: 10.1210/endo.140.11.7090. [DOI] [PubMed] [Google Scholar]
- 28.Ahonen TJ, Harkonen PL, Rui H, Nevalainen MT. PRL signal transduction in the epithelial compartment of rat prostate maintained as long-term organ cultures in vitro. Endocrinology. 2002;143:228–38. doi: 10.1210/endo.143.1.8576. [DOI] [PubMed] [Google Scholar]
- 29.Li H, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–82. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 30.Dagvadorj A, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–101. doi: 10.1210/en.2006-1761. [DOI] [PubMed] [Google Scholar]
- 31.Reiter E, et al. Growth hormone and prolactin stimulate androgen receptor, insulin-like growth factor-I (IGF-I) and IGF-I receptor levels in the prostate of immature rats. Mol Cell Endocrinol. 1992;88:77–87. doi: 10.1016/0303-7207(92)90011-t. [DOI] [PubMed] [Google Scholar]
- 32.Steger RW, Chandrashekar V, Zhao W, Bartke A, Horseman ND. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology. 1998;139:3691–5. doi: 10.1210/endo.139.9.6209. [DOI] [PubMed] [Google Scholar]
- 33.Robertson FG, et al. Prostate development and carcinogenesis in prolactin receptor knockout mice. Endocrinology. 2003;144:3196–205. doi: 10.1210/en.2003-0068. [DOI] [PubMed] [Google Scholar]
- 34.Nevalainen MT, et al. Epithelial defect in prostates of Stat5a-null mice. Lab Invest. 2000;80:993–1006. doi: 10.1038/labinvest.3780105. [DOI] [PubMed] [Google Scholar]
- 35.Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000;14:2410–34. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- 36.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 37.Van Coppenolle F, et al. Effects of hyperprolactinemia on rat prostate growth: evidence of androgeno-dependence. Am J Physiol Endocrinol Metab. 2001;280:E120–9. doi: 10.1152/ajpendo.2001.280.1.E120. [DOI] [PubMed] [Google Scholar]
- 38.Wennbo H, Kindblom J, Isaksson OG, Tornell J. Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology. 1997;138:4410–5. doi: 10.1210/endo.138.10.5461. [DOI] [PubMed] [Google Scholar]
- 39.Kindblom J, et al. Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology. 2003;144:2269–78. doi: 10.1210/en.2002-0187. [DOI] [PubMed] [Google Scholar]
- 40.Kindblom J, Dillner K, Ling C, Tornell J, Wennbo H. Progressive prostate hyperplasia in adult prolactin transgenic mice is not dependent on elevated serum androgen levels. Prostate. 2002;53:24–33. doi: 10.1002/pros.10113. [DOI] [PubMed] [Google Scholar]
- 41.Syms AJ, Harper ME, Griffiths K. The effect of prolactin on human BPH epithelial cell proliferation. Prostate. 1985;6:145–53. doi: 10.1002/pros.2990060204. [DOI] [PubMed] [Google Scholar]
- 42.Leav I, et al. Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol. 1999;154:863–70. doi: 10.1016/S0002-9440(10)65333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rannikko S, Adlercreutz H. Plasma estradiol, free testosterone, sex hormone binding globulin binding capacity, and prolactin in benign prostatic hyperplasia and prostatic cancer. Prostate. 1983;4:223–9. doi: 10.1002/pros.2990040302. [DOI] [PubMed] [Google Scholar]
- 44.Colao A, et al. Prolactin and prostate hypertrophy: a pilot observational, prospective, case-control study in men with prolactinoma. J Clin Endocrinol Metab. 2004;89:2770–5. doi: 10.1210/jc.2003-032055. [DOI] [PubMed] [Google Scholar]
- 45.Nickel JC. Inflammation and benign prostatic hyperplasia. Urol Clin North Am. 2008;35:109–15. vii. doi: 10.1016/j.ucl.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Yang JR, Yang LY, Liu ZT. Chronic inflammation in benign prostatic hyperplasia: implications for therapy. Med Hypotheses. 2008;70:1021–3. doi: 10.1016/j.mehy.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 47.Tangbanluekal L, Robinette CL. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology. 1993;132:2407–16. doi: 10.1210/endo.132.6.8504745. [DOI] [PubMed] [Google Scholar]
- 48.Gilleran JP, et al. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144:2046–54. doi: 10.1210/en.2002-0038. [DOI] [PubMed] [Google Scholar]
- 49.McPherson SJ, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–67. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 50.Yatkin E, Bernoulli J, Talvitie EM, Santti R. Inflammation and epithelial alterations in rat prostate: impact of the androgen to oestrogen ratio. Int J Androl. 2009;32:399–410. doi: 10.1111/j.1365-2605.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 51.Stattin P, et al. Plasma prolactin and prostate cancer risk: A prospective study. Int J Cancer. 2001;92:463–5. doi: 10.1002/ijc.1191. [DOI] [PubMed] [Google Scholar]
- 52.Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–26. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 53.Eaton NE, Reeves GK, Appleby PN, Key TJ. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999;80:930–4. doi: 10.1038/sj.bjc.6690445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melck D, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–26. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 55.Janssen T, et al. In vitro characterization of prolactin-induced effects on proliferation in the neoplastic LNCaP, DU145, and PC3 models of the human prostate. Cancer. 1996;77:144–9. doi: 10.1002/(SICI)1097-0142(19960101)77:1<144::AID-CNCR24>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Kreye E, Kuo CB, Walker AM. A molecular mimic of phosphorylated prolactin markedly reduced tumor incidence and size when du145 human prostate cancer cells were grown in nude mice. Cancer Res. 2001;61:6098–104. [PubMed] [Google Scholar]
- 57.Peirce SK, Chen WY, Chen WY. Quantification of prolactin receptor mRNA in multiple human tissues and cancer cell lines by real time RT-PCR. J Endocrinol. 2001;171:R1–4. doi: 10.1677/joe.0.171r001. [DOI] [PubMed] [Google Scholar]
- 58.Ahonen TJ, et al. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–92. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- 59.Suwa T, Nyska A, Haseman JK, Mahler JF, Maronpot RR. Spontaneous lesions in control B6C3F1 mice and recommended sectioning of male accessory sex organs. Toxicol Pathol. 2002;30:228–34. doi: 10.1080/019262302753559560. [DOI] [PubMed] [Google Scholar]
- 60.Lawson DA, et al. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci U S A. 107:2610–5. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neilson LM, et al. Coactivation of janus tyrosine kinase (Jak)1 positively modulates prolactin-Jak2 signaling in breast cancer: recruitment of ERK and signal transducer and activator of transcription (Stat)3 and enhancement of Akt and Stat5a/b pathways. Mol Endocrinol. 2007;21:2218–32. doi: 10.1210/me.2007-0173. [DOI] [PubMed] [Google Scholar]
- 64.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 65.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 66.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 67.Mui A, et al. Function of the common beta subunit of the GM-CSF/IL-3/IL-5 receptors. Adv Exp Med Biol. 1994;365:217–23. doi: 10.1007/978-1-4899-0987-9_22. [DOI] [PubMed] [Google Scholar]
- 68.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92:8831–5. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu JX, Mietz J, Modi WS, John S, Leonard WJ. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem. 1996;271:10738–44. [PubMed] [Google Scholar]
- 70.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–12. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 71.Vinkemeier U. Getting the message across, STAT! Design principles of a molecular signaling circuit. J Cell Biol. 2004;167:197–201. doi: 10.1083/jcb.200407163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y. Interferon-gamma-dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996;271:31017–20. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- 73.Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. Embo J. 1997;16:7067–77. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawashima T, et al. A Rac GTPase-activating protein, MgcRacGAP, is a nuclear localizing signal-containing nuclear chaperone in the activation of STAT transcription factors. Mol Cell Biol. 2009;29:1796–813. doi: 10.1128/MCB.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marg A, et al. Nucleocytoplasmic shuttling by nucleoporins Nup153 and Nup214 and CRM1-dependent nuclear export control the subcellular distribution of latent Stat1. J Cell Biol. 2004;165:823–33. doi: 10.1083/jcb.200403057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soldaini E, et al. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol Cell Biol. 2000;20:389–401. doi: 10.1128/mcb.20.1.389-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT. Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res. 2008;14:1317–24. doi: 10.1158/1078-0432.CCR-07-2024. [DOI] [PubMed] [Google Scholar]
- 78.Li H, et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863–8. doi: 10.1158/1078-0432.CCR-05-0562. [DOI] [PubMed] [Google Scholar]
- 79.Kazansky AV, Spencer DM, Greenberg NM. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res. 2003;63:8757–62. [PubMed] [Google Scholar]
- 80.Gu L, et al. Transcription factor Stat3 stimulates metastatic behavior of human prostate cancer cells in vivo, whereas Stat5b has a preferential role in the promotion of prostate cancer cell viability and tumor growth. Am J Pathol. 2010;176:1959–72. doi: 10.2353/ajpath.2010.090653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu L, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17:481–93. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan SH, et al. Transcription factor Stat5 synergizes with androgen receptor in prostate cancer cells. Cancer Res. 2008;68:236–48. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
- 83.Isaacs JT, Isaacs WB. Androgen receptor outwits prostate cancer drugs. Nat Med. 2004;10:26–7. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- 84.Thomas C, et al. Transcription factor Stat5 knockdown enhances androgen receptor degradation and delays castration-resistant prostate cancer progression in vivo. Mol Cancer Ther. 10:347–59. doi: 10.1158/1535-7163.MCT-10-0850. [DOI] [PubMed] [Google Scholar]
- 85.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 86.Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26:400–22. doi: 10.1210/er.2004-0016. [DOI] [PubMed] [Google Scholar]
- 87.Llovera M, et al. Human prolactin (hPRL) antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation. Oncogene. 2000;19:4695–705. doi: 10.1038/sj.onc.1203846. [DOI] [PubMed] [Google Scholar]
- 88.Tallet E, et al. Rational design of competitive prolactin/growth hormone receptor antagonists. J Mammary Gland Biol Neoplasia. 2008;13:105–17. doi: 10.1007/s10911-008-9066-8. [DOI] [PubMed] [Google Scholar]
- 89.Wu W, Ginsburg E, Vonderhaar BK, Walker AM. S179D prolactin increases vitamin D receptor and p21 through up-regulation of short 1b prolactin receptor in human prostate cancer cells. Cancer Res. 2005;65:7509–15. doi: 10.1158/0008-5472.CAN-04-3350. [DOI] [PubMed] [Google Scholar]
- 90.Rao YP, Buckley DJ, Buckley AR. Rapid activation of mitogen-activated protein kinase and p21ras by prolactin and interleukin 2 in rat Nb2 node lymphoma cells. Cell Growth Differ. 1995;6:1235–1244. [PubMed] [Google Scholar]
- 91.Erwin RA, Kirken RA, Malabarba MG, Farrar WL, Rui H. Prolactin activates Ras via signaling proteins SHC, growth factor receptor bound 2, and son of sevenless. Endocrinology. 1995;136:3512–8. doi: 10.1210/endo.136.8.7628388. [DOI] [PubMed] [Google Scholar]
- 92.Buckley AR, Rao YP, Buckley DJ, Gout PW. Prolactin-induced phosphorylation and nuclear translocation of MAP kinase in Nb2 lymphoma cells. Biochem Biophys Res Commun. 1994;204:1158–64. doi: 10.1006/bbrc.1994.2584. [DOI] [PubMed] [Google Scholar]
- 93.Clevenger CV, Medaglia MV. The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol Endocrinol. 1994;8:674–81. doi: 10.1210/mend.8.6.7935483. [DOI] [PubMed] [Google Scholar]
- 94.Berlanga JJ, Fresno Vara JA, Martin-Perez J, Garcia-Ruiz JP. Prolactin receptor is associated with c-src kinase in rat liver. Mol Endocrinol. 1995;9:1461–7. doi: 10.1210/mend.9.11.8584023. [DOI] [PubMed] [Google Scholar]
- 95.Too CK, Shiu RP, Friesen HG. Cross-linking of G-proteins to the prolactin receptor in rat NB2 lymphoma cells. Biochem Biophys Res Commun. 1990;173:48–52. doi: 10.1016/s0006-291x(05)81019-8. [DOI] [PubMed] [Google Scholar]
- 96.Berlanga JJ, et al. Prolactin activates tyrosyl phosphorylation of insulin receptor substrate 1 and phosphatidylinositol-3-OH kinase. J Biol Chem. 1997;272:2050–2. doi: 10.1074/jbc.272.4.2050. [DOI] [PubMed] [Google Scholar]
- 97.al-Sakkaf KA, Dobson PR, Brown BL. Activation of phosphatidylinositol 3-kinase by prolactin in Nb2 cells. Biochem Biophys Res Commun. 1996;221:779–84. doi: 10.1006/bbrc.1996.0673. [DOI] [PubMed] [Google Scholar]
- 98.Tefferi A, Gilliland DG. The JAK2V617F tyrosine kinase mutation in myeloproliferative disorders: status report and immediate implications for disease classification and diagnosis. Mayo Clin Proc. 2005;80:947–58. doi: 10.4065/80.7.947. [DOI] [PubMed] [Google Scholar]
- 99.Gu LMT, Alanen K, Edmonston T, Nevalainen MT. JAK2 V617F Mutation is Absent in Advanced Human Prostate Cancers. Cellular Oncology. 2009;2010 In Press. [Google Scholar]
- 100.Faderl S, et al. Atiprimod blocks phosphorylation of JAK-STAT and inhibits proliferation of acute myeloid leukemia (AML) cells. Leuk Res. 2007;31:91–5. doi: 10.1016/j.leukres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 101.Hedvat M, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guh JY, et al. Advanced glycation end product-induced proliferation in NRK-49F cells is dependent on the JAK2/STAT5 pathway and cyclin D1. Am J Kidney Dis. 2001;38:1096–104. doi: 10.1053/ajkd.2001.28616. [DOI] [PubMed] [Google Scholar]
- 103.Nagel-Wolfrum K, et al. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res. 2004;2:170–82. [PubMed] [Google Scholar]
- 104.Turkson J, et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–55. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 105.Turkson J, et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–9. [PubMed] [Google Scholar]
- 106.Numata A, et al. Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway. J Biol Chem. 2005;280:12621–9. doi: 10.1074/jbc.M408442200. [DOI] [PubMed] [Google Scholar]
- 107.Malin S, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–9. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–54. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 109.Goffin V, Kelly PA. The prolactin/growth hormone receptor family: structure/function relationships. J Mammary Gland Biol Neoplasia. 1997;2:7–17. doi: 10.1023/a:1026313211704. [DOI] [PubMed] [Google Scholar]
- 110.Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991;12:235–51. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- 111.Hu ZZ, Meng J, Dufau ML. Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J Biol Chem. 2001;276:41086–94. doi: 10.1074/jbc.M102109200. [DOI] [PubMed] [Google Scholar]
- 112.Arden KC, Boutin JM, Djiane J, Kelly PA, Cavenee WK. The receptors for prolactin and growth hormone are localized in the same region of human chromosome 5. Cytogenet Cell Genet. 1990;53:161–5. doi: 10.1159/000132919. [DOI] [PubMed] [Google Scholar]
- 113.Hu ZZ, Meng J, Dufau ML. Isolation and characterization of two novel forms of the human prolactin receptor generated by alternative splicing of a newly identified exon 11. J Biol Chem. 2001;22:22. doi: 10.1074/jbc.M102109200. [DOI] [PubMed] [Google Scholar]
- 114.Kelly PA, et al. The growth hormone/prolactin receptor family. Recent Prog Horm Res. 1993;48:123–64. doi: 10.1016/b978-0-12-571148-7.50009-9. [DOI] [PubMed] [Google Scholar]
- 115.Boutin JM, et al. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- 116.Okamura H, Zachwieja J, Raguet S, Kelly PA. Characterization and applications of monoclonal antibodies to the prolactin receptor. Endocrinology. 1989;124:2499–508. doi: 10.1210/endo-124-5-2499. [DOI] [PubMed] [Google Scholar]
- 117.Ferrag F, et al. Homodimerization of IL-2 receptor beta chain is necessary and sufficient to activate Jak2 and downstream signaling pathways. FEBS Lett. 1998;421:32–6. doi: 10.1016/s0014-5793(97)01529-9. [DOI] [PubMed] [Google Scholar]
- 118.Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269:5364–8. [PubMed] [Google Scholar]
- 119.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–51. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 120.Chen X, et al. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–39. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- 121.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–37. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 122.Decker T, Kovarik P. Transcription factor activity of STAT proteins: structural requirements and regulation by phosphorylation and interacting proteins. Cell Mol Life Sci. 1999;55:1535–46. doi: 10.1007/s000180050393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–94. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 124.Franklin RB, Costello LC. Prolactin directly stimulates citrate production and mitochondrial aspartate aminotransferase of prostate epithelial cells. Prostate. 1990;17:13–8. doi: 10.1002/pros.2990170103. [DOI] [PubMed] [Google Scholar]