Abstract
Short Telomere Syndromes (STS) are accelerated aging syndromes often caused by inheritable gene mutations resulting in decreased telomere lengths. As a consequence, organs systems with increased cell turnover such as the skin, bone marrow, lungs, and the gastrointestinal tract are commonly affected. Due to diverse clinical presentations, STS pose a diagnostic challenge, with bone marrow failure and idiopathic pulmonary fibrosis being frequent manifestations; occurring in association with gene mutations involving DKC1 (dyskerin), TERT (telomerase reverse transcriptase) and TERC (telomerase RNA component) amongst others. Inherited STS demonstrate genetic anticipation, occurring at an earlier age with more severe manifestations in the affected progeny. Telomere lengths can be assessed in peripheral blood granulocytes and lymphocytes using a sensitive technique called flow-FISH (fluorescence in-situ hybridization), while mutational analysis can be performed using next generation sequencing assays. In ~40% of patients with shortened telomere lengths, gene mutations cannot be identified either due to the fact that all STS-associated genes have not yet been defined, or due to alternative mechanisms of telomere shortening. Danazol, an anabolic steroid, has been associated with hematological responses in patients with STS and associated bone marrow failure; however, its reported ability to increase telomerase activity and reduce telomere attrition needs further elucidation. Organ transplantation is reserved for patients with end organ failure and is a procedure associated with significant morbidity and mortality. In this review, we summarize the clinical and laboratory characteristics of STS and offer a stepwise approach to diagnose and manage complications in affected patients.
Keywords: Telomere, premature aging, bone marrow failure, idiopathic pulmonary fibrosis, cirrhosis
Introduction
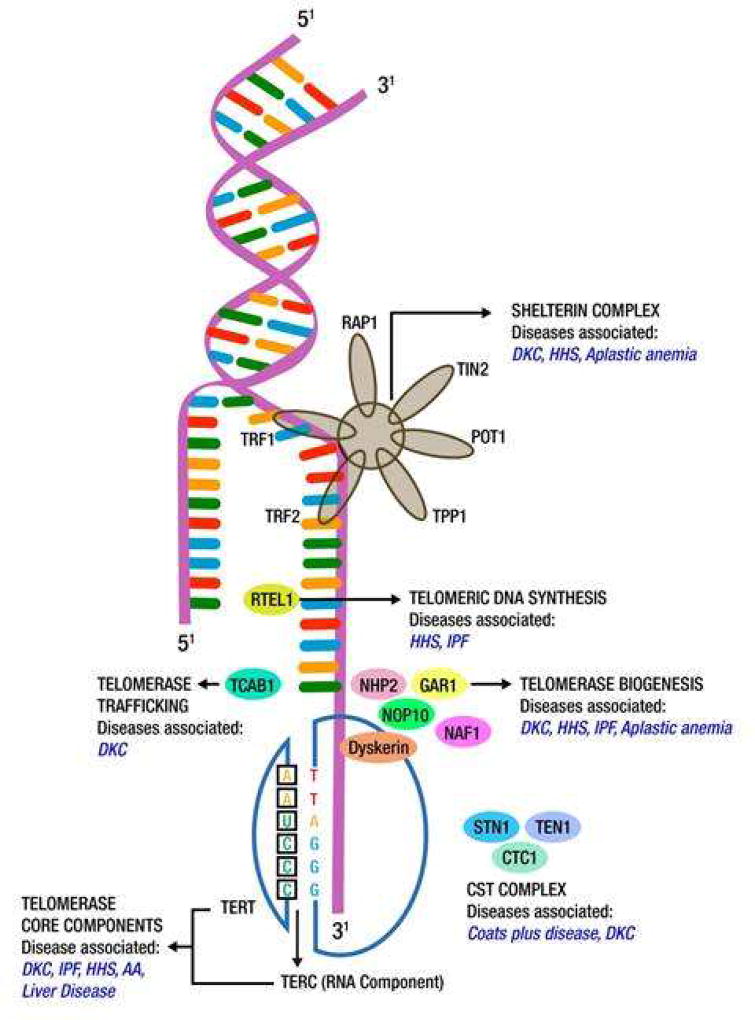
Telomeres are hexanucleotide tandem repeats (TTAGGG) present at the ends of chromosomes, protecting them from gradual degradation in the process of aging.1, 2 Physiologic telomere losses occur over time, contributing to the process of ageing. Once telomeres become critically short, the ends of chromosomes are exposed as double-stranded DNA breaks, resulting in DNA damage response, p53 activation and apoptosis/senescence. This forms the basis for telomere lengths representing the “molecular clock” in human beings. Telomeres are distinguished by a 30–400 nucleotide long overhang of a guanosine-rich strand, also known as the G-strand overhang, which folds back to a double-stranded area, thereby forming a T-loop and a displacement or D- loop. Telomeres are flanked by special regions called the shelterin complex, that regulate telomere lengths and protect them from DNA damage response.1 The shelterin complex is comprised of: telomeric repeat binding factors 1 and 2 (TRF1 and TRF2), protection of telomeres protein 1 (POT1), TRF1-interacting protein 2 (TIN2), repressor activator protein 1 (RAP1) and POT1- interacting protein (TPP1).3
Telomerase is a ribonucleoprotein enzyme complex (DNA polymerase) that builds new telomere sequences onto the ends of chromosomes. It is made up of the telomerase reverse transcriptase (TERT) and its RNA template (TERC). In addition, there are associated proteins such as dyskerin, NHP2 (nuclear protein family A, member 2), NOP10 (nuclear protein family A, member 3), NAF1 (nuclear assembly factor 1) and GAR1 (nuclear protein family A, member 1) that help in telomere assembly, trafficking, recruitment and stability (Figure 1).2, 4
Figure 1.
Constituents of the human telomere complex and associated genetic anomalies (Key: DKC-Dyskeratosis congenita, HHS-Hoyeraal-Hreidarsson syndrome, IPF-Idiopathic pulmonary fibrosis, AA: Aplastic anemia RAP1-repressor activator protein 1, TIN2-TFR-1interacting protein 2, TFR-telomeric repeat binding factor, TPP-POT1-interacting protein, RTEL1-regulator of telomere length 1, TCAB1- Telomerase Cajal body protein1, NHP2- nuclear protein family A, member 2, NOP10-nuclear protein family A, member 3, GAR1-nuclear protein family A, member 1, TERT-telomerase reverse transcriptase, TERC-telomerase RNA component, CTC1-conserved telomere maintenance component 1).
The aforementioned telomere repair process is not foolproof and physiological telomere attrition occurs at an estimated rate of 50–150 base pairs/cell division, resulting in a gradual decrease in median telomere lengths with increasing age.5 Germ line mutations that impact the telomere complex can lead to telomere loss during cell replication, thus shortening the stem-cell pool. Regenerative replicative stresses that occur in patients with bone marrow failure, post chemotherapy or hematopoietic stem cell transplantation (HCT) can enhance telomere shortening, secondary to the increased mitotic activity. Finally reactive oxygen species generated secondary to inflammation, toxins or radiation exposure can cause DNA damage and telomere loss.2
Secondary to advances in genomics, shortened telomere lengths have now been identified to play a critical role in the pathogenesis of a variety of multisystemic disorders, resulting in clinical manifestations such as bone marrow failure, primary immunodeficiency, enterocolitis, idiopathic pulmonary fibrosis (IPF), premature onset emphysema, cryptogenic cirrhosis of the liver, nodular regenerative hyperplasia of the liver (NRH), premature graying of hair, fibrous cartilage dysplasia, osteoporosis and cancer predisposition syndromes (epithelial and hematological malignancies).6–8 In this critical review, we discuss the implications and management strategies for patients with Short Telomere Syndromes (STS).
Clinical features of short telomere syndromes
STS can involve multiple organ systems and are clinically defined by premature loss of progenitor stem cells, thereby impacting the regenerative capacity of involved cells/organs. An important characteristic of telomere diseases is the phenomenon of genetic anticipation; wherein the disease manifestations tend to occur at an earlier age in subsequent generations, with severe manifestations. This is largely due to the fact that offspring inherit, not only the telomere related mutation, but also shortened germ line telomere lengths.8
i) Shortened telomeres and bone marrow failure
Shortened telomeres are commonly associated with bone marrow failure syndromes including aplastic anemia. Amongst these, the classical telomere biology disorder that commonly presents in early childhood is dyskeratosis congenita (DKC). DKC is characterized by the presence of a clinical triad of nail dysplasia, oral leukoplakia and abnormal skin pigmentation, associated with bone marrow failure.2 Associated features can include pulmonary fibrosis, emphysema, cryptogenic liver cirrhosis, lacrimal ductal, esophageal and urethral stenosis, premature graying of hair, avascular necrosis of hips and shoulder, periodontal disease and an increased predisposition towards epithelial and hematological malignancies. Types of malignancies encountered in DKC include head and neck cancers (~70 fold), anogenital squamous cell carcinomas (~50 fold), myelodysplastic syndromes (~500 fold) and acute myeloid leukemia (~70 fold).9 Although DKC is characterized by extremely short telomere lengths, the genetic basis and mode of inheritance is variable. Thus far, several mutant genes have been identified impacting the telomerase or the telomere protein complex, resulting in a DKC phenotype. These include DKC1, TINF2, TERT, TERC, NHP2, NOP10, TCAB1 and RTEL1.8 Hoyeraal Hriedarsson (HH) syndrome is a severe manifestation of DKC, presenting with concomitant cerebellar hypoplasia, usually occurring secondary to mutations involving DKC1 or RTEL1.10 Revesz syndrome consists of a severe DKC phenotype with associated retinal pathology, usually secondary to dominant mutations in TINF2.11 Mutations involving TERT and TERC can be associated with bone marrow failure in adolescents and adults (autosomal dominant), with manifestations being more severe with TERC mutations, potentially due to a greater impact on telomerase activity.12, 13 Telomere related adult onset marrow failure is hard to distinguish from idiopathic aplastic anemia, with many patients presenting with macrocytosis and having similar initial responses to immunosuppressive therapies; however, very often these responses are not durable.
ii) Idiopathic pulmonary fibrosis, emphysema and interstitial pneumonitis
Idiopathic pulmonary fibrosis (IPF) is the most frequent pulmonary manifestation seen in patients with STS (70%) and can occur in the setting of familial IPF (25%) or sporadic IPF (1–3%).6, 14, 15 In addition to IPF, additional pulmonary issues that can be encountered include bronchiolitis obliterans with organizing pneumonia, chronic hypersensitivity pneumonitis, interstitial pneumonitis and emphysema.16 Familial interstitial pneumonia (FIP), a disease entity clinically defined by the diagnosis of an idiopathic interstitial pneumonia (IIP) or IPF in ≥2 relatives of common ancestry;17is characterized by deleterious mutations not only in surfactant production genes such as surfactant protein A2 [SFTPA2], surfactant protein C [SFTPC] and adenosine triphosphate–binding cassette subfamily A member 3 [ABCA3]) among others, but also telomere biology-associated genes such TERT, TERC, RTEL1, TINF2 and PARN.17, 18 Pulmonary fibrosis secondary to telomere dysfunction usually presents in adulthood and is accelerated in patients that smoke. In fact, smokers can present with a mixed restrictive/obstructive pulmonary syndrome with overlapping features of emphysema and IPF.19–21 A diagnosis of IPF is usually made in the clinical context with the help of pulmonary function testing and high resolution computed tomography scans of the chest (inspiratory and expiratory views). In approximately 10% of patients who present with ‘sporadic IPF’, a paternal or maternal relative is likely to be diagnosed with IIP during subsequent years, emphasizing the importance of taking a detailed family history.
iii) Hepatobiliary manifestation of STS
Hepatic manifestations were first noted in approximately 7% of patients with DKC and since then have been identified as critical disease manifestations under the spectrum of STS. These include hepatic parenchymal inflammation, hepatic fibrosis, cryptogenic cirrhosis of the liver, NRH and portal hypertension.14, 22 In a comprehensive study assessing liver pathology findings in patients with STS, recurrent manifestations included; inflammatory and fibrotic components (hepatocyte necrosis and bridging fibrosis) with several cases of non-cirrhotic portal fibrosis (NCPF), cirrhosis, NRH and iron accumulation in the absence of a history of blood transfusions or hemochromatosis gene mutations.22
iv) Gastrointestinal manifestations
Gastrointestinal manifestations occur in roughly 16% of patients with STS, and can be the initial, often fatal presentation in young children.23 Common manifestations include esophageal stenosis, B-cell immunodeficiency with enterocolitis, and a celiac like enteropathy (telomere-mediated stem cell failure).23 The intestinal mucosa in affected patients often displays villous atrophy, apoptosis and anaphase bridging pointing to defects in regeneration in the gastrointestinal epithelium. Gastrointestinal manifestations tend to be most severe in patients with the HH syndrome.
v) Primary immunodeficiency syndromes
Telomere shortening causes early senescence of B and T lymphocytes and a spectrum of immune defects. Immunodeficiency is most severe in children with the HH syndrome, whereas milder defects can be seen in other genetic variants. A unique entity associated with short telomeres is the immunodeficiency, centromeric region stability and facial anomalies type 1 (ICF) syndrome, caused by mutations in DNA methyltransferase 3B gene, thus leading to severe hypomethylation of subtelomeric regions, critically short telomere lengths and increased levels of telomeric-repeat-containing RNA (TERRA).24, 25 Furthermore, telomere-dependent replicative senescence contributes to immune dysregulation in certain patients with common variable immunodeficiency.26 The association between altered immunity and telomere shortening is further highlighted by the fact that telomere attrition is thought to be responsible for diminished natural killer cell function in the elderly.27
Clinical measurement of telomere length
Several methods have been described to measure telomere length, namely fluorescence in-situ hybridization or FISH [Flow FISH and Quantitative (Q)-FISH28–30), quantitative polymerase chain reaction or Q PCR-based techniques31 (including single-cell telomere length measurement32), monochrome multiplex Q PCR (MMQ PCR), optical techniques (surface enhanced Raman scattering33), hybridization protection assay34 and telomere restriction fragment length analysis.35 Although no single technique is ideal for use in all clinical and research scenarios, the telomere restriction fragment length analysis is considered the ‘gold standard’ largely due to the simplicity of its protocol and accuracy, however its widespread use is limited as it requires large amounts of DNA for analysis.32, 36 Despite the low cost, Q PCR-based approaches are limited by their high inter-laboratory variability and technical challenges.36 For Q PCR methodologies, telomeres are amplified using primers that attach to both telomeric C- and G-rich areas with mismatches, which thereby reduce primer-dimer formation, and amplification at telomeric templates only happens at low annealing temperatures. Discrepancy in pipetting volume among telomere and control reactions, and a possible consequent inaccuracy in estimating telomere lengths is a major limitation of the Q PCR technique, thereby limiting its use in routine clinical practice.36
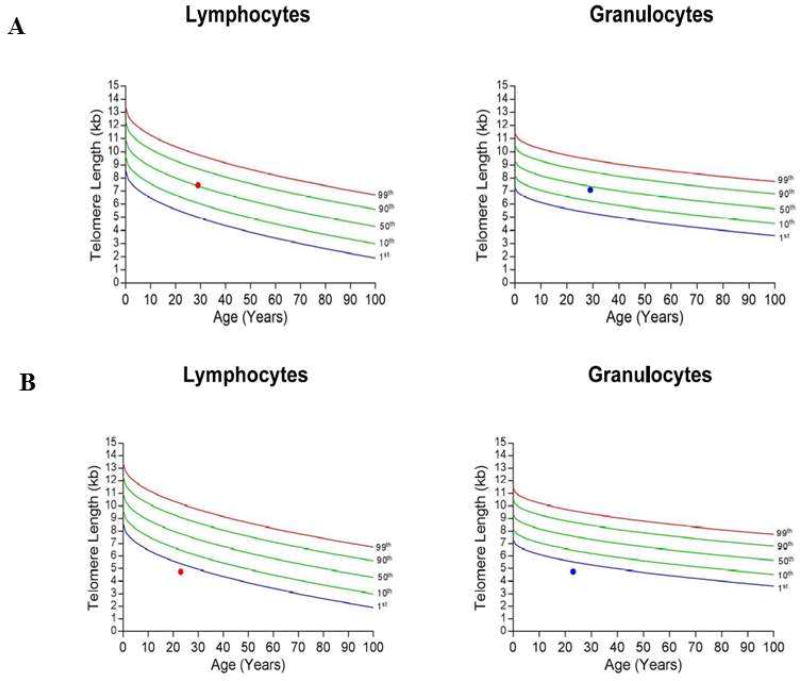
On the other hand, flow-FISH is an extremely accurate technique and can be used for assessing large numbers of samples, making it our preferred testing modality to assess telomere lengths.36, 37 Flow-FISH involves the use of a fluorescent probe which tags on to telomeres and thereby allows flow cytometry-based assessment of telomere fluorescence in distinct cell populations, such as granulocytes and lymphocytes. The ability to assess telomere lengths in different cell types is particularly advantageous in distinguishing malignancy-associated telomere attrition from a true STS. For example, a patient with myelodysplastic syndrome will have shorter age-adjusted telomere lengths in granulocytes and therefore, telomere lengths need to be assessed in lymphocytes to diagnose a true underlying STS. A flow-FISH report provides information on telomere lengths relative to age, which is important as telomere attrition is part of the normal ageing process (Figure 2). Figure 2A describes a control with telomere lengths in granulocytes and lymphocytes at the 50th percentile, which is interpreted as a telomere length value similar to ~50 per cent of the normal population for the same age range. Figure 2B describes a patient with telomere lengths <1st percentile in granulocytes and lymphocytes, highly suggestive of a STS. The main disadvantages of the flow-FISH technique is the requirement of large viable cell populations and expertise for transporting, processing and analyzing clinical samples.32 Flow-FISH testing has had a significant impact on the management of bone marrow failure syndromes, with a recent study demonstrating that approximately 24% of tested patients had shortened telomere lengths (Alder JK et al. BioRxiv reported/unpublished manuscript), influencing treatment and transplant decision making.38
Figure 2.
Figure showing flow-FISH reports for a normal and suspected STS patient. 2A shows the report for a normal patient with telomere lengths in both lymphocytes and granulocytes around 50th centile. 2B shows the report for a patient with telomere lengths less than 1st centile, highly suggestive for a STS (Key: FISH-fluorescence in-situ hybridization, STS: short telomere syndrome).
Telomere mutation analysis
Several genes have been associated with STS and can be sub-categorized based on their normal physiological role in telomere biology. Inheritance modes are usually X-linked recessive, autosomal recessive and autosomal dominant. Genes associated with telomerase biogenesis include DKC1.39 Telomerase core component genes include hTERT, TERC, while TCAB1 is involved in telomerase trafficking. TINF2 and ACD (encoding TPP1) are part of the shelterin complex.2, 6, 7 In DKC, an X-linked recessive gene called ‘dyskerin’ involved in telomerase biogenesis, is the most frequent causative genomic aberration.39 Other DKC-associated mutations include hTERT40, TERC41 and TINF211 which are inherited in an autosomal dominant pattern, while the autosomal recessive mutations include NOP1042, NHP243 and TCAB144. A comprehensive list of STS-associated mutations, along with modes of inheritance is detailed in Table 1.
Table 1.
Table summarizing short telomere syndromes, associated genes and their respective modes of inheritance.
| Disease | Inheritance | Associated genes | Reference |
|---|---|---|---|
|
| |||
| Bone marrow failure associated with short telomere syndromes | |||
|
| |||
| a Dyskeratosis congenita | X- linked recessive (more common) | Telomerase biogenesis: | |
| DKC1 | Heiss NS et al. Nat Gent 1998.39 | ||
|
| |||
| Autosomal dominant | Telomerase core components: | ||
| TERT | Vulliamy T et al. Nature 2001.63 | ||
| TERC | Armanios M et al. PNAS 2005.40 | ||
| Vulliamy TJ et al. Blood 2006.41 | |||
| Shelterin component: | |||
| TIN2 | Savage SA et al. Am J Hum Genet 2008.11 | ||
|
| |||
| Autosomal recessive | Telomerase biogenesis: | ||
| NOP10 (NOLA3) | Walne AJ et al. Hum Mol Genet 200742 | ||
| NHP2 | Vulliamy T et al. Proc Natl Acad 2008.43 | ||
| Telomerase trafficking: | |||
| TCAB1 | Zhong F et al. Genes Dev 2011.44 | ||
|
| |||
| b Hoyeraal-Hreidarsson syndrome | X-linked recessive | Telomerase biogenesis: | |
| DKC1 | Glousker et al. Br J Hematol 2015.64 | ||
| Autosomal dominant | Shelterin components: | Kocak H et al. Gene&Dev 2014.65 | |
| TIN2 | |||
|
| |||
| Autosomal recessive | Telomerase core components: | ||
| TERT | |||
| Shelterin components: | |||
| ACD (encoding TPP1), | |||
| Telomeric DNA synthesis: | |||
| RTEL1 | |||
|
| |||
| Revesz syndrome | Autosomal dominant | Shelterin component: | |
| TIN2 | Savage SA et al. Am J Human Genet 2008.11 | ||
|
| |||
| Cerebroretinal microangiopathy with calcifications and cysts (Coats plus disease) | Autosomal recessive | Telomeric DNA synthesis: | |
| CTC1, STN1 (part of the CTC complex) | Anderson et al. Nat Gent 2012.66 | ||
| Simon AJ et al. JEM 2016.67 | |||
|
| |||
| Aplastic anemia | Autosomal dominant | Telomerase core components: | |
| TERT | Guo Y et al. Blood 2014.56 | ||
| TERC | Martinez P et al. JCB 2017.1 | ||
|
|
|||
| X-linked recessive Autosomal recessive | Telomerase biogenesis: | Joksic I et al. Genome Integr 2012.68 | |
| DKC1 | |||
| NOP10 | |||
| NHP2 | |||
|
| |||
| Fanconi anemia | Autosomal dominant | Shelterin components: | |
| ACD | |||
|
| |||
| Autosomal recessive | FANCD2 | ||
|
| |||
| Pulmonary short telomere syndromes | |||
|
| |||
| Idiopathic pulmonary fibrosis; or familial lung fibrosis or fibrotic idiopathic interstitial pneumonias. | Autosomal dominant | Telomerase core components: | |
| TERC | Armanios MY et al. NEJM 2007.69 | ||
| TERT | Fingerlin TE et al. Nat Gen 2013.18 | ||
| Telomerase biogenesis: | |||
| NAF1 | Stanley SE et al. Sci Transl Med 2016.4 | ||
|
| |||
| GI short telomere syndromes | |||
|
| |||
| Cryptogenic cirrhosis Or Nodular regenerative hyperplasia | Autosomal dominant | Telomerase core components: | Calado RT et al. Plos One 2009.22 |
| TERT | |||
| TERC | |||
|
| |||
| Others | |||
|
| |||
| Rothmund-Thomson syndrome | Autosomal recessive | c RECQL4 | Ghosh et al. J Biol Chem 2012.70 |
|
| |||
| ICF (Immunodeficiency, centromeric region instability and facial anomalies type 1) | Autosomal recessive | dDNMT3B | Xu GL et al. Nature 1999.24 |
| Yehezkel et al. Frontiers in Oncology 2013.25 | |||
|
| |||
| Facioscapulohumeral muscular dystrophy | Autosomal dominant | DUX4 | Stadler G et al. Nat Struct Mol Biol 2013.71 |
~40% patients with DKC still have un-identified mutations;
Considered a severe variant of DKC;
Plays a role in telomere maintenance;
Causes abnormally short telomeres, hypomethylation of subtelomeric regions and elevated levels of abnormal telomeric transcripts known as TERRA.
Several targeted exome or next generation sequencing (NGS) panels are currently available, both in commercial and research settings to detect causative mutations in patients with clinical suspicion for STS. In our experience, mutations are identified in ~40% of clinically suspected cases, suggesting that there are several yet to be characterized genetic and epigenetic mechanisms of telomere length regulation.
Therapeutic options for patients with STS
Organ transplantation remains the mainstay for treatment of organ failure associated with STS. Allogeneic HCT for DKC and STS-related bone marrow failure syndromes, lung transplantation for IPF and/or emphysema and liver transplantation for end-stage cryptogenic cirrhosis of the liver have been performed with significant morbidity and mortality.45–48 For patients with STS-associated bone marrow failure syndromes, we use reducing intensity conditioning regimens for allogeneic HCT, so as to minimize pulmonary toxicity associated with exposure to ionizing radiation and high doses of cytotoxic chemotherapy.49 Details on modalities and outcomes of organ transplantation for STS are outside the scope of this review.
For several years, androgens have been used with success in patients with aplastic anemia with reported hematological response rates of ~ 50%.50–53 In vitro and animal model studies have shown that androgens upregulate telomerase gene expression, thus slowing the rate of telomere attrition and enhancing cell regeneration.54–56 In 2016, Townsley et al. reported findings from a phase 1/2 clinical trial which included 27 patients with age-adjusted telomere lengths <1st percentile or a known STS mutation with clinical manifestations such as cytopenias, pulmonary fibrosis or both, treated with danazol at an oral dose of 800 mg, administered twice daily. Telomere length attrition was reduced in 12/27 (44%) patients after 12 months of use, with adverse effects including hepatic transaminitis (41%), muscle cramps (33%), edema (26%) and lipid (26%) abnormalities.57 At 3 months, 19/24 (79%) showed a hematologic response, while by 24 months, 10/12 (83%) had an improvement in their blood counts and red blood cell transfusion independence was documented in 12 of 13 (92%) transfusion-dependent patients; while a mean increase in neutrophil counts by 300 per mm3 and in platelet counts by 14,250 per mm3, was documented in responders. At last follow up (~ 2 years), 10 of 12 (83%) evaluable patients had an ongoing hematological response.57 Danazol therapy also resulted in stability in pulmonary functions in patients with pulmonary fibrosis. Liver fibrosis was objectively measured using ultrasound-mediated transient elastometry (FibroScan) at baseline and at 2 years in 4/6 patients with an established diagnosis of cirrhosis; with 3 demonstrating a substantial reduction in the degree and extent of liver fibrosis.57 In this study, the authors used a Q PCR technique to measure telomere lengths and found that danazol had the ability to reduce telomere length attrition, presumably by increasing telomerase activity. This finding remains unsubstantiated and is further fraught with technical inaccuracies associated with the Q PCR technique. In a prior study, Khincha P et al. found that telomere lengths (measured at baseline and at a median of 2.6 years on therapy), when assessed by the more sensitive flow-FISH technique in 4 androgen responsive patients with DKC (oxymetholone- 3, fluoxymesterone-1), were consistently the same or declined after androgen exposure.58 Hence, more evidence is needed before the routine use of danazol can be recommended to reduce telomere attrition rates in clinically affected and asymptomatic patients with STS. Danazol should be used with caution in older men due to the risk of potentially worsening prostatic hypertrophy and stimulating the growth of preexisting prostatic adenocarcinoma. In addition, longer term follow up with danazol is much needed in order to mitigate concerns about potentially stimulating malignant clonal hematopoiesis in patients with bone marrow failure.
Retrospective studies in DKC have also shown clinical benefit with other anabolic steroids such as oxymetholone, fluoxymesterone and nandrolone.58 Exciting new therapeutic options such as gene therapy are currently being studied in animal models and are outlined in table 2.59, 60 TA-65, is a small molecule that has been shown to activate telomerase activity in mice, thereby effectively increasing telomere lengths.59 Adeno-associated virus (AAV) 9 gene therapy vectors-induced telomerase activation has also shown efficacy in two independent mouse models of aplastic anemia.61 These studies need additional safety and efficacy measures before they can transition to in human trials, but are exciting prospects for affected patients.
Table 2.
Table summarizing investigational treatment strategies for short telomere syndromes.
| Type of study |
Inclusion criteria |
Drug | Dose | No. of pts |
Response rate |
Adverse effects |
Reference |
|---|---|---|---|---|---|---|---|
| Phase 1/2 clinical trial | Age-adjusted telomere length ≤ 1st percentile, or known telomere associated mutation, or both, PLUS either cytopenias, or pulmonary fibrosis, or both. | Danazol | 800 mg/day | 27 | Primary endpoint: Reduction in telomere attrition length; 12/27 (44%) at 12 months. | 41% Transaminitis, 33% muscle cramps, 26% edema, 26% lipid abnormalities. | Townsley et al. NEJM 2016.57 |
| Retrospective | DKC | Oxymetholone (14), fluoxyme sterone (1), nandrolone (1) | For oxymetholone: 0.5–1 mg/kg (starting dose); 0.5–2.7 mg/kg/day for maintenance dose | 16 | 70% patients achieved a hematologic response | Hyperlipid emia (Data not available on all patients) | Khincha PP et al. Br J Haematol 2014.58 |
| Pre-clinical | Terc+/− mice, Terc−/− mice as controls | TA-65 | Not established | N/A | N/A | N/A | de Jesus BB et al. Aging Cell 2011.59 |
| Pre-clinical | Mouse models of aplastic anemia | Tert gene therapy (AAV9-Tert delivery) | Not established | N/A | N/A | N/A | Bär C et al. F1000Re search 2016.60 |
Our approach for management of telomere disorders
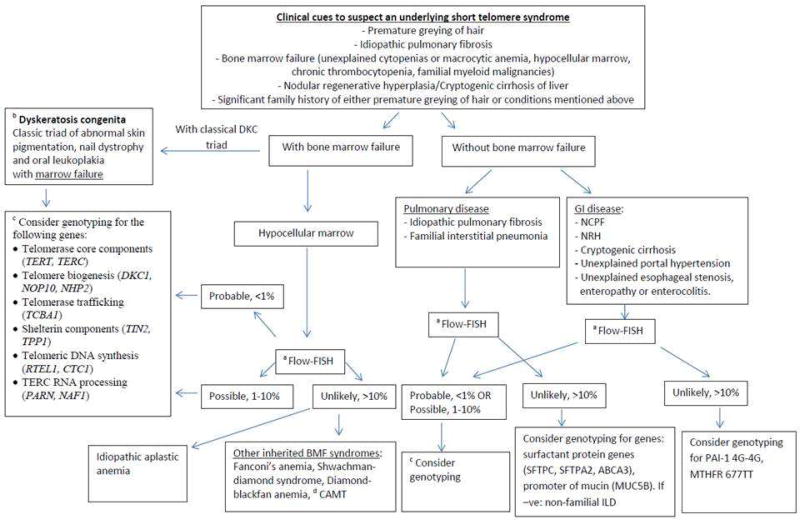
At the Mayo Clinic, with the help of the Center for Individualized Medicine and the Division of Hematology, we have established an Inherited Bone Marrow Failure Precision Genomics Clinic (ClinicalTrials.gov Identifier: NCT02958462), to aid with the diagnosis and management of inherited bone marrow failure syndromes, including STS. We suspect STS when patients present with, or have a strong family history of premature greying of hair (defined by consensus as onset of graying of hair before age 30 years), IPF, premature emphysema or IIP, unexplained cytopenias or bone marrow failure, NRH or cryptogenic cirrhosis of the liver, along with other stigmata of STS such as esophageal, lacrimal ductal and urethral stenosis. We obtain a detailed personal and family history along with a thorough physical examination, followed by genetic counsellor-assisted pedigree charting and counselling. We then assess telomere lengths in these individuals by using the peripheral blood flow-FISH assay. If telomere lengths are <1st percentile in granulocytes and lymphocytes, then probability of STS is high and we proceed with mutational analysis by NGS [DKC1, NOP10 (NOLA3), NHP2 (NOLA2), RTEL1, TERC (hTR), TERT, WRAP53 (WDR79), TINF2, TCAB1 and NAF1]. Patients with telomere lengths >10th percentile are unlikely to have an STS, while those with telomere lengths in the 1–10th percentile range could possibly have an STS. For these patients with indeterminate telomere lengths, we proceed with mutational analysis based on the strength of clinical suspicion (pretest probability). For patients where NGS testing returns negative, we do have a research protocol (Mayo Clinic IRB# 16-004173) that allows us to proceed with advanced genomic techniques such as whole exome sequencing and RNA sequencing after obtaining a written informed consent. In patients with cytopenias or bone marrow failure, we recommend a bone marrow aspiration and biopsy with cytogenetic studies and myeloid relevant NGS assays to assess for clonal evolution, if indicated. We work closely with our colleagues in pulmonary medicine and gastroenterology to assess and screen for additional organ involvement. We obtain a baseline set of pulmonary function tests and a baseline high resolution computed tomography scan of the chest with inspiratory and expiratory views. We assess the hepatobiliary tree with a base line ultrasound and Doppler study of the abdomen and the portal venous system. If there is suspicion for liver involvement, additional studies such as magnetic resonance elastography and liver biopsies are undertaken. Annual head and neck, and anogenital screening for cancers is recommended, and in age-eligible patients without significant immunodeficiency, we strongly recommend the human papilloma virus vaccine.62 Special focus is placed on bone health and risk for avascular necrosis of the hips and shoulder joints. Appropriate subspecialty and organ transplant referrals are made in a timely manner, and family members are screened after extensive counselling. Our schematic approach to manage a suspected STS is summarized in Figure 3.
Figure 3.
Figure summarizing various disorders associated with telomere shortening and steps in management (Key: DKC-dyskeratosis congenita, FISH-fluorescence in-situ hybridization, TERT-telomerase reverse transcriptase, TERC-telomerase RNA component, NOP10- nuclear protein family A, member 3, NHP2- nuclear protein family A, member 2, TCAB1- Telomerase Cajal body protein 1, TIN2- TFR-1 interacting protein 2, TPP1-POT1-interacting protein, RTEL1-regulator of telomere length 1, CTC1- Conserved telomere maintenance component 1, PARN-Poly(A)-specific ribonuclease, NAF1-Nuclear Assembly Factor 1 Ribonucleoprotein, BMF-bone marrow failure, CAMT- Congenital amegakaryocytic thrombocytopenia, NCPF- non-cirrhotic portal fibrosis, NRH- nodular generative hyperplasia, SFTPC-surfactant protein C, SFTPA2-surfactant protein A2, ABCA3-ATP binding cassette subfamily A member 3, MUC5B-Mucin 5B, PAI-1-plasminogen activator inhibitor 1, MTHFR-methylenetetrahydrofolate reductase).
Conclusion
STS occur secondary to premature shortening of telomere lengths, resulting in multisystemic disease, often associated with significant morbidity and mortality. Telomere shortening results in accelerated ageing of the stem cell pool with predominant manifestations seen in organs with high cell turn over. The classical STS often seen in pediatric patients is DKC, characterized by a lacy skin rash, nail and hair dystrophy, oral leukoplakia and bone marrow failure; while milder versions secondary to different gene mutations can occur at all ages. Prominent manifestation of STS include bone marrow failure, IPF, premature emphysema, cryptogenic cirrhosis of the liver, NRH with portal hypertension, premature greying of hair, luminal stenosis (esophageal, lacrimal ductal and urethral) and immune deregulation. Telomere lengths can be assessed using the flow-FISH methodology, whereas gene mutations, seen in ~40% of affected patients, can be identified using NGS techniques. Organ transplantation is reserved for patients with end organ dysfunction, while androgen therapy with drugs such as danazol has been associated with clinical benefit. Advances in understanding telomere biology and the use of gene therapy to augment impaired telomere functioning are exciting future prospects.
Highligts.
Short telomere syndromes are multisystem disorders with widespread clinical manifestations.
Organs with high cell turn-over such as the bone marrow, liver, lungs and immune system are commonly affected.
Key clinical cues to suspect short telomeres in a patient are: personal of family history of premature greying of hair (at age<30 years), unexplained cytopenias, idiopathic pulmonary fibrosis, cryptogenic cirrhosis.
Flow-FISH is the initial screening test, followed by genetic sequencing.
Treatment requires a multidisciplinary approach.
Acknowledgments
The authors acknowledge the time, support and constructive feedback from Dr. Mary Y. Armanios, MD.
This work has received grant supports from ‘The Gerstner Family Career Development Award’, Mayo Clinic Center for Individualized Medicine, and CTSA (Grant# KL2 TR000136) from the National Center for Advancing Translational Science. Its contents do not necessarily represent the official views of the U.S NIH and contents are the role responsibility of the authors.
Abbreviation list
- STS
Short telomere syndromes
- DKC1 (gene)
Dyskerin
- TERT
Telomerase reverse transcriptase
- TERC
Telomerase RNA component
- FISH
Fluorescence in-situ hybridization
- DNA
deoxy ribonucleic acid
- TRF1 and 2
telomeric repeat binding factors 1 and 2
- TIN2
TRF1-interacting protein 2
- POT1
protection of telomeres protein 1
- TPP1
TIN2, POT1- interacting protein (TPP1)
- RAP1
repressor activator protein 1
- NHP2
nuclear protein family A, member 2
- NOP10
nuclear protein family A, member 3
- NAF1
nuclear assembly factor 1
- GAR1
nuclear protein family A, member 1
- HCT
Hematopoietic stem cell transplantation
- IPF
idiopathic pulmonary fibrosis
- NRH
Nodular regenerative hyperplasia
- DKC
Dyskeratosis congenita
- HH
Hoyeraal Hreidarsson
- IPF
Idiopathic pulmonary fibrosis
- FIP
Familial interstitial pneumonia
- IIP
Idiopathic interstitial pneumonia
- SFTPC
Surfactant protein C
- SFTPA2
Surfactant protein A2
- ABCA
adenosine triphosphate-binding cassette subfamily A member 3
- NCPF
Non-cirrhotic portal fibrosis
- ICF
Immunodeficiency, centromeric region stability and facial anomalies type 1
- TERRA
telomeric-repeat-containing RNA
- Q PCR
quantitative polymerase chain reaction
- MMQ PCR
monochrome multiplex PCR
- NGS
next generation sequencing
- AAV
adeno-associated virus
- MUC5B
Mucin 5B
- PAI-1
Plasminogen activator inhibitor 1
- MTHFR
methylenetetrahydrofolate reductase
- TCAB1
Telomerase Cajal body protein 1
- ACD
adrenocortical dysplasia
- RTEL1
regulator of telomere length 1
- CTC1
conserved telomere maintenance component 1
- FANCD2
Fanconi Anemia Complementation Group D2
- RECQL4
Adenosine triphosphate-dependent DNA helicase Q4
- DNMT3B
DNA methyltransferase 3B
- DUX4
Double homeobox 4
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no conflict of interest relevant to the current manuscript.
References
- 1.Martinez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol. 2017;216:875–887. doi: 10.1083/jcb.201610111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775–2783. doi: 10.1182/blood-2014-05-526285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes. Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 4.Stanley SE, Gable DL, Wagner CL, et al. Loss-of-function mutations in the RNA biogenesis factor <em>NAF1</em> predispose to pulmonary fibrosis–emphysema. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aaf7837. 351ra107-351ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JC, Warner JK, Erdmann N, Lansdorp PM, Harrington L, Dick JE. Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc Natl Acad Sci U S A. 2005;102:14398–14403. doi: 10.1073/pnas.0504161102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armanios M. Telomerase mutations and the pulmonary fibrosis-bone marrow failure syndrome complex. N Engl J. Med. 2012;367:384. doi: 10.1056/NEJMc1206730. author reply 384. [DOI] [PubMed] [Google Scholar]
- 7.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat. Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight SW, Heiss NS, Vulliamy TJ, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 11.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirwan M, Beswick R, Walne AJ, et al. Dyskeratosis congenita and the DNA damage response. Br J Haematol. 2011;153:634–643. doi: 10.1111/j.1365-2141.2011.08679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vulliamy TJ, Kirwan MJ, Beswick R, et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PLoS. One. 2011;6:e24383. doi: 10.1371/journal.pone.0024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calado RT, Young NS. Telomere diseases. N Engl J. Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alder JK, Cogan JD, Brown AF, et al. Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS genetics. 2011;7:e1001352. doi: 10.1371/journal.pgen.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz de Leon A, Cronkhite JT, Yilmaz C, et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140:753–763. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kropski JA, Young LR, Cogan JD, et al. Genetic Evaluation and Testing of Patients and Families with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care. Med. 2017;195:1423–1428. doi: 10.1164/rccm.201609-1820PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingerlin TE, Murphy E, Zhang W, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanley SE, Chen JJ, Podlevsky JD, et al. Telomerase mutations in smokers with severe emphysema. The Journal of clinical investigation. 2015;125:563–570. doi: 10.1172/JCI78554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care. Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz de Leon A, Cronkhite JT, Katzenstein AL, et al. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS. One. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calado RT, Regal JA, Kleiner DE, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS. One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonassaint NL, Guo N, Califano JA, Montgomery EA, Armanios M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell. 2013;12:319–323. doi: 10.1111/acel.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu GL, Bestor TH, Bourc'his D, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 25.Yehezkel S, Segev Y, Viegas-Pequignot E, Skorecki K, Selig S. Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions. Hum Mol Genet. 2008;17:2776–2789. doi: 10.1093/hmg/ddn177. [DOI] [PubMed] [Google Scholar]
- 26.Visentini M, Cagliuso M, Conti V, et al. Telomere-dependent replicative senescence of B and T cells from patients with type 1a common variable immunodeficiency. European journal of immunology. 2011;41:854–862. doi: 10.1002/eji.201040862. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. Telomere length in human natural killer cell subsets. Annals of the New York Academy of Sciences. 2007;1106:240–252. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 28.Poon SS, Martens UM, Ward RK, Lansdorp PM. Telomere length measurements using digital fluorescence microscopy. Cytometry. 1999;36:267–278. doi: 10.1002/(sici)1097-0320(19990801)36:4<267::aid-cyto1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.O'Sullivan JN, Finley JC, Risques RA, et al. Telomere length assessment in tissue sections by quantitative FISH: image analysis algorithms. Cytometry. Part A : the journal of the International Society for Analytical Cytology. 2004;58:120–131. doi: 10.1002/cyto.a.20006. [DOI] [PubMed] [Google Scholar]
- 30.Cukusic Kalajzic A, Vidacek NS, Huzak M, Ivankovic M, Rubelj I. Telomere Q-PNAFISH--reliable results from stochastic signals. PLoS. One. 2014;9:e92559. doi: 10.1371/journal.pone.0092559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Callaghan N, Dhillon V, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. BioTechniques. 2008;44:807–809. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Pan X, Kalmbach K, et al. Robust measurement of telomere length in single cells. Proc Natl Acad Sci U S A. 2013;110:E1906–1912. doi: 10.1073/pnas.1306639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong S, Wang Z, Chen H, Cui Y. Assessing telomere length using surface enhanced Raman scattering. Scientific reports. 2014;4:6977. doi: 10.1038/srep06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura Y, Hirose M, Matsuo H, Tsuyama N, Kamisango K, Ide T. Simple, rapid, quantitative, and sensitive detection of telomere repeats in cell lysate by a hybridization protection assay. Clinical chemistry. 1999;45:1718–1724. [PubMed] [Google Scholar]
- 35.Kimura M, Stone RC, Hunt SC, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nature protocols. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 36.Aubert G, Hills M, Lansdorp PM. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat. Res. 2012;730:59–67. doi: 10.1016/j.mrfmmm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH) Nature protocols. 2006;1:2365–2376. doi: 10.1038/nprot.2006.263. [DOI] [PubMed] [Google Scholar]
- 38.Alder JK, Hanumanthu VS, Strong MA, et al. Diagnostic utility of telomere length measurement in a hospital setting. bioRxiv. 2017 [Google Scholar]
- 39.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 40.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 42.Walne AJ, Vulliamy T, Marrone A, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vulliamy T, Beswick R, Kirwan M, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhong F, Savage SA, Shkreli M, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes. Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Blood. 2017;130:2257–2264. doi: 10.1182/blood-2017-05-781799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silhan LL, Shah PD, Chambers DC, et al. Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. The European respiratory journal. 2014;44:178–187. doi: 10.1183/09031936.00060014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donati B, Valenti L. Telomeres, NAFLD and Chronic Liver Disease. International journal of molecular sciences. 2016;17:383. doi: 10.3390/ijms17030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marmur J, Bergquist A, Stal P. Liver transplantation of patients with cryptogenic cirrhosis: clinical characteristics and outcome. Scandinavian journal of gastroenterology. 2010;45:60–69. doi: 10.3109/00365520903384742. [DOI] [PubMed] [Google Scholar]
- 49.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone marrow transplantation. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camitta BM, Thomas ED, Nathan DG, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–514. [PubMed] [Google Scholar]
- 51.Najean Y. Long-term follow-up in patients with aplastic anemia. A study of 137 androgen-treated patients surviving more than two years. Joint Group for the Study of Aplastic and Refractory Anemias. The American journal of medicine. 1981;71:543–551. doi: 10.1016/0002-9343(81)90204-7. [DOI] [PubMed] [Google Scholar]
- 52.Jaime-Perez JC, Colunga-Pedraza PR, Gomez-Ramirez CD, et al. Danazol as first-line therapy for aplastic anemia. Annals of hematology. 2011;90:523–527. doi: 10.1007/s00277-011-1163-x. [DOI] [PubMed] [Google Scholar]
- 53.Facon T, Walter MP, Fenaux P, et al. Treatment of severe aplastic anemia with antilymphocyte globulin and androgens: a report on 33 patients. Annals of hematology. 1991;63:89–93. doi: 10.1007/BF01707279. [DOI] [PubMed] [Google Scholar]
- 54.Bar C, Huber N, Beier F, Blasco MA. Therapeutic effect of androgen therapy in a mouse model of aplastic anemia produced by short telomeres. Haematologica. 2015;100:1267–1274. doi: 10.3324/haematol.2015.129239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayne S, Liu JP. Hormones and growth factors regulate telomerase activity in ageing and cancer. Molecular and cellular endocrinology. 2005;240:11–22. doi: 10.1016/j.mce.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Guo Y, Kartawinata M, Li J, et al. Inherited bone marrow failure associated with germline mutation of ACD, the gene encoding telomere protein TPP1. Blood. 2014;124:2767–2774. doi: 10.1182/blood-2014-08-596445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsley DM, Dumitriu B, Liu D, et al. Danazol Treatment for Telomere Diseases. N Engl J. Med. 2016;374:1922–1931. doi: 10.1056/NEJMoa1515319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khincha PP, Wentzensen IM, Giri N, Alter BP, Savage SA. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol. 2014;165:349–357. doi: 10.1111/bjh.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernardes de Jesus B, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bar C, Blasco MA. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Res. 2016;5 doi: 10.12688/f1000research.7020.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar C, Povedano JM, Serrano R, et al. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood. 2016;127:1770–1779. doi: 10.1182/blood-2015-08-667485. [DOI] [PubMed] [Google Scholar]
- 62.Alter BP, Giri N, Pan Y, Savage SA, Pinto LA. Antibody response to human papillomavirus vaccine in subjects with inherited bone marrow failure syndromes. Vaccine. 2014;32:1169–1173. doi: 10.1016/j.vaccine.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 64.Glousker G, Touzot F, Revy P, Tzfati Y, Savage SA. Unraveling the pathogenesis of Hoyeraal-Hreidarsson syndrome, a complex telomere biology disorder. Br J Haematol. 2015;170:457–471. doi: 10.1111/bjh.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kocak H, Ballew BJ, Bisht K, et al. Hoyeraal-Hreidarsson syndrome caused by a germline mutation in the TEL patch of the telomere protein TPP1. Genes. Dev. 2014;28:2090–2102. doi: 10.1101/gad.248567.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson BH, Kasher PR, Mayer J, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nat Genet. 2012;44:338–342. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- 67.Simon AJ, Lev A, Zhang Y, et al. Mutations in STN1 cause Coats plus syndrome and are associated with genomic and telomere defects. J. Exp Med. 2016;213:1429–1440. doi: 10.1084/jem.20151618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joksic I, Vujic D, Guc-Scekic M, et al. Dysfunctional telomeres in primary cells from Fanconi anemia FANCD2 patients. Genome Integr. 2012;3:6. doi: 10.1186/2041-9414-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 70.Ghosh AK, Rossi ML, Singh DK, et al. RECQL4, the protein mutated in Rothmund-Thomson syndrome, functions in telomere maintenance. J Biol Chem. 2012;287:196–209. doi: 10.1074/jbc.M111.295063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadler G, Rahimov F, King OD, et al. Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat Struct Mol Biol. 2013;20:671–678. doi: 10.1038/nsmb.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]