Abstract
Background & Aims
There is an urgent need for safe treatments for irritable bowel syndrome (IBS) that relieve treatment-refractory symptoms and their societal and economic burden. Cognitive behavior therapy (CBT) is an effective treatment that has not been broadly adopted into routine clinical practice. We performed a randomized controlled trial to assess clinical responses to home-based CBT compared with clinic-based CBT and patient education.
Methods
We performed a prospective study of 436 patients with IBS, based on Rome III criteria, at 2 tertiary centers from August 23, 2010 through October 21, 2016. Subjects (41.4±14.8 y old; 80% female) were randomly assigned groups that received: standard CBT (S-CBT, n=146, comprising 10 weekly, 60-min sessions that emphasized the provision of information about brain–gut interactions; self-monitoring of symptoms, their triggers, and consequences; muscle relaxation; worry control; flexible problem solving; and relapse prevention training), or 4 sessions of primarily home-based CBT requiring minimal therapist contact (MC-CBT, n=145), in which patients received home-study materials covering same procedures as S-CBT), or 4 sessions of IBS education (EDU, n=145) that provided support and information about IBS and the role of lifestyle factors such as stress, diet, exercise. The primary outcome was global improvement of IBS symptoms, based on the IBS-version of the Clinical Global Impressions-Improvement Scale. Ratings were performed by patients and board-certified gastroenterologists blinded to treatment allocation. Efficacy data were collected 2 weeks, 3 months, and 6 months after treatment completion.
Results
A higher proportion of patients receiving MC-CBT reported moderate to substantial improvement in gastrointestinal symptoms 2 weeks after treatment (61.0% based on ratings by patients and 55.7% based on ratings by gastroenterologists) than those receiving EDU (43.5% based on ratings patients and 40.4% based on ratings by gastroenterologists) (P<.05). Gastrointestinal symptom improvement, rated by gastroenterologists, 6 months after the end of treatment also differed significantly between the MC-CBT (58.4%) and EDU groups (44.8%) (P= .05). Formal equivalence testing applied across multiple contrasts indicated that MC-CBT is at least as effective as S-CBT in improving IBS symptoms. Patients tended to be more satisfied with CBT vs EDU (P<.05) based on immediate post-treatment responses to the client satisfaction questionnaire. Symptom improvement was not significantly related to concomitant use of medications.
Conclusions
In a randomized controlled trial, we found that a primarily home-based version of CBT produced significant and long-term gastrointestinal symptom improvement for patients with IBS compared to education. Clinicaltrials.gov no. clinicaltrials.gov/ct2/show/NCT00738920
Keywords: functional gastrointestinal disorder, disease management, value-based healthcare, brain-gut interactions
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder that affects up to 15% percent of adults worldwide1. While only 10% of IBS sufferers seek medical attention2, it is one of the commonest diseases gastroenterologists and primary care physicians treat. There is neither a reliable biomarker nor a uniformly effective medical treatment for the full range of IBS symptoms (abdominal pain/discomfort, constipation and/or diarrhea). The development of effective pharmacotherapies has been impeded by withdrawal of several FDA-approved IBS drugs due to safety concerns. There is an urgent need for effective treatments that relieve IBS symptoms and their societal and economic burden estimated at $US28 billion annually3.
Various practice guidelines support the efficacy of a cognitive behavior therapy (CBT)4–6, a specific psychological intervention that targets putative factors maintaining IBS. Despite comparatively strong levels of empirical support of clinic-based CBT7, only a small fraction of people receive it in accordance with practice guidelines. Barriers to adoption include cost, limited therapist availability beyond select metropolitan areas, stigma, and logistical challenges such as transportation and time. In a value-based healthcare environment, there is a demand for treatments that retain the efficacy profile of “gold standard” therapies but are more efficient to implement and disseminate. One strategy for achieving this goal is decreasing therapist contact time through the use of primarily self-administered or “home-based” treatments8.
The immediate and sustained efficacy of a primarily home-based CBT was investigated in the context of a multicenter randomized trial addressing methodological shortcomings (e.g., small-scale studies from single investigative teams, inadequate blinding) of prior trials. Patients with moderate-to-severe IBS symptoms were assigned to clinic-based CBT (Standard-CBT), a home-based version of CBT requiring minimal therapist contact (Minimal Contact-CBT), or an active comparator (EDU) that emphasized IBS education but excluded CBT techniques. The aim was to assess clinical response of CBT-treated patients 2 weeks, 3 months, and 6 months after a 10-week treatment phase. Based on our pilot work8, our primary hypothesis was that Minimal -Contact-CBT would deliver a comparable clinical response to Standard-CBT and superior response to IBS Education on a primary endpoint of global symptom improvement featured in previous NIH and industry-funded FGID trials.
METHODS
Study Design and Participants
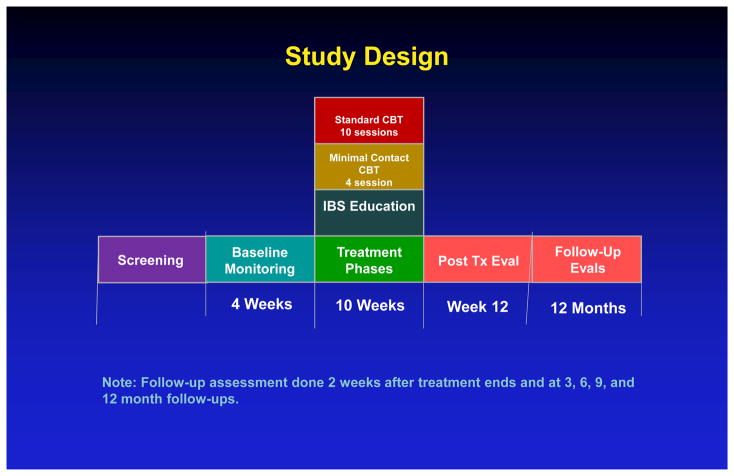
As shown in Figure 1. the IBSOS is a randomized controlled, parallel group trial that allocated patients into one of three conditions at two sites (University at Buffalo, Northwestern University). Additional details regarding the rationale and methodology of the Irritable Bowel Syndrome Outcome Study (IBSOS) protocol are detailed elsewhere9. Patients were recruited from referrals from health care professionals, advertising and word of mouth (first assessment: 8/23/2010–12/30/2015; last follow-up: 10/21/2016). Recruitment ended when accrual goals were met. This study presents immediate post-treatment, 3- and 6-month follow-up data. All authors had access to these data and reviewed and approved final manuscript.
Figure 1.
Study Design
Note: Follow-up assessment done 2 weeks after treatment ends and at 3, 6, 9, and 12 month follow-ups.
Adults (18–70 years) suffering from IBS as defined by Rome III criteria10 were included provided GI symptoms were at least moderately severe (i.e., they occurred at least twice weekly and caused some life interference). IBS diagnosis was established by study gastroenterologists at baseline assessment. Patients were excluded if they presented evidence of current structural/biochemical abnormalities or other primary GI disease that better explained gastrointestinal symptoms; had been diagnosed with a malignancy other than localized basal or squamous cell carcinomas of the skin in the past 5 years; were undergoing IBS-targeted psychotherapy; could not commit to completing all scheduled follow up visits; had an unstable extraintestinal condition or a major psychiatric disorder (e.g., depression with severe suicidality, psychotic disorder); reported a current gastrointestinal infection or an infection within 2 weeks before evaluation; used a gut-sensitive antibiotic during the 12 weeks prior to baseline assessment.
Study Oversight
Institutional Review Boards at each site approved the protocol. An independent Data Safety Monitoring Board the NIDDK Project Scientist appointed monitored the trial on a bi-annual basis for participant safety, study conduct, and progress. Bi-annual external quality assurance audits verified the trial was conducted in accordance with protocol.
Randomization and Masking
Simple randomization without constraints was performed using a centralized web-based allocation scheme (1:1:1) overseen by a study coordinator without patient care responsibilities. Study gastroenterologists masked to treatment assignment functioned as independent evaluators of improvement at immediate and 6 month follow-ups. Participants were blind to treatment assignment through pretreatment baseline period. Patient-reported expectation of IBS symptom improvement11 by end of treatment was 60.2% across conditions
Treatments
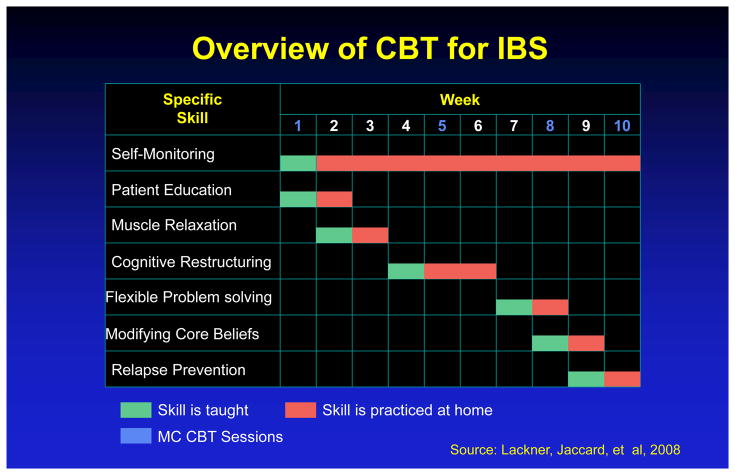
Standard-CBT (S-CBT8) involves 10 weekly, 60-minute face-to-face sessions and emphasizes the provision of information regarding brain-gut interactions; self-monitoring of GI symptoms, their antecedents and consequences; muscle relaxation to dampen physiological arousal and increase control over GI symptoms; worry control to challenge and dispute negatively skewed thinking patterns; flexible problem solving to aid in the deployment of more effective ways of managing realistic stressors; and relapse prevention training. As a learning-based program, CBT assigns home exercises to facilitate acquisition of symptom self-management skills introduced in session through didactic instruction. Because Minimal Contact-CBT (MC-CBT8) requires only four clinic visits over the 10 week period, it relies more extensively on home study materials12 to cover the same procedures S-CBT introduces at each session. Figure 2 describes the structure and format of CBT of IBS. The education condition (EDU9) was equivalent to MC-CBT in time, attention and receipt of home study materials13. EDU sessions were structured around education, support and reflection. Content included information about IBS, its clinical features, epidemiology, diagnostic criteria, medical tests, and treatment options as well as the role of stress in IBS, diet and physical activity. Clinicians were prohibited from prescribing relevant behavior changes (e.g., stress management skills). To mimic receipt of the workbook MC-CBT patients, EDU patients received a copy of IBS: Learn to Take Charge Of It 13 which emphasizes the “empowering” value of patient education. All content referencing CBT strategies were extracted through a special printing of the book. As such, the EDU condition represents a viable treatment protocol in its own right and whose procedures did not overlap with those deemed critical to CBT for IBS. This design allowed rigorous evaluation of the incremental value of the technical features of CBT over and above the contribution of state-of-the art educational protocols. It creates a much higher standard of comparison than designs that feature wait-list control or active controls with clinically inert activities such as receiving attention from someone. By emphasizing education and support, EDU incorporated lifestyle recommendations that are regarded as “of great importance in the management of patients with …IBS”14 and featured in practice guidelines 15 and was therefore more clinically robust and ecologically valid than attention control conditions whose main goal is to control for nonspecific factors (attention, expectancy)
Figure 2.
Overview of CBT for IBS
Outcomes
Consistent with recommendations for efficacy assessment for functional gastrointestinal and chronic pain trials16,17, the a priori primary endpoint was global IBS symptom improvement based on the IBS version8 of the Clinical Global Impressions-Improvement Scale (CGI-I)18: “Compared to how you felt prior to entering the study, how would you rate the IBS symptoms for which you sought treatment during the past week?” (1 = substantially worse, 2 = moderately worse 3, slightly worse, 4= no change, 5 = slightly improved, 6 = moderately improved, 7 = substantially improved). We adopted the practice of classifying patients whose symptoms were rated as “substantially improved” or “moderately improved” as treatment responders. Study gastroenterologists completed a physician-version of the CGI19. Use of “blind” physician ratings is advantageous because they are not subject to patient reporting bias and they represent judgments by trained professionals with extensive experience in IBS. Data source triangulation (using evidence about an endpoint from different data sources) is a notable study strength because it lends verification and validity to outcome data. The IBS Symptom Severity Scale (IBS-SSS20) served as a secondary index of efficacy (0–500 scale, ≥300 = Severe). Quality of care was measured at immediate post-treatment with the 8-item Client Satisfaction Questionnaire (CSQ21, range = 8–32, higher scores signifying greater patient satisfaction with treatment).
Quality Assurance
To ensure treatment fidelity, therapists received extensive training in the components of each treatment under expert supervision before assigned study patients. Delivery was optimized by treatment manuals that provided detailed session-by-session guidance to standardize intervention among therapists, the completion of checklists for session protocols after each session; and regularly scheduled supervision with senior clinicians. Sessions were audio taped 20% of which were randomly selected per patient and rated for protocol adherence (1= ineffective, 5 = extremely effective). Overall therapist adherence ratings were 4.45 (SD = .50). Clinicians rated weekly patient adherence to home exercises using a 6-point scale ranging from 1 (0%) to 6 (>100%): MC-CBT, 68%, S-CBT, 57%, EDU, 71%
Statistical Analyses
Because both per-protocol (PP) and intent-to-treat (ITT) frameworks provide unique perspectives on efficacy profiles of treatments22,23, both were applied as specified a priori in the statistical analysis plan approved by the sponsor and DSMB prior to unblinding. ITT data included all randomized patients who completed 4-week baseline period. Given the disease chronicity (17 years) of our sample, ITT patients who dropped out during treatment (9%) were assigned a score of no improvement (CGI =4; IBS-SSS = baseline score) at posttest and two follow-ups. Small normally-distributed random perturbances were added to the imputed scores (including the IBS-SSS imputed values) to remove downward bias of within-condition variability (in sensitivity analyses, different residual distributional forms were explored and did not affect conclusions). Attrition was negligible (see below). For treatment completers with missing data at a given follow-up, two strategies were explored for purposes of sensitivity analysis. The first used multiple imputatons with chained equations and the second used listwise deletion, which was justified given the small amount of missing data and the lack of any evidence for systematic missing data bias across a very wide range of covariates. Each approach makes assumptions that can be questioned, so sensitivity analyses are called for24,25. Core conclusions for the two methods were comparable, with the few exceptions noted.26
Primary analyses focused on within-time single degree of freedom (df) contrasts between the three treatment conditions. For dichotomous outcomes, between-group comparisons used a modified linear probability model with Huber-White robust estimators27; for between-group mean comparisons, single df contrasts used Huber-White robust estimators. These analyses included site as a covariate as well as covariates representing medication status (patient using medication for abdominal pain, bowel symptoms, or for multiple IBS symptoms versus not), and patient ethnicity (White versus non-White, see below). For dichotomous outcomes, contrasts were replicated using logistic regression for sensitivity analyses. All such contrasts were between-subjects in nature, not repeated measure based. For posttest/follow-up versus baseline analyses, contrasts used difference scores with non-pooled error terms. Marginal probabilities instead of odds ratios are reported in the interest of interpretability. 95% confidence intervals are reported as margins of error, i.e., the maximum absolute half width for the lower versus upper confidence limit relative to the parameter estimate.
Power analyses used an alpha of 0.05, two-tailed test, and a desired power of 0.80. The effect size sensitivity (ESS) for a between-condition contrast for a sample size of 145 per condition yields a Cohen ESS of d = 0.33. For between-group differences in proportions where one proportion is set to 0.50, the ESS is 0.16 (i.e., a group difference of 16%). For posttest/follow-up versus baseline contrasts, the ESS assuming a correlation of 0.40 between measures yields Cohen’s d = 0.26. For a within-time, across-condition proportions, ESS is 0.12 (or 12%), assuming a 0.40 phi coefficient.
A study goal was to evaluate the comparability of the 4-session MC-CBT versus the 10-session S-CBT. Supplemental analyses used Two One-Sided Test (TOST) equivalence tests between these conditions requiring 95% confidence intervals of condition differences to be fully contained within a priori defined equivalence intervals. For dichotomous outcomes, the a priori equivalence interval was set at plus or minus 10%. For the IBS-SSS, the accepted equivalence threshold is ±50 points20. Because no guidelines for defining equivalence thresholds for CGI means exist, we conducted preliminary analyses (blind to and collapsing across treatment condition) and determined a reasonable interval was ± 0.50.
RESULTS
As Table 1 shows, of 652 individuals assessed for eligibility, 436 (66%) completed 4-week baseline and were randomized to S-CBT (N=146); MC-CBT (N=145), or EDU (N=145). Table 1 presents self-reported sociodemographic characteristics for the sample. All baseline characteristics were comparable across treatment groups. Mean (SD) age was 41.4 years (14.8). Participants were predominantly female (80.3%) and non-Hispanic White (89.4%). GI symptoms were generally severe, longstanding, disruptive, and unresponsive to conventional medical therapies (i.e. treatment refractory4). Medication use was common. While medication use was unrelated to outcome, it was included as a covariate in all analyses.
Table 1.
Baseline Sociodemographic and Clinical Characteristics by Treatment Condition
| Characteristic | Overall (n = 436) | MC-CBT (n = 145) | S-CBT (n = 146) | EDU (n = 145) |
|---|---|---|---|---|
| Age, mean (SD) | 41.4 (14.8) | 40.9 (14.6) | 41.1 (14.4) | 42.2 (15.4) |
| Women, N (%) | 350 (80.3%) | 124 (85.5%) | 112 (76.7%) | 114 (79.2%) |
| Race/ethnicity N (%) | ||||
| Non-Hispanic white | 390 (89.4%) | 133 (91.7%) | 128 (87.7%) | 129 (89.0%) |
| African-American | 28 (6.4%) | 8 (5.5%) | 9 (6.2%) | 11 (7.6%) |
| Other or missing | 18 (4.2%) | 4 (2.8%) | 9 (6.2%) | 5 (3.5%) |
| Marital status, N (%) | ||||
| Never married | 185 (42.4%) | 61 (44.1%) | 60 (41.1%) | 64 (44.1%) |
| Married | 185 (42.4%) | 68 (46.9%) | 58 (39.7%) | 59 (40.7%) |
| Separated/Divorced | 57 (13.1%) | 11 (7.6%) | 26 (17.8%) | 20 (13.8%) |
| Widowed | 9 (2.1%) | 5 (3.4%) | 2 (1.4%) | 2 (1.4%) |
| Income ($), mean (SD) | 74.0 (54.2) | 77.9 (56.4) | 73.1 (52.2) | 71.3 (54.0) |
| Education, N(%) | ||||
| High school or less | 99 (22.7%) | 31 (21.4%) | 30 (20.5%) | 38 (26.2%) |
| Associate or Vo-tech | 65 (14.9%) | 25 (17.2%) | 22 (15.1%) | 18 (12.4%) |
| College degree | 142 (32.6%) | 54 (37.2%) | 41 (28.1%) | 47 (33.1%) |
| Post-grad degree | 127 (29.1%) | 35 (24.1%) | 52 (35.6%) | 40 (27.6%) |
| Missing | 3 (0.7%) | 0 | 1 (0.7%) | 2 (1.4%) |
| Employment status, N (%) | ||||
| Employed full- or part-time | 277 (63.5%) | 92 (63.4%) | 91 (62.3%) | 94 (64.8%) |
| Unemployed | 109 (25.0%) | 38 (26.2%) | 40 (27.4%) | 31 (21.4%) |
| Homemaker | 13 (3.0%) | 4 (2.8%) | 5 (3.4%) | 4 (2.8%) |
| Retired | 33 (7.6%) | 9 (6.2%) | 9 (6.2%) | 15 (10.3%) |
| Missing | 4 (0.9%) | 2 (1.4%) | 1 (0.7%) | 1 (0.7%) |
| Predominant bowel type, N (%) | ||||
| Constipation | 130 (29.8%) | 43 (29.7%) | 40 (27.4%) | 47 (32.4%) |
| Diarrhea | 188 (43.1%) | 59 (40.7%) | 67 (45.9%) | 62 (42.8%) |
| Mixed | 98 (22.5%) | 33 (22.8%) | 35 (24.0%) | 30 (20.7%) |
| Undifferentiated | 20 (4.6%) | 10 (6.9%) | 4 (2.7%) | 6 (4.1%) |
| Years with IBS, mean (SD) | 17.1 (14.4) | 15.7 (13.3) | 17.7 (13.3) | 17.7 (16.4) |
| Received medical care for IBS (lifetime), N (%) | 328 (75.2%) | 107 (73.8%) | 116 (79.5%) | 105 (72.4%) |
| IBS treatment-naïve, N (%) | 10 (2.2%) | 4 (2.6%) | 3 (1.9%) | 3 (1.9%) |
| Assessment scores, mean (SD) | ||||
| IBS Symptom Severity Scalea | 281.9 (72.1) | 278.0 (68.6) | 285.1 (76.7) | 282.4 (71.0) |
| Brief Symptom Inventory35,a | ||||
| Anxiety | 4.50 (4.50) | 4.22 (4.26) | 4.27 (4.41) | 5.02 (4.81) |
| Depression | 3.97 (4.29) | 4.07 (4.47) | 3.82 (4.33) | 4.03 (4.09) |
| Somatization | 4.22 (3.93) | 4.16 (4.31) | 4.00 (3.56) | 4.54 (3.91) |
| Global Severity Index | 12.7 (11.0) | 12.4 (11.6) | 12.1 (10.5) | 13.6 (10.8) |
| Medical comorbidities36, # | 4.6 (4.9) | 4.8 (5.2) | 4.3 (4.7) | 4.8 (5.0) |
| Psychiatric comorbidities37, # | 1.2 (1.6) | 1.1 (1.5) | 1.3 (1.7) | 1.2 (1.7) |
| Medication use for IBS symptoms, (N, %) | 292 (67.0%) | 94 (64.8%) | 95 (65.1%) | 103 (71.0%) |
| Pain medication | 35 (8.0%) | 9 (6.2%) | 13 (8.9%) | 13 (9.0%) |
| Bowel medication | 271 (62.2%) | 86 (59.3%) | 87 (59.6%) | 98 (67.6%) |
| Multi-symptom medication | 20 (4.6%) | 6 (4.1%) | 7 (4.8%) | 7 (4.8%) |
| Psychiatric medication | 26 (6.0%) | 8 (5.5%) | 12 (8.2%) | 6 (4.1%) |
notes:
Higher scores indicate more severe symptoms; IBS-SSS ≥300 = Severe
Attrition
Nine percent of patients dropped out during treatment (no statistically significant percent differences between conditions). Dropout was unrelated to a range of demographic, psychological, and IBS-related variables measured at baseline, with one exception: an 8% treatment dropout rate for Whites versus a 22% rate for non-Whites (p < 0.05). Non-whites represented only 10% of the sample. This difference did not vary significantly by treatment condition. Eighty-nine percent of the sample received a minimally sufficient dosage of their assigned treatment, defined a priori as completion of 8 of 10 for S-CBT sessions and 3 of 4 for MC-CBT and EDU. This percent did not vary significantly by condition. Attrition between posttest and 3-month follow-up was 5.1%. Attrition between 3- and 6-month follow-ups was 4.1%. None of these rates varied significantly by condition. There were no statistically significant differences between those lost to attrition versus those retained on multiple demographic and clinical variables assessed at baseline, nor as a function of outcome variables at immediate posttest.
Analyses of Outcomes
Table 2 presents per protocol and intent-to-treat results for the percent of treatment responders for the CGI as reported by patients and study gastroenterologists. Table 3 presents comparable data based on mean CGIs. Table 4 presents data for the IBS-SSS and CSQ.
Table 2.
Percent of Responders on CGI as Reported by Patients and Physicians
| Per Protocol | Intent-To-Treat | |||||
|---|---|---|---|---|---|---|
| Condition | Immediate | 3 Month | 6 Month | Immediate | 3 Month | 6 Month |
| Patient-Report CGI | ||||||
| MC-CBT | 68.1% ± 8.1 | 64.0% ± 8.8 | 63.9% ± 8.8 | 61.0% ± 8.0 | 57.0% ± 8.4 | 56.6% ± 8.4 |
| S-CBT | 64.6% ± 8.9 | 64.6% ± 9.3 | 58.1% ± 9.4 | 54.5% ± 8.4 | 53.3% ± 8.6 | 48.1% ± 8.6 |
| EDU | 46.7% ± 8.8 | 49.5% ± 9.3 | 51.0% ± 9.6 | 43.5% ± 8.4 | 45.8% ± 9.0 | 47.0% ± 9.2 |
| MC-CBT – S-CBT | 3.5% ± 12.0 | <1% ± 12.7 | 5.9% ± 12.9 | 6.5% ± 11.6 | 3.7% ± 12.0 | 8.5% ± 12.0 |
| MC-CBT – EDU | 21.5% ± 12.0** | 14.5% ± 12.8* | 12.9% ± 13.0a,b | 17.6% ± 11.6** | 11.2% ± 12.3a,b | 9.6% ± 12.5 |
| S-CBT – EDU | 18.1% ± 12.5** | 15.0% ± 13.1* | 7.1% ± 13.4 | 11.1% ± 11.9a,b | 7.5% ± 12.2 | 1.1% ± 12.6 |
| MC-CBT – S-CBT CI | −8.5% to 15.5% | −13.2% to 12.5% | −7.0% to 18.7% | −5.1% to 18.0% | −8.3% to 15.8% | −3.6% to 20.5% |
| Physician-Rated CGI | ||||||
| MC-CBT | 63.0% ± 9.0 | - | 66.4% ± 9.1 | 55.7% ± 8.6 | - | 58.4% ± 8.9 |
| S-CBT | 61.0% ± 9.4 | - | 64.0% ± 9.9 | 50.6% ± 8.7 | - | 51.6% ± 9.2 |
| EDU | 43.6% ± 9.2 | - | 49.6% ± 10.5 | 40.4% ± 8.8 | - | 44.8% ± 10.0 |
| MC-CBT – S-CBT | 2.0% ± 13.0 | - | 2.4% ± 13.5 | 5.1% ± 12.2 | - | 6.8% ± 12.8 |
| MC-CBT – EDU | 19.4% ± 12.9** | - | 16.8% ± 13.9* | 15.2% ± 12.3** | - | 13.7% ± 13.4* |
| S-CBT – EDU | 17.4% ± 13.1* | - | 14.4% ± 14.5a,b | 10.1% ± 12.4 | - | 6.8% ± 13.6 |
| MC-CBT – S-CBT CI | −10.0% to 15.0% | - | −11.1% to 15.9% | −7.1% to 17.3% | - | −5.9% to 19.6 % |
notes:
p < 0.05,
p < 0.01,
p < 0.08;
trend p value using listwise deletion is p < 0.05 for chained equation missing data algorithm; MC-CBT - S-CBT CI = 95% CI for difference; numbers after ± are margins of error (half widths of 95% CIs); IBSOS was not designed to collect blind ratings at 3 month follow up
Table 3.
Means on CGI as Reported by Patients and Physicians
| Per Protocol | Intent-To-Treat | |||||
|---|---|---|---|---|---|---|
| Condition | Immediate | 3 Month | 6 Month | Immediate | 3 Month | 6 Month |
| Patient-Report CGI | * | |||||
| MC-CBT | 5.79 ± 0.22 | 5.75 ± 0.23 | 5.70 ± 0.25 | 5.61 ± 0.21 | 5.55 ± 0.23 | 5.51 ± 0.24 |
| S-CBT | 5.76 ± 0.23 | 5.67 ± 0.24 | 5.64 ± 0.23 | 5.49 ± 0.22 | 5.38 ± 0.23 | 5.35 ± 0.22 |
| EDU | 5.43 ± 0.21 | 5.36 ± 0.23 | 5.42 ± 0.24 | 5.33 ± 0.20 | 5.2 ± 0.22 | 5.31 ± 0.24 |
| MC-CBT – S-CBT | 0.03 ± 0.31 | 0.08± 0.34 | 0.05 ± 0.34 | 0.12 ± 0.30 | 0.17 ± 0.32 | 0.16 ± 0.32 |
| MC-CBT – EDU | 0.36 ± 0.30* | 0.39 ± 0.32* | 0.28 ± 0.35 | 0.28 ± 0.29a,b | 0.29 ± 0.32a,b | 0.20 ± 0.34 |
| S-CBT – EDU | 0.33 ± 0.31* | 0.31 ± 0.33a | 0.22 ± 0.34 | 0.16 ± 0.29 | 0.12 ± 0.32 | 0.04 ± 0.32 |
| MC-CBT – S-CBT CI | −0.28 to 0.35 | −0.26 to 0.42 | −0.29 to 0.39 | −0.18 to 0.43 | −0.15 to 0.49 | −0.17 to 0.48 |
| Physician-Rated CGI | ||||||
| MC-CBT | 5.69 ± 0.19 | - | 5.91 ± 0.18 | 5.50 ± 0.19 | - | 5.67 ± 0.20 |
| S-CBT | 5.70 ± 0.20 | - | 5.70 ± 0.24 | 5.42 ± 0.20 | - | 5.37 ± 0.23 |
| EDU | 5.28 ± 0.19 | - | 5.38 ± 0.24 | 5.20 ± 0.19 | - | 5.26 ± 0.24 |
| MC-CBT – S-CBT | −0.01 ± 0.28 | - | 0.21 ± 0.30 | 0.08 ± 0.28 | - | 0.30 ± 0.30* |
| MC-CBT – EDU | 0.41 ± 0.27** | - | 0.53 ± 0.30** | 0.30 ± 0.27* | - | 0.42 ± 0.31** |
| S-CBT – EDU | 0.42 ± 0.28** | - | 0.31 ± 0.34a,b | 0.22 ± 0.27 | - | 0.11 ± 0.33 |
| MC-CBT – S-CBT CI | −0.29 to 0.26 | - | −0.09 to 0.51 | −0.20 to 0.35 | - | 0.01 to 0.61 |
notes:
p < 0.05,
p < 0.01,
p < 0.08;
trend p value using listwise deletion is p < 0.05 for chained equation missing data algorithm; MC-CBT - S-CBT CI = 95% CI for difference; numbers after ± are margins of error (half widths of 95% CIs)
Table 4.
Mean Change in IBS-SSS from Baseline and Patient Satisfaction
| Per Protocol | Intent-To-Treat | |||||
|---|---|---|---|---|---|---|
| Condition | Immediate | 3 Month | 6 Month | Immediate | 3 Month | 6 Month |
| IBS-SSS | ||||||
| MC-CBT | −89.35 ±17.0 | −105.9 ±17.7 | −111.3 ±17.9 | −80.69 ± 15.9 | −95.27 ± 16.7 | −99.09 ±17.0 |
| S-CBT | −80.99 ±17.0 | −103.2 ±16.4 | −103.5 ±17.7 | −67.57 ± 15.3 | −87.10 ± 15.3 | −85.16 ± 16.4 |
| EDU | −84.43 ±14.6 | −88.53 ±14.6 | −97.93 ±15.4 | −78.88 ± 14.3 | −82.19 ± 14.2 | −91.37 ± 14.9 |
| MC-CBT – S-CBT | −8.39 ±24.0 | −2.71 ±24.2 | −7.74 ± 24.9 | −13.11 ± 22.1 | −8.16 ± 22.7 | −13.93 ± 23.6 |
| MC-CBT – EDU | −4.95 ±22.4 | −17.41 ±22.9 | −13.34 ±23.4 | −1.81 ± 21.4 | −13.08 ± 21.9 | −7.72 ± 22.6 |
| S-CBT – EDU | 3.44 ±22.4 | −14.70 ±22.0 | −5.60 ±23.1 | 11.31 ± 21.0 | −4.91 ± 21.0 | 6.21 ± 22.4 |
| MC-CBT – S-CBT CI | −32.4 to 15.6 | −26.9 to 21.5 | −32.7 to 17.2 | −35.2 to 9.0 | −30.8 to 14.5 | −38.0 to 9.7 |
| Patient Satisfaction | ||||||
| MC-CBT | 28.9 ± 0.6 | - | - | - | - | - |
| S-CBT | 28.4 ± 0.7 | - | - | - | - | - |
| EDU | 26.6 ± 0.9 | - | - | - | - | - |
| MC-CBT – S-CBT | 0.70 ± 1.0 | - | - | - | - | - |
| MC-CBT – EDU | 2.27 ± 1.0** | - | - | - | - | - |
| S-CBT – EDU | 1.79 ± 1.1** | - | - | - | - | - |
| MC-CBT – S-CBT CI | −0.3 to 1.6 | - | - | - | - | - |
notes:
p < 0.05; mean IBS-SSS at baseline across conditions = 281.9 with a margin of error of ± 7.4; all changes from baseline are statistically significant, p < 0.01; Unadjusted standard deviations for IBS-SSS change scores at each time point are about 19.0, indicating substantial standardized effects (Cohen’s d) relative to baseline; MC-CBT - S-CBT CI = 95% CI for difference; Cohen’s d for patient satisfaction for MC-CBT-EDU = 0.53 and for S-CBT- EDU = 0.37; numbers after ± are margins of error (half widths of 95% CIs
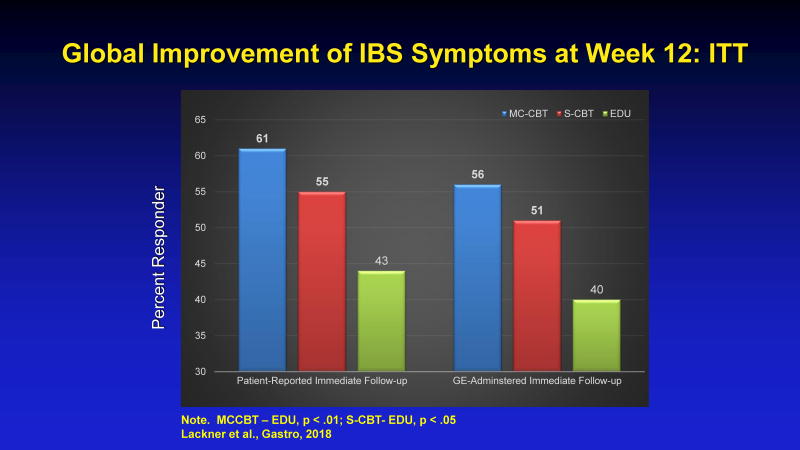
MC-CBT produced a statistically significantly larger percent of treatment responders than IBS education (EDU) at immediate posttest for the patient reports (per-protocol (PP) = 68.1% versus 46.7%. ITT = 61.0% versus 43.5%) and for gastroenterologist assessments (PP = 63.0% versus 43.6%, ITT = 55.7% versus 40.4%). ITT data are graphically represented in Figure 3. Gastroenterologist-reported CGI differences retained significance at 6 months for both PP and ITT analyses and this also was true for the patient reported analyses using chained equation imputation for the PP analysis but not the ITT analyses (thought a trend was evident).
Figure 3.
Global Improvement of IBS Sym ptoms at Week 12: ITT
MCCBT – EDU, p < .01; S-CBT-EDU, p < .05
There was a tendency for mean values of the CGI to be more positive in the MC-CBT condition than the EDU condition, although patterns of statistical significance were less consistent beyond two-week follow up. Table 4 shows, substantial change in the IBS-SSS from baseline to posttest and follow-ups (>80 points where 50 points is clinically significant) for all conditions but no statistically significant differences between the three groups at any time point. The magnitude of changes on the secondary endpoint (IBS-SSS) for all conditions corresponded to a Cohen’s d ≥ 3.5 (large). Both CBT dosages yielded significantly higher patient satisfaction than EDU (Cohen’s d for MC-CBT= 0.53).
For equivalence tests for the parameter MC-CBT minus S-CBT, the critical result is whether confidence interval limits are completely within the equivalence interval (or, for the present study, if the CI lower limit of the difference is larger than the lower limit of the equivalence interval, to affirm S-CBT is not superior to MC-CBT). This generally was the case, suggesting that MC-CBT is, at minimum, as efficacious as S-CBT.
Adverse events
One patient reported an adverse event (suicide attempt) but it was unrelated to treatment protocol and resolved
Discussion
In this multisite study, a brief, primarily home-based version of CBT yielded comparable results to the “gold standard” clinic-based version of CBT in improving chronic, severe, and treatment-refractory GI symptoms of IBS. Within-CBT gains were clinically meaningful and substantial with negligible erosion (~5%) 6 months after treatment ended. A 10-session, clinic-based version of CBT does not appear to confer incremental advantage over a 4-session, home-based version, even though the latter required 60% less clinician delivery time. Symptomatic improvement was achieved without risk of safety to patients which is notable given reported adverse effects of most medical therapies for IBS28
While both CBT conditions outperformed IBS education on the primary measure of symptomatic improvement at immediate follow up, we found no significant between-group differences on the IBS-SSS. All 3 conditions yielded significant IBS symptom severity reductions that persisted over time. The discrepancy between CGI and IBS-SSS outcome data may relate to the nature of the IBS-SSS, which is heavily weighted by sensory (e.g., pain, bloating) symptoms29. Because the CGI requires patients to rate any symptomatic improvement on the basis of both sensory and defecatory symptoms, it may be a more sensitive IBS endpoint.
CBT improvement rates post treatment are among the highest in the IBS outcome literature when examined on an absolute level. To put these data in context, treatment response of FDA-approved pharmacological agents using global IBS symptom improvement scales range from 17–40%30,31. CBT improvement rates compare favorably to the immediate and sustained efficacy profile of available medical or dietary therapy from over 100 trials. A less robust but still important effect is evident when comparing the degree of therapeutic gain (absolute % benefit increase) of CBT. At immediate follow-up, the magnitude of therapeutic gain of CBT over an active education/support comparator met or exceeded (11–21%) the threshold (10–15%) for defining clinical significance of novel pharmacotherapies in placebo-controlled trials32. Because these contrasts isolate treatment effects attributable to therapeutic ingredients specific to CBT rather than the overall effect that also includes the influence of factors common across all therapies such as the formation of a collaborative doctor-patient relationship, the somewhat diminished effect size is expected.
CBT appears to improve IBS symptoms in a way that cannot be explained solely by nonspecific factors such as placebo, patient support, or education. First, CBT response far exceeded the 30–40% placebo response rate reported in IBS trials33,34. Second, unlike a placebo response, CBT gains generally persisted with negligible erosion after treatment cessation through 6 month follow ups35. Third, the overall pattern of response on primary outcome did not appreciably differ when reported by patients or blind assessors whose ratings were immune from bias from any expectation or allegiance favoring a specific condition. Fourth, while education/support leads to symptomatic improvement in a sizable minority of patients, it falls short of the response rate of the two CBT conditions particularly at immediate post-treatment which represents the critical follow up period for gauging efficacy of a Phase II trial. Patient education and support may be insufficient for some patients to achieve more immediate symptomatic improvement. For these patients, optimizing treatment response may involve learning strategies to correct faulty threat appraisals that can dysregulate brain-gut interactions36.
While response rate for CBT is generally strong, it is by no means complete. At immediate follow up, less than half (42%) of CBT-treated patients who reported symptomatic improvement (28%, EDU) met remission criteria as defined by having no to mild IBS symptoms on the gastroenterologist-administered CGI-Illness Severity Scale19. Combination treatments of CBT and medical therapies that target both central and peripheral mechanisms of IBS may have therapeutic advantage over monotherapies for patients whose symptoms do not improve or whose improvement falls short of registering as clinically meaningful. Effectively reducing the societal and economic burden of IBS, however, calls for more than clinically proven treatments regardless of how they are configured or how well they work. Innovative direct-to-patient delivery systems are needed to transport evidence-based learning content key to symptom self-management to a broader number of individuals than more time-intensive, face-to-face encounters with specially trained professionals in select clinical settings reach. This study is a step in this direction.
While the primary aim of the IBSOS was to characterize short-term efficacy of CBT, data speak to durability of treatment effects of which there are two vantage points. Using a within-condition perspective, the percent of symptom responders for the patient- reported CGI for the M-CBT at the immediate posttest was 61% and at 6 months it was 57%, suggesting treatment durability. For the (blinded) physician assessments, the corresponding percents were 56% and 58%, also suggesting durability. Neither of these changes in percents between the immediate posttest and FU6 were statistically significant. For the IBS-SSS, the mean change at 6 months was about −99 units, which is substantial relative to standards in the field (a 50-point pre-post change is considered clinically significant) and did not degrade from prior levels of change (see Table 4) From a between-condition peprspective, the MC-CBT versus EDU comparisons focused not so much on the durability of the overall treatments and all that they entail but rather the durability of improvement attributed to the technical components of CBT. For the physician assessments, the increment in the percent responders in M-CBT over and above EDU was 17% at the immediate posttest and 14% ar the 6 month follow-up. Both increments were statistically significantly different from EDU. For the patient reports, the incremental percent of responders at the immediate posttest in M-CBT beyond EDU was 18% and at the 6-month follow-up it was 10%. This change in incremental rates over time was not statistically significant, but the latter 10% increment by M-CBT over EDU was the one durability comparison that was not statistically significant (although it trended in the expected direction). It is possible that CBT has a more powerful catalytic effect on initiating rather than maintaining GI symptom relief. However, the broad pattern of the above data suggest that CBT has a relatively durable effect particularly in comparison to medical therapies whose efficacy diminishes with treatment withdrawal. Nonetheless, we suggest that further efforts be devoted to determine how to optimize CBT’s maintenance effects, perhaps through brief, phone-based booster sessions to keep CBT skills salient and help patients troubleshoot around difficulties they previously had in relieving GI symptoms
Study limitations include relatively few male patients for examining gender differences. Reliance on volunteers most of whom were White females may limit generalizability. Supplemental analyses such as those based on machine learning principles using targeted maximum likelihood for purposes of sensitivity analyses might be of value.37,38 While an inert placebo comparator may have enhanced the interpretability of findings, this was neither feasible nor ethical given study demands extending months after treatment discontinuation to characterize maintenance effects. Like all patient-reported outcomes, the global endpoint approach we adopted at the recommendation of the Rome Foundation16 is subjective and vulnerable to potential biases of self-report. Such biases should, however, operate equally across treatments, rendering any observed between-group differences as clinical meaningful. Further, blind ratings not subject to the same biases yielded results comparable to patient-reported ratings, suggesting findings are robust across data source. A focus on dichotomous improvement judgments also has inherent limitations, although this is a standard metric for gauging efficacy in the field. Strengths of the design include extended follow-up assessments, methodologically rigorous trial architecture that minimized risk of multiple biases that obscures study findings and supports their reproducibility, negligible attrition and missing data, and a relatively large, well-characterized sample from two sites that rendered comparable data.
In conclusion, a primarily home-based version of CBT produced substantial gains in the percent of patients reporting moderate to substantial improvement of GI symptoms. GI symptom improvement is not explained away by nonspecific effects such as support, patient education, or attention.
Acknowledgments
Grant Support: Research reported in this manuscript was supported by the NIH/NIDDK Grant 77738 (Dr. Lackner).
The investigators would like to thank the volunteers who enrolled in the IBSOS. We gratefully acknowledge the following individuals for their instrumental roles in the design and conduct of the IBSOS:
Abbreviations
- IBSOS
Irritable Bowel Syndrome Outcome Study
- IBS
Irritable Bowel Syndrome
- CBT
Cognitive Behavior Therapy
- S-CBT
Standard-Cognitive Behavior Therapy
- MC-CBT
Minimal Contact-Cognitive Behavior Therapy
- EDU
Education condition
- CGI
Clinical Global Impressions Scale
- CGI-I
Clinical Global Impressions-Improvement Scale
- IBS-SSS
Irritable Bowel Syndrome Symptom Severity Scale
- CSQ
Client Satisfaction Questionnaire
- PP
Per-protocol
- ITT
Intent-to-treat
- df
degrees of freedom
- ESS
Effect size sensitivity
- TOST
Two One-Sided Test
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Conflict of interest: None reported.
Role of the Funder/Sponsor: NIDDK scientists (Drs Frank Hamilton and Patricia Robuck) contributed to the design and conduct of the study, which included the collection and management of data. The project scientist from the NIDDK served as a member of the IBSOS steering committee. The decision to publish was made by the IBSOS steering committee in consultation with all authors, with no restrictions imposed by the sponsor. As a coauthor, the NIDDK scientist contributed to the interpretation of the data and the preparation, review, and approval of the manuscript
NIDDK: Sherry R. Hall, M.S., Patricia Robuck, Ph.D., MPH (past Project Scientist), Rebecca Torrance, R.N., M.S.N., Aynur Unalp-Arida, MD, MSc, Ph.D (Program Officer), Rebekah Van Raaphorst
Data and Safety Monitoring Board: Jennifer A. Haythornthwaite, Ph.D., Brian E. Lacy, MD, Ph.D. (Chair), Donald B. Penzien, Ph.D., Catherine Spino, D.Sc., Arnold Wald, MD.
Data Coordinating Center: Mark Byroads, Kenneth Wood (Frontier Science).
Study Sites: University at Buffalo: Deana Bervoets, Adam Booth, Alicia Brasel, Abbey Braun, Ann Marie Carosella, Ph.D., Ray Dannenhoffer, Ph.D., Paul Dressel, Gary Iacabucci, Justin Kimber, Pat Klinck, Jessica Perkins, Cathrine Powell, Tatyana Raby, Mark Schneggenberger, Camille Simonetti, Amanda Smith, Emily Smith, Christopher Sova, Travis Stewart, Andrew Wurl and the staff of the UB Clinical and Translational Research Center. Northwestern: Caroline E. Artz, Sarah K. Ballou, Ph.D., Jason R. Bratten, MBA, Beth Doerfler, RD, Kate Eident, Sarah Kinsinger, Ph.D., Sarah E. Quinton, PsyD, Tiffany Taft, PsyD.,
Collaborators: Kathleen Carroll, Ph.D. (Yale University); Mel C. Wilcox, MD, MSPH (University of Alabama); Bruce Naliboff, Ph.D., Emeran Mayer, MD (UCLA); Nancy Norton (International Foundation for Functional GI Disorders)
Author Contributions: Dr. Lackner and Dr. Jaccard had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lackner, Firth, Jaccard, Gudleski, Katz, Krasner, Keefer, Brenner, Sitrin, Hamilton
Acquisition, analysis, or interpretation of data: All authors
Drafting of the manuscript: Lackner, Jaccard
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Jaccard
Obtained funding: Lackner, Jaccard, Firth, Keefer, Sitrin
Administrative, technical, or material support: All authors
Study supervision: Lackner, Firth, Jaccard, Keefer, Brenner, Hamilton, Ma
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hungin APS, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharm Ther. 2005;21(11):1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Choi MG. Review article: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11(1):3–15. doi: 10.1046/j.1365-2036.1997.84256000.x. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–754. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376(26):2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 5.Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med. 2008;358(16):1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford AC, Quigley EMM, Lacy BE, et al. Effect of Antidepressants and Psychological Therapies, Including Hypnotherapy, in Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2014;109(9):1350–1365. doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 7.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Short-term and Long-term Efficacy of Psychological Therapies for Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Clin Gastroenterol H. 2016;14(7):937–947. e934. doi: 10.1016/j.cgh.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Holroyd K. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6(8):899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lackner JM, Keefer L, Jaccard J, et al. The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self-versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33(6):1293–1310. doi: 10.1016/j.cct.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead W. Rome III. The functional gastrointestinal disorders: Diagnosis, pathophysiology and treatment: A multinational consensus. 2. McLean, VA: Degnon Associates; 2006. [Google Scholar]
- 11.Deviliya GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 12.Lackner JM, Holroyd KA. Breaking the bonds of IBS: A step-by-step guide. 2. Buffalo, NY: University at Buffalo, SUNY; 2010. [Google Scholar]
- 13.Gordon D. IBS: Learn to take charge of it. New York: Barnes & Noble; 2004. [Google Scholar]
- 14.Ringstrom G, Storsrud S, Posserud I, Lundqvist S, Westman B, Simren M. Structured patient education is superior to written information in the management of patients with irritable bowel syndrome: a randomized controlled study. Eur J Gastroenterol Hepatol. 2010;22(4):420–428. doi: 10.1097/MEG.0b013e3283333b61. [DOI] [PubMed] [Google Scholar]
- 15.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology Monograph on the Management of Irritable Bowel Syndrome and Chronic Idiopathic Constipation. The American Journal Of Gastroenterology. 2014;109:S2. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 16.Irvine EJ, Whitehead WE, Chey WD, et al. Design of Treatment Trials for Functional Gastrointestinal Disorders. Gastroenterology. 2006;130(5):1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Klein KB. Assessment of treatment outcome in the functional gastrointestinal disorders. In: Corazziari E, editor. Approach to the patient with chronic gastrointestinal disorders. Milano, Italy: Messaggi; 1999. [Google Scholar]
- 19.Guy W. US Department of Heath E, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, editor. Rockville, MD: 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- 20.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11(2):395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 21.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Evaluation and program planning. 1979;2(3):197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 22.Feinman RD. Intention-to-treat. What is the question? Nutrition & Metabolism. 2009;6:1–1. doi: 10.1186/1743-7075-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallinckrodt C, Molenberghs G, Rathmann S. Choosing estimands in clinical trials with missing data. Pharmaceutical statistics. 2017;16(1):29–36. doi: 10.1002/pst.1765. [DOI] [PubMed] [Google Scholar]
- 24.Allison PD. Multiple Imputation for Missing Data: A Cautionary Tale. Sociological Methods & Research. 2000;28(3):301–309. [Google Scholar]
- 25.Cheema J. Some general guidelines for choosing missing data handling methods in educational research. Journal of Modern Applied Statistical Methods. 2014;13:53–75. [Google Scholar]
- 26.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 27.Angrist J, Pischke J. Mostly harmless econometrics: An empiricist’s companion. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- 28.Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of Harm in the Pharmacotherapy of Irritable Bowel Syndrome. The American Journal of Medicine. 125(4):381–393. doi: 10.1016/j.amjmed.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Lackner JM, Jaccard J, Baum C, et al. Patient-Reported Outcomes for Irritable Bowel Syndrome Are Associated With Patients’ Severity Ratings of Gastrointestinal Symptoms and Psychological Factors. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(11):957–964. e951. doi: 10.1016/j.cgh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The Effect of a Nonabsorbed Oral Antibiotic (Rifaximin) on the Symptoms of the Irritable Bowel Syndrome. Annals of Internal Medicine. 2006;145(8):557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 31.Drossman DA, Chey WD, Johanson JF, et al. Clinical trial: lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment Pharmacol Ther. 2009;29(3):329–341. doi: 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 32.Corazziari E, Bytzer P, Delvaux M, et al. Clinical trial guidelines for pharmacological treatment of irritable bowel syndrome. Aliment Pharm Ther. 2003;18(6):569–580. doi: 10.1046/j.1365-2036.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- 33.Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharm Ther. 2010;32(2):144–158. doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]
- 34.Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastro Hepat. 2015;12(8):472–485. doi: 10.1038/nrgastro.2015.117. [DOI] [PubMed] [Google Scholar]
- 35.Jones J, Boorman J, Cann P, et al. British Society of Gastroenterology guidelines for the management of the irritable bowel syndrome. Gut. 2000;47(suppl 2):ii1–ii19. doi: 10.1136/gut.47.suppl_2.ii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore KL, van der Laan MJ. Covariate adjustment in randomized trials with binary outcomes: targeted maximum likelihood estimation. Stat Med. 2009;28(1):39–64. doi: 10.1002/sim.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Laan MJ, Gruber S. Collaborative double robust targeted maximum likelihood estimation. The international journal of biostatistics. 2010;6(1) doi: 10.2202/1557-4679.1181. Article 17. [DOI] [PMC free article] [PubMed] [Google Scholar]