Abstract
Liver ischemia-reperfusion injury (IRI) represents a risk factor for early graft dysfunction and an obstacle to expanding donor pool in orthotopic liver transplantation (OLT). We have reported on the crucial role of macrophage Notch1 signaling in mouse warm hepatic IRI model. However, its clinical relevance or therapeutic potential remain unknown. Here, we used Serelaxin (SER), to verify Notch1 induction and putative hepatoprotective function in IR-stressed OLT. C57BL/6 mouse livers subjected to extended (18hr) cold storage were transplanted to syngeneic recipients. SER treatment at reperfusion ameliorated IRI, improved post-OLT survival, decreased neutrophil/macrophage infiltration, suppressed pro-inflammatory cytokine programs, while simultaneously increasing Notch1 intracellular domain (NICD) and hairy and enhancer of split-1 (Hes1) target genes. In bone marrow-derived macrophage cultures, SER suppressed pro-inflammatory while enhancing anti-inflammatory gene expressions concomitantly with increased NICD and Hes1. Hepatic biopsies from twenty-one adult primary liver transplant patients (2h post-reperfusion) were divided into low-NICD (n=11) and high-NICD (n=10) expression groups (Western blots). Consistent with our murine findings, human livers characterized by high-NICD were relatively IRI resistant, as shown by sALT levels at day 1 post-OLT. Our study documents the efficacy of SER – Notch1 signaling in mouse OLT and highlights the protective function of Notch1 in liver transplant patients.
Introduction
Orthotopic liver transplantation (OLT) has become the standard care for patients with end-stage liver disease and those with hepatic malignancies. Liver ischemia-reperfusion injury (IRI), an innate immune-driven inflammation response leading to hepatocellular death, is an inevitable consequence of multiple clinical conditions, including trauma, sepsis, hepatectomy and liver transplantation. Indeed, hepatic IRI has been recognized as a major risk factor for delayed early graft function, acute and chronic rejection as well as a key obstacle to expanding the donor pool. However, despite clinical importance, mechanisms that account for liver IRI are partially understood and there are no effective strategies to combat IRI in humans (1).
Relaxin, a group of low-molecular-weight peptide hormone of the insulin-growth factor family, consists of seven members, with relaxin-2 (RLX-2) accounting for most of the biological effects in humans and animals (2). Originally isolated from ovary and named for its ability to “relax” pubic symphysis during pregnancy (3), relaxin plays a role in hemodynamic change, such as decreasing systemic vascular resistance, increasing cardiac output, and improving global arterial compliance (4). Experiments demonstrating cytoprotective (5), anti-inflammatory (6) and anti-fibrotic (7) functions, possibly due to antioxidant effects, underline increasing interest in RLX-2 as a pharmacotherapeutic agent. Indeed, a recent phase III randomized clinical trial showed the efficacy, safety and tolerability of Serelaxin (SER, recombinant human relaxin-2) in acute heart failure patients (8).
By binding with high affinity to cognate receptor relaxin family peptide receptor-1 (RXFP1), the majority of RLX-2 related signal transduction involves RXFP1 molecule (9). RXPF1 is expressed in reproductive tissues, heart, kidney, lung and brain (10), while protective effects of RLX-2 induction were described in heart (2), kidney (11) and lung (12) IRI models. Although barely detectable in normal rat liver, RXFP1 is predominantly expressed in fibrotic lesion after chronic carbon-tetrachloride treatment (13). The mechanisms and putative clinical relevance of apparent RLX-2 protection seen in ex-vivo isolated rat livers (14) remain to be elucidated.
Notch1 is a highly conserved transmembrane receptor involved in cell fate decision, proliferation, differentiation and regeneration (15). Upon ligand binding, the Notch intracellular domain (NICD) is released by proteolytic cleavage operated by γ-secretase complex, translocates into nucleus followed by the activation of Notch1 target genes, including hairy and enhancer of split-1 (Hes1) (16). Disruption of Notch1 transcription factor RBP-J increased cell apoptosis/necrosis and inflammatory response leading to aggravated liver IRI (17). We have shown that myeloid-specific Notch1 deficiency augmented macrophage activation and hepatocellular damage in a mouse warm IRI model (18). The clinical relevance of Notch1 signaling in OLT is unknown and therapeutic potential of Notch1 induction has not been tested to date. Although SER may stimulate Notch1 in cardiac fibroblast (19), cardiomyocyte (20) and HUVEC (21) systems, whether SER can modulate Notch1 signaling in liver transplantation remains to be defined.
The present translational study was designed to investigate the therapeutic potential of SER and determine the role of Notch1 in SER-mediated hepatoprotection in a murine IRI-OLT model. In a clinical arm, we assessed the relevance of Notch1 in liver transplant patients. Our findings document the significance of Notch1 in hepatoprotection/innate immune regulation, and highlight SER-mediated therapeutic potential of Notch1 activation in liver transplantation.
Materials and Methods
Animals
C57BL/6 mice at 6-8 weeks of age were used (Jackson Laboratory, Bar Harbor, ME). Animals were housed in UCLA animal facility under specific pathogen-free conditions, received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH publication 86-23 revised 1985). All studies were reviewed and approved by the UCLA Animal Research Committee.
Reagents
Serelaxin (SER; recombinant form of human relaxin-2, RLX030) was provided by Novartis International AG (East Hanover, NJ). Lipopolysaccharides (LPS) and Interleukin-4 (IL4) were obtained from Sigma Aldrich (St. Louis, MO).
Mouse liver transplantation
We used an established mouse model of ex-vivo hepatic cold storage and OLT (22). To mimic “marginal” human OLT setting, donor livers were stored in UW solution at 4°C for 18h prior to transplantation into syngeneic mice. Animals were treated i.v. with SER (5μg/kg) or lactate ringer solution (control) at the time of reperfusion. Optimal dose of SER was determined in a preliminary experiment comparing the effects of 0/1/3/5/10 μg/kg given at the time of reperfusion (n=2/group, data not shown). Liver and serum samples were collected at 6h, the peak of hepatocellular damage in this model. Separate groups of OLT recipients were monitored for 14-day survival. The sham group underwent the same procedures except for OLT.
Hepatocellular function assay
Serum alanine aminotransferase (sALT) and aspartate aminotransferase (sAST) levels were measured with Infinity™ ALT and AST Liquid Stable Reagent (Thermo Scientific, Rockford, IL) and validated with Validate®GC3 (Maine Standards Company, LLC, ME).
Liver histology and IRI grading
Formalin-fixed paraffin-embedded liver sections (5μm) were stained with hematoxylin and eosin (H&E). The severity of IRI was graded using Suzuki's criteria (22).
TdT-mediated dUTP nick end labeling (TUNEL) assay
Cell death in formalin-fixed paraffin-embedded liver sections (5μm) was detected by Apop Tag Plus Peroxidase in Situ Apoptosis Kit (Millipore, Temecula, CA). Results were scored semi-quantitatively by blindly counting the number of positive cells in 10 HPF/section.
Immunohistochemistry
The expression of RXFP1 (liver and heart) was examined using rabbit anti-RXFP1 Ab (Santa Cruz Biotechnology, Santa Cruz, CA). Immunostaining signals were visualized with a labeled polymer in the EnVision+ system horseradish-peroxidase kit (Dako, Carpinteria, CA). Liver infiltrating neutrophils and macrophages were detected with rat anti-Ly6G Ab (BD Biosciences, San Jose, CA) and rabbit anti-CD11b Ab (Abcam, Cambridge, MA), respectively. Signals were visualized with secondary Alexa Fluor 488 IgG. Results were scored semi-quantitatively by blindly counting the number of positive cells in 10 HPF/section (×400).
Cell isolations and in vitro cultures
Femurs and tibias were removed from WT mice, and bone marrow-derived macrophages (BMDM) were generated, as described (22). In some experiments, cells pretreated with SER (5μg/ml, 24h) were stimulated with LPS (100ng/ml, 6h) or IL4 (10ng/ml, 24h).
Quantitative RT-PCR analysis
RNA was extracted from livers or cell samples using RNAse Mini Kit (Qiagen, Germantown, MD). A total of 5.0μg of RNA was reverse-transcribed into cDNA. Quantitative PCR was performed using Quant studio 3 (Applied Biosystems, Foster city, CA). The primers sequences are listed (Table S1). The expression of the target gene was normalized to the housekeeping HPRT or β2M.
Western blot assay
Proteins were extracted from liver or cell samples, followed by concentration measurement (BCA Protein Assay Kit, Thermo Scientific). Equal amount of protein was electrophoresed, blotted, and incubated with primary Ab, secondary HRP-conjugated Ab, and developed. Primary Ab detecting NICD, pIκBα (Ser32), cleaved caspase-3, GR, β-actin (Cell Signaling Technology, Danvers, MA), Hes1, RXFP1 (Santa Cruz Biotechnology) and CD206 (R&D systems, Minneapolis, MN) were used.
Clinical liver transplantation study
Twenty-one adult OLT recipients recruited between May 2013 and August 2015 received routine standard of care and immunosuppressive therapy, as specified by UCLA liver transplant protocols (23). Study data were managed using REDCap electronic data capture tools. Recipient blood was collected prior to the transplant and at post-operative day 1 (POD1). Liver function was evaluated by sALT/sAST. Post-transplant biopsies (Bx) were collected from left liver lobe at 2h after portal reperfusion (prior to the abdominal closure) followed by Western blot analyses. To compare target protein expression in multiple human OLT samples, densitometry quantification was conducted using a reference sample and normalization with β-actin, as reported (23).
Statistical analysis
In mouse experiments, group comparisons were performed using a Student t-test. For human data, continuous values were analyzed by Mann-Whitney U test and categorical variables by Fisher's exact test. The cumulative survival rate was analyzed by Kaplan-Meier method, and differences between groups were compared using a log-rank test. JMP for Windows 8.0 (SAS Institute, Cary, NC) was used for statistical analyses. A p-value of <0.05 was considered statistically significant.
Results
SER ameliorates hepatic IRI and improves OLT survival
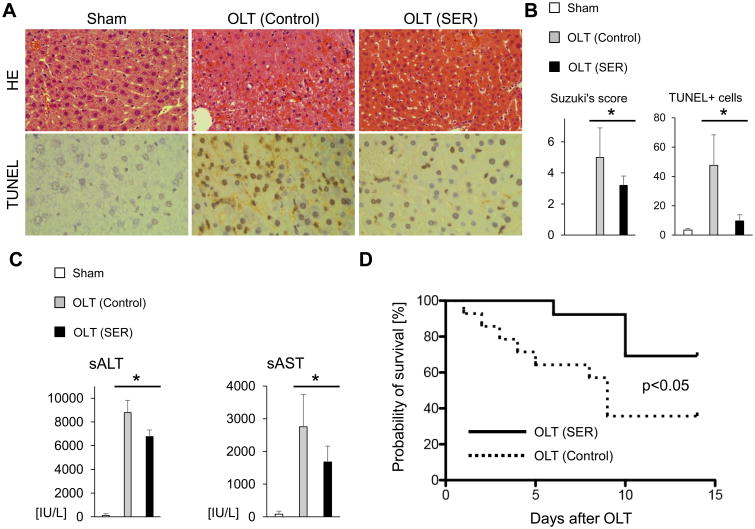
We first aimed to determine the efficacy of SER treatment in mouse OLT with extended cold storage (18h), which mimics marginal human liver transplantation. Recipient mice (n=5/group) were infused i.v. with SER (5μg/kg) or lactate ringer (control) at the completion of surgery (immediately prior to reperfusion). At 6h post-transplant, SER treated OLT displayed attenuated sinusoidal congestion, edema/vacuolization and hepatocellular necrosis as compared with controls (Fig. 1A). These correlated with decreased Suzuki's histological grading of liver IRI (control-OLT=5.0±1.9 vs. SER-OLT=3.2±0.6; p=0.030, Fig. 1B); attenuated hepatocellular damage (sALT: control-OLT= 8791±1032 vs. SER-OLT=6762±551 IU/L; p=0.005; sAST: control-OLT=2754±992 vs. SER-OLT=1676±486 IU/L; p=0.042; Fig. 1C); and decreased number of TUNEL+ cells (control-OLT=47.6±20.8 vs. SER-OLT=9.6±4.2; p=0.008, Fig. 1A/B). These effects of SER at 6h post-reperfusion correlated with improved (p=0.030) overall OLT survival in SER-OLT (69.2%; n=13) as compared to control-OLT (35.7%; n=14) recipient groups at 14 days post-transplant (Fig. 1D). Thus, SER treatment ameliorated IR-induced hepatocellular injury and improved OLT outcomes.
Figure 1. SER attenuates hepatic IRI and improves mouse OLT survival.
C57/Bl6 donor livers subjected to extended cold storage (18h) were transplanted to syngeneic mice. OLT recipients were treated i.v. with SER (5μg/kg) or equivalent amount of lactate ringer solution (control) at reperfusion. Liver and serum samples were collected at 6h post-surgery. (A) Representative H&E (upper panels, original magnification, ×100) and TUNEL (lower panels, original magnification, ×400) staining. (B) Suzuki's histological grading of liver IRI and quantification of TUNEL+ cells/HPF (n=5/group). (C) Serum ALT and AST levels (IU/L, n=5/group). Data shown as mean±SD (*p<0.05, Student t-test). (D) Animals were monitored for 14 days and cumultive survival was analyzed by Kaplan-Meier method. Solid line indicates SER-treated (n=13) while dotted line control (n=14) mice (*p<0.05, log-rank test).
Marginal hepatic RXFP1 expression in OLT with/without SER treatment
Having shown the hepatoprotective effect of SER treatment in IR-stressed OLT, we next assessed RXFP1 (cognate receptor of SER) expression, aiming to gain insight to underlying SER cytoprotection. Consistent with previous reports (13), unlike in normal murine heart (positive control), RXFP1 was undetectable by immunohistology in sham-operated livers (Fig. S1A). Neither OLT nor SER treatment affected hepatic RXFP1 expression, data confirmed by Western blots (Fig. S1B). Moreover, SER treatment failed to enhance RXFP1 levels in IR-stressed OLT. Hence, it is unlikely that RXFP1 signaling is essential in SER hepatoprotection.
SER mitigates leukocyte infiltration in IR-stressed OLT
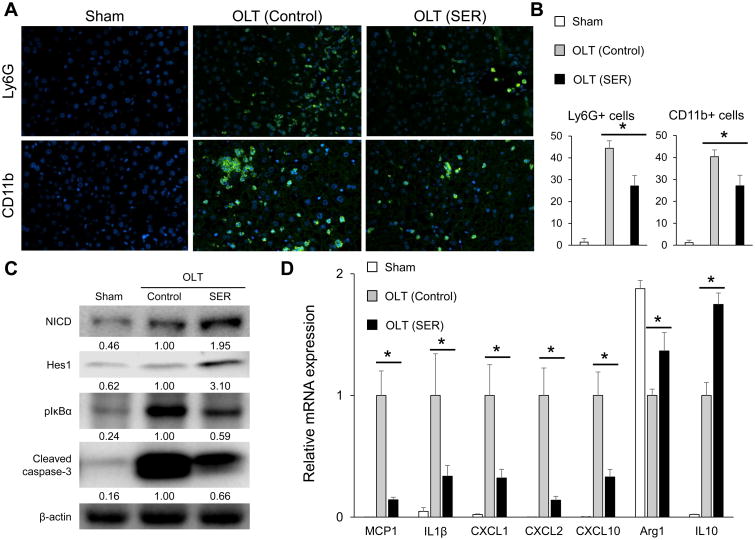
As cytokine/chemokine producing leukocytes in OLT accelerate IR-damage, we next evaluated the SER effect on neutrophil (Ly6G) and macrophage (CD11b) infiltration (n=5/group). As shown in Fig. 2A/B, decreased numbers of Ly6G+ and CD11b+ cells were observed in SER treated as compared with control OLT (Ly6G: control-OLT=44.4±3.5 vs. SER-OLT= 27.2±4.8/HPF; p=0.001; CD11b: control-OLT=39.6±2.2 vs. SER-OLT=26.6±5.7/HPF, p=0.002).
Figure 2. SER alleviates IR-inflammation and induces Notch1 signaling in mouse OLT.
C57/Bl6 donor livers subjected to extended cold storage (18h) were transplanted to syngeneic mice. OLT recipients were treated i.v. with SER or equivalent amount of lactate ringer solution (control) at reperfusion, followed by sampling (6h post-reperfusion). (A) Representative immunohistochemical staining of OLT-infiltrating Ly6G+ (neutrophil) and CD11b+ (macrophage) cells (B) Quantification of Ly6G+ and CD11b+ cells/HPF (n=5/group). (C) Western blot-assisted detection of NICD, Hes1, pIκBα (Ser32) and cleaved caspase-3. β-actin expression served as an internal control for normalization. The values under the bands represent the relative ratio of normalized intensity compared to OLT (Control). Representative of three experiments is shown. (D) Quantitative RT-PCR-assisted detection of mRNA coding for MCP1, IL1β, CXCL1, CXCL2, CXCL10, Arg1 and IL10. Data were normalized to HPRT gene expression (n=4/group). Data are shown as mean±SD (*p<0.05, Student t-test).
SER suppresses pro-inflammatory IR-signature and induces Notch1 signaling in OLT
As our recent study has identified the regulatory function of Notch1 for innate immune activation in liver IRI (18), we next asked whether SER may promote Notch1 signaling in OLT. Indeed, as shown in Fig. 2C, SER treatment was accompanied by upregulation of NICD (Notch-1 intracellular domain) and Hes1 (one of downstream Notch1 target genes). In addition, SER decreased pIκBα expression (Fig. 2C), depressed mRNA levels of pro-inflammatory MCP1, IL1β, CXCL1, CXCL2, CXCL10, and increased mRNA levels of anti-inflammatory Arg1, IL10 (Fig. 2D). Consistent with Fig. 1A/B findings, SER treatment decreased cleaved caspase-3 expression (terminal apoptosis executer) in OLT. Thus, hepatoprotective (Fig. 1) and anti-inflammatory (Fig. 2A/B/D) function of SER was accompanied by Notch1 induction in IR-stressed OLT.
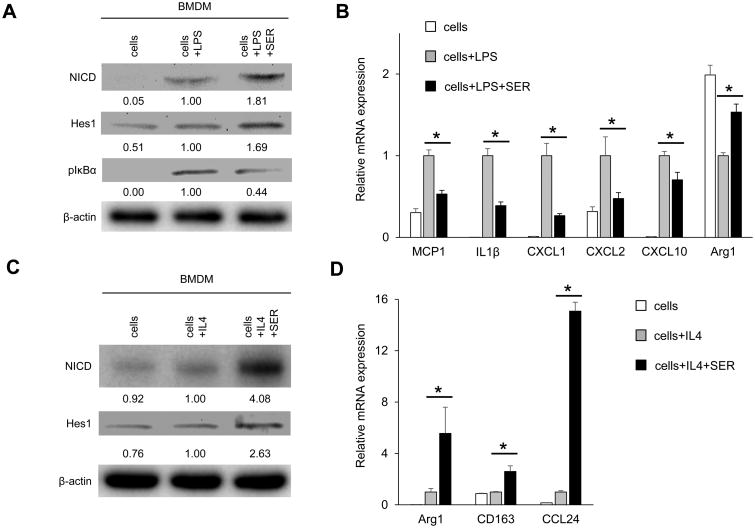
SER induces Notch1 signaling and inhibits LPS-stimulated macrophage activation in vitro
Since macrophages play a key role in innate immune activation in IR-stressed OLT, we next evaluated the impact of SER on bone marrow-derived macrophage (BMDM) cultures under LPS stimulation. Consistent with Fig. 2C/D data, SER enhanced Notch1 signaling (NICD and Hes1, Fig. 3A); decreased pIκBα protein expression (Fig. 3A); suppressed pro-inflammatory gene program (MCP1, IL1β, CXCL1, CXCL2, CXCL10); and increased immunoregulatory Arg1 signaling in LPS-stimulated BMDM (Fig. 3B). As shown in Fig. S2, increasing dose of SER treatment upregulated NICD, CD206 while decreasing pIκBα levels in cultured BMDM. Of note, the expression of RXFP1 and glucocorticoid receptor (GR, another SER receptor) remained unchanged, implying the importance of Notch1 signaling in macrophage regulation.
Figure 3. SER inhibits pro-inflammatory but enhances anti-inflammatory signature and up-regulates Notch1 signaling in BMDM cultures.
(A/B) BMDM stimulated with LPS (100ng/ml, 6h) were pretreated with or without SER (5μg/ml, 24h). (A) Western blot-assisted detection of NICD, Hes1 and pIκBα (Ser32). β-actin expression serves as an internal control for normalization. The values under the bands represent the relative ratio of normalized intensity compared to cells + LPS. Representative of three experiments is shown. (B) Quantitative RT-PCR-assisted detection of mRNA coding for MCP1, IL1β, CXCL1, CXCL2, CXCL10 and Arg1. Data were normalized to β2M gene expression (n=4/group). (C/D) BMDM stimulated with IL4 (10ng/ml, 24h) were pretreated with or without SER (5μg/ml, 24h). (C) Western blot-assisted detection of NICD and Hes1. β-actin expression served as an internal control for normalization. The values under the bands represent the relative ratio of normalized intensity compared to cells + IL4. Representative of three experiments is shown. (D) Quantitative RT-PCR-assisted detection of mRNA coding for Arg1, CD163 and CCL24. Data were normalized to β2M gene expression (n=4/group). Data are shown as mean±SD (*p<0.05, Student t-test).
SER enhances anti-inflammatory gene expression in IL4-stimulated macrophage cultures
We next examined whether SER may affect anti-inflammatory gene programs in vitro. Consistent with Fig. 2C/3A data, SER increased NICD and Hes1 expression in IL4-stimulated BMDM (Fig. 3C). In parallel, SER adjunct enhanced mRNA levels coding for anti-inflammatory Arg1, CD163 and CCL24 (Fig. 3D). These findings, consistent with our studies on critical role of Notch1 in macrophage immunoregulation (18), imply Notch1 in SER-mediated macrophage regulation.
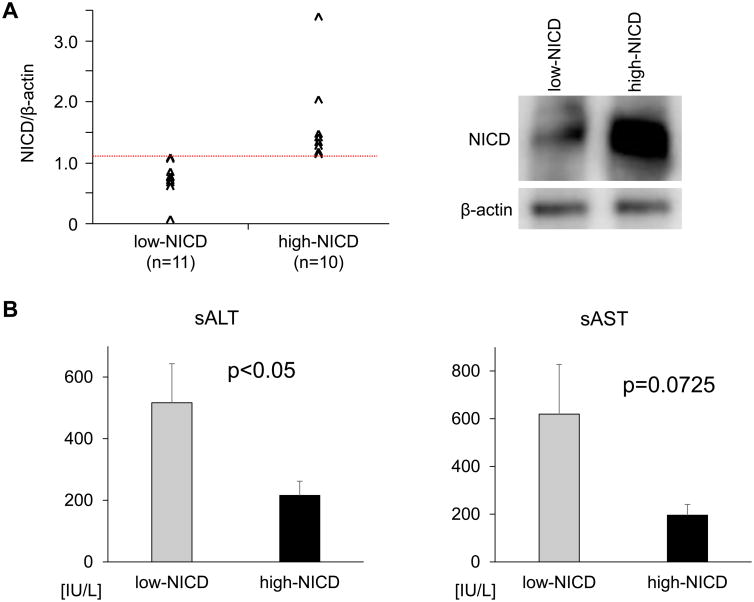
Increased NICD expression correlates with attenuated liver damage in human OLT
Having shown the regulatory function of Notch1 in SER-mediated hepatoprotection in mouse OLT, we next aimed to determine the clinical relevance of Notch1 signaling in human OLT. Post-reperfusion liver Bx from twenty-one human OLTs screened by Western blots, were classified into low-NICD (n=11) and high-NICD (n=10) expression groups (Fig. 4A left column; representative Western blots are shown in right column). The patients' demographic data and clinical parameters are shown (Table S2). There was no correlation between NICD expression and donor/graft background, including age, gender, weight, BMI cold ischemia time or pre-procurement blood tests (ALT, AST, T-Bil, INR). We found no correlation between NICD expression and recipient factors, including age, gender, weight, BMI, race, disease etiology, presence of HCC, ABO-compatibility, MELD score or pre-transplant blood tests. Notably, high-NICD expression group had significantly lower sALT (216±46 vs. 517±127 IU/L, mean±SEM, p=0.0486) and lower sAST (196±45 vs. 619±208 IU/L, mean±SEM, p=0.0725) levels at POD1 (Fig. 4B), indicating OLTs with increased NICD expression were relatively more resistant to IR-damage. To examine the relationship between NICD graft levels and clinical outcomes, we analyzed post-operative hospital stay and cumulative post-transplant survival with median follow-up of 712 days (range, 27-1009 days). None of the patients underwent secondary liver transplantation. Consistently, high-NICD expression group exhibited shorter post-OLT hospital stay (32.4±4.3 vs. 40.5±10.4 days, p=0.5253) and better post-OLT survival (500 days: 93.3% vs. 80.0%, p=0.3673). However, these differences did not reach statistical significance.
Figure 4. NICD levels correlate with the hepatocellular function in human OLT.
(A) Based on Western blot-assisted relative protein expression, twenty-one human OLT biopsies were classified into low-NICD (n=11) and high-NICD (n=10) groups (left panel). Representative NICD expression profile is shown in the right panel. (B) Hepatocellular function, assessed by serum ALT and AST levels at POD1. Data are shown as mean±SEM (Mann–Whitney U test).
Discussion
A recent phase III randomized clinical trial in a cohort of 1,161 patients with acute heart failure demonstrated that SER treatment was associated with an improvement of patients' symptoms and significant reduction of cardiovascular mortality through day 180 (8). By documenting the hepatoprotective effects of SER in a clinically relevant mouse IRI-OLT model, our findings support a new application of SER in organ transplantation. Indeed, SER given at the time of reperfusion in mice, mitigated liver damage as evidenced by preserved hepatic architecture and decreased hepatocellular death (Fig. 1A-C); improved post-OLT survival (Fig. 1D); inhibited local neutrophil/macrophage infiltration (Fig. 2A/B); and depressed pro-inflammatory signature (Fig. 2C/D). The aforementioned clinical trial has also demonstrated that except for a controllable hypotension, SER treatment was well tolerated and safe, with equivalent of adverse effects compared with placebo. With no adverse effects during SER treatment and improved OLT survival, our murine findings fortify its tolerable therapeutic use.
Liver IRI represents an innate immune-driven local inflammation, followed by hepatocellular death. By recognizing initial danger-associated molecular patterns and triggering TLR4 mediated inflammation, macrophages are key components of this immune response (1). Macrophage-derived MCP1 promotes monocyte/macrophage recruitment in IR-stressed OLT, which in turn accelerates local inflammatory cell death (24). Macrophages also produce neutrophil chemoattractant (CXCL1, CXCL2), which triggers neutrophil infiltration, reactive oxidative species diffusion, homeostasis disturbances and cell death (25). Consistent with these findings, in our current study, SER treatment suppressed MCP1/CXCL1/CXCL2 expression in macrophage cultures (Fig. 3B) and IR-stressed OLTs (Fig. 2D); attenuated macrophage/neutrophil infiltration into OLTs (Fig. 2A/B); and alleviated hepatocellular death (Fig. 1A/B), indicating immunoregulatory role on macrophage is one of the central features of SER-mediated hepatoprotection. On the other hand, we recently reported that myeloid Notch1-Hes1 signaling axis suppressed pro-inflammatory (MCP1/I1β) but enhanced anti-inflammatory (Arg1) gene programs, and as a consequence myeloid Notch1 KO mice suffered aggravated liver damage in warm IRI model (18). In our current study, SER-mediated in vivo hepatoprotection and in vitro macrophage regulation was accompanied by induction of Notch1-Hes1 and Arg1 (Fig. 2/3), while SER failed to alter RXFP1 or GR receptor expression in BMDM cultures (Fig. S2). These findings identify Notch1-Hes1 axis as the key pathway underlining macrophage regulation by SER. With previously reported contradictory RXFP1/GR function in macrophage regulation and divergent responses to relaxin between different macrophage origins (26) (27), their regulatory mechanisms await future in-depth studies.
To the best of our knowledge, our study is the first to document the beneficial impact of Notch1 induction in liver transplant patients. Indeed, high-NICD levels in hepatic Bx at the time of reperfusion correlated with lower sALT levels at POD1 (Fig. 4B left panel), with no correlation between NICD expression and donor/recipient baseline parameters (Table S2). As our failure to detect significant differences in sAST levels (Fig. 4B right panel), post-OLT hospital stay and post-OLT patients' survival might be due to a limited patient cohort (n=21), future studies with a larger number of OLT recipients are warranted.
RXFP1 was barely detectable in mouse livers, and neither OLT nor SER treatment altered its marginal hepatic expression (Fig. S1A/B). Indeed, Fallowfield reported that no RXPF1 protein was detected in naive human or rat livers, while RXFP1 became detectable on stellate cells/myofibroblasts and sinusoidal endothelial cells in fibrotic livers (13). However, despite minimal RXFP1 expression in our study, we cannot exclude a possibility that RXFP1 signaling may function as a component of the beneficial molecular crosstalk of SER in OLT. For example, one of the distinct actions of relaxin is mediated by RXFP1-PI3K-Akt-eNOS axis on artery and endothelial cells, whereas relaxin dilated sinusoid in un-stressed rat liver via NO-depending manner (28). Therefore, endothelial RXFP1 signal induction followed by sinusoid dilatation might be a part of hepatoprotective SER mechanism in OLT. Studies using RXFP1 KO mice or chemically modified RXFP1-inactive relaxin (29) are needed to substantiate the involvement of RXFP1 in SER-mediated hepatoprotection.
Notch1 signal deficiency by genetic RBP-J disruption decreased manganese superoxide dismutase while increasing ROS and hepatocyte apoptosis (17). In line with this finding, SER mediated Notch1 upregulation may exert direct cytoprotection in hepatocytes. On the other hand, SER primed hepatocyte GR signaling may also promote anti-apoptotic pathway (30). Indeed, SER cytoprotection was abolished after GR silencing using siRNA in mouse primary hepatocyte cultures, indicating dominant contribution of GR in hepatocyte protection by SER.
In conclusion, our preclinical study documents the efficacy of SER in graft protection and survival via Notch1 signaling in mouse OLT. Parallel clinical screening highlights protective function of Notch1 in liver transplant patients. In the context of a recent phase III clinical trial demonstrating promising outcomes of SER in patients with acute heart failure (8), our translational study supports the rationale for using SER in clinical liver transplantation.
Supplementary Material
Figure S1: Marginal liver RXFP1 expression is unaffected by OLT or SER treatment. (A) Representative (n=3) immunohistochemical staining of RXFP1 in mouse liver (Sham, control OLT and SER-treated OLT) and normal heart (positive control). (B) Representative (n=4) Western blot-assisted detection of RXFP1 in control OLT, SER-treated OLT and normal heart. α-Tubulin expression served as an internal control.
Figure S2: SER mitigates macrophage activation by inducing Notch1 signaling but not altering RXFP1/GR expression in vitro. Bone marrow-derived macrophages (BMDM) pretreated with SER (1-5μg/ml, 24h) were stimulated with LPS (100ng/ml) for 6h. Representative Western blot-assisted expression of NICD, RXFP1, GR, p-IκBα, IκBα and CD206. β-actin expression serves as an internal control and used for normalization. The values under the bands represent the relative ratio of normalized intensity compared to that of cells+LPS. Representative of at least three is shown.
Table S1: Primer sequences used for Real-Time Quantitative PCR
Table S2: Donor and recipient demographics. Post-transplant liver biopsy samples collected from twenty-one human liver transplant cases were analyzed by Western-bolts. Based on Western-blot assisted NICD expression, 21 cases were divided into low-NICD (n=11) and high-NICD (n=10) groups. Correlations between donor and recipient demographic parameters and post-transplant NICD expression were analyzed using Fisher's exact test for categorical variables and Mann–Whitney U test for continuous values. Values are expressed as median (range).
Acknowledgments
This work was supported by Novartis International AG; NIH grants PO1 AI120944; RO1 DK062357, DK107533, DK102110 (to JWKW); and The Dumont Research Foundation. We thank Ko Takanashi (UCLA-TPCL) and Damla Oncel (undergrad UCLA student) for immunohistochemical assistance; and Dr. Takahiro Ito and Antony Aziz for helping with clinical data collection.
Abbreviations
- BMDM
marrow-derived macrophages
- Bx
biopsy
- GR
glucocorticoid receptor
- Hes1
hairy and enhancer of split-1
- IL4
interleukin-4
- IRI
ischemia-reperfusion injury
- LPS
lipopolysaccharides
- NICD
Notch intracellular domain
- OLT
orthotopic liver transplantation
- POD1
post-operative day 1
- RLX-2
relaxin-2
- RXFP1
relaxin family peptide receptor-1
- sALT
serum alanine aminotransferase
- sAST
serum aspartate aminotransferase
- SER
Serelaxin
- TUNEL
TdT-mediated dUTP nick end labeling
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information: Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature reviews Gastroenterology & hepatology. 2013;10(2):79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Lascio G, Harmelin G, Targetti M, Nanni C, Bianchi G, Gasbarri T, et al. Cellular retrograde cardiomyoplasty and relaxin therapy for postischemic myocardial repair in a rat model. Texas Heart Institute journal. 2012;39(4):488–499. [PMC free article] [PubMed] [Google Scholar]
- 3.Sherwood OD, Crnekovic VE, Gordon WL, Rutherford JE. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- 4.Teichman SL, Unemori E, Teerlink JR, Cotter G, Metra M. Relaxin: review of biology and potential role in treating heart failure. Current heart failure reports. 2010;7(2):75–82. doi: 10.1007/s11897-010-0010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodhi RS, Nakabayashi K, Suzuki K, Yamada AY, Hazama R, Ebina Y, et al. Relaxin has anti-apoptotic effects on human trophoblast-derived HTR-8/SV neo cells. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2013;29(12):1051–1054. doi: 10.3109/09513590.2013.829444. [DOI] [PubMed] [Google Scholar]
- 6.Nistri S, Chiappini L, Sassoli C, Bani D. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(14):2109–2111. doi: 10.1096/fj.03-0216fje. [DOI] [PubMed] [Google Scholar]
- 7.Lekgabe ED, Kiriazis H, Zhao C, Xu Q, Moore XL, Su Y, et al. Relaxin reverses cardiac and renal fibrosis in spontaneously hypertensive rats. Hypertension (Dallas, Tex : 1979) 2005;46(2):412–418. doi: 10.1161/01.HYP.0000171930.00697.2f. [DOI] [PubMed] [Google Scholar]
- 8.Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet (London, England) 2013;381(9860):29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 9.Du XJ, Bathgate RA, Samuel CS, Dart AM, Summers RJ. Cardiovascular effects of relaxin: from basic science to clinical therapy. Nature reviews Cardiology. 2010;7(1):48–58. doi: 10.1038/nrcardio.2009.198. [DOI] [PubMed] [Google Scholar]
- 10.Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiological reviews. 2013;93(1):405–480. doi: 10.1152/physrev.00001.2012. [DOI] [PubMed] [Google Scholar]
- 11.Collino M, Rogazzo M, Pini A, Benetti E, Rosa AC, Chiazza F, et al. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. Journal of cellular and molecular medicine. 2013;17(11):1494–1505. doi: 10.1111/jcmm.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexiou K, Matschke K, Westphal A, Stangl K, Dschietzig T. Relaxin is a candidate drug for lung preservation: relaxin-induced protection of rat lungs from ischemia-reperfusion injury. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2010;29(4):454–460. doi: 10.1016/j.healun.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Fallowfield JA, Hayden AL, Snowdon VK, Aucott RL, Stutchfield BM, Mole DJ, et al. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology (Baltimore, Md) 2014;59(4):1492–1504. doi: 10.1002/hep.26627. [DOI] [PubMed] [Google Scholar]
- 14.Boehnert MU, Hilbig H, Armbruster FP. Relaxin as an additional protective substance in preserving and reperfusion solution for liver transplantation, shown in a model of isolated perfused rat liver. Annals of the New York Academy of Sciences. 2005;1041:434–440. doi: 10.1196/annals.1282.065. [DOI] [PubMed] [Google Scholar]
- 15.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nature reviews Genetics. 2008;9(1):49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 16.Bray SJ. Notch signalling: a simple pathway becomes complex. Nature reviews Molecular cell biology. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 17.Yu HC, Qin HY, He F, Wang L, Fu W, Liu D, et al. Canonical notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology (Baltimore, Md) 2011;54(3):979–988. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 18.Yue LuL, Jiang S, Li L, Zhu C, Ke QM, et al. Myeloid Notch1 Deficiency Activates RhoA/ROCK Pathway and Aggravates Hepatocellular Damage In Mouse Ischemic Livers. Hepatology (Baltimore, Md) 2017 doi: 10.1002/hep.29593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassoli C, Chellini F, Pini A, Tani A, Nistri S, Nosi D, et al. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-beta/Smad3 signaling. PloS one. 2013;8(5):e63896. doi: 10.1371/journal.pone.0063896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boccalini G, Sassoli C, Formigli L, Bani D, Nistri S. Relaxin protects cardiac muscle cells from hypoxia/reoxygenation injury: involvement of the Notch-1 pathway. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(1):239–249. doi: 10.1096/fj.14-254854. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Chen X, Cai JJ, Chen LZ, Gong YS, Wang LX, et al. Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway. Drug design, development and therapy. 2015;9:4599–4611. doi: 10.2147/DDDT.S85399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K, Kageyama S, Yue S, Huang J, Fujii T, Ke B, et al. Heme Oxygenase-1 Regulates Sirtuin-1 - Autophagy Pathway in Liver Transplantation: From Mouse-to-Human. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017 Nov 14; doi: 10.1111/ajt.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Zhang M, Kageyama S, Ke B, Fujii T, Sosa R, et al. Macrophage HO-1–SIRT1–p53 Axis Regulates Sterile Inflammation in Liver Ischemia–Reperfusion Injury. Journal of hepatology. 2017;67(6):1232–1242. doi: 10.1016/j.jhep.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology (Baltimore, Md) 2008;48(5):1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, et al. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology (Baltimore, Md) 2008;48(5):1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiredo KA, Mui AL, Nelson CC, Cox ME. Relaxin stimulates leukocyte adhesion and migration through a relaxin receptor LGR7-dependent mechanism. The Journal of biological chemistry. 2006;281(6):3030–3039. doi: 10.1074/jbc.M506665200. [DOI] [PubMed] [Google Scholar]
- 27.Horton JS, Yamamoto SY, Bryant-Greenwood GD. Relaxin modulates proinflammatory cytokine secretion from human decidual macrophages. Biology of reproduction. 2011;85(4):788–797. doi: 10.1095/biolreprod.110.089201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bani D, Nistri S, Quattrone S, Bigazzi M, Bani Sacchi T. The vasorelaxant hormone relaxin induces changes in liver sinusoid microcirculation: a morphologic study in the rat. The Journal of endocrinology. 2001;171(3):541–549. doi: 10.1677/joe.0.1710541. [DOI] [PubMed] [Google Scholar]
- 29.Dschietzig T, Bartsch C, Baumann G, Stangl K. RXFP1-inactive relaxin activates human glucocorticoid receptor: further investigations into the relaxin-GR pathway. Regulatory peptides. 2009;154(1-3):77–84. doi: 10.1016/j.regpep.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Bailly-Maitre B, de Sousa G, Zucchini N, Gugenheim J, Boulukos KE, Rahmani R. Spontaneous apoptosis in primary cultures of human and rat hepatocytes: molecular mechanisms and regulation by dexamethasone. Cell death and differentiation. 2002;9(9):945–955. doi: 10.1038/sj.cdd.4401043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Marginal liver RXFP1 expression is unaffected by OLT or SER treatment. (A) Representative (n=3) immunohistochemical staining of RXFP1 in mouse liver (Sham, control OLT and SER-treated OLT) and normal heart (positive control). (B) Representative (n=4) Western blot-assisted detection of RXFP1 in control OLT, SER-treated OLT and normal heart. α-Tubulin expression served as an internal control.
Figure S2: SER mitigates macrophage activation by inducing Notch1 signaling but not altering RXFP1/GR expression in vitro. Bone marrow-derived macrophages (BMDM) pretreated with SER (1-5μg/ml, 24h) were stimulated with LPS (100ng/ml) for 6h. Representative Western blot-assisted expression of NICD, RXFP1, GR, p-IκBα, IκBα and CD206. β-actin expression serves as an internal control and used for normalization. The values under the bands represent the relative ratio of normalized intensity compared to that of cells+LPS. Representative of at least three is shown.
Table S1: Primer sequences used for Real-Time Quantitative PCR
Table S2: Donor and recipient demographics. Post-transplant liver biopsy samples collected from twenty-one human liver transplant cases were analyzed by Western-bolts. Based on Western-blot assisted NICD expression, 21 cases were divided into low-NICD (n=11) and high-NICD (n=10) groups. Correlations between donor and recipient demographic parameters and post-transplant NICD expression were analyzed using Fisher's exact test for categorical variables and Mann–Whitney U test for continuous values. Values are expressed as median (range).