Abstract
Long-term success of heart transplantation is hindered by humoral and cell-mediated immune responses. We studied preexisting antibodies to two cardiac self-antigens, myosin and vimentin, and exosomes induced by antibodies to self-antigens in eliciting immune responses to cardiac grafts. After syngeneic heterotopic murine heart transplantation, rabbit anti-myosin or normal rabbit immunoglobulin was administered at days 0 or 7. Sera were collected after heartbeat cessation and cellular infiltration was analyzed, and exosomes were isolated from sera. Histopathology of controls' transplanted hearts demonstrated normal architecture, and their sera demonstrated neither antibodies to self-antigens nor exosomes expressing self-antigens. Administration of antibodies to cardiac myosin immediately post-transplant (day 0) but not day 7 triggered graft failure on day 7, and histopathology revealed marked cellular infiltration with neutrophils and lymphocytes. Rejected heart histopathology also demonstrated myocyte damage and sera had increased antibodies to myosin and vimentin, and development of exosomes expressing self-antigens. Administration of exosomes isolated from failed grafts containing self-antigens induced graft dysfunction; exosomes isolated from stable mice did not induce graft failure. Antibodies to self-antigens can induce exosomes containing self-antigens, initiating an immune response and causing graft failure after cardiac transplantation.
Introduction
Organ transplantation is a therapeutic option for patients with end-stage organ disease. Chronic rejection is a major impediment to the long-term survival of heart allografts following heart transplantation (HTx), and results from a recurrent immune response to the transplanted organ, affecting mainly the arteries and capillaries (1). During the rejection process, normal tissue architecture is replaced by fibrous scar tissue (2). Cardiac allograft vasculopathy (CAV) is a feature of chronic rejection, characterized by occlusive narrowing of coronary vessels within the intramyocardial microvasculature (3, 4). Studies have demonstrated that CAV develops in about 50% of transplant recipients within 7 years (5), and the development of CAV can be either antigen-dependent or antigen-independent. Recent studies have demonstrated that autoimmune responses may play an important role in the pathogenesis of CAV (6-8).
Antibody-mediated rejection can result in allograft dysfunction after HTx, and is characterized by capillary injury, C4d deposition, and presence of CD68-positive cells in endomyocardial biopsies. Development of donor-specific antibodies (DSA) to mismatched donor human leukocyte antigen (HLA) has also been reported to be involved in chronic rejection.(9, 10) A strong correlation exists between de novo development of DSA and both acute and chronic cardiac allograft rejection (9, 11-13). In addition, recent reports strongly support that acute and chronic rejection after transplantation of the heart (14), lungs (15), and kidneys (14, 16) may be associated with immune response to non-HLA antigens. Furthermore, preexisting antibodies (Abs) to tissue-restricted self-antigens (SAgs) have been associated with primary graft dysfunction, de novo development of Abs to donor HLA, and increased risk of chronic rejection after human lung transplantation (17). Preexisting Abs to angiotensin 2 receptor have also been shown to lead to antibody-mediated rejection following human kidney transplantation (18).
Cardiac myosin (MYO) is an autologous contractile protein, a SAg that is recognized by both T- and B-cells during rejection.(19) Murine HTx models have shown that sensitization with MYO before transplantation can lead to accelerated rejection of not only allogeneic grafts, but also of syngeneic heart grafts (19). Another tissue-specific SAg implicated in the development of CAV is the intermediate filament protein vimentin (VIM), which is found in cells of mesenchymal origin (20). Recent studies have demonstrated that increased levels of Abs to MYO and VIM are significantly associated with the development of DSA and CAV after HTx (21).
Recent findings indicate that exosomes may play a role in tissue rejection. Exosomes are vesicles that measure roughly 40-100 nm in diameter, originated by endocytic pathway and released into body fluids with mRNA, cytosolic proteins, and micro RNA (miRNA) (22-25). Abs to SAgs can lead to exosome formation, and the role of Abs to SAgs in inducing graft rejection remains to be determined. The aim of this study was to determine the role of Abs to MYO in inducing exosomes, and to define their role in graft failure after murine syngeneic HTx. We demonstrate that exosomes are induced following administration of Abs to cardiac MYO, and that the circulating exosomes express not only SAg MYO but also VIM, and can result in the failure of heterotopically transplanted syngeneic hearts. Further immunization with exosomes containing cardiac SAgs can also result in graft failure.
Materials and Methods
Mice
Six- to twelve-week-old male C57BL/6 (H-2b) mice were obtained from the Jackson Laboratory and housed in the animal care facility at Norton Thoracic Institute. These studies were approved by the Institutional Animal Care Committee at our institution. Five animals per group were used for all of the experiments
Heterotopic cardiac transplantation
Syngeneic (C57BL/6 to C57BL/6) heterotopic HTx was performed in the abdominal cavity, as previously described (26). Briefly, the donor's ascending aorta and the pulmonary trunk from the heart graft was anastomosed end-to-side to the recipient's intrarenal abdominal aorta and inferior vena cava, respectively, using 10–0 sutures. Cold ischemic times were less than 30 minutes.
Administration of rabbit anti-MYO Abs
Rabbit anti-MYO Abs was prepared by immunization of purified cardiac MYO (New England Peptide LLC, Gardner, MA). Anti-MYO immunoglobulin G (IgG) was purified using Protein A column, according to manufacturer protocol (Sigma Aldrich, St. Louis, MO). Titer of rabbit anti-MYO analyzed by enzyme-linked immunosorbent assay (ELISA) was 1:64,000. Abs were tested for endotoxins (<1.67 Eu/mg) using the by limulus amebocyte lysate assay. Purified rabbit anti-MYO IgG (200μg/ml in 100 μl) was administrated intraperitoneally following syngeneic murine HTx on days 0 and 7. Failure of the transplanted organ was defined by cessation of a palpable heartbeat, and was confirmed by histology.
Assessment of histological changes
Cardiac grafts were obtained following cessation of palpable beat (day 7) or from functioning syngeneic transplants (day 30) and were fixed with paraformaldehyde and embedded in paraffin. 5 μm-sections were stained with hematoxylin and eosin to assess histopathological changes.
Detection of Abs to SAgs (MYO and VIM)
Serum concentration of Abs to cardiac SAgs (ie, MYO and VIM) was measured by ELISA. Human and murine MYO is highly conserved (27), therefore, we used human NYO as antigen. In brief, 96 well plates (ThermoFisher Scientific, Waltham, MA) were coated with 1μg/ml of human MYO (Sigma Aldrich) or recombinant purified VIM (prepared in our laboratory). Sera collected from failed and functioning grafts were added to the plate (1:100 dilution) and were incubated at 4°C overnight. Rabbit anti-MYO and anti-VIM (Santa Cruz Biotechnology, Dallas, TX) were used as positive controls. A secondary Ab conjugated by horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added and developed with tetramethylbenzidine substrate (Millipore, Billerica, MA). The ELISA plates were read at 460 nm wave length and the concentration of Abs was calculated based on a standard curve run in the same plate. The positivity of the sample was calculated with 2× mean±standard deviation concentration of Abs to MYO and VIM in the control; this was used as cutoff to determine positive titers of Abs in experimental samples.
ELISpot
The frequency of cells secreting SAg-specific IFN-γ, TNF-α and IL-17 cytokines were determined by ELISpot as described previously (17, 28). In brief, Abs to IFN-γ, TNFα, IL-17, and IL-10 (5 μg/ml) were coated in the 96 well plate and incubated at 4°C overnight (BD Biosciences, San Jose, CA). The plates were washed and blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS). CD4 T cells isolated from spleen were incubated in the presence of either MYO (20 μg/ml) or VIM (20 μg/ml) for 48 hours (IL-10, IFN-γ, and TNFα) and 72 hours (IL-17). Biotinylated Abs (3μg/ml) specific for IL-10, IFN-γ, TNFα, and IL-17 (BD Biosciences) were added, followed by horseradish-peroxidase-labeled streptavidin (1:300; BD Biosciences Pharmingen, San Diego, CA). The spots were developed using amino-9-ethylcarbazole solution (BD Biosciences Pharmingen). Spots were then analyzed (ImmunoSpot Series 1; Cellular Technology Limited, Cleveland, OH) and the results were reported as spots per million (spm).
Exosome isolation from serum by ultracentrifugation
Exosomes from sera were isolated using the ultracentrifugation method (29). Briefly, sera were centrifuged at 2,000g for 30 minutes at 4°C and thereafter at 10,000g for 30 minutes at 4°C to remove cells and cell debris. Sera were diluted with 1X PBS and centrifuged at 10,000g for 90 minutes at 4°C. The pelleted exosomes were solubilized with 1X PBS and used for further analysis. Exosome protein concentration was quantified using the Pierce bicinchoninic acid protein assay kit (ThermoFisher Scientific, Waltham, MA). Exosome purity was verified by exosome-specific marker CD63 by immunoblot (30). Exosomes were validated by using sucrose cushion method as described by Gunasekaran et al (31) and also by size determination (40-100nm) using transmission electron microscopy.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis and Immunoblot
Serum exosomes were resuspended in radioimmunoprecipitation assay buffer. Exosome proteins were diluted in 4X Laemmli buffer (Bio-Rad Laboratories, Hercules, CA) and incubated at 95°C for 5 minutes. Samples were loaded onto 10-12% pre-cast polyacrylamide gel (Bio-Rad) and bands were electroblotted to a polyvinylidene fluoride membrane (Bio-Rad). For analysis of MYO and VIM, membranes were probed with Abs to MYO and VIM (Santa Cruz Biotechnology). Membranes were probed with specific secondary Abs conjugated with horseradish peroxidase. Membranes were exposed to x-ray films to detect staining.
Immunogold staining
For immunogold staining of exosomes, formvar carbon-coated copper grids were probed with mouse anti-CD63 Ab (1:100) or mouse anti-MYO or mouse anti-VIM (1:50) for 30 minutes, followed by specific secondary Ab conjugated to 18 nm colloidal gold (Jackson ImmunoResearch Laboratories). Grids were washed and stained with uranyl acetate and staining was observed by transmission electron microscopy (JEOL USA Inc., Peabody, MA).
miRNA profiling
Exosomes were isolated from pooled sera (collected from 5 mice). The mirVana miRNA isolation kit (Applied Biosystems, Foster City, CA) was used to isolate miRNA from exosomes. Isolated miRNAs were subjected to the GeneChip miRNA array (ThermoFisher Scientific) for miRNA profiling. Partek Genomics Suite 6.6 software (St. Louis, MO) was used to analyze differentially expressed exosomal miRNAs. The heat map for miRNA in rejected and stable samples was generated by R statistics software.
TaqMan miRNA reverse transcription polymerase chain reaction
Total RNA from sera exosomes was isolated using mirVana miRNA isolation kit (Applied Biosystems) and reverse-transcribed using TaqMan miRNA Reverse Transcription Kit (Applied Biosystems). Real-time polymerase chain reaction (RT-PCR) was done by TaqMan miRNA assays as described by Xu et al (32).
Immunization of mice with exosomes
Exosomes were isolated from the sera of mice after cessation of heartbeat following HTx or from mice with stable syngeneic grafts. Exosomes (100 μl of 100 μg/ml) were injected into mice subcutaneously with incomplete adjuvants on days 0, 3, and 7 following syngeneic HTx. Sera was collected on days 3, 7, and 15 and was used to measure Abs to SAgs. Following cessation of the heartbeat, spleens were isolated for detecting SAgs specific to cellular immune responses and induced cytokines.
Statistical analysis
Statistical data were expressed as mean±standard deviation. All the p values were calculated using two tail T test using graph pad prism 5. P values less than 0.05 were considered statistically significant.
Results
Administration of Abs to cardiac MYO immediately after syngeneic HTx leads to graft failure
To determine the role of Abs to cardiac MYO in HTx, we performed syngeneic heterotopic HTx in C57BL/6 mice, and rabbit anti-cardiac MYO or normal rabbit sera (200μg/ml of purified IgG) were administrated immediately after the transplant (day 0). As shown in Figure 1–A1, administration of normal rabbit IgG had no effect on syngeneic heart grafts, and palpable heartbeats were noted until the time of sacrifice (30 days). In contrast, mice immunized with anti-MYO IgG administered immediately after transplantation induced inflammatory cell infiltration and damage to cardiac myocytes, resulting in graft failure and cessation of heartbeat by day 7 (Figure 1–A2). Therefore, Abs to SAgs present at the time of transplantation may activate an inflammatory cascade, leading to graft failure. Furthermore, Abs administered on day 7 after syngeneic HTx had no effect on transplanted hearts, as these grafts remained functional up to 30 days following transplantation (Figure 1–A3). Anti-MYO administration had no effect on the native hearts, which showed normal architecture without any cellular infiltration. Morphometric analysis was used to quantitate cellular infiltration which demonstrated increased number of lymphocytes in rejected mice when compare to control (Supporting Information Table 1). Survival curve (Figure 1B) showed that median survival of mice with anti-MYO on day 0 is day 7 and for other group is day 30 when mice were sacrificed with functioning hearts.
Figure 1. Histopathology and development of immune response after administration of anti-MYO and rejection of syngeneic hearts.
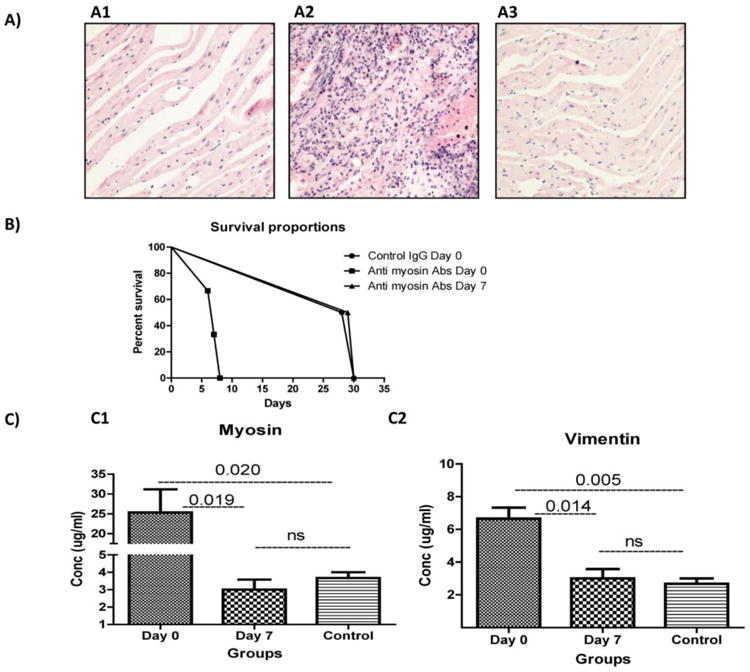
(A) Hematoxylin and eosin staining of donor heart tissues were analyzed after graft failure. Normal immunoglobulin G (IgG) (A1) and administration of anti-MYO IgG on day 7 (A3) showed normal architecture of the heart. Day 0 (A2) showed cellular infiltration and myocyte damage. (B) Survival curve: Survival comparison for three groups: control IgG on day 0 versus anti-MYO on days 0 and 7.
(C) Serum of recipient mice on day 0 developed significantly higher titers of MYO (B1) and VIM (B2) compared to the serum of recipient mice on day 7. All data were normalized with (results from normal mice subtracted from results obtained from transplant mice). Five animals/group.
Development of Abs to SAgs in mice injected with anti-MYO Abs
In order to determine whether Abs to SAgs developed in mice with cessation of heartbeat and in isograft controls following injection of Abs to SAgs and isotype controls, ELISA was used to measure rabbit Ab titer and Abs to SAgs in sera collected from both groups. Sera collected on day 2 following transplantation of mice had a rabbit anti-MYO titer of greater than 1:1000 by ELISA using anti-rabbit as a secondary Ab. Further, our results showed that mice injected with rabbit anti-MYO IgG after HTx experienced development of murine Abs not only to cardiac MYO 25ug/ml (Figure 1–C1) but also to VIM 8 μg/ml (Figure 1–C2) compared with mice injected with anti-MYO on day 7 (p=.0190 in MYO; p=.0142 in VIM). Specificity of the reaction to cardiac SAg is evident since the sera did not react to other tissue restricted SAgs, ie, kidney associated SAgs collagen type IV and fibronectin (data not shown).
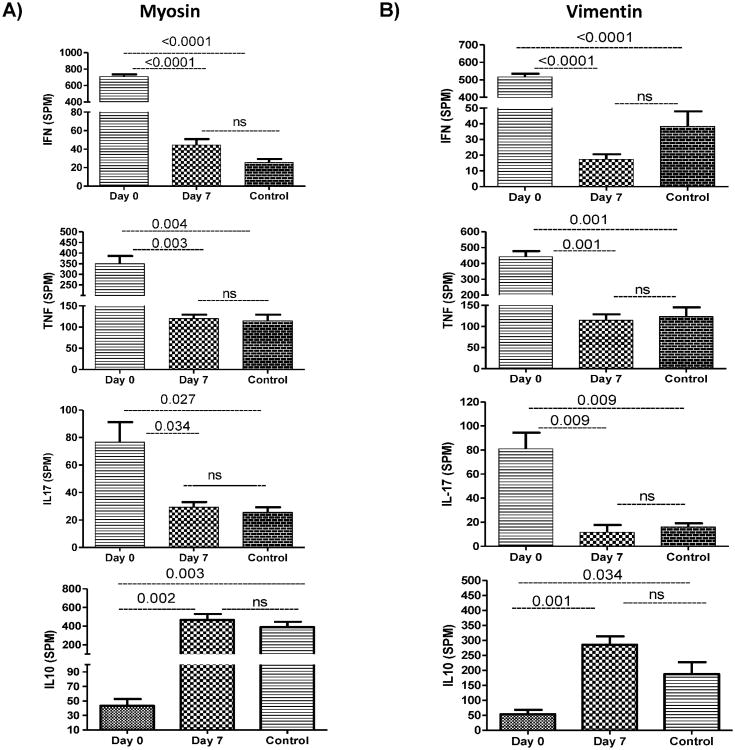
Increased frequency of cardiac-SAg-specific IFN-γ, TNF-α, and IL-17-secreting CD4 T cells
The frequency of cytokine-producing cells against SAgs in mice with cessation of heartbeat and isograft controls was enumerated by ELISpot. Figure 2A demonstrates increased MYO-specific secretion of IFN-γ (708 spm; p=<.001), TNF-α (349 spm; p=.004), Th17 (81 spm; p=.003) and decreased IL-10 (43 spm; p=.003). Figure 2B shows VIM-specific secretion of IFN-γ (517 spm; p<.001), TNF-α (442 spm; p=.001), IL-17 (55 spm; p=.002) cells, and significant decreases in IL-10 (53 spm; p=.002) after administration of rabbit anti-MYO IgG immediately following transplantation. Animals administered with anti-MYO on day 7 showed very little SAg-specific secretion of IFN-γ (MYO: 18 spm; VIM: 17 spm), TNF-α (MYO: 120 spm; VIM 114 spm) and IL-17 (MYO: 11 spm; VIM: 7 spm) cells, with significant increase in IL-10 (MYO: 465 spm; VIM: 285 spm).
Figure 2. Increased frequency of cells secreting SAg-specific T-cells.
(A) Recipient who received anti-MYO IgG on day 0 showed increased frequency of TNF-α, IFN-γ, and IL-17, as well as decreased frequency of IL-10-secreting CD4+ T-cells specific to MYO compared with a recipient who received anti-MYO IgG on day 7. (B) Recipient with administration of anti-MYO IgG on day 0 showed increased frequency of TNF-α, IFN-γ, and IL-17, as well as decreased frequency of IL-10-secreting CD4+ T-cells specific to VIM compared with recipient who received anti-MYO IgG on day 7. All data were normalized with normal mice data (results from normal mice subtracted from results obtained from transplant mice). Five animals/group.
Exosomes are induced following administration of anti-MYO
In our analysis of whether exosomes are induced following administration of anti-MYO Abs in mice with cessation of heartbeat and in stable isograft controls, Western blot analysis demonstrated the presence of MYO and VIM (Figure 3A). Significant expressions of MYO and VIM were observed in exosomes isolated from mice with graft failure, and markedly lower levels of the same were seen in the controls. Densitometry data (Figure 3B) demonstrate a threefold change in expression of SAgs in the isolated exosomes. Result of sucrose cushion method demonstrated that exosomes are pure and having size of 40-100nm by TEM and expressed exosome specific marker CD63. (Supporting Information Figure 1)
Figure 3. Expression of cardiac SAgs in exosomes.
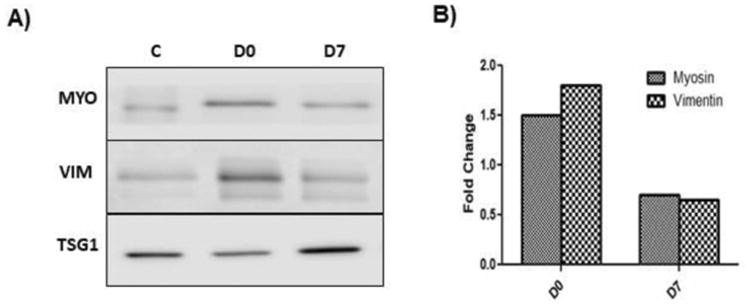
(A) Serum-derived exosomes were isolated from a recipient who received anti-MYO IgG on day 0. Western blot analysis showed the presence of the cardiac SAgs MYO and VIM compared to exosomes isolated from recipient mice who received anti-MYO IgG on day 7 and normal IgG. (B) Densitometry analysis showed increased fold changes of MYO and VIM expression. All data were normalized with normal mice data. C: Control exosomes isolated from mice who received normal IgG on Day 0. D0: Exosomes isolated from recipient mice who received anti-MYO IgG on day 0. D7: Exosomes isolated from recipient mice who received anti-MYO IgG on day 7.
Cardiac SAgs MYO and VIM are detectable on the surfaces of exosomes
Exosomes isolated from mice following heartbeat cessation after administration of anti-MYO on day 0 and exosomes isolated from control mice (control or anti-MYO administered on day 7) were analyzed for the surface expression of SAgs by immunogold transmission electron microscopy. Exosomes were less than 100 nm in diameter and were stained specifically by the exosome-specific marker CD63. Exosomes isolated from sera following graft failure expressed cardiac SAgs MYO and VIM, which are undetectable on exosomes isolated from controls (Figure 4). These results indicate that anti-MYO induces exosomes that contain cardiac SAgs MYO and VIM. Intriguingly, SAgs present on the surface of exosomes may play an important role in eliciting immune responses to SAgs leading to graft failure following HTx.
Figure 4. Exosome Immunogold staining.
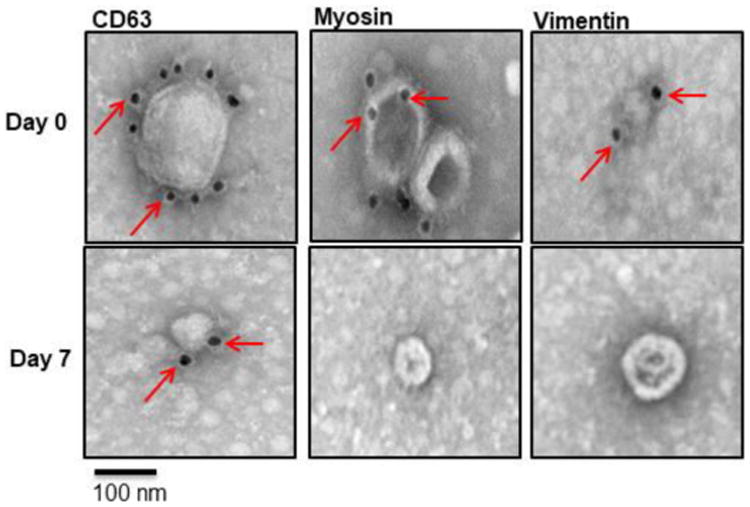
Immunogold transmission electron microscopy demonstrated that exosomes from day 0 recipient mice expressed MYO and VIM on their surface when compared with exosomes from day 7 recipient mice. CD63 is the exosome marker used as control.
Immunoregulatory miRNAs in exosomes
To investigate the potential contribution of serum-derived exosomic miRNA involved in the induction of inflammation that leads to heart graft failure, we identified the miRNA content of exosomes isolated from sera of mice with failed grafts (mice with anti-MYO on day 0) and from controls (normal IgG on day 0 mice and rabbit anti-MYO on day 7). Heat map comparisons of exosomes following graft failure and controls are shown in Figure 5A miRNA analysis demonstrates that 107 miRNAs were detectable in exosomes isolated from rejected mice after administration of the anti-MYO Ab. All 107 miRNAs were elevated after graft failure following administration of anti-MYO on day 0 when compared with levels seen in the controls (ie, anti-MYO administered on day 7 after HTx). We selected 6 miRNAs (miR-10, miR-155, miR-142-5p, and miR-133b, miR-21, and miR-31) based on literature, which were significantly increased in the anti-MYO-induced animals with graft failure compared with the controls.
Figure 5. Presence of miRNA involved in regulation of immune responses.
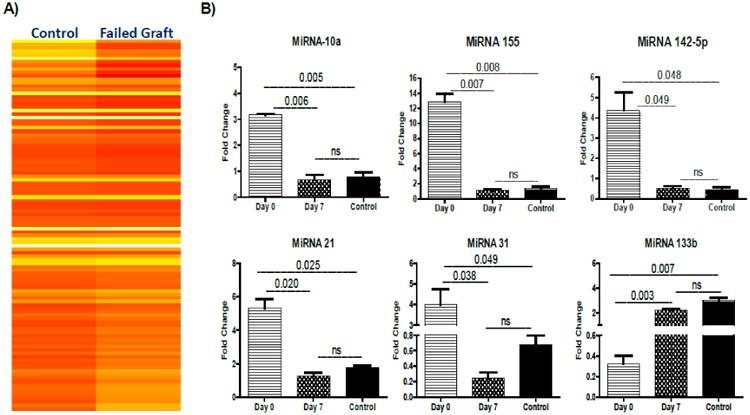
(A) miRNA analysis in failed graft and in stable mice (exosomes isolated from HTx mice injected at day 0 with anti-MYO IgG versus control (normal IgG plus sera from anti-MYO given on day 7) is shown in the heat map. The expression of miRNA refers to relative fold change between graft failure and control HTx recipients. The color code range from yellow (negative) to red (positive) indicates the value of fold difference.(B) Differential miRNAs validation: Expressions of miRNA involved in inflammation (miR-10b, miR-155, miR-142-5p) and fibrosis (miR-21, miR-31, miR-133b) were quantified by TaqMan RT-PCR. Relative expressions of these miRNAs were higher in exosomes from day 0 recipient mice expressing MYO and VIM on their surface compared with exosomes from day 7 recipient mice.
Quantitative RT-PCR was performed for specific miRNAs to validate the higher levels observed in miRNA profiling array: miR-10, miR-155, miR-142-5p, and miR-133b, miR-21, and miR-31. We found significant upregulation of miRNAs involved in inflammation (miR-10a [3.14-fold, p=.006], miR-142-5p [3.48-fold, p=.049], and miR-155 [13.93-fold, p=.007]) in mice following graft failure compared with controls (Figure 5B). Furthermore, we observed significant upregulation of miRNAs involved in fibrosis (miRNA 133b [6.25-fold, p=.003], miR-21 [4.76-fold, p=.020], and miR-31 [3.25-fold, p=.038]). Exosomes from control animals did not show significant changes (miR-10: 0.48-fold, miR-142-5p: 0.62-fold, miR-155: 1.62-fold, miR-133b: 2.38-fold, miR-21: 1.87-fold, miR-31: 0.55-fold). These results indicate that miRNAs that have been shown to be involved in inflammation and fibrosis were significantly increased in the exosomes isolated from mice that experienced cardiac grafts failure induced by anti-MYO administered day 0. This finding suggests that biologically active miRNA transferred through circulatory exosomes may contribute to inflammation and fibrosis.
Administration of exosomes from failed syngeneic grafts following the administration of anti-MYO-induced rejection of syngeneic cardiac grafts
To determine the immunogenicity of exosomes expressing SAgs, we administered exosomes containing SAgs, isolated from sera following cessation of heartbeat induced by anti-MYO and compared these exosomes to those of the controls (normal or anti-MYO on day 7). The results demonstrated that exosomes containing SAgs isolated from mice after cessation of heart function (ie, anti-MYO administered on day 0), induced cellular infiltration and myocyte damage to syngeneic heart grafts, resulting in cessation of heartbeat on day 7 (Figure 6A). Quantification of infiltration showed presence of higher number of neutrophils with lymphocytes (data shown in Supporting Information Table 1)
Figure 6. Immunization with exosomes isolated from sera of rejected syngeneic graft induces rejection and immune responses to SAgs.
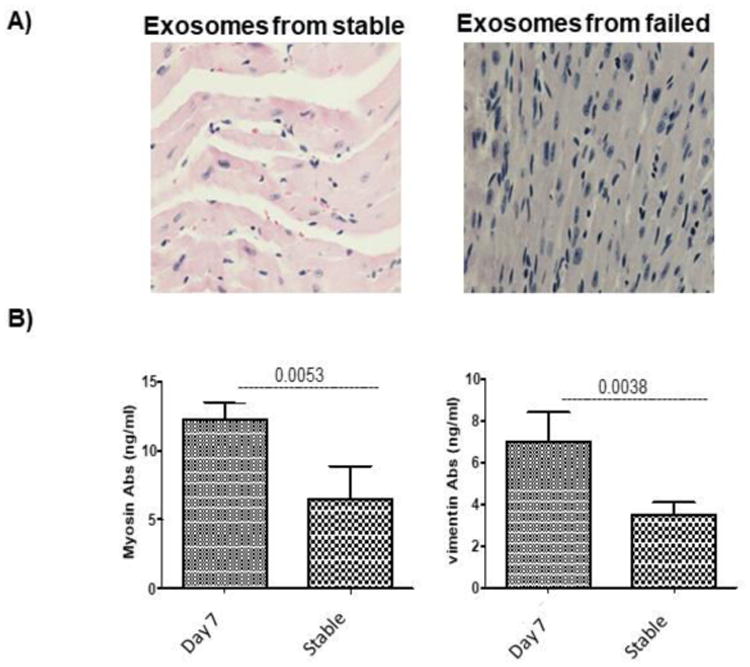
Exosomes containing the SAgs MYO and VIM were isolated from sera of rejected mice and exosomes from stable mice immediately following HTx. (A) Administration of exosomes from the sera of mice who experienced graft rejection induced cellular infiltration and myocyte damage to syngeneic heart grafts, resulting in rejection on day 7 (the controls did not experience rejection). (B) De novo development of Abs to SAgs in sera shows that exosomes containing SAgs induced Ab development to cardiac SAgs MYO and VIM in mice who experienced graft failure compared with control mice who did not experience graft failure. Five animals/group.
Furthermore, the addition of exosomes induced higher levels of Abs to cardiac SAgs MYO (12 μg/ml vs 6.5ug/ml, p=.005) and VIM (7 μg/ml vs 3.5ug/ml, p=.003) when compared with controls (Figure 6B). We also administered exosomes prepared from rejected animals on days 6 and 7 without adjuvant which resulted in rejection of HTx by day 7, similar to the results obtained when anti-MYO was given immediately following heterotopic HTx (data not shown).
Discussion
Several reports have demonstrated that mice receiving cardiac allografts not only developed immune responses to alloantigens, but also to cardiac MYO, a tissue-restricted SAg (19, 33, 34). Furthermore, prior sensitization to cardiac MYO led to accelerated rejection of the murine cardiac allografts, indicating a pathogenic role for autoimmune responses in the rejection process (19, 33, 34). Similar results have been reported in a preclinical miniature swine model in which pre-transplant activation of cardiac MYO-specific T-cells has accelerated rejection (35). In this study, we found T cells to accelerate rejection and also found that administration of Abs specific to the cardiac SAg MYO can lead to graft failure following syngeneic HTx. Our data demonstrate, for the first time, that administration of Abs to cardiac MYO immediately after syngeneic HTx results in induction of exosomes from the transplanted organ containing cardiac SAgs, which augments immune responses to SAgs and results in graft failure.
To determine the mechanisms contributing to failure of syngeneic cardiac grafts following administration of anti-MYO, we administered anti-MYO either immediately after transplantation (on day 0) or on day 7 after transplantation. In mice who received Abs to MYO on day 0, we found that the grafts stopped functioning on day 7. However, the grafts did not stop functioning when Abs to MYO was administered on day 7. In addition, we did not find any cellular infiltration or myocyte damage to the native heart. These results suggest that ischemia-reperfusion injury and exposure of immunogenic epitopes of cardiac MYO to MYO Abs leads to activation of an immune cascade, ultimately resulting in rejection. These results have important clinical ramifications, as it is likely that patients awaiting HTx, including those with left ventricular assist devices, may develop Abs to cardiac SAgs (36). Patients with preexistingAbs after HTx may experience immediate graft dysfunction and may be predisposed to development of Abs to mismatched donor HLA, leading to rejection. In this context, studies from our laboratory have demonstrated that preexisting Abs to two lung SAgs (ie, collagen V and Kα1 Tubulin) can predispose individuals to primary graft dysfunction, and these patients are at increased risk for development of Abs to mismatched donor HLA as well as chronic rejection (17).
To further define the mechanisms leading to development of immune responses to cardiac SAgs resulting in graft failure, we analyzed the development of Abs to SAgs from sera collected from both mice following cessation of heartbeat and from stable syngeneic grafts. We found that administration of anti-MYO induced de novo Abs to cardiac MYO, but also to VIM—indicating a spread of immune responses to multiple cardiac SAgs (Figure 1). In addition, we observed activation of T-cell immune responses to MYO and VIM, as measured by the high frequency of cells secreting antigen-specific IFN-γ, TNF-α and IL-17 (Figure 2). Furthermore, SAg-specific IL- 10 responses were significantly reduced, suggesting that peripheral tolerance to SAgs is lost by the augmentation of pro-inflammatory cytokines, leading to graft failure.
Exosomes isolated from both groups were used to study the presence of exosomes on the surface by transmission electron microscopy. The results demonstrate the presence of nanovesicles (mean diameter: 80 nm) expressing CD63- and TSG-101-exosome-specific markers on the surface as well as cardiac SAgs MYO and VIM. Exosomes isolated from both stable HTx recipients (anti-MYO on day 7 and normal rabbit IgG-stable cardiac transplants) demonstrated exosome-specific markers (CD63, TSG1), but did not express cardiac SAgs (Figure 4). These results suggest that, ischemia-reperfusion injury may be responsible for exposing self-antigens epitope. These SAgs augment immune responses due to the immunoregulatory miRNA as well as the costimulatory molecules and proteasomes known to be present in the exosomes. We demonstrated that exosomes isolated from sera of failed grafts contained miRNA involved in inflammation (ie, miR-10a, miR-155 and miR-142-5p) and fibrosis (ie, miR-21, miR-31, and miR-133b) (31, 32). RT-PCR results also confirmed upregulation of immunoregulatory miRNAs, including miR-155, miR-21, and miR-31 in exosomes from these mice but not from stable mice, supporting the concept that exosomes with cardiac SAgs can augment immune responses.
The role of exosomes in inducing the immune responses that lead to graft failure was investigated by administration of exosomes from mice with failed grafts and control mice following syngeneic HTx. We found that exosomes containing or expressing SAgs induced immune responses against the cardiac SAgs MYO and VIM, resulting in graft failure by day 7. In addition, these mice developed Abs and cellular immune responses to cardiac SAgs. Figures 6A and B demonstrate that exosomes isolated from mice with failed grafts can induce immune responses in normal mice, leading to development of Abs to cardiac SAgs MYO and VIM. These results support the concept that exosomes are indeed immunogenic and play an important role in graft failure induced by anti-MYO after syngeneic HTx.
In conclusion, the results presented in this communication demonstrate that administration of Abs to cardiac MYO after heterotopic syngeneic HTx in mice results in cardiac graft failure. This rejection is mediated by induction of exosomes containing immune-regulatory miRNAs and cardiac SAgs, activating immune responses and increasing production of SAg-specific pro-inflammatory cytokines with concomitant loss of peripheral regulation to SAgs.
Supplementary Material
Figure S1: Purity of Exosomes
Table S1: Quantification of Infiltrate Cells
Acknowledgments
Supported by NIH R21AI123034 (TM) and R01HL092514 (TM). The authors wish to acknowledge Billie Glasscock and Clare Prendergast for assistance with preparation and submission of manuscript, and the Transmission Electron Microscope Facility at Arizona State University.
Abbreviations
- Ab
antibody
- CAV
cardiac allograft vasculopathy
- DSA
donor-specific antibody
- ELISA
enzyme-linked immunosorbent assay
- HLA
human leukocyte antigen
- HTx
heart transplantation
- IgG
immunoglobulin G
- miRNA
micro RNA
- MYO
myosin
- PBS
phosphate-buffered saline
- RT-PCR
real-time polymerase chain reaction
- SAg
self-antigen
- spm
spots per million
- VIM
vimentin
Footnotes
Author Contributions: MS, TM: Research Design
MS, WL, MG: Perform research
MS, SP; TM: Data analysis
MS, TM: Writing of manuscript
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information: Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Kushner YB, Colvin RB. In: Organ transplantation: A clinical guide. A KA, J LC, C MJ, editors. Cambridge (UK): Cambridge University Press; 2011. pp. 38–45. [Google Scholar]
- 2.Nath DS, Basha HI, Mohanakumar T. Antihuman leukocyte antigen antibody-induced autoimmunity: role in chronic rejection. Current opinion in organ transplantation. 2010;15(1):16–20. doi: 10.1097/MOT.0b013e3283342780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiemann NE, Wellnhofer E, Knosalla C, Lehmkuhl HB, Stein J, Hetzer R, Meyer R. Prognostic impact of microvasculopathy on survival after heart transplantation: evidence from 9713 endomyocardial biopsies. Circulation. 2007;116(11):1274–82. doi: 10.1161/CIRCULATIONAHA.106.647149. [DOI] [PubMed] [Google Scholar]
- 4.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circulation research. 2006;99(8):801–15. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 5.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011;30(10):1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. The American journal of pathology. 2007;170(4):1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010;29(11):1277–85. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss MJ, Madsen JC, Rosengard BR, Allan JS. Mechanisms of chronic rejection in cardiothoracic transplantation. Frontiers in bioscience: a journal and virtual library. 2008;13:2980–8. doi: 10.2741/2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2005;24(11):1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Uber WE, Self SE, Van Bakel AB, Pereira NL. Acute antibody-mediated rejection following heart transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(9):2064–74. doi: 10.1111/j.1600-6143.2007.01900.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaczmarek I, Deutsch MA, Kauke T, Beiras-Fernandez A, Schmoeckel M, Vicol C, Sodian R, Reichart B, Spannagl M, Ueberfuhr P. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2008;6(3):229–35. [PubMed] [Google Scholar]
- 12.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, Rodriguez ER, Rose M, Stewart S, Suciu-Foca N, et al. Acute antibody-mediated rejection of cardiac transplants. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2006;25(2):153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Stastny P, Ring S, Lu C, Arenas J, Han M, Lavingia B. Role of immunoglobulin (Ig)-G and IgM antibodies against donor human leukocyte antigens in organ transplant recipients. Human immunology. 2009;70(8):600–4. doi: 10.1016/j.humimm.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Suciu-Foca N, Reed E, Marboe C, Harris P, Yu PX, Sun YK, Ho E, Rose E, Reemtsma K, King DW. The role of anti-HLA antibodies in heart transplantation. Transplantation. 1991;51(3):716–24. doi: 10.1097/00007890-199103000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-alpha1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180(7):4487–94. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee PC, Chen YL, Wang WM, Tu WC, Chen HY. Clinical relevance of pre- and post-transplant HLA antibodies, donor-specific, and nondonor-specific HLA antibodies detected by ELISA in renal transplantation. Clinical transplants. 2013:385–91. [PubMed] [Google Scholar]
- 17.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86(1):189–95. doi: 10.1016/j.athoracsur.2008.03.073. discussion 96-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352(6):558–69. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 19.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162(11):6836–42. [PubMed] [Google Scholar]
- 20.Kobashigawa JA. Cardiac allograft vasculopathy in heart transplant patients: pathologic and clinical aspects for angioplasty/stenting. Journal of the American College of Cardiology. 2006;48(3):462–3. doi: 10.1016/j.jacc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Mahesh B, Leong HS, Nair KS, McCormack A, Sarathchandra P, Rose ML. Autoimmunity to vimentin potentiates graft vasculopathy in murine cardiac allografts. Transplantation. 2010;90(1):4–13. doi: 10.1097/TP.0b013e3181dfa694. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Sun X, Scicluna BJ, Coleman BM, Hill AF. Characterization and deep sequencing analysis of exosomal and non-exosomal miRNA in human urine. Kidney international. 2014;86(2):433–44. doi: 10.1038/ki.2013.502. [DOI] [PubMed] [Google Scholar]
- 23.Manterola L, Guruceaga E, Gallego Perez-Larraya J, Gonzalez-Huarriz M, Jauregui P, Tejada S, Diez-Valle R, Segura V, Sampron N, Barrena C, et al. A small noncoding RNA signature found in exosomes of GBM patient serum as a diagnostic tool. Neuro-oncology. 2014;16(4):520–7. doi: 10.1093/neuonc/not218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer cell. 2014;26(5):707–21. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 26.Smith CR, Jaramillo A, Liu W, Tu Y, Kaleem Z, Swanson CJ, Mohanakumar T. CD4+ T cell recognition of a single discordant HLA-A2-transgenic molecule through the indirect antigen presentation pathway induces acute rejection of murine cardiac allografts. Transplantation. 2001;71(11):1640–8. doi: 10.1097/00007890-200106150-00025. [DOI] [PubMed] [Google Scholar]
- 27.Weiss A, McDonough D, Wertman B, Acakpo-Satchivi L, Montgomery K, Kucherlapati R, Leinwand L, Krauter K. Organization of human and mouse skeletal myosin heavy chain gene clusters is highly conserved. PNAS. 1999;96:2958–63. doi: 10.1073/pnas.96.6.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83(2):150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 29.Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76(10):1503–10. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- 30.Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, Perdicchio M, Marino ML, Federici C, Iessi E, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PloS one. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T. Donor-derived exosomes with lung self-antigens in human lung allograft rejection. Am J Transpl. 2017;17(2):474–84. doi: 10.1111/ajt.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Nayak D, Yang W, Baskaran G, Ramachandran S, Sarma N, Aloush A, Trulock E, Hachem R, Patterson GA, et al. Dysregulated MicroRNA Expression and Chronic Lung Allograft Rejection in Recipients With Antibodies to Donor HLA. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(7):1933–47. doi: 10.1111/ajt.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgun A, Shulzhenko N, Unterkircher CS, Diniz RV, Pereira AB, Silva MS, Nishida SK, Almeida DR, Carvalho AC, Franco M, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2004;23(2):204–9. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 34.Fedoseyeva EV, Kishimoto K, Rolls HK, Illigens BM, Dong VM, Valujskikh A, Heeger PS, Sayegh MH, Benichou G. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169(3):1168–74. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 35.Veillette GR, Sahara H, Meltzer AJ, Weiss MJ, Iwamoto Y, Kim KM, Rosengard BR, Allan JS, Houser SL, Sachs DH, et al. Autoimmune sensitization to cardiac myosin leads to acute rejection of cardiac allografts in miniature swine. Transplantation. 2011;91(11):1187–91. doi: 10.1097/TP.0b013e318218415d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banan B, Phelan D, Medhat A, Ewald G, Mohanakumar T. Increased Sensitization To HLA and To Cardiac Self-Antigens (Myosin and Vimentin) in Patients Waiting for Cardiac Transplantation With Left Ventricular Assisting Device (LVAD) The Journal of Heart and Lung Transplantation. 33(4):S25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Purity of Exosomes
Table S1: Quantification of Infiltrate Cells