Abstract
Oral rabies vaccination (ORV) is an effective tactic for wildlife rabies control, particularly for containment of disease spread along epizootic fronts. As part of the continuing evaluation of the ORV program in free-ranging raccoons in the US, 37 raccoons from ORV-baited areas in Pennsylvania were live-trapped and transferred to captivity to evaluate protection against rabies in animals with varying levels of existing neutralizing antibodies, expressed in international units per milliliter (IU/mL). Among the 37 raccoons at the date of capture, 24% (9/37) of raccoons were seronegative (<0.05 IU/mL), 22% (8/37) were low positive (≥0.05–0.11 IU/mL), 27% (10/37) were medium positive (>0.11–,0.5 IU/mL), and 27% (10/37) were high positive (≥0.5 IU/mL). Raccoons were held for 86–199 d between the date of capture and rabies virus challenge. At challenge, 68% (25/37) raccoons were seronegative. The overall survival rate among challenged animals was 46% (17/37). Based on the antibody titers at the time of challenge, survivorship was 24% (6/25) among seronegative animals, 100% (4/4) among low positive animals, 83% (5/6) among medium positive animals, and 100% (2/2) among high positive animals. Evidence of high-titer seroconversion after vaccination is a good surrogate indicator of rabies survival; however, survival rates of approximately 45% (15/35) were found among raccoons with detectable titers below 0.5 IU/mL. In contrast, any detectable titer at the time of challenge (>3 mo after vaccination) appeared to be a surrogate indicator of survival. Overall, we illustrated significant differences in the value of specific titers as surrogates for survival based on the timing of measurement relative to vaccination. However, survivorship was generally greater than 45% among animals with any detectable titer regardless of the timing of measurement. These findings suggest that lower titer cutoffs may represent a valid approach to measuring immunization coverage within ORV management zones, balancing both sensitivity and specificity for estimating herd immunity.
Keywords: Neutralizing antibodies, Procyon lotor, rabies, raccoon, vaccination, virus
Introduction
Rabies is an invariably fatal encephalitis caused by a Lyssavirus (Family Rhabdoviridae). Globally, rabies is responsible for more than 60,000 human deaths each year, largely due to uncontrolled canine rabies circulating in Africa and Asia (Hampson et al. 2015). While canine rabies has been eliminated in the US, wildlife rabies remains a significant source of exposure to humans and domestic animals and represents a high financial and social cost of coexistence (Uhaa et al. 1992; Kemere et al. 2000; Velasco-Villa et al. 2008). The expansive raccoon rabies epizootic along the eastern coast of the US is associated with most animal rabies cases and human exposures (Christian et al. 2009; Monroe et al. 2016). While raccoon rabies was largely restricted to the southeast US prior to the 1970s, the translocation of infected animals into the Mid-Atlantic region resulted in its rapid spread throughout the Northeast. By the early 2000s, the distribution of this rabies virus (RV) variant ranged from Alabama and Florida northward along the Appalachian Mountains into Maine and southeastern Canada (Nettles et al. 1979; CDC 2000; Blanton et al. 2008).
While live attenuated oral rabies vaccines had been developed in the US in the 1960s and were successfully used to control rabies in red foxes in Europe by the late 1970s, they were not approved for use in the US at the time raccoon rabies began to expand into the Mid-Atlantic states (Wandeler et al. 1988). Subsequently, large-scale interventions to control rabies in the raccoon population did not begin until the recombinant vacciniarabies glycoprotein (V-RG) vaccine became available for oral rabies vaccination (ORV) in the 1990s (Rupprecht et al. 1988; Hanlon et al. 1998). Since then, the raccoon ORV program in the US has expanded to include more than 16 states, and, along with special contingency actions, it is credited with preventing appreciable westward expansion of the raccoon RV variant (Slate et al. 2009).
While ORV has been in place for 20 yr in some areas, there has not been a sufficient combination of efficacious vaccine, bait matrix, and application strategies to move toward elimination of the raccoon RV variant within the raccoon population. The US Department of Agriculture (USDA) monitors post-ORV rabies virus neutralizing antibody (RVNA) titers in baited regions as an operational metric of performance for the national ORV program. Despite multiple applications of ORV across these regions, the overall seroprevalence of RVNA among trapped raccoons has averaged around 30%, with some spatiotemporal variability (Slate et al. 2009).
While the efficacy of V-RG has been documented in captive animals to comply with regulatory requirements, many issues may impact the effectiveness of the vaccine when distributed in the field. These factors include design of the bait, timing of vaccinebait distribution, nontarget species uptake, animal nutrition levels, and exposure to other infections such as wild orthopoxviruses that might interfere with response to V-RG (Root et al. 2008; Gallardo-Romero et al. 2016). The relative usefulness of serologic evaluation in relation to protection against RV challenge is not well understood for animals receiving ORV under natural conditions. Following one field administration of ORV, survival against RV challenge of wild-caught raccoons was reported in 78% of raccoons captured (Rupprecht et al. 1993). In that study, approximately 40% of the animals had high RVNA titers (>0.5 IU/mL) when challenged nearly 7 mo post-ORV (Rupprecht et al. 1993). Antibodies developing following ORV exposure and the subsequent anamnestic response to infection are presumed to be correlates of survival and have been used as markers of ORV effectiveness. We evaluated survivorship among raccoons captured in an ORV zone with a range of RVNA titers to evaluate correlations between titer level and survival against RV challenge. The objective was to identify potential antibody titer cutoffs that might represent a valid surrogate marker of protection when monitoring seroprevalence of adequate response in ORV management areas.
Materials and Methods
Raccoons were cage-trapped (Model 54130 live traps, Safeguard Products, Inc., New Holland, Pennsylvania, USA) during June 2005 in an ORV zone in western Pennsylvania (Westmoreland, Somerset, and Indiana Counties) where RABO-RAL V-RG® (Merial Ltd., Athens, Georgia, USA) had been distributed. Traps were tended to daily. Nontarget species and juvenile raccoons were immediately released.
All raccoons sampled for blood and other biologic information were sedated based on weight with an intramuscular injection with a mixture of 10.0 mg/kg ketamine hydrochloride (Fort Dodge Laboratories, Inc., Overland Park, Kansas, USA) and 2.0 mg/kg xylazine hydrochloride (Mobay Corp., Shawnee, Kansas, USA). Five-milliliter to 7-mL samples of blood were collected from a jugular vein from each sedated raccoon. Sex, reproductive status, relative age, weight, and other pertinent information were recorded. Blood was centrifuged (1,000 × G), and serum was collected, stored in labeled cryovials the day of capture, and shipped by express mail on dry ice to the Centers for Disease Control and Prevention (CDC) Rabies Laboratory to determine baseline RVNA titers. Test results were returned within 48–72 h, and 37/195 raccoons with a range of RVNA titers were selected for further study. OpenEpi was used to calculate study sample size assuming a survival rate following challenge with rabies virus of <20% in the animal cohorts with no detectable titer and >80% in animal cohorts with detectable titers (Dean et al. 2009). We estimated a sample size of approximately 10 animals in each group to find statistical significance between experimental groups. The primary effect measured was survival after challenge with rabies virus. The Fisher's exact test was used to assess differences in survival between study groups. A probability value of <0.05 was considered significant. Rate ratios (RR) and 95% confidence intervals (CIs) of survival were calculated for comparisons between groups.
The remaining raccoons were released at their original site of capture. Raccoons held for one night or longer were placed in dog kennels at a site secure from public access and monitored to ensure water was available ad libitum, and they were fed a commercial dry dog food. Kennels were cleaned daily. Selected animals were transported to the CDC Rabies Laboratory animal holding facility for RV challenge studies. Raccoons transferred to CDC custody were individually caged and offered commercial food and water ad libitum for a minimum quarantine period of 30 d for general health observations.
Following the quarantine period, raccoons were routinely sedated, and blood (2 to 4 mL) was sampled as above. Serum was separated at low-speed centrifugation (1,000 × G), collected, and stored at −20 C until testing. Levels of RVNA were determined by use of the rapid fluorescent focus inhibition test (RFFIT; Smith et al. 1973) and expressed in international units per milliliter (IU/mL).
All captured raccoons were grouped based on their RVNA titers at capture. Cutoff points for these groups were based on existing recommended levels by the US Advisory Committee on Immunization Practices (0.11 IU/mL) and World Health Organization (0.5 IU/mL) and are commonly used for determining human and animal vaccination recommendations (Manning et al. 2008; WHO 2013). These values are also approximately equivalent to complete neutralization of virus at a 1:5 serum dilution (0.12 IU/mL) or 1:25 serum dilution (0.5 IU/mL) in the RFFIT. Seronegative animals were designated as animals with RVNA below the threshold of detection for the RFFIT test at CDC (<0.05 IU/mL); a value of 0.01 IU/ml was used for these animals in geometric mean titer calculations.
Raccoons were challenged in three groups between 86 to 199 d after capture. At challenge, raccoons were inoculated in the right and left masseter muscle with 0.5 mL each of submandibular salivary gland suspensions of RV obtained from naturally infected eastern raccoons (1 × 104.9 mouse intracerebral lethal dose 50%). After inoculation, raccoons were observed daily for onset of clinical signs of rabies. A postchallenge titer was determined 7 d after challenge. When signs consistent with RV infection were observed, raccoons were sedated and then euthanized by intracardiac administration of a phenytoin-pento-barbital mixture (Beuthansia©-D, Merck Animal Health, Madison, New Jersey, USA). Postmortem tissue collection included brain stem and serum. A diagnosis of rabies was confirmed by the direct fluorescent antibody test on fresh brain tissue samples, done at CDC. All animal care and experimental procedures were performed in compliance with the CDC Institutional Animal Care and Use Guidelines (Number: 1364RU-PRACL).
Results
In total, 24% (9/37) of the raccoons were seronegative (<0.05 IU/mL), 22% (8/37) had a low RVNA titer (≥0.05–0.11 IU/mL), 27% (10/37) had a medium RVNA titer (>0.11–<0.5 IU/mL), and 27% (10/37) had a high RVNA titer (≥0.5 IU/mL) at time of capture (Table 1). The mean length of time between baseline serum collection and challenge with RV was 141 d (SD: 33.7, range: 86–199 d). Between baseline titer collection and challenge, 100%, 82%, and 9% of the low, medium, and high RVNA titer animals became seronegative, respectively (Fig. 1). Overall, the median RVNA titer decline among raccoons with a detectable titer at baseline was 0.81 IU/mL (range: 0.04–10.39 IU/mL). At challenge, 68% (25/37) of the raccoons were seronegative, 11% (4/37) had a low RVNA titer, 16% (6/37) had a medium RVNA titer, and 5% (2/37) had a high RVNA titer (Table 1).
Table 1.
Rabies virus neutralizing antibodies at baseline, challenge, and 7 d after challenge among raccoons (Procyon lotor) captured in an area where oral rabies vaccine was distributed.
| Rabies virus neutralizing antibody titer (IU/mLb) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Animal no. | Titer groupa | Days from capture to challenge | At baselinec | At challengec | At 7 d postchallengec | Survivald |
| 125 | Negative | 105 | 0.01 | 0.01 | 0.01 | N |
| 5199 | Negative | 105 | 0.01 | 0.01 | 0.01 | N |
| 5142 | Negative | 107 | 0.01 | 0.01 | 0.01 | N |
| 5143 | Negative | 107 | 0.01 | 0.01 | 0.01 | N |
| 5162 | Negative | 107 | 0.01 | 0.01 | 0.01 | N |
| 5714 | Negative | 107 | 0.01 | 0.01 | 0.01 | N |
| 5038 | Negative | 151 | 0.01 | 0.01 | 0.01 | N |
| 5044 | Negative | 198 | 0.01 | 0.01 | 0.10 | N |
| 123 | Negative | 108 | 0.01 | 0.01 | 0.01 | N |
| 5198 | Low | 105 | 0.05 | 0.01 | 0.01 | N |
| 5716 | Low | 107 | 0.06 | 0.01 | 0.01 | N |
| 5718 | Low | 107 | 0.06 | 0.01 | 9.00 | Y |
| 5667 | Low | 86 | 0.08 | 0.01 | 0.68 | Y |
| 122 | Low | 108 | 0.07 | 0.01 | 0.01 | N |
| 5672 | Low | 129 | 0.08 | 0.01 | 0.07 | Y |
| 5745 | Low | 129 | 0.11 | 0.01 | 0.01 | Y |
| 5167 | Low | 151 | 0.11 | 0.01 | 0.01 | N |
| 5227 | Medium | 128 | 0.13 | 0.01 | 0.55 | Y |
| 5938 | Medium | 128 | 0.13 | 0.07 | 0.55 | Y |
| 5749 | Medium | 128 | 0.14 | 0.01 | 0.55 | Y |
| 5164 | Medium | 151 | 0.19 | 0.01 | 0.55 | N |
| 5459 | Medium | 152 | 0.22 | 0.01 | 0.07 | N |
| 5047 | Medium | 149 | 0.23 | 0.01 | 0.01 | N |
| 5160 | Medium | 151 | 0.36 | 0.01 | 0.10 | N |
| 5032 | Medium | 152 | 0.37 | 0.01 | 0.01 | N |
| 5195 | Medium | 149 | 0.43 | 0.10 | 0.13 | Y |
| 5043 | Medium | 149 | 0.40 | 0.13 | 0.05 | N |
| 5936 | High | 129 | 0.50 | 0.16 | 0.01 | Y |
| 5668 | High | 130 | 0.50 | 0.55 | 0.55 | Y |
| 4104 | High | 152 | 0.62 | 0.22 | 0.55 | Y |
| 5145 | High | 151 | 0.76 | 0.40 | 0.55 | Y |
| 5194 | High | 198 | 0.90 | 0.08 | 37.50 | Y |
| 5186 | High | 198 | 1.70 | 0.45 | 37.50 | Y |
| 5046 | High | 198 | 2.20 | 0.36 | 37.50 | Y |
| 5040 | High | 199 | 2.50 | 2.00 | 37.50 | Y |
| 5183 | High | 198 | 4.30 | 0.10 | 1.16 | Y |
| 5189 | High | 198 | 10.40 | 0.01 | 0.01 | N |
Group categories based on animal's rabies virus neutralizing antibody level at baseline: negative: <0.05 IU/mL, low: ≥0.05–0.11 IU/mL, medium: >0.11–<0.5 IU/mL, high: ≥0.5 IU/mL.
IU/mL = international units/milliliter.
Animal blood sampling time periods: baseline = day animal captured in the wild, challenge = day animal challenged with rabies virus.
Survival status of animals: Y= survived rabies challenge, N = developed signs consistent with rabies and was euthanized. All euthanized animals were confirmed rabid by direct fluorescent antibody.
Figure 1.
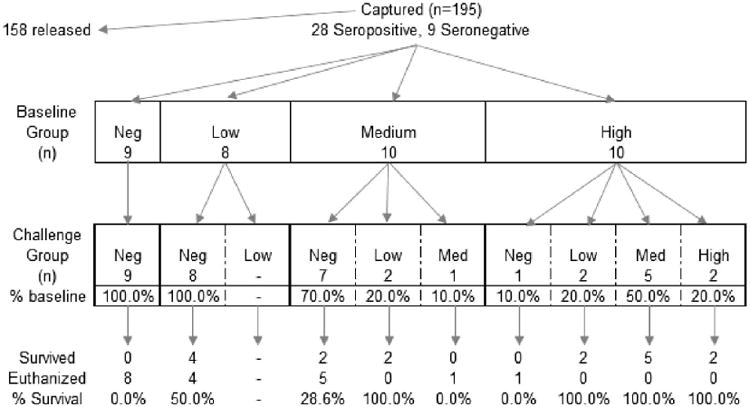
Sampling of wild-caught raccoons (Procyon lotor) from an area where oral rabies vaccine was distributed for inclusion in study, selected animals by rabies virus neutralizing antibody category, and change in group distribution between animal capture and challenge with rabies virus. Categories based on animal's rabies virus neutralizing antibody level: negative: <0.05 IU/mL, low: ≥0.05–0.11 IU/mL, medium: >0.11–<0.5 IU/mL, high: ≥0.5 IU/mL. Blood sampling time periods occurred at baseline (when animal was captured in the wild) and at challenge (when animal was challenged with rabies virus).
All nine raccoons that were seronegative at capture (baseline) developed signs of rabies and were euthanized. Overall, 46% (17/37) of the raccoons survived challenge. Regardless of the timing of titer determination in relation to RV challenge, higher titers were correlated with survival (Table 2). A total of 16 raccoons had a detectable RVNA titer at baseline but had become seronegative at challenge. Among those, 37% (6/16) survived rabies challenge. In comparison, 92% (11/12) of the raccoons that still had a detectable titer at challenge survived.
Table 2.
Survivorship following rabies virus challenge among wild-caught raccoons (Procyon lotor) by rabies virus neutralizing antibody level.
| Titer group at baselineb | Titer group at challengeb | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Titergroupa | Survived | Total | % Survival | Survived | Total | % Survival |
| Negative | 0 | 9 | 0 | 6 | 25 | 24 |
| Low | 4 | 8 | 50 | 4 | 4 | 100 |
| Medium | 4 | 10 | 40 | 5 | 6 | 83 |
| High | 9 | 10 | 90 | 2 | 2 | 100 |
| All seropositive | 17 | 28 | 61 | 11 | 12 | 92 |
| Total | 17 | 37 | 46 | 17 | 37 | 46 |
Group categories based on animal's rabies virus neutralizing antibody level: negative: <0.05 IU/mL, low: ≥0.05–0.11 IU/mL, medium: >0.11–<0.5 IU/mL, high: ≥0.5 IU/mL.
Animal blood sampling time periods: baseline = day animal captured, challenge = day animal challenged with rabies virus.
Raccoons with a detectable titer (≥0.05 IU/mL) at capture were significantly more likely to survive challenge (Fisher exact test, P=0.001) after an average of 145 d after capture. Assuming animal vaccination status was accurately classified based on serologic status at baseline (i.e., a detectable titer is associated with history of vaccination), this would represent a vaccine efficacy of approximately 61%. Based on the titer measurement at challenge, the rate of survival for animals with a titer ≥0.05 IU/mL was nearly four times higher than those without a measurable RVNA titer (RR=3.8, 95% CI=1.9–7.8). The rate of survival in animals that demonstrated an anamnestic response to RV challenge (≥2-fold increase in titer) 7 d after challenge was three times higher (RR=3.2, 95% CI=1.3–7.1).
Discussion
The raccoons in this study were likely vaccinated during the ORV period ending on 29 April 2005. Approximately 2 mo passed between the period when the raccoons may have consumed ORV baits and when they were captured, and baseline antibody titers were determined. This is consistent with the period during which USDA routinely conducts serologic monitoring after distributing baits. Animals were challenged over three periods, resulting in a total of 7–10 mo between possible oral vaccination and challenge with RV. Approximately 68% of the seropositive animals became negative over this period. Prior studies of vaccination of dogs with parenterally administered modified live virus vaccines found high antibody titers persisted for most animals for more than 1 yr (Coyne et al. 2001). A study of oral vaccination of foxes with an adenovirus vectored rabies vaccine similarly reported duration of immunity over 1 yr, but it also identified peak immune response at 7 wk followed by a general decline, resulting in more than 50% of the titers of vaccinated animals falling below 0.5 IU/mL by 6 mo postchallenge (Brown et al. 2014).
Antibody titer level as a surrogate value for protection is not well defined and can be difficult to determine given multiple sources of variation (e.g., host immune response, environmental factors, and diagnostic test variability). To reflect this variability and to account for concerns over false-positive serology results, higher cutoffs to document seroconversion have been suggested for seroprevalence studies and post-ORV monitoring (Bahloul et al. 2005). In the current study, approximately 45% of animals with low and medium level titers at baseline survived challenge 7–10 mo later, suggesting that the use of high titer cutoff values for seroprevalence surveys may misclassify many animals that would be protected against rabies challenge. However, the use of a very low cutoff value (i.e., ≥0.05 IU/mL) should be construed as an upper threshold of population immunity at most.
Few studies (Rupprecht et al. 1993) have measured longitudinal changes in titers for raccoons exposed to ORV under field conditions, but the current study suggests that titer levels may decay quickly (e.g., less than 1 yr). This could have a significant impact on cross-sectional surveys of RVNA titers and dynamics related to the critical vaccination coverage necessary to eliminate rabies. Measurement of high antibody titers (i.e., >0.5 IU/mL) 2 mo after ORV appears to be a strong indicator of protection; however, maintenance of any detectable titer (i.e., ≥0.05 IU/mL) at the point of RV exposure has a higher correlation with survival. If serologic monitoring is conducted at periods greater than 2 mo after ORV distribution, the use of lower RVNA cutoffs as a surrogate of protection appears to be warranted.
We encountered a few limitations during the study. The most notable limitation was the assumption regarding the study animals' vaccination and rabies exposure histories. While these raccoons had the potential to be vaccinated by ORV, we cannot exclude that some may have received parenteral vaccination, been exposed to rabies, or had ingested multiple ORV baits over multiple years. Serologic surveillance among raccoons in enzootic areas of the US Southeast where ORV had not been applied varied between 2% and 22% seropositive depending on the epizootic cycle (McLean 1971). No noted epizootics had been reported in the areas where these Pennsylvania raccoons were collected.
In addition to the assumptions regarding the vaccination status of animals, there are underlying assumptions regarding the specificity of neutralization observed in the RFFIT, particularly at low titers. The RFFIT is a cell culture–based assay, and performance is susceptible to variation from laboratory and operator conditions, cytotoxicity, and other factors (e.g., presence of complement) that might impact interpretation of results. In this case, it is possible that low positive titers (≥0.05–0.1 IU/mL) were false or nonspecific and did not represent virus neutralization due to RVNA in the serum. Previous studies have suggested higher thresholds up to 1 IU/mL be used to assess serum collected from field animals. These conclusions were based in part on studies that identified high rates of seropositive dogs in areas with low vaccination rates or that were believed to be free of rabies (Cleaveland et al. 1999; Bahloul et al. 2005). In these previous studies, there was concern about the interpretation of lower titers as an adequate surrogate for immunity. However, in the current study, nearly 50% of the animals with a detectable titer (≥0.05 IU/mL) at capture survived RV challenge. Of the 10 raccoons that did not survive, three produced an anamnestic response within 7 d of challenge. Overall, this would suggest that specific RVNA was detected in 43% of the low, 73% of the medium, and 82% of the high titer groups as indicated by survival or anamnestic response postchallenge.
The detection of RVNA antibodies from raccoons in an ORV zone appears to be an adequate surrogate marker of protection and may help with the assessment of ORV coverage and effectiveness. Many factors affect antibody levels in a free-ranging population. A critical consideration is the timeliness of sample collection following bait distribution, because antibody kinetics from ORV in raccoons may lead to a narrow window of detection (Brown et al. 2012). The presence of any level of antibody indicates a reasonable probability of immunity to a lethal RV challenge for the purposes of serologic monitoring of ORV programs. Increasing the cutoff to >0.11 IU/mL may improve specificity while maintaining a high sensitivity.
Additional research regarding the use of other assays for serologic monitoring (e.g., enzyme-linked immunosorbent assay) should be conducted to determine if they are also closely correlated with protection against RV challenge. These tests are frequently easier to perform than the RFFIT and may be more cost-effective for monitoring ORV in wildlife. In addition, further evaluations of the antibody decay rate in wild raccoons where ORV is distributed should be further explored.
Acknowledgments
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the positions of the US government or other affiliations. Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services or US Department of Agriculture. We thank the rabies staffs at Centers for Disease Control and Prevention and US Department of Agriculture for their substantial contributions to this research.
Literature Cited
- Bahloul C, Taieb D, Kaabi B, Diouani MF, Ben Hadjahmed S, Chtourou Y, Imen B'chir B, Dellagi K. Comparative evaluation of specific ELISA and RFFIT antibody assays in the assessment of dog immunity against rabies. Epidemiol Infect. 2005;133:749–757. doi: 10.1017/s095026880500381x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton JD, Palmer D, Christian KA, Rupprecht CE. Rabies surveillance in the United States during 2007. J Am Vet Med Assoc. 2008;233:884–897. doi: 10.2460/javma.233.6.884. [DOI] [PubMed] [Google Scholar]
- Brochier B, Godfroid J, Costy F, Blancou J, Pastoret PP. Vaccination of young foxes (Vulpes vulpes, L.) against rabies: Trials with inactivated vaccine administered by oral and parenteral routes. Ann Rech Vet. 1985;16:327–333. [PubMed] [Google Scholar]
- Brown LJ, Rosatte RC, Fehlner-Gardiner C, Bachmann P, Ellison JA, Jackson FR, Taylor JS, Davies C, Donovan D. Oral vaccination and protection of red foxes (Vulpes vulpes) against rabies using ONRABt, an adenovirus-rabies recombinant vaccine. Vaccine. 2014;32:984–989. doi: 10.1016/j.vaccine.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Rosatte RC, Fehlner-Gardiner C, Taylor JS, Davies JC, Donovan D. Immune response and protection in raccoons (Procyon lotor) following consumption of baits containing ONRAB®, a human adenovirus rabies glycoprotein recombinant vaccine. J Wildl Dis. 2012;48:1010–1020. doi: 10.7589/2012-01-023. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Update: Raccoon rabies epizootic—United States and Canada, 1999. Morb Mortal Wkly Rep. 2000;49:31–35. [PubMed] [Google Scholar]
- Christian KA, Blanton JD, Auslander M, Rupprecht CE. Epidemiology of rabies post-exposure prophylaxis—United States of America, 2006–2008. Vaccine. 2009;27:7156–7161. doi: 10.1016/j.vaccine.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Barrat J, Barrat MJ, Selve M, Kaare M, Esterhuysen J. A rabies serosurvey of domestic dogs in rural Tanzania: Results of a rapid fluorescent focus inhibition test (RFFIT) and a liquid-phase blocking ELISA used in parallel. Epidemiol Infect. 1999;123:157–164. doi: 10.1017/s0950268899002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Burr JH, Yule TD, Harding MJ, Tresnan DB, McGavin D. Duration of immunity in dogs after vaccination or naturally acquired infection. Vet Rec. 2001;149:509–515. doi: 10.1136/vr.149.17.509. [DOI] [PubMed] [Google Scholar]
- Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. [Accessed February 2018];2009 http://www.openepi.com.
- Gallardo-Romero NF, Arechiga-Ceballos N, Emerson GL, Martinez-Martinez FO, Doty JB, Nakazawa YJ, Rendon-Franco E, Munoz-Garcia CI, Villanueva-Garcia C, Ramirez-Cid C, et al. Endemic orthopoxvirus circulating in procyonids in Mexico. J Wildl Dis. 2016;52:609–615. doi: 10.7589/2015-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Niezgoda M, Hamir AN, Schumacher C, Koprowski H, Rupprecht CE. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J Wildl Dis. 1998;34:228–239. doi: 10.7589/0090-3558-34.2.228. [DOI] [PubMed] [Google Scholar]
- Kemere P, Liddel MK, Evagelou P, Slate D, Osmek S. Economic analysis of a large scale oral vaccination program to control raccoon rabies. In: Clark L, Hone J, Shivik JA, Watkins RA, Vercauteren KC, Yoder JC, editors. Proceedings: Third NWRC Special Symposium: Human conflicts with wildlife: economic considerations. Fort Collins, Colorado: US Department of Agriculture, Animal and Plant Health Inspection Service, National Wildlife Research Center; 2000. Aug 1–3, pp. 109–116. [Google Scholar]
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al. Human rabies prevention—United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;200857:1–28. [PubMed] [Google Scholar]
- McLean RG. Rabies in raccoons in the southeastern United States. J Infect Dis. 1971;123:680–681. doi: 10.1093/infdis/123.6.680. [DOI] [PubMed] [Google Scholar]
- Monroe BP, Yager P, Blanton J, Birhane MG, Wadhwa A, Orciari L, Petersen B, Wallace R. Rabies surveillance in the United States during 2014. J Am Vet Med Assoc. 2016;248:777–788. doi: 10.2460/javma.248.7.777. [DOI] [PubMed] [Google Scholar]
- Nettles VF, Shaddock JH, Sikes RK, Reyes CR. Rabies in translocated raccoons. Am J Public Health. 1979;69:601–602. doi: 10.2105/ajph.69.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JJ, McLean RG, Slate D, MacCarthy KA, Osorio JE. Potential effect of prior raccoonpox virus infection in raccoons on vaccinia-based rabies immunization. BMC Immunol. 2008;9:57. doi: 10.1186/1471-2172-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht CE, Hamir AN, Johnston DH, Koprowski H. Efficacy of a vaccinia-rabies glycoprotein recombinant virus vaccine in raccoons (Procyon lotor) Rev Infect Dis. 1988;10(Suppl 4):S803–S809. doi: 10.1093/clinids/10.supplement_4.s803. [DOI] [PubMed] [Google Scholar]
- Rupprecht CE, Hanlon CA, Niezgoda M, Buchanan JR, Diehl D, Koprowski H. Recombinant rabies vaccines: Efficacy assessment in free-ranging animals. Onderstepoort J Vet Res. 1993;60:463–468. [PubMed] [Google Scholar]
- Slate D, Algeo TP, Nelson KM, Chipman RB, Donovan D, Blanton JD, Niezgoda M, Rupprecht CE. Oral rabies vaccination in North America: Opportunities, complexities, and challenges. PLoS Negl Trop Dis. 2009;3:e549. doi: 10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Yager PA, Baer GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull World Health Organ. 1973;48:535–541. [PMC free article] [PubMed] [Google Scholar]
- Uhaa IJ, Dato VM, Sorhage FE, Beckley JW, Roscoe DE, Gorsky RD, Fishbein DB. Benefits and costs of using an orally absorbed vaccine to control rabies in raccoons. J Am Vet Med Assoc. 1992;201:1873–1882. [PubMed] [Google Scholar]
- Velasco-Villa A, Reeder SA, Orciari LA, Yager PA, Franka R, Blanton JD, Zuckero L, Hunt P, Oertli EH, Robinson LE, et al. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg Infect Dis. 2008;14:1849–1854. doi: 10.3201/eid1412.080876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler AI, Capt S, Kappeler A, Hauser R. Oral immunization of wildlife against rabies: Concept and first field experiments. Rev Infect Dis. 1988;10(Suppl 4):S649–S653. doi: 10.1093/clinids/10.supplement_4.s649. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) World Health Organization Technical Report Series No 982. World Health Organization; Geneva, Switzerland: 2013. WHO expert consultation on rabies Second report; p. 139. [PubMed] [Google Scholar]


