Abstract
T-regulatory (Treg) cells are like other cells present throughout the body in being subject to biochemical modifications in response to extracellular signals. An important component of these responses involves changes in post-translational modifications (PTMs) of histones and many non-histone proteins, including phosphorylation/dephosphorylation, ubiquitination/deubiquitination and acetylation/deacetylation. Foxp3, the key transcription factor of Tregs, is constantly being rapidly turned over, and a number of these PTMs determine its level of expression and activity. Of interest in the transplant setting, modulation of the acetylation or deacetylation of key lysine residues in Foxp3 can promote the stability and function, leading to increased Treg production and increased Treg suppressive activity. This mini-review focuses on recent data concerning the roles that histone/protein deacetylases (HDACs) play in control of Treg function, and how small molecule HDAC inhibitors can be used to promote Treg-dependent allograft survival in experimental models. These data are discussed in the light of increasing interest in the identification and clinical evaluation of isoform-selective HDAC inhibitors, and their potential application as tools to modulate Foxp3+ Treg cell numbers and function in transplant recipients.
1. INTRODUCTION
While it was known for several decades that neonatal thymectomy could induce T cell-mediated autoimmunity1, the finding that normal individuals contain a small population of CD4+CD25+ T cells that, upon adoptive transfer, prevented the development of autoimmunity,2 renewed interest in what became known as T-regulatory (Treg) cells. The field was further legitimized when 3 groups collectively reported that Tregs expressed the transcription factor, Foxp3, and that Foxp3 mutations caused IPEX syndrome in humans and Scurfy disease in mice.3–5 Since then, various aspects of Treg biology were elucidated and their importance for allograft acceptance in experimental models was recognized. However, the development and functions of Foxp3+ Treg are impaired by calcineurin inhibitors (CNI), a key component of most post-transplant immunosuppressive protocols.6 This effect probably involves multiple mechanisms, but key actions include the effect of CNI on NFAT phosphorylation and translocation in both Tregs and conventional T cells, given the importance of NFAT/Foxp3 complexes in maintaining the suppressor program of Tregs, and the associated inhibition of paracrine IL-2 production by conventional T cells that is essential for Treg activity and survival.7–9 Here, we provide an updated review of the biochemical mechanisms, and pharmacologic agents, by which Treg function can be enhanced, including after organ transplantation.10 Such agents might be delivered intermittently or continuously and have long-term benefits that include effects on the incidence of both acute and chronic allograft rejection.
2. POST-TRANSLATIONAL REGULATION OF FOXP3
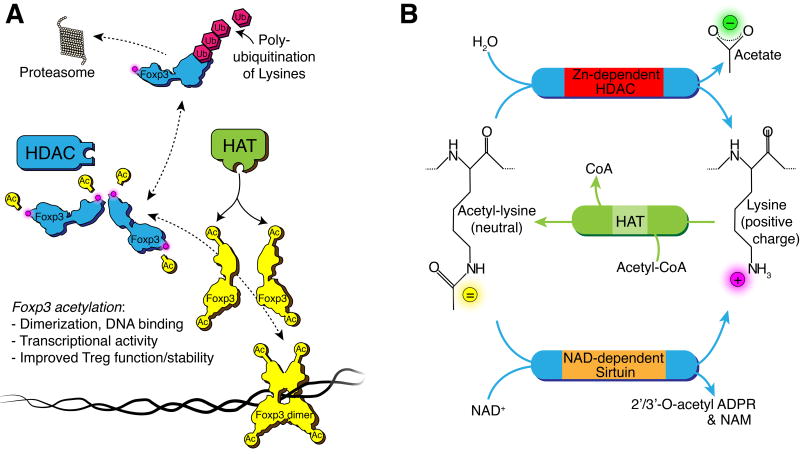
Foxp3 binds to DNA and regulates the transcription of more than 700 genes in Treg cells, with roughly equal numbers of genes being induced or repressed. To do this, Foxp3 forms dimers and higher order oligomers, allowing long-range interactions with non-adjacent loops of DNA that help coordinate the expression of target genes.11 Assembly of Foxp3 complexes is dependent upon acetylation of ε-amino groups on several key lysine residues,12 and inhibition of their acetylation prevents dimerization, allowing the lysines to be ubiquitinated and Foxp3 protein to be degraded via the proteasome (Fig. 1A).13–15 Acetylation of additional lysines promotes the DNA binding of Foxp3, as well as the interactions of a region in the N-terminal domain of Foxp3 with a number of other proteins.16–18 Acetylation and deacetylation are dynamic events catalyzed by histone acetyltransferases (HATs) and histone/protein deacetylases (HDACs), respectively; these enzyme families are also known as lysine acetyltransferases (KATs) and lysine deacetylases (KDACs), so as to deemphasize the focus on histones and to highlight regulation of non-histone proteins, such as occurs with Foxp3 (Fig. 1B). The actions of these enzymes are rapid, allowing for dynamic regulation of both chromatin remodeling and protein interactions within minutes.19, 20
Figure 1. HDAC reaction and Foxp3.
(A) Lysine deacetylation renders the Foxp3 transcriptionally inactive and prone to poly-ubiquitination and proteasomal degradation. (B) Lysine deacetylation reaction by Zn2+ and NAD+ dependent HDACs and acetylation by HATs. The deacetylation reaction removes acetate from the ε-amino group, changing the polarity of the lysine side chain from neutral to a positive charge, and altering the properties of the client protein. HATs catalyze the reverse of this reaction and acetylate the ε-amino group. Abbreviations: NAD, nicotinamide adenine dinucleotide; NAM, nicotinamide; ADPR, adenosine diphosphate riboside; Ub, ubiquitin; Ac, acetate or acetyl-group; CoA, Coenzyme A; HAT, histone acetyl-transferase.
Tregs express multiple HAT enzymes whose gene deletion or pharmacologic targeting impairs Foxp3 acetylation and Treg suppressive function, including p300, CBP and, especially, Tip60.21–23 Tip60 is the only HAT whose deletion leads to the rapid onset of lethal autoimmunity in the early post-natal period, whereas p300 and CBP, as well as PCAF and GCN5, appear to be able to compensate for the loss of each other. In contrast, Foxp3+ Treg cells express all 11 classical Zn-dependent HDAC enzymes, and several members of the Sirtuin family of NAD-dependent HDACs, though Sirt1 has received most of the attention to date.
3. HDAC ENZYMES AND TREG CELLS
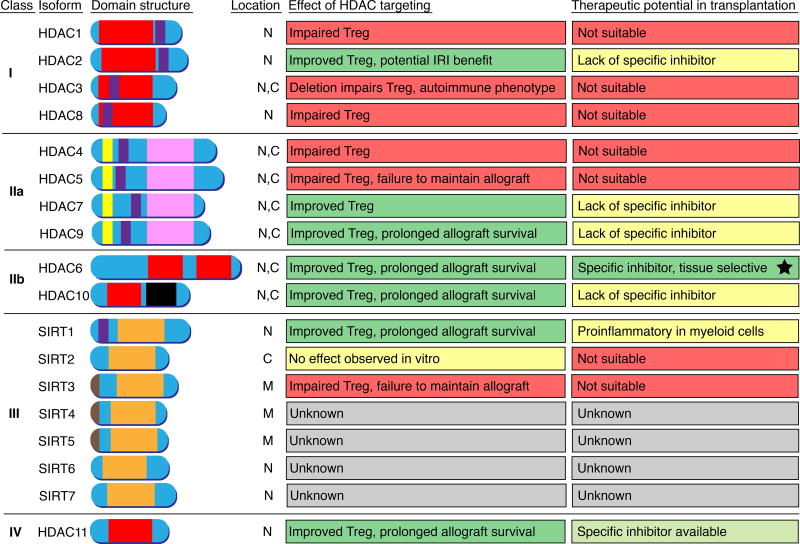
There are 18 HDAC enzymes that are divided into 4 classes (Fig. 2), with the 11 classical Zn-dependent HDAC enzymes constituting class 1 (HDACs 1, 2, 3 and 8); class II, divided into IIa (HDACs 4, 5, 7 and 9) and IIb (HDACs 6 and 10); and class IV (HDAC11). The 7 Sirtuin enzymes constitute the class III HDAC family.
Figure 2. Role of HDACs in Treg function and their therapeutic potential in transplantation.
Deletion and pharmacologic targeting of HDAC2, HDAC7, HDAC9, HDAC6, HDAC10, HDAC11, as well as Sirtuin-1 (Sirt1) improves Treg function. Of those, HDAC6 is the most promising candidate since HDAC6 knockout mice are normal, and HDAC6 selective pharmacologic inhibitors are being tested in clinical trials for other indications. Domain color codes: Red, classic Zn2+ dependent HDAC catalytic domain; Pink, class IIa HDAC catalytic domain (histidine to tyrosine substitution compared to class I, IIb, and IV HDAC); orange, NAD+ dependent class III HDAC (Sirtuin) catalytic domain; black, inactive leucine rich domain (HDAC10); purple, nuclear localization sequence; brown, mitochondrial targeting sequence; yellow, MEF2 binding site. Abbreviations: N, nucleus; C, cytoplasm; M, mitochondria.
3.1 Class I HDACs
Class I HDACs are essential for cell function and their deletion during development results in lethality.24 HDACs 1 and 2 are structurally highly homologous (83% amino acid identity) and are present within the CoREST, NuRD and Sin3 multiprotein nuclear repressor complexes that regulate gene transcription. In addition to HDACs 1 and 2, the core members of each complex are as follows. CoREST complexes contain CoREST1 and CoREST2, an H3K4 demethylase, Lsd1, and the scaffolding protein CoREST. NuRD complexes contain the histone-binding proteins RbAp46 and RbAp48, the metastasis-associated proteins MTA1 (or MTA2/MTA3), the methyl-CpG-binding domain protein MBD3 (or MBD2) and the chromodomain-helicase-DNA-binding protein CHD3 (aka Mi-2alpha) or CHD4 (aka Mi-2beta). Sin3 complexes contain Sin3A or Sin3B, RBBP4 and RBBP7, SDS3, and the SIN3-associated proteins SAP18 and SAP30.
Very little is yet known of the functions of these complexes within the immune system, including Treg cells, though there are some interesting initial findings, especially in the case of the CoREST complex. Treg deletion of HDAC1 impairs Treg function and abrogates the efficacy of Treg-dependent costimulation blockade in allograft models, whereas Treg deletion of HDAC2 promotes Treg function, including in allograft recipients.25 As a result, inhibition of HDAC1 and HDAC2 in a Treg-dependent colitis model, using MS-275 (Etinostat), that inhibits HDAC1-3 with IC50 values of 0.19 μM, 0.41 μM, and 0.95 μM, respectively26, has no significant effects on Treg function.27 Individual pharmacologic targeting of Hdac1 vs. Hdac2 is considered very difficult given the nearly identical nature of the their active sites and surrounding surfaces, but there are reports of small molecules that show different dissociation rates for Hdac1 vs. Hdac2; such kinetically-selective Hdac2 inhibitors enhance Treg function in vitro but have not been tested in vivo.28, 29 An alternate approach is to target the functions of the HDAC-associated complex, though the core components and their partnering molecules may vary by cell type and function.30 An example of this approach, in the case of the CoREST complex, was the development of dual inhibitors of the HDAC and demethylase activities of the complex,31 and these inhibitors are under investigation for their effects on Tregs.25 Unexpected results can also be generated, as was the case of targeting of Mbd2, a component of the NuRD complex. This protein is known to bind to methylated DNA and by recruiting the NuRD complex is thought to promote gene repression. However, in Tregs, Mbd2 was shown to function as a Foxp3 demethylase, such that its targeting led to increased methylation of the TSDR/CNS2 site, decreased Foxp3 expression and impaired Treg function.32 There are no data available yet as to the effects of targeting Sin complexes in Treg cells.
HDAC3 occurs as part of the NCoR/SMRT complex, which is recruited by class IIa HDAC enzymes (discussed in the next section), and can traffic in and out of the nucleus.33 The deacetylase activity of HDAC3 is dependent upon its interaction with NCoR and SMRT, and the complex is key to regulation of multiple nuclear hormone receptors.24 However, conditional deletion of HDAC3 in Foxp3+ Treg cells leads to lethal autoimmunity, in association with derepression of IL-2 production and disruption of Treg development and suppressive functions.33 The last of the class I HDACs, HDAC8, is unusual among class I HDACs in not being dependent upon association with other nuclear proteins for its activity, and due to the availability of HDAC8 isoform-selective inhibitors. However, our ongoing studies indicate that conditional deletion of HDAC8 in Foxp3+ Treg cells, or use of HDAC8 inhibitors, impairs Treg function and Treg-dependent allograft survival, and promotes anti-tumor immunity.
Taken together, these data indicate that class I HDACs are vital to multiple cellular functions and that their targeting either directly or as part of nuclear complexes, with the possible exception of HDAC2, is unlikely to be of use in promoting allograft survival.
3.2 Class IIa HDACs
Class IIa HDACs have interesting properties compared to other HDAC enzymes. They shuttle in and out of the nucleus, have a long N-terminal extension (~500 amino acids) that favors interactions with multiple transcription factors and is subject to numerous post-translational modification (Fig. 2), and they have a restricted tissue distribution, being markedly enriched in the brain, heart, striated muscle and cells of the immune system.34 Perhaps most remarkable of all, these HDACs have a histidine to tyrosine substitution in their catalytic domains, conserved from yeast to mammals, which renders them essentially inactive when assayed using conventional substrates. This enigmatic property suggests they serve primarily as acetyl-lysine binding proteins that, like other bromodomain readers, can recruit and bind HDAC3, NCoR and SMRT, or that their real targets have yet to be identified.35 Both may be true, and class IIa HDACs do show deacetylase activity using non-conventional trifluoroacetylated lysine substrates, though these substrates are unknown in nature. Their ability to function as lysine deacylases, removing fatty acid modifications from proteins, has not been reported, though other HDACs with limited deacetylase activity, such as HDAC8, 10 and 11, are active in this regard.36
Class IIa HDACs interact with, and suppress the functions of, many transcription factors but upon cell activation undergo phosphorylation-dependent conformational changes, with binding to the 14-3-3 proteins and nuclear export. In the cytoplasm, the class IIa HDACs may be then degraded via the proteasome. or be dephosphorylated by PP1 and PP2A and re-enter the nucleus to once again form multiprotein complexes with HDAC3/NCoR/SMRT. This trafficking allows for immediate and reversible responses to environmental conditions. Despite considerable homology, studies of Foxp3+ Tregs from mice with individual deletions of each HDAC show important differences. Thus, HDAC7 and HDAC9 can be co-precipitated with Foxp3 and their deletion enhances Treg function, whereas HDACs 4 and 5 appear necessary for optimal Treg function.10, 16, 37
Deletion of HDAC7 or HDAC9 increases Foxp3 acetylation. In the case of HDAC7, deletion is associated with derepression of Nur77, and Tregs from Nur77 transgenic mice, or Tregs treated with Nur77 inducers, have enhanced Treg function. Levels of Tregs in peripheral lymphoid tissues of Nur77 transgenic mice approach 50% of CD4 T cells, and these mice spontaneously accepted fully MHC-mismatched cardiac allografts, whereas grafts are rejected if the Tregs are depleted using CD25 mAb therapy.38 Such data provide clues as to the high bar required for Tregs alone to mediate allograft acceptance.
HDAC9 deficient (HDAC9−/−) mice also accept cardiac graft long-term but require a brief, peritransplant 14 days course of low dose rapamycin that by itself only extends graft survival for a few days.16 HDAC9 deletion promotes iTreg development and Treg proliferation and suppressive function, in conjunction with increased expression of the chaperone protein, HSP70, which co-associated with Foxp3.27 In addition, studies with Tregs from HSP70−/− or HSP70 transgenic mice showed that HSP70 was required for optimal Foxp3 expression and resistance to apoptosis.27 Deletion of HDAC9 in Tregs also stabilizes the acetylation, phosphorylation, and transcriptional activity of STAT5.39
Collectively, these data indicate that HDAC7 and 9 targeting may be of therapeutic value, but how to achieve this without also impairing the actions of the closely related HDAC4 and 5 proteins that appear necessary for Treg function is a problem, not to mention the negligible HDAC activity of class IIA HDACs such that pharmacologic options are few. The available data suggest that attention to the pathways modulated by deletion of each HDAC may be informative, for example with regard to increasing expression of Nur77 (HDAC7) or HSP70 (HDAC9) or promoting STAT5 acetylation (HDAC9) in Foxp3+ Treg cells.
3.3. Class IIb HDACs
In contrast to the very limited options for class IIa HDAC targeting, HDAC6 and to a lesser extent, HDAC10, offer considerable therapeutic potential. HDAC6 has two deacetylase domains (DD1, DD2), though the biological significance of DD1 is not well understood. HDAC6 resides primarily in the cytoplasm where it regulates the acetylation levels of α-tubulin, HSP90, cortactin, beta-catenin, glucocorticoid receptors and other proteins. Upon activation of Tregs, HDAC6 can migrate into the nucleus and regulate the acetylation levels of Foxp3, with which it can co-associate. Encouraging aspects of HDAC6 biology are that HDAC6 null mice are essentially normal, there are potent and isoform specific inhibitors such as Tubastatin A, and there are mAbs to acetylated α-tubulin which can serve as a biomarker (as can Treg suppressive activity) of the efficacy of HDAC6 targeting.40 HDAC6 deletion or pharmacologic inhibition of HDAC6 promotes HSP90 acetylation and release of client proteins, including HSF-1, and upregulation of heat-shock proteins. As noted with the upregulation of HSP70 in HDAC9−/− Tregs, the expression of heat shock proteins can promote Treg functions, but there are also HSP90-independent effects of HDAC6 targeting in Tregs, likely as a function of the ability of HDAC6 to enter the nucleus in Tregs and deacetylate Foxp3, as well as other actions.39, 40
The second class IIb HDAC, HDAC10, can also deacetylate Foxp3, such that HDAC10 deletion promotes Foxp3 acetylation and Foxp3-dependent gene expression (suppression of IL-2 and enhancement of IL-10, CTLA4 and other gene expression), and Treg suppressive functions in allograft and colitis models.41 Analogous data are seen with small molecules active against HDAC10, though these are currently less selective than those available for HDAC6.41
Thus, the most compelling evidence to date that targeting any single HDAC is possible, feasible and useful in the context of Treg biology and therapeutic effects arises from the studies of HDAC6.
3.4 Class III HDACs (Sirtuins)
These enzymes are present within the nucleus (Sirt1, Sirt6, Sirt7), mitochondria (Sirt3,4 and 5) or cytosol (Sirt2). Their actions differ from Zn-dependent HDACs1-11, in that their deacetylase activity is linked with NAD hydrolysis and hence the energy level of the cell. Most of the relevant data around the Sirtuin family involves studies of Sirt1. This enzyme deacetylates Foxp3 and many other proteins, and Sirt1 deletion in T cells or specifically in Tregs markedly prolongs heterotopic cardiac and orthotopic renal allograft survival, and suppresses autoimmune colitis, as does use of a Sirt1-selective inhibitor (EX-527).42–44 These data are notable given the life-supporting nature of the renal allograft model in mice that that undergone bilateral nephrectomy. Combination therapy with HDAC6 and Sirt1 inhibitors is more effective in enhancing Treg suppressive function than use of either one alone.39 In contrast to the effects of Sirt1, deletion of the mitochondria-associated Sirt3 in Tregs impairs Treg function in vitro and in vivo, abrogating Treg-dependent allograft survival.45
Despite the encouraging data for Sirt1 targeting, this enzyme promotes inflammation when deleted in myeloid cells, and pharmacologic inhibitors have dose-dependent effects such that low doses promote Treg function, but higher doses can also affect metabolically active organs, such as renal transplant, and yield systemic toxicity. Thus, it is not easy to recommend clinical applications of Sirt1 inhibitors.
3.5 Class IV HDAC
HDAC11, the sole HDAC class IV member, has been studied by undertaking cardiac transplants in mice with full gene deletion or conditional deletion in host Foxp3+ Tregs, as well as in wild-type treated with pharmacologic inhibitors.46 HDAC11 co-associates with Foxp3, leading to its deacetylation, whereas HDAC11 targeting promoted chromatin remodeling at the Foxp3 locus, with increased Foxp3 promoter binding of Fos and Jun that dimerize to form the AP-1 transcription factor, and increased expression of Foxp3 and Foxp3-dependent genes. HDAC11 targeting protected against development of acute rejection in fully MHC-disparate mice, and also blocked the development of transplant arteriosclerosis seen in class II-mismatched models.46 Hence, initial data for HDAC11 targeting in allograft recipients are encouraging.
4. PRACTICAL CONSIDERATIONS
4.1 Are there data with human Treg cells?
HDAC expression and the effects of various HDAC inhibitors on purified human Tregs, and expanded human Tregs, were studied under resting and activating conditions.47 Baseline levels of 3 of the 4 class I HDACs (HDAC1, 2 and 3) were comparable in Tregs and conventional T cells, whereas Tregs showed higher baseline expression of the remaining class I HDAC (HDAC8). In addition, Tregs showed higher levels than conventional T cells of all 5 class II HDACs (HDAC4, 5, 6, 7 and 9), with HDAC9 showing the most notable difference, consistent with murine Treg data.16 Pan-HDAC inhibitors and HDAC6-selective inhibitors were shown to enhance human Treg suppressive function in vitro.47, 48
4.2 What drugs are available for clinical use?
Apart from valproic acid, that has weak HDAC inhibitory activity and is used as an anti-epileptic and mood-stabilizing drug, there are now 4 clinically approved HDACi compounds in the US. Three (Vorinostat, Romidepsin, Belinostat) were licensed for treatment of cutaneous or peripheral T cell lymphoma. The fourth, Panobinostat, was licensed for treatment of multiple myeloma. All 4 compounds are pan-HDAC inhibitors, which given the lack of catalytic activity of class IIa HDACs, means that they block class I HDACs and at least HDAC6 (data on HDAC10 and 11 are unavailable).35 Pharmacophore analysis shows most HDAC inhibitors have a zinc-binding group (ZBG), a linker to the outside of the HDAC catalytic site, and a “cap” interacting group that can promote HDAC-selective interactions (Fig. 3).10 ZBG that include hydroxamic acids (hydroxamates) are usually the most potent compounds, whereas 2-aminobenzamides are usually selective for class I HDACs. Three of the 4 clinically approved HDAC inhibitors are hydroxamates, with Romidepsin being a cyclic depsipeptide. All 4 enhance Treg function, presumably through effects on HDAC6 rather than class I HDACs, and in a clinical trial in patients receiving allogeneic stem cell transplants, Vorinostat was shown to increase Treg numbers and decrease the incidence and severity of GVHD.49 However, the clinical applications of these 4 compounds rely on direct inhibition of tumor growth rather than modulation of antitumor immunity.
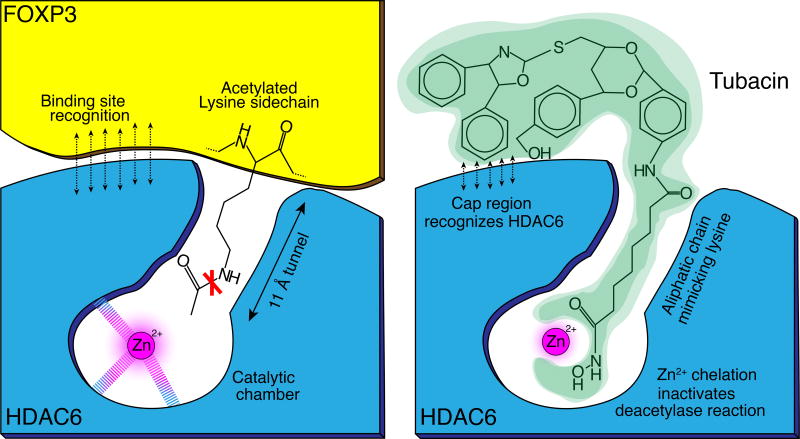
Figure 3. HDAC6 inhibitor design.
Schematic representation of how HDAC6 deacetylates Foxp3 and how this process can be inhibited with an HDAC6 inhibitor, e.g. Tubacin. The aliphatic lysine sidechain fits into a tunnel, leading to a catalytic chamber containing a zinc ion that is essential for the deacetylation reaction. HDAC inhibitors take advantage of this principle and are designed to mimic the aliphatic lysine side chain to fit into the tunnel. Instead of an acetyl group, the HDAC6 inhibitor has a zinc binding group, chelating the Zn2+ ion and blocking the deacetylation reaction. HDAC-isoform specificity is achieved through the ‘cap region’ of the molecule fitting to the surrounding amino acid side chains specific to HDAC6.
There are ~700 clinical trials of HDACi listed at clinicaltrials.gov. Multiple trials of relatively HDAC6-selective inhibitors (all hydroxamates) are underway in patients with solid tumors, multiple myeloma or melanoma, respectively, and initial data from the myeloma studies are sufficiently encouraging that a licensing application is anticipated. With regard to potential benefits from Treg modulation, pan-HDACi are currently in clinical trials in patients with Crohn’s disease, GVHD, juvenile arthritis and other autoimmune diseases. However, an important practical concern in the context of potential application in transplantation rather than oncology is that hydroxymates are typically Ames test-positive and are thereby considered potentially genotoxic. Hence, the recent development of new generation non-hydroxamic acid HDAC6-selective inhibitors that promote Treg function in vitro and in vivo, and are Ames test-negative, is an important advance.50, 51
4.3 Clinical applications and future directions
Based on the above considerations, an appropriately specific and potent HDAC6 inhibitor appears a good candidate for application in transplantation (Fig. 2), with the potential to modulate Treg function on a long-term basis as desired, and there is commercial interest in this point.52, 53 Additional benefits may come from targeting HDAC10 and HDAC11, and HDAC11 inhibitors are in development.46 Further applications for HDAC inhibitors in transplant settings may come through their utility in suppressing ischemia/reperfusion injury,54 and through use in stabilizing the Treg phenotype for cell therapy. Beyond the scope of this review, components of the gut microbiome release short-chain fatty acids that have HDAC inhibitory activity and promote Treg development and function within gut lymphoid tissues, such that therapeutic modulation may also be possible. In the current era, however, application of HDACi therapy in conjunction with rapamycin, rather than a CNI-based protocol, appears the most productive, and may be especially suited to the growing field of vascularized composite allotransplantation where maintenance CNI and the side effects thereof may be a deterrent to expansion of this approach to a broader patient cohort.
Acknowledgments
National Institutes of Health Grant/Award Numbers: R01CA177852, R01AI123241, R01DK106243, R43CA174037 and K08AI095353.
Abbreviations
- CNI
calcineurin inhibitor
- HAT
histone acetyltransferase
- HDAC
histone/protein deacetylase
- KAT
lysine acetyltransferase
- KDAC
lysine deacetylase
- PTM
posttranslational modification
- Treg
T-regulatory
- ZBG
zinc-binding group.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Akimova T, Kamath BM, Goebel JW, Meyers KE, Rand EB, Hawkins A, et al. Differing effects of rapamycin or calcineurin inhibitor on T-regulatory cells in pediatric liver and kidney transplant recipients. Am J Transplant. 2012;12:3449–3461. doi: 10.1111/j.1600-6143.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 8.Lozano T, Villanueva L, Durantez M, Gorraiz M, Ruiz M, Belsue V, et al. Inhibition of FOXP3/NFAT interaction enhances T cell function after TCR stimulation. J Immunol. 2015;195:3180–3189. doi: 10.4049/jimmunol.1402997. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Rowell EA, Thomas RM, Hancock WW, Wells AD. Transcriptional regulation by Foxp3 is associated with direct promoter occupancy and modulation of histone acetylation. J Biol Chem. 2006;281:36828–36834. doi: 10.1074/jbc.M608848200. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nat Rev Drug Discov. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chen C, Zhang Z, Liu CC, Johnson ME, Espinoza CA, et al. DNA binding by FOXP3 domain-swapped dimer suggests mechanisms of long-range chromosomal interactions. Nucleic Acids Res. 2015;43:1268–1282. doi: 10.1093/nar/gku1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X, Li B, Xiao Y, Chen C, Wang Q, Liu Y, et al. Structural and biological features of FOXP3 dimerization relevant to regulatory T cell function. Cell Rep. 2012;1:665–675. doi: 10.1016/j.celrep.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Kumar S, Dahiya S, Wang F, Wu J, Newick K, et al. Ubiquitin-specific protease-7 inhibition impairs Tip60-dependent Foxp3+ T-regulatory cell function and promotes antitumor immunity. EBioMedicine. 2016;13:99–112. doi: 10.1016/j.ebiom.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Wang L, Wu J, Sokirniy I, Nguyen P, Bregnard T, et al. Active site-targeted covalent irreversible inhibitors of USP7 impair the functions of Foxp3+ T-regulatory cells by promoting ubiquitination of Tip60. PLoS One. 2017;12:e0189744. doi: 10.1371/journal.pone.0189744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Wang L, Han R, Beier UH, Hancock WW. Two lysines in the forkhead domain of foxp3 are key to T regulatory cell function. PLoS One. 2012;7:e29035. doi: 10.1371/journal.pone.0029035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katan-Khaykovich Y, Struhl K. Dynamics of global histone acetylation and deacetylation in vivo: rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes Dev. 2002;16:743–752. doi: 10.1101/gad.967302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dancy BM, Crump NT, Peterson DJ, Mukherjee C, Bowers EM, Ahn YH, et al. Live-cell studies of p300/CBP histone acetyltransferase activity and inhibition. Chembiochem. 2012;13:2113–2121. doi: 10.1002/cbic.201200381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Wang L, Predina J, Han R, Beier UH, Wang LC, et al. Inhibition of p300 impairs Foxp3+ T regulatory cell function and promotes antitumor immunity. Nat Med. 2013;19:1173–1177. doi: 10.1038/nm.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wang L, Han R, Beier UH, Akimova T, Bhatti T, et al. Two histone/protein acetyltransferases, CBP and p300, are indispensable for Foxp3+ T-regulatory cell development and function. Mol Cell Biol. 2014;34:3993–4007. doi: 10.1128/MCB.00919-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Nagai Y, Deng G, Ohtani T, Zhu Z, Zhou Z, et al. Dynamic interactions between TIP60 and p300 regulate FOXP3 function through a structural switch defined by a single lysine on TIP60. Cell Rep. 2014;7:1471–1480. doi: 10.1016/j.celrep.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harbor perspectives in biology. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Samanta A, Levine MH, Beier UH, Han R, Kalin J, et al. Vital role of the CoREST complex as a master regulator of Foxp3+ T-regulatory cell gene expression and suppressive function. Am J Transplant. 2017;17(Suppl 3) [Google Scholar]
- 26.Leus NG, van den Bosch T, van der Wouden PE, Krist K, Ourailidou ME, Eleftheriadis N, et al. HDAC1-3 inhibitor MS-275 enhances IL10 expression in RAW264.7 macrophages and reduces cigarette smoke-induced airway inflammation in mice. Sci Rep. 2017;7:45047. doi: 10.1038/srep45047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Zoeten EF, Wang L, Sai H, Dillmann WH, Hancock WW. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138:583–594. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner T, Kiweler N, Wolff K, Knauer SK, Brandl A, Hemmerich P, et al. Sumoylation of HDAC2 promotes NF-kappaB-dependent gene expression. Oncotarget. 2015;6:7123–7135. doi: 10.18632/oncotarget.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, Li M, Chen N, Wang S, Luo HB, Zhang Y, et al. Computational design of a time-dependent histone deacetylase 2 selective inhibitor. ACS Chem Biol. 2015;10:687–692. doi: 10.1021/cb500767c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millard CJ, Watson PJ, Fairall L, Schwabe JW. Targeting class I histone deacetylases in a "complex" environment. Trends Pharmacol Sci. 2017;38:363–377. doi: 10.1016/j.tips.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Kalin JH, Wu M, Gomez AV, Song Y, Das J, Hayward D, et al. Targeting the CoREST complex with dual histone deacetylase and demethylase inhibitors. Nat Commun. 2018;9:53. doi: 10.1038/s41467-017-02242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Liu Y, Han R, Beier UH, Thomas RM, Wells AD, et al. Mbd2 promotes foxp3 demethylation and T-regulatory-cell function. Mol Cell Biol. 2013;33:4106–4115. doi: 10.1128/MCB.00144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T, et al. FOXP3+ regulatory T cell development and function require histone/protein deacetylase 3. J Clin Invest. 2015;125:1111–1123. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Giorgio E, Brancolini C. Regulation of class IIa HDAC activities: it is not only matter of subcellular localization. Epigenomics. 2016;8:251–269. doi: 10.2217/epi.15.106. [DOI] [PubMed] [Google Scholar]
- 35.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang H, Zhang X, Chen X, Aramsangtienchai P, Tong Z, Lin H. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem Rev. 2018 doi: 10.1021/acs.chemrev.6b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao H, Jiao J, Wang L, O'Brien S, Newick K, Wang LC, et al. HDAC5 controls the functions of Foxp3(+) T-regulatory and CD8(+) T cells. Int J Cancer. 2016;138:2477–2486. doi: 10.1002/ijc.29979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao R, Hancock WW. Resistance of Foxp3+ regulatory T cells to Nur77-induced apoptosis promotes allograft survival. PLoS ONE. 2008;3:e2321. doi: 10.1371/journal.pone.0002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Zoeten EF, Wang L, Butler K, Beier UH, Akimova T, Sai H, et al. Histone deacetylase 6 and heat shock protein 90 control the functions of Foxp3(+) T-regulatory cells. Mol Cell Biol. 2011;31:2066–2078. doi: 10.1128/MCB.05155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahiya S, Wang L, Beier UH, Angelin A, Han R, Wallace DC, et al. Mechanistic insights into how targeting of HDAC10 promotes Foxp3+ Treg cell suppressive activity, gene expression and metabolism, and enhances allograft survival. Am J Transplant. 2016;16(suppl 3) [Google Scholar]
- 42.Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31:1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine MH, Wang Z, Xiao H, Jiao J, Wang L, Bhatti TR, et al. Targeting Sirtuin-1 prolongs murine renal allograft survival and function. Kidney Int. 2016;89:1016–1026. doi: 10.1016/j.kint.2015.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akimova T, Xiao H, Liu Y, Bhatti TR, Jiao J, Eruslanov E, et al. Targeting sirtuin-1 alleviates experimental autoimmune colitis by induction of Foxp3+ T-regulatory cells. Mucosal Immunol. 2014;7:1209–1220. doi: 10.1038/mi.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beier UH, Angelin A, Akimova T, Wang L, Liu Y, Xiao H, et al. Essential role of mitochondrial energy metabolism in Foxp3+ T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J, Wang L, Dahiya S, Beier UH, Han R, Samanta A, et al. Histone/protein deacetylase 11 targeting promotes Foxp3+ Treg function. Sci Rep. 2017;7:8626. doi: 10.1038/s41598-017-09211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol. 2010;136:348–363. doi: 10.1016/j.clim.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akimova T, Levine MH, Beier UH, Hancock WW. Standardization, evaluation and area-under-curve analysis of human and murine Treg suppressive function. Methods Mol Biol. 2016;1371:43–78. doi: 10.1007/978-1-4939-3139-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hancock WW. Effects of histone deacetylase inhibitors on alloresponses. Lancet Oncol. 2014;15:10–11. doi: 10.1016/S1470-2045(13)70537-0. [DOI] [PubMed] [Google Scholar]
- 50.Kalin JH, Butler KV, Akimova T, Hancock WW, Kozikowski AP. Second-generation histone deacetylase 6 inhibitors enhance the immunosuppressive effects of Foxp3+ T-regulatory cells. J Med Chem. 2012;55:639–651. doi: 10.1021/jm200773h. [DOI] [PubMed] [Google Scholar]
- 51.Segretti MC, Vallerini GP, Brochier C, Langley B, Wang L, Hancock WW, et al. Thiol-based potent and selective HDAC6 inhibitors promote tubulin acetylation and T-regulatory cell suppressive function. ACS Med Chem Lett. 2015;6:1156–1161. doi: 10.1021/acsmedchemlett.5b00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellis JD, Neil DAH, Inston NG, Jenkinson E, Drayson MT, Hampson P, et al. Inhibition of histone deacetylase 6 reveals a potent immunosuppressive effect in models of transplantation. Transplantation. 2016;100:1667–1674. doi: 10.1097/TP.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 53.Hancock WW. Isoform-selective HDAC inhibitor therapy for transplantation: Are we ready for HDAC6? Transplantation. 2016;100:1597–1598. doi: 10.1097/TP.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine MH, Wang Z, Wang Y, Bhatti TR, McNeal S, Liu Y, et al. Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Transplantation. 2015;15:965–973. doi: 10.1111/ajt.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]