Abstract
While many patients have substantial residual kidney function (RKF) when initiating hemodialysis (HD), most patients with end stage renal disease in the United States are initiated on a three-times per week conventional HD regimen, with little regard to RKF or patient preference. RKF is associated with many benefits including survival, volume control, solute clearance and reduced inflammation. Several strategies have been recommended to preserve RKF after HD initiation, including an incremental approach to HD initiation. Incremental HD prescriptions are personalized to achieve adequate volume control and solute clearance with consideration to a patient’s endogenous renal function. This allows the initial use of less frequent and/or shorter HD treatment sessions. Regular measurement of RKF is important because HD frequency needs to be increased as RKF inevitably declines. We narratively review the results of 12 observational cohort studies of twice weekly compared to thrice weekly HD. Incremental HD is associated with several benefits including preservation of RKF as well as extending the event-free life of arteriovenous fistulas and grafts. Patient survival and quality of life, however, has been variably associated with incremental HD. Serious risks must also be considered, including increased hospitalization and mortality perhaps related to fluid and electrolyte shifts after a long inter-dialytic interval. Based on the above literature review, and our clinical experience, we suggest patient characteristics which may predict favorable outcomes with an incremental approach to HD. These include substantial RKF, adequate volume control, lack of significant anemia/electrolyte imbalance, satisfactory health related quality of life, low comorbid disease burden and good nutritional status without evidence of hypercatabolism. Clinicians should engage patients in on-going conversations to prepare for incremental HD initiation and to ensure a smooth transition to thrice weekly HD when needed.
Keywords: residual kidney function, end-stage renal disease, incremental hemodialysis
INTRODUCTION
In the United States, there are currently over 450,000 prevalent hemodialysis (HD) patients, and a million more are expected to initiate HD in the next decade1. Many patients who initiate HD have substantial residual kidney function (RKF)2. In the United States, more than 90% of end stage renal disease (ESRD) patients new to hemodialysis initiate a standardized three-times per week HD prescription3 with little consideration to RKF, patient preference or lifestyle. This is despite the continuing evolution of medical care towards a personalized and individual approach.
The dogma of three times a week HD frequency is based on a target Kt/Vurea (urea clearance normalized to its volume of distribution). This concept of dialysis adequacy was developed in the early 1980s. Patient outcomes were studied initially in the NCDS study4, and subsequently in the HEMO trial5. Both of these landmark trials used a thrice-weekly HD prescription for all study arms, and enrolled study patients had little or no RKF (creatinine clearance ≤ 3 ml/minute in the NCDS study and urea clearance ≤ 1.5 ml/min per 35 L body water in the HEMO study). Yet results from these landmark studies led to adequacy guidelines6,7 which were clinically applied to most incident HD patients, many who had substantial RKF2. While the most recent KDOQI adequacy guidelines now advise that HD frequency may be reduced in the presence of substantial RKF8, clinical practice continues to lag behind and the vast majority of HD patients remain on a thrice weekly HD regimen9,10.
The prognosis for ESRD patients on dialysis remains grim. While mortality has modestly improved in the past decade, maintenance dialysis patients still have an approximately 6 to 8 times higher risk of mortality than the general Medicare population11. The median life expectancy for an incident HD patient is only 3 years1. Hospitalizations in patients on HD also remain high, with an average of 1.7 hospitalizations per year and a 35% risk of 30-day readmission to hospital12, more than double that of the general Medicare population13. Health related quality of life is also substantially lower in ESRD patients than the general population14, a finding which remained consistent across 3 continents in one study from the Dialysis Outcomes and Practice Patterns Study (DOPPS).
Given the poor prognosis and lack of convincing evidence that a HD prescription based solely on raising Kt/Vurea can benefit patient mortality and quality of life, there should be a shift away from a “one size fits all” protocolized HD initiation toward a more personalized approach to account for unique patient factors including RKF. In this review, we will first discuss the importance of RKF to improve patient outcomes, and predictors for loss of RKF. We will then summarize the available evidence for incremental HD, and discuss the potential benefits and risks. Finally, we will provide our opinion on specific patient characteristics which may predict for favorable outcomes with incremental HD initiation.
THE IMPORTANCE OF RESIDUAL KIDNEY FUNCTION
RKF has been associated with numerous patient benefits, including survival, volume control and reduced inflammation. While HD by nature is intermittent, native kidney function is continuous. For this reason, even a small amount of residual kidney function contributes to reduced plasma levels of solutes that are cleared poorly by HD, such as B2-microglobulin and protein bound solutes15–18. RKF has long been utilized in determining the optimal dose among patients receiving peritoneal dialysis (PD)19 and has been reported in observational studies as an independent predictor of survival in PD20–23.
In HD patients, an understanding of the importance of RKF is still emerging. Less than 5% of HD patients have measured RKF24, which may be related to difficulties with accurate inter-dialytic urine collection24. Despite this, several observational studies25–29 have found independent associations with measures of increased RKF and survival. For example, in a recent longitudinal cohort of 5686 patients initiating maintenance dialysis, higher RKF at 1 year was associated with better survival, with a linear association between mortality and both renal urea clearance and urine volume29.
In another prospective study of 1191 HD and 609 PD patients initiating dialysis, anuria was found to be associated with a 1.5 times higher risk of mortality than patients with RKF. Importantly, this survival benefit did not differ significantly between PD and HD patients27. HD patients with RKF also have the advantage of improved volume control. This may benefit patients in several ways, including lower ultrafiltration volumes during each dialysis session, less intradialytic hypotension, myocardial stunning30,31, and subsequent reduction in cardiovascular mortality32,33.
RKF may also play a role in reduction of inflammatory markers34,35, including C-reactive protein and interleukin-636; this has been observed in nephrectomized rats through a reduction in clearance of inflammatory markers37,38. RKF has been associated with several other benefits to HD patients, including better quality of life39, better overall nutritional status40, less anemia with less use of epoetin alpha36, and better control of serum phosphorus41.
A number of factors can affect the rate of RKF decline once dialysis has been initiated, and these can be broadly classified as demographic characteristics, comorbid diseases, or HD prescription characteristics. Much of this literature is limited in comparability due to lack of a standard definition for RKF and retrospective study designs. With respect to demographic characteristics, non-white race has been associated with faster RKF decline24. Gender has a variable association, with one analysis of USRDS data reporting female sex associated with faster RKF decline24 while another study reported male sex predicted decline in RKF42. Co-morbid conditions of diabetes43, poorly controlled hypertension and cardiovascular disease have all been reported to predict loss of RKF. Intradialytic hypotension during the first 3 months of dialysis is also associated with RKF decline, as is the presence of proteinuria, even after 6 months after dialysis initiation25.
Certain HD prescription characteristics, including use of high-flux, biocompatible dialysis membranes44–46, ultrapure dialysate47 and online hemodiafiltration,48 may slow the decline of RKF. Finally, high frequency of dialysis treatments accelerates RKF decline. In a secondary analysis of the Frequent Hemodialysis Network (FHN) study49, 67% of incident HD patients randomized to frequent nocturnal HD (i.e > 4 times per week) had urine output decline to zero, compared to only 32% of control patients on thrice weekly HD.
INCREMENTAL HEMODIALYSIS – WHAT IS IT AND WHAT IS THE EVIDENCE?
While robust estimates of the incidence and prevalence of incremental HD in current practice patterns are lacking, a historic United States cohort documented 6.1% of incident and 2.7% of prevalent HD patients were treated with a twice-weekly HD regimen in 19939. A more recent DOPPS study compared the prevalence of twice weekly HD across several countries, and found one quarter of Chinese patients were treated with twice weekly HD in 2011, compared to < 5% across 11 other countries including the United States, Canada, France, Italy and Japan10.
Incremental HD prescriptions are personalized to achieve adequate volume control and solute clearance with consideration of a patient’s endogenous renal function. This individualization of the HD prescription allows for the initial use of shorter duration, less frequent and less intense dialysis (i.e. smaller dialyzer surface areas and lower blood and dialysate flows)50–52. RKF, along with patient symptoms and inter-dialytic weight gains, must be regularly monitored, with adjustment to the HD prescription as RKF declines and/or a change in patient factors. Using the recommended fixed target stdKt/V (dialysis + residual renal) of 2.38, a conceptual scheme for transition to thrice weekly dialysis using an incremental approach is shown in Figure 1. Once or twice-weekly dialysis can also be considered as part of a decremental approach to HD in terminally ill patients as they transition to end-of-life palliative care52,53.
Figure 1.
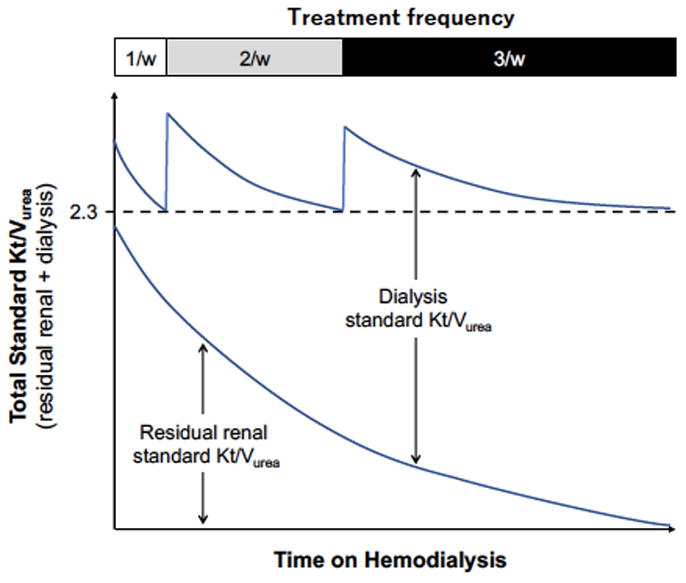
A conceptual scheme for an incremental hemodialysis regimen with adjustment of hemodialysis frequency based on residual renal urea clearance. (used with permission, Kidney International Reports and the International Society of Nephrology)
While RKF has a long history of inclusion into the overall calculation of peritoneal dialysis adequacy, it has been largely ignored when initiating and prescribing HD. This may be in part due to the HD urea-based “adequacy” targets set forth by the Centers for Medicare and Medicaid Services Quality Incentive Program, which do not include residual urea clearance (KRU). As such, historical interest in individualizing the HD prescription based on RKF, as well research assessing the efficacy and outcomes of incremental HD, has been lacking.
There are several small single center cohort studies comparing twice and thrice weekly HD that suggest that twice weekly HD may be associated with a slower decline in RKF compared to thrice-weekly HD54–57 (Table 1). For example, in a Shanghai based study55, 30 HD patients initiated on a twice-weekly HD prescription were compared to 55 patients initiated on thrice-weekly HD. RKF loss was significantly higher in the thrice-weekly group compared to the twice-weekly group, with an odds ratio of RKF loss for each additional HD treatment per week of 7.2, suggesting that thrice-weekly HD during the first year of dialysis was associated with a 7 times higher risk of RKF loss than twice weekly HD. However, small sample sizes and lack of reporting on patient survival complicate interpretation of these results and limit application to clinical practice.
Table 1.
Summary of Comparative Studies Evaluating Patient Outcomes in with Twice vs Thrice weekly hemodialysis
| Author | Year | Study Design | Study Duration (years) | Twice-weekly N | Thrice-weekly N | RKF Metric | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| RKF | Mortality | Other | |||||||
| Hanson9 | 1999 | Retrospective Cohort | 3 | 570 | 14497 | eGFR | Lower mortality for twice weekly group (RR=0.76, p=0.02), but when adjusted for eGFR at time of dialysis start, mortality was similar between two groups (RR 0.85, p=0.31) | ||
| Supasyndh79 | 2009 | Cross-sectional study | N/A | 82 | 60 | Nutritional status similar in both groups measured by bio-impedance | |||
| Lin54 | 2009 | Prospective Cohort | 8 | 23 | 51 | UOP and residual GFR* | Twice weekly group had higher mean urine output than thrice weekly group (1.7L vs 0.61L; p=0.001) and residual GFR (1.9mL/min vs 0.71mL/min; p=0.001) | -Less frequent hospitalization in twice weekly group (63% vs 33%; p=0.012 -No difference in nutrition or inflammation indices between groups |
|
| Stankuviene80 | 2010 | Retrospective Cohort | 8 | Total cohort N=2428 58.5% 3x week 36.2% 2x week 5.3% 1x week |
Higher mortality in twice weekly group (RR 1.98 (95% CI 1.64, 2.40; p<0.001) | ||||
| Lin81 | 2012 | 1041 | 1531 | Similar survival in both groups (RR 0.78; 95% CI 0.55,1.09; p=0.145) | |||||
| Elamin82 | 2012 | Prospective Cohort | 2 | Total cohort N=1011 74.8% 2x week |
Similar one year mortality in twice weekly group (85% vs 89%, p=0.06) | ||||
| Fernandez-Lucas83 | 2012 | Prospective Cohort | 5 | 41 | 54 | loss of UOP/24 hours | -loss of UOP/24 hours was greater in thrice weekly group compared to twice weekly group (206mL/month vs 91mL/month | -Survival greater in twice weekly group (log-rank 3.96; p-0.04) | |
| Zhang55 | 2014 | Prospective Cohort | 1 | 30 | 55 | RKF loss, defined as < 200mL/day of urine output | RKF loss reported in 60% (n=18) in twice weekly vs 82% (n=45) in thrice weekly group | ||
| Caria56 | 2014 | Prospective Cohort^ | 2 | 38 (with very low protein diet) | 30 | GFR* loss per month | -GFR loss of −0.13mL/min/month in twice weekly vs −1.53mL/min/month in thrice weekly group weekly group (p=NS). | -Survival 95% vs 87% in twice vs thrice | -Hospitalization in 24 months was 3.7 days/patient in twice weekly vs 6.1 days/patient in thrice weekly group |
| Obi28 | 2016 | Retrospective Cohort | 4 | 351 | 8068 | UOP and KRU | - Slower RKF decline over time in twice vs thrice weekly group (UOP to ≤ 600mL/day RR 1.15 (95% CI, 1.02–1.30, p<0.001) | -Similar overall survival between groups (HR1.11; 95% CI, 0.89–1.38; P=0.3). | |
| Hwang84 | 2016 | Prospective Cohort | 3 | 113 | 572 | KRU corrected for 1.73m2 body surface area | -RKF at 36 months 2.9mL/min in twice weekly vs 1.0 mL/min in thrice weekly; p<0.001) | -Higher mortality in twice weekly group compared to thrice weekly group (HR 4.2; 95% CI 1.02–17.32; p0.04) | |
| Mathew58 | 2016 | Retrospective Cohort | 5 | 434 | 50162 | -Similar survival between groups (HR 0.88, 95% CI 0.72, 1.08), after adjustment for RKF | |||
includes once-weekly and twice weekly HD patients, 5.3% and 36.2% of total cohort, respectively
calculated as arithmetic mean of residual urea and creatinine clearances
Abbreviations: RKF=residual kidney function; eGFR=estimated glomerular filtration rate; UOP=urine output; KRU=residual urea clearance; GFR=glomerular filtration rate; RR=relative risk; CI=confidence interval
Recent shifts in clinical practice towards a new paradigm of “personalized medicine”, and the 2015 KDOQI adequacy guidelines which advise consideration of incremental HD in the presence of substantial RKF8, have prompted larger observational studies with more rigorous analysis (Table 1). For example, a large cohort study of 351 incremental HD patients and 8068 matched thrice-weekly HD patients assessed the outcomes of decline in RKF and mortality28. Substantial RKF was defined as renal urea clearance >3.0mL/min/1.73m2, or urine volume of >600mL/day. Older patients and non-Hispanic white patients were more likely, whereas non-Hispanic black patients and those with a CVC were less likely, to receive twice weekly HD. After matching, variables which remained imbalanced included weekly IDWG, dialysis treatment time, standard Kt/V delivered by dialysis. The results demonstrated that compared with the thrice weekly patients, twice-weekly HD patients had 16% (95% CI, 5%–28%) and 15% (95% CI, 2%–30%) more preserved renal urea clearance and urine volume, respectively.
The presence of RKF also modified the association of an incremental HD regimen with mortality. Incremental HD patients showed similar survival among patients with substantial RKF at baseline (HR, 0.99; 95% CI, 0.76–1.28), but higher mortality risk if they had inadequate baseline renal urea clearance (≤ 3.0 mL/min/1.73 m2; HR, 1.61; 95% CI, 1.07–2.44). Results were similar with stratification by baseline urine volume of 600 mL/d. The authors concluded that in patients with substantial RKF, incremental HD can be considered as a safe option and is associated with greater preservation of RKF. However, use of incremental HD in patients with inadequate RKF may prove harmful.
In another recent large observational study58 of 434 incremental HD patients matched to 50,162 thrice weekly HD patients, the outcome of mortality adjusted for RKF was assessed. Incremental HD patients were older and had with less co-morbidity than thrice-weekly HD patients. RKF was defined as residual renal urea clearance and calculated using the Daugirdas approach59,60. After matching, incremental HD patients compared to thrice weekly patients still had higher renal urea clearance (5.4 vs 3.1 mL/min/1.73m2). Mortality was similar in the incremental versus thrice-weekly HD groups (HR 0.88; 95% CI 0.72,1.08). In a pre-specified subgroup analysis of patients with a high Charleston Co-morbidity index ≥ 5, incremental HD patients had a 1.7-fold higher mortality than thrice weekly HD patients (HR 1.77, 95% CI 1.20, 2.62).
While these larger studies provide a more rigorous analytic approach, the observation design has inherent limitations including residual confounding by indication and lack of prospective data collection of all important variables. A randomized controlled trial has not yet been conducted comparing twice to thrice weekly HD, and would shed light on the safety and efficacy of incremental HD in select patient populations.
POTENTIAL BENEFITS OF INCREMENTAL HD
Incremental HD has many potential benefits to patients, clinicians and health systems. Preservation of RKF is important in incident HD patients and is associated with many benefits including patient survival, better quality of life, improved overall nutritional status and less anemia (see above, The Importance of Residual Kidney Function). Another benefit of incremental HD is longevity of vascular access related to less frequent arteriovenous fistula or graft cannulations. In an analysis from the FHN study, more frequent HD reduced the composite endpoint of vascular access loss, repair or access-related hospitalization. The risk for a first access event was 76% higher with daily HD than with conventional HD (HR 1.76; 95% CI, 1.11–2.79; P=0.017)61. Economic benefits must also be considered with less frequent HD treatment regimens. Conventional thrice weekly HD treatments costs approximately $89,000 per patient annually in the United States, with a total annual cost of $42 billion ($34 billion paid by Medicare, the remainder by Medicaid, private insurance or out-of-pocket payments)62.
Perhaps through the mechanism of RKF preservation, longer patient survival has been observed with incremental HD in some studies, and survivals similar to those of thrice weekly HD have been noted in other studies (see Table 1). This variable effect of incremental HD on mortality may be related to a beneficial modifying effect of RKF28, which is not accounted for in all studies. However, it is important to note that these associations have only been reported in observational studies.
Clinicians may intuit that patients choose twice weekly HD for convenience and improved quality of life. While robust prospective studies are lacking, a recent DOPPS study examined HD patient characteristics and health related quality of life (HRQOL) in China (where over one quarter of HD patients are dialyzed twice weekly). In 304 patients on a twice weekly HD regimen and 982 patients on a thrice weekly regimen, there was no significant difference in HRQOL, measured using the KDQOL Short Form 1210.
POTENTIAL RISKS AND BARRIERS TO INCREMENTAL HD
Without careful evaluation and discussion between physician and patient, the broad use of incremental HD can potentially be associated with several risks. In addition to less solute clearance, of particular concern is the longer inter-dialytic interval with less frequent HD63–65. In the landmark study by Foley et al64 of 32,065 patients on conventional thrice weekly HD, all-cause mortality was significantly higher on the day after the long, 2-day inter-dialytic interval compared to other days (22.1 vs 18.0 deaths per 100 person years, p<0.001). This increased mortality is presumably related to rapid fluid shifts, with subsequent myocardial stunning and cardiac adverse events66,67. Large inter-dialytic weight gains over the 2-day interval would necessitate rapid ultrafiltration rates (≥10mL/kg/hr)68, which has been independently associated with mortality.
Similarly, rapid electrolyte shifting is also associated with adverse outcomes. Brunelli et al recently studied 52,734 thrice-weekly HD patients69, and reported elevated serum potassium levels of 5.5 to <6.0 mEq/L obtained on Friday were associated with highest magnitude of hospitalization risk within 4 days of measurement (OR, 1.68, 95% CI, 1.22–2.30), compared to levels obtained on Monday or Wednesday.
It is important to note that the study cohort in both of these analyses was comprised on prevalent HD patients, who likely had minimal or non-existent RKF. Presence of substantial RKF in a patient on HD may contribute to fluid and electrolyte control on non-dialysis days, and mitigate the rapid ultrafiltration and electrolyte shifts after a long inter-dialytic interval.
While there has been recent renewed interest in preservation of RKF in ESRD patients, barriers remain for widespread use of an incremental approach to HD. Firstly, there are alternative means to potentially slow the decline of RKF once HD is initiated, including: 1) avoidance of nephrotoxins70,71 (aminoglycosides, non-steroidal anti-inflammatories, radiocontrast dye), 2) control hypertension while minimizing intradialytic hypotension 72,73, 3) adjustment of the HD prescription (high-flux biocompatible dialyzer membranes and ultra-pure dialysate water)44,74,75, and 4) possible consideration of a low protein diet (0.6 to 0.8g/kg/day) on non-dialysis days56,76,77.
We believe these tactics are important, and should be used whenever possible and appropriate. However, the modifying effect of RKF on the association of incremental HD and survival 28 provides a potent rationale for incorporating this incremental HD strategy into an attentive and thoughtful approach for RKF preservation.
Secondly, clinicians may have concerns about the practicalities on how or when to increase HD frequency, especially related to patient adherence with changing HD treatment frequency. Clinicians must actively engage patients and their caregiver(s) in the shared decision-making process with incremental HD transitions with ongoing conversations over multiple sessions. Clear expectations prior to HD initiation and each frequency change are critical in order to ensure a smooth patient transition from twice to thrice weekly HD. Golper provides a practical approach, with a case example dialogue, regarding the need to increase HD frequency from twice to three times per week50. In addition, an HD patient’s RKF must be monitored regularly while on a twice-weekly regimen. Monthly timed urine collections for residual creatinine and urea clearance are advised, although some experts recommend urine volume may be an appropriate surrogate measure52.
Finally, some HD outpatient facilities in the private sector may perceive loss of dialysis treatment income with a shift to twice weekly treatments, and pose barriers to its implementation. Pragmatic and innovative solutions, such as scheduling shifts, can accommodate the same number of HD treatments for incremental HD patients. For example, 3 twice weekly patients could be scheduled on Mon-Thu, Tue-Fri and Wed-Sat in lieu of 2 thrice-weekly patients78.
OUR OPINION
Incremental HD is not suitable for all patients with ESRD, and requires a judicious and attentive clinical approach for successful implementation. The KDOQI 2015 clinical practice guidelines update for HD adequacy do not provide a clear approach for its use 8. The ungraded KDOQI recommendations are: 1) In patients with significant residual native kidney function (Kr), the dose of HD may be reduced provided Kr is measured periodically; and 2) For HD schedules other than thrice weekly, a target standard Kt/V of 2.3 volumes per week with a minimum delivered dose of 2.1 using a method of calculation that includes the contributions of ultrafiltration and residual kidney function. Based on our clinical experience and review of the literature, we provide our opinion on treatment criteria and for incremental HD in order to clarify the current clinical practice guidelines.
Treatment Criteria for Twice Weekly HD
Careful patient selection for initiation of an incremental approach to HD is crucial to maximize treatment success and maintain patient quality of life. These recommendations are based on clinical criteria set forth by Kalantar-Zadeh et al52 where decision support is provided for incremental HD.
Substantial RKF: We believe this is the most important clinical criteria to determine HD frequency. The 2006 KDOQI guidelines suggest a minimum session single pool Kt/V which can be reduced in patients with KRU of > 2 ml/min per 1.73 m2, but that twice-weekly HD is not recommended unless KRU is > 3 ml/min per 1.73 m2. Monthly measurements of RKF are important to avoid under-dosing HD as RKF is lost over time. Monthly timed urine collections with KRU calculations can be cumbersome, and consideration can be given to a more practical approach for monitoring urine volume (i.e target urine volume > 0.5L/day), along with other important markers of adequacy such as anemia and fluid gains. The results from a contemporary cohort study also supported the cutoff of 3 ml/min per 1.73 m2 for KRU while suggesting 0.6 L/day as an alternative target for the urine volume-based approach28.
Infrequent hospitalization and manageable co-morbid conditions: It is important that patients being considered for infrequent HD regimens are otherwise in good health. Patients with high co-morbid disease burden may have no benefit, and may incur harm from a twice weekly HD regimen.
Infrequent electrolyte imbalance: We suggest that hyperkalemia (K.5.5mEq/L) and hyperphosphatemia (P>5.5mg/dL) are infrequent and readily manageable. This provides physicians with clinical evidence of substantial RKF and patient adherence.
Lack of profound anemia: We suggest Hb >8g/L, along with appropriate responsiveness to therapy for anemia, as another indicator of good health and substantial RKF.
Manageable volume status: We believe this can be assessed by limited fluid retention between two consecutive HD treatments 3 days apart of <2.5kg (or 5% of ideal dry weight), in addition to limited or readily manageable cardiovascular/pulmonary symptoms of fluid overload.
Good nutritional status and lack of hypercatabolic state: Since clinicians may choose to implement a low-protein diet along with twice weekly HD to preserve RKF, underlying good nutritional status is important. In addition, we believe that infrequent dialysis in the setting of unmitigated catabolism will have negative consequences on HD adequacy, since solutes and toxins are produced and retained beyond those from dietary protein intake.
Satisfactory health related quality of life: We believe that a detailed assessment of patients’ quality of life prior to initiation of an incremental approach to HD is vital. A patient’s underlying psychological well-being may help alleviate a negative response when frequency of HD is increased.
CONCLUSION
In summary, incremental HD provides patients with an individualized approach to the initiation of HD. This approach has many potential benefits, but may increase health-related risks in patients who are not judiciously selected, educated and will participate in a shared decision-making process with their treating nephrologist. While several large observational studies have demonstrated benefit in select populations, well-designed clinical trials are still needed to determine the safety, efficacy and optimal patient characteristics to optimize outcomes with an incremental HD approach.
Acknowledgments
Funding Source: YO is supported by the Uehara Memorial Foundation Research Fellowship.
CR is supported by NIDDK grant K23-DK102903.
KKZ is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK095668 and K24-DK091419, as well as philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee, and AVEO.
Footnotes
Relevant Potential Conflict of Interest:
KK-Z has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, the American Society of Nephrology, Astra-Zeneca, Aveo, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, the National Institutes of Health, the National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma.
The other authors declare no conflicts of interest.
References
- 1.United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. [Google Scholar]
- 2.Shafi T, Mullangi S, Toth-Manikowski SM, Hwang S, Michels WM. Residual Kidney Function: Implications in the Era of Personalized Medicine. Semin Dial. 2017;30:241–5. doi: 10.1111/sdi.12587. [DOI] [PubMed] [Google Scholar]
- 3.O’Hare AM, Wong SP, Yu MK, et al. Trends in the Timing and Clinical Context of Maintenance Dialysis Initiation. J Am Soc Nephrol. 2015;26:1975–81. doi: 10.1681/ASN.2013050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney International. 1985;28:526–34. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 5.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–9. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 6.National Kidney Foundation. K/DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1998 doi: 10.1016/s0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 7.European Best Practice Guidelines Expert Group on Haemodialysis. European best practice guidelines for haemodialysis (Part 1) Nephrol Dial Transplant. 2002;17(suppl 7):S16–S31. [PubMed] [Google Scholar]
- 8.National Kidney F. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Hanson JA, Hulbert-Shearon TE, Ojo AO, et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19:625–33. doi: 10.1159/000013533. [DOI] [PubMed] [Google Scholar]
- 10.Bieber B, Qian J, Anand S, et al. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics and Quality of Life in the China Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant. 2014;29:1770–7. doi: 10.1093/ndt/gft472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saran R, Robinson B, Shahinian V. US Renal Data System 2016 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.12.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Accessed June 12, 2015, 2015];USRDS 2014 Hospitalizations. 2015 at http://www.usrds.org/2014/view/v2_04.aspx.
- 13.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Health-related quality of life among dialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;64:1903–10. doi: 10.1046/j.1523-1755.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- 15.Kabanda A, Jadoul M, Pochet JM, Lauwerys R, van Ypersele de Strihou C, Bernard A. Determinants of the serum concentrations of low molecular weight proteins in patients on maintenance hemodialysis. Kidney Int. 1994;45:1689–96. doi: 10.1038/ki.1994.221. [DOI] [PubMed] [Google Scholar]
- 16.Stompór T, Sułowicz W, Anyszek T, Kuśnierz B, Fedak D, Naskalski JW. Dialysis adequacy, residual renal function and serum concentrations of selected low molecular weight proteins in patients undergoing continuous ambulatory peritoneal dialysis. Med Sci Monit. 2003;9:CR500–4. [PubMed] [Google Scholar]
- 17.Delaney MP, Stevens PE, Al Hasani M, Stowe HJ, Judge C, Lamb EJ. Relationship of serum cystatin C to peritoneal and renal clearance measures in peritoneal dialysis: a cross-sectional study. Am J Kidney Dis. 2008;51:278–84. doi: 10.1053/j.ajkd.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Marquez IO, Tambra S, Luo FY, et al. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6:290–6. doi: 10.2215/CJN.06100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrotra R, Nolph KD, Gotch F. Early initiation of chronic dialysis: role of incremental dialysis. Perit Dial Int. 1997;17:426–30. [PubMed] [Google Scholar]
- 20.Bargman JM, Thorpe KE, Churchill DN, Group CPDS. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–62. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999;33:523–34. doi: 10.1016/s0272-6386(99)70190-3. [DOI] [PubMed] [Google Scholar]
- 22.Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int. 2000;58:446–57. doi: 10.1046/j.1523-1755.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 23.Termorshuizen F, Dekker FW, van Manen JG, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–70. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 24.Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–64. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 25.Jansen MA, Hart AA, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–53. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 26.Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal WM, Noordzij M, Dekker FW, et al. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26:2978–83. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 28.Obi Y, Streja E, Rhee CM, et al. Incremental Hemodialysis, Residual Kidney Function, and Mortality Risk in Incident Dialysis Patients: A Cohort Study. Am J Kidney Dis. 2016;68:256–65. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obi Y, Rhee CM, Mathew AT, et al. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol. 2016;27:3758–68. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorairajan S, Chockalingam A, Misra M. Myocardial stunning in hemodialysis: what is the overall message? Hemodial Int. 2010;14:447–50. doi: 10.1111/j.1542-4758.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood Purif. 2010;29:105–10. doi: 10.1159/000245634. [DOI] [PubMed] [Google Scholar]
- 32.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-Induced Cardiac Injury: Determinants and Associated Outcomes. Clinical Journal of the American Society of Nephrology. 2009;4:914–20. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–31. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palomo-Piñón S, Mora-Villalpando CJ, Del Carmen Prado-Uribe M, et al. Inflammation and myocardial damage markers influence loss of residual renal function in peritoneal dialysis patients. Arch Med Res. 2014;45:484–8. doi: 10.1016/j.arcmed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Wang AY, Wang M, Woo J, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15:2186–94. doi: 10.1097/01.ASN.0000135053.98172.D6. [DOI] [PubMed] [Google Scholar]
- 36.Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–58. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bemelmans MH, Gouma DJ, Buurman WA. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J Immunol. 1993;150:2007–17. [PubMed] [Google Scholar]
- 38.Poole S, Bird TA, Selkirk S, et al. Fate of injected interleukin 1 in rats: sequestration and degradation in the kidney. Cytokine. 1990;2:416–22. doi: 10.1016/1043-4666(90)90050-4. [DOI] [PubMed] [Google Scholar]
- 39.Termorshuizen F, Korevaar JC, Dekker FW, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003;41:1293–302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 40.Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2000;15:396–401. doi: 10.1093/ndt/15.3.396. [DOI] [PubMed] [Google Scholar]
- 41.Penne EL, van der Weerd NC, Grooteman MP, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:281–9. doi: 10.2215/CJN.04480510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429–38. [PubMed] [Google Scholar]
- 43.Eriguchi R, Obi Y, Rhee CM, et al. Changes in urine volume and serum albumin in incident hemodialysis patients. Hemodial Int. 2017;21:507–18. doi: 10.1111/hdi.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKane W, Chandna SM, Tattersall JE, Greenwood RN, Farrington K. Identical decline of residual renal function in high-flux biocompatible hemodialysis and CAPD. Kidney Int. 2002;61:256–65. doi: 10.1046/j.1523-1755.2002.00098.x. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy JT, Jenson BM, Squillace DP, Williams AW. Improved preservation of residual renal function in chronic hemodialysis patients using polysulfone dialyzers. Am J Kidney Dis. 1997;29:576–83. doi: 10.1016/s0272-6386(97)90341-3. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann J, Fricke H, Schiffl H. Biocompatible membranes preserve residual renal function in patients undergoing regular hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1997;30:366–73. doi: 10.1016/s0272-6386(97)90281-x. [DOI] [PubMed] [Google Scholar]
- 47.Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant. 2002;17:1814–8. doi: 10.1093/ndt/17.10.1814. [DOI] [PubMed] [Google Scholar]
- 48.Penne EL, van der Weerd NC, van den Dorpel MA, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST) Am J Kidney Dis. 2010;55:77–87. doi: 10.1053/j.ajkd.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 49.Daugirdas JT, Greene T, Rocco MV, et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–58. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golper TA. Incremental Hemodialysis: How I Do It. Semin Dial. 2016;29:476–80. doi: 10.1111/sdi.12530. [DOI] [PubMed] [Google Scholar]
- 51.Kalantar-Zadeh K, Casino FG. Let us give twice-weekly hemodialysis a chance: revisiting the taboo. Nephrol Dial Transplant. 2014;29:1618–20. doi: 10.1093/ndt/gfu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Unruh M, Zager PG, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–6. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obi Y, Eriguchi R, Ou SM, Rhee CM, Kalantar-Zadeh K. What Is Known and Unknown About Twice-Weekly Hemodialysis. Blood Purif. 2015;40:298–305. doi: 10.1159/000441577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin YF, Huang JW, Wu MS, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology (Carlton) 2009;14:59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang M, Wang M, Li H, et al. Association of Initial Twice-Weekly Hemodialysis Treatment with Preservation of Residual Kidney Function in ESRD Patients. American Journal of Nephrology. 2014;40:140–50. doi: 10.1159/000365819. [DOI] [PubMed] [Google Scholar]
- 56.Caria S, Cupisti A, Sau G, Bolasco P. The incremental treatment of ESRD: a low-protein diet combined with weekly hemodialysis may be beneficial for selected patients. BMC Nephrol. 2014;15:172. doi: 10.1186/1471-2369-15-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández-Lucas M, Teruel-Briones JL, Gomis-Couto A, Villacorta-Pérez J, Quereda-Rodríguez-Navarro C. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32:767–76. doi: 10.3265/Nefrologia.pre2012.Jul.11517. [DOI] [PubMed] [Google Scholar]
- 58.Mathew A, Obi Y, Rhee CM, et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90:1071–9. doi: 10.1016/j.kint.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 59.Daugirdas J, Blake P, Ing T. Handbook of dialysis. Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 60.Daugirdas JT, Leypoldt JK, Akonur A, Greene T, Depner TA, Group FT. Improved equation for estimating single-pool Kt/V at higher dialysis frequencies. Nephrol Dial Transplant. 2013;28:2156–60. doi: 10.1093/ndt/gfs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suri RS, Larive B, Sherer S, et al. Risk of vascular access complications with frequent hemodialysis. Journal of the American Society of Nephrology. 2013;24:498–505. doi: 10.1681/ASN.2012060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2014. [Google Scholar]
- 63.Foley RN, Herzog CA. How can we reduce sudden cardiac death in cardiorenal syndrome? Dialogues in Cardiovascular Medicine. 2011;16:267–76. [Google Scholar]
- 64.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 65.Krishnasamy R, Badve SV, Hawley CM, et al. Daily Variation in Death in Patients Treated by Long-term Dialysis: Comparison of In-Center Hemodialysis to Peritoneal and Home Hemodialysis. American Journal of Kidney Diseases. 2013;61:96–103. doi: 10.1053/j.ajkd.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Semin Dial. 2010;23:449–51. doi: 10.1111/j.1525-139X.2010.00782.x. [DOI] [PubMed] [Google Scholar]
- 67.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney International. 2011;79:250–7. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM. Serum Potassium and Short-term Clinical Outcomes Among Hemodialysis Patients: Impact of the Long Interdialytic Interval. Am J Kidney Dis. 2017;70:21–9. doi: 10.1053/j.ajkd.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 70.Janousek R, Krajina A, Peregrin JH, et al. Effect of intravascular iodinated contrast media on natural course of end-stage renal disease progression in hemodialysis patients: a prospective study. Cardiovascular and interventional radiology. 2010;33:61–6. doi: 10.1007/s00270-009-9715-3. [DOI] [PubMed] [Google Scholar]
- 71.Dittrich E, Puttinger H, Schillinger M, et al. Effect of radio contrast media on residual renal function in peritoneal dialysis patients--a prospective study. Nephrol Dial Transplant. 2006;21:1334–9. doi: 10.1093/ndt/gfi023. [DOI] [PubMed] [Google Scholar]
- 72.James SH, Meyers AM, Milne FJ, Reinach SG. Partial recovery of renal function in black patients with apparent end-stage renal failure due to primary malignant hypertension. Nephron. 1995;71:29–34. doi: 10.1159/000188670. [DOI] [PubMed] [Google Scholar]
- 73.Xydakis D, Papadogiannakis A, Sfakianaki M, et al. Residual renal function in hemodialysis patients: the role of Angiotensin-converting enzyme inhibitor in its preservation. ISRN Nephrol. 2013;2013:184527. doi: 10.5402/2013/184527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stannat S, Bahlmann J, Kiessling D, Koch KM, Deicher H, Peter HH. Complement activation during hemodialysis. Comparison of polysulfone and cuprophan membranes. Contrib Nephrol. 1985;46:102–8. [PubMed] [Google Scholar]
- 75.Schiffl H, Lang SM, Fischer R. Effects of high efficiency post-dilution on-line hemodiafiltration or conventional hemodialysis on residual renal function and left ventricular hypertrophy. Int Urol Nephrol. 2013;45:1389–96. doi: 10.1007/s11255-012-0336-4. [DOI] [PubMed] [Google Scholar]
- 76.Jiang N, Qian J, Sun W, et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant. 2009;24:2551–8. doi: 10.1093/ndt/gfp085. [DOI] [PubMed] [Google Scholar]
- 77.Shah AP, Kalantar-Zadeh K, Kopple JD. Is there a role for ketoacid supplements in the management of CKD? Am J Kidney Dis. 2015;65:659–73. doi: 10.1053/j.ajkd.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 78.Kalantar-Zadeh K. Incremental Dialysis: Can it make a difference for residual kidney function? [Accessed November 20, 2017];Neumann M, editor. Nephrology News & Issues. 2017 May; https://www.nephrologynews.com/incremental-dialysis-can-make-difference-residual-renal-function/
- 79.Supasyndh O, Satirapoj B, Seenamngoen S, Yongsiri S, Choovichian P, Vanichakarn S. Nutritional status of twice and thrice-weekly hemodialysis patients with weekly Kt/V > 3. 6. J Med Assoc Thai. 2009;92:624–31. [PubMed] [Google Scholar]
- 80.Stankuviene A, Ziginskiene E, Kuzminskis V, Bumblyte IA. Impact of hemodialysis dose and frequency on survival of patients on chronic hemodialysis in Lithuania during 1998–2005. Medicina (Kaunas) 2010;46:516–21. [PubMed] [Google Scholar]
- 81.Lin X, Yan Y, Ni Z, et al. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33:66–72. doi: 10.1159/000334634. [DOI] [PubMed] [Google Scholar]
- 82.Elamin S, Abu-Aisha H. Reaching target hemoglobin level and having a functioning arteriovenous fistula significantly improve one year survival in twice weekly hemodialysis. Arab journal of nephrology and transplantation. 2012;5:81–6. [PubMed] [Google Scholar]
- 83.Fernandez-Lucas M, Teruel-Briones JL, Gomis-Couto A, Villacorta-Perez J, Quereda-Rodriguez-Navarro C. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32:767–76. doi: 10.3265/Nefrologia.pre2012.Jul.11517. [DOI] [PubMed] [Google Scholar]
- 84.Hwang HS, Hong YA, Yoon HE, et al. Comparison of Clinical Outcome Between Twice-Weekly and Thrice-Weekly Hemodialysis in Patients With Residual Kidney Function. Medicine (Baltimore) 2016;95:e2767. doi: 10.1097/MD.0000000000002767. [DOI] [PMC free article] [PubMed] [Google Scholar]


