Abstract
Pancreatic ductal adenocarcinoma (PDAC) is resistant to T-cell mediated immunotherapy. We engineered T cells to transiently express an mRNA encoding a chimeric antigen receptor (CAR) specific for mesothelin—a protein that is over-expressed by PDAC cells. We performed a phase 1 study to evaluate the safety and efficacy of adoptive cell therapy with autologous mesothelin-specific CAR T cells (CARTmeso cells) in 6 patients with chemotherapy-refractory metastatic PDAC. Patients were given intravenous CARTmeso cells 3 times weekly for 3 weeks. None of the patients developed cytokine release syndrome or neurologic symptoms and there were no dose limiting toxicities. Disease stabilized in 2 patients, with progression-free survival times of 3.8 and 5.4 months. We used FDG-positron emission tomography/computed tomography imaging to monitor the metabolic active volume (MAV) of individual tumor lesions. The total MAV remained stable in 3 patients and decreased by 69.2% in 1 patient with biopsy-proven mesothelin expression; in this patient, all liver lesions had a complete reduction in FDG uptake at 1 month compared to baseline, although there was no effect on the primary PDAC. Transient CAR expression was detected in patients’ blood after infusion and led to expansion of new immunoglobulin G proteins. Our results provide evidence for the potential anti-tumor activity of mRNA CARTmeso cells, as well as PDAC resistance to the immune response. Keywords: immune response heterogeneity, immune therapy, anti-tumor immunity, pancreatic cancer treatment
MAIN TEXT
Pancreatic ductal adenocarcinoma (PDAC) has demonstrated striking resistance to T cell immunotherapy 1–3. This resistance may reflect PDAC’s lack of strong immunogenic neoantigens 4 or ineffective priming of endogenous T cells in vivo 5,6. To circumvent these issues, T cells can be synthetically modified to express a chimeric antigen receptor (CAR) which activates T cells upon engagement with its cognate cell surface target protein 7. However, the translation of CAR T cells to solid malignancies has been hampered by on-target off-tumor toxicities as a result of target antigen expression by normal healthy tissues 8, 9. To address this concern, we have used mRNA electroporation to engineer T cells to transiently express a CAR, so as to limit exposure and potential for toxicity. Here, we conducted a phase I study to evaluate adoptive cell therapy with autologous T lymphocytes (CARTmeso cells) genetically modified with mRNA to express a CAR recognizing mesothelin 10, a protein that is over-expressed in most surgically-resected PDACs 11, 12 but also found on some normal tissues including the lining of the peritoneum, pleura, and pericardium.
Six patients with chemotherapy-refractory metastatic PDAC received CARTmeso cells administered intravenously three times weekly for three weeks (Figure 1A, Suppl Figure 1 and Suppl Methods). 53 of 54 planned CARTmeso cell infusions were administered. None of the patients experienced cytokine release syndrome or neurologic symptoms (Supplementary Table 1). There were no dose limiting toxicities. Safety and tolerability of the infusions are described in Supplementary Methods.
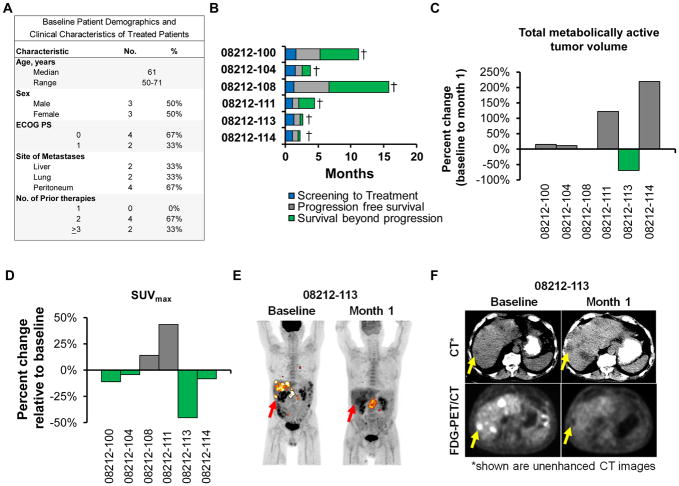
Figure 1. Clinical responses.
(A) Baseline patient demographics. (B) Swimmer plot showing patient outcomes. Percent change at 1 month relative to baseline in (C) total MAV and (D) SUVmax for tumor lesions detected using FDG-PET/CT imaging. (E) Sequential coronal maximum intensity projection PET images and (F) sequential unenhanced CT and FDG-PET images for patient 08212-113. In (E), red and yellow arrows indicate liver and primary pancreatic lesions, respectively. In (F), yellow arrows mark a representative liver lesion.
The best overall response achieved with mRNA CARTmeso cells was stable disease by RECIST v1.1, with two patients (08212-100 and 08212-108) demonstrating progression-free survival lasting 3.8 and 5.4 months, respectively (Figure 1B and Supplementary Figure 1). We used 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (FDG-PET/CT) imaging to monitor the metabolically active volume (MAV) and maximum standardized uptake value (SUVmax) of individual tumor lesions (Figure 1C, D). We found that the total MAV remained stable in three patients but decreased by 69.2% in one patient (08212- 113). For 08212-113, SUVmax for all lesions was found to decrease with a complete reduction in FDG uptake seen in all liver lesions at Month 1 compared to baseline, despite an increase in MAV within the primary pancreatic lesion (Figure 1E, F). This dramatic reduction in FDG uptake is atypical for the course of this disease and thus, this observation is highly suggestive of the potential anti-tumor activity of mRNA CARTmeso cells.
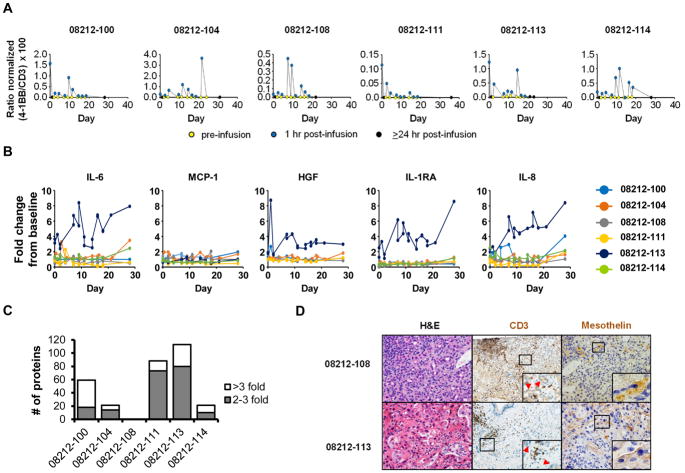
CAR expression is transient in T cells when introduced as mRNA 13. Consistent with this, we detected CAR transcripts transiently in the blood after each infusion in all patients (Figure 2A). To determine the impact of CARTmeso cell infusion on endogenous immune responses, we first examined serum cytokine levels within the peripheral blood after infusion. We found elevated levels of several inflammatory cytokines, including IL-6, HGF, IL-1RA, and IL-8, after starting therapy in patient 08212-113, but not in any of the other patients (Figure 2B). We also detected no significant levels of human anti-mouse antibodies (HAMA) in patients at baseline or at multiple defined time points after CARTmeso infusion. In contrast, human anti-chimeric antibodies (HACA) were seen in some patients (Supplementary Figure 2A). We next analyzed serum from patients at baseline and 1–2 months after beginning CARTmeso therapy for the presence of IgG antibodies reactive to an array of >9000 proteins. For all but one patient (08212-108), antibodies levels against multiple protein targets were found to increase, with some against previously unrecognized proteins (Figure 2C, Supplementary Tables 2 and 3). Gene ontology analysis of differentially recognized proteins from all patients identified oxidative stress and leukocyte activation and proliferation as the most significantly upregulated protein function groups (Supplementary Figure 2C). Increases in IgG antibodies reactive against six proteins, including three immune-related proteins (BCMA, PD-1, and PD-L1), were detected in multiple patients (Supplementary Figure 2D).
Figure 2. Pharmacodynamic analyses.
(A) RT-qPCR analysis to detect CAR expression in whole blood after CARTmeso cell infusion. (B) Longitudinal measurements of serum soluble factors after CARTmeso cell infusion. (C) Differentially recognized proteins detected by self-reactive IgG antibodies in the serum of each patient at 1-2 months after CARTmeso cell infusion compared to baseline. (D) Representative images of H&E staining (40x) and IHC staining to detect CD3 (20x) and mesothelin (40x) expression in metastatic liver lesions analyzed from patient 08212-108 and 08212-113. Red arrowheads indicate malignant cells adjacent to CD3+ cells.
We next considered the possibility that exclusion of T cells from the tumor microenvironment, lack of mesothelin expression on malignant cells, or both might limit the efficacy of CARTmeso cell therapy. Tumor biopsies at 3–7 days after the last infusion were available from patients 08212-104 and 08212-108 who demonstrated disease progression and prolonged stable disease, respectively. CAR transcripts were not detected in either biopsy, excluding the presence of CARTmeso cells within tumors at this time point. Baseline tumor biopsies were available for patients 08212-108 and 08212-113 and showed CD3+ T cells amongst malignant cells in both specimens suggesting the potential of T cells to penetrate the tumor microenvironment in these patients (Figure 2D). For patient 08212-108, mesothelin expression was confined to the cytoplasm of malignant cells, whereas for patient 08212-113, malignant cells showed mesothelin expression on the cell surface, a pre-requisite for CARTmeso effector activity (Figure 2D).
Our findings reveal the safety, feasibility and therapeutic potential of autologous T cells redirected with a CAR recognizing mesothelin for the treatment of PDAC. The mixed metabolic response detected in one patient with biopsy-proven cell surface expression of mesothelin implies distinct biology associated with primary and metastatic lesions in this patient. We hypothesized that CARTmeso cells may produce a vaccine effect by inducing tumor cell death and the release of tumor-associated antigens. Consistent with this hypothesis, we found that therapy induced a spreading of antibody responses against multiple proteins, including immunoregulatory molecules (e.g. PD-1, PD-L1, and BCMA). Further, we have recently reported that mRNA CARTmeso cell infusion stimulates clonal expansion of T cell clones, which may contribute to the anti-tumor potential of CAR T cells 14. The ultimate fate of tumor-infiltrating T cells, though, is determined by signals received within the tumor microenvironment. In our study, we were unable to evaluate the fate of adoptively transferred CARTmeso cells due to transient CAR expression in vivo. In conclusion, we propose that CARTmeso adoptive cell therapy can serve as a novel tool for probing the immunobiology of PDAC, without issues of T cell priming, and in doing so, guide the development of effective T cell immunotherapies in this disease.
Supplementary Material
Acknowledgments
Financial support: This work was supported by a research grant funded by the Lustgarten Foundation and Cancer Research Institute. Additional support was provided in part by a Doris Duke Charitable Foundation Clinical Investigator Award (to G.L. Beatty) and by grant number K08 CA138907 (to G.L. Beatty).
The authors thank the nurses of the Clinical Trials Research Center, Don Siegel Director of Transfusion Medicine and Therapeutic Pathology, and the staff of the Apheresis Unit at the Hospital of the University of Pennsylvania for their outstanding and dedicated patient care and careful data collection. We also acknowledge Natalka Kengle for cytokine luminex assays, Jeffrey Finklestein, and Minnal Gupta for sample processing, Vanessa Gonzalez for data management and quality control, and Andrea Brennan and the staff of the Clinical Cell and Vaccine Production Facility for cell manufacturing and testing. We also thank the Lustgarten Foundation and Cancer Research Institute for funding and support of this study. Additional support was provided in part by a Doris Duke Charitable Foundation Clinical Investigator Award (to G.L. Beatty) and by grant number K08 CA138907-02 (to G.L. Beatty) and 5R01 CA120409 (to C.H.J.). B.L.L., C.H.J., G.L.B., G.P., S.F.L., and J.J.M. are inventors of intellectual property related to CAR T cells that is licensed by the University of Pennsylvania to Novartis.
Footnotes
Conflict of Interest Statement: B.L.L., C.H.J., G.L.B., G.P., S.F.L., and J.J.M. are inventors of intellectual property related to CAR T cells that is licensed by the University of Pennsylvania to Novartis. All other authors declare no conflict.
Author Contributions: Study concept and design: G.L.B., G.P., and C.H.J.; acquisition of data: G.L.B., M.H.O., D.A.T., M.M., A.M.N.; analysis and interpretation of data: all authors contributed; drafting of manuscript: G.L.B.; critical revision of the manuscript for important intellectual content: all authors contributed; statistical analysis: G.L.B., F.N., S.F.L., F.C.; obtained funding: G.L.B., C.H.J.; study supervision: G.L.B., G.P., C.H.J. All authors edited and approved the final manuscript.
This manuscript presents original results of a phase I study of autologous mesothelin-specific chimeric antigen receptor modified T cells in patients with chemotherapy-refractory metastatic pancreatic ductal adenocarcinoma. Preliminary findings have been reported at the 51st Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2015, Chicago, IL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le DT, et al. J Clin Oncol. 2017;35 abstract 345. [Google Scholar]
- 2.Brahmer JR, et al. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Royal RE, et al. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandrov LB, et al. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, et al. J Clin Oncol. 2015;33:1325–33. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty GL, et al. Gastroenterology. 2015;149:201–10. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill S, et al. Immunol Rev. 2015;263:68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 8.Morgan RA, et al. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamers CH, et al. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 10.Beatty GL, et al. Pharmacol Ther. 2016;166:30–9. doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastan I, et al. Cancer Res. 2014;74:2907–12. doi: 10.1158/0008-5472.CAN-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Argani P, et al. Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 13.Beatty GL, et al. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim RH, et al. Journal of Clinical Oncology. 2017;35:3011–3011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.