Epicardial adipose tissue (EAT) is a visceral adipose tissue depot and, as such, has been investigated in several metabolic and inflammatory disorders. EAT has been associated with coronary artery disease and myocardial dysfunction, and it remains a burgeoning subject of research due to its potential as a therapeutic target. No endorsed guidelines exist on the measurement of EAT, with studies predominantly reporting linear thickness according to transthoracic echocardiography (EAT-TTE) or area/volume measured by using cardiac computed tomography (EAT-CT). TTE is advantageous because of its rapid performance at the bedside and low cost, but its reproducibility and accuracy may be limited by the effects of probe angulation on 2-dimensional imaging, as well as an inability to quantify periatrial fat or total EAT volume. CT imaging has the drawback of radiation exposure but allows 3-dimensional volumetric quantification. Because EAT is not uniformly distributed around the heart, volumetric quantification is arguably preferred (1). Few data compare agreement between these modalities, and we aimed to compare EAT-TTE versus volumetric EAT-CT by using commonly described methods.
We studied 106 consecutive patients who underwent clinically indicated CT scanning for suspected coronary artery disease who also had TTE performed within 30 days. CT imaging was performed on a 320-row scanner by using a previously described protocol (2), and EAT-CT images were measured by using a research-specific tool (QFAT 2.0, Cedars-Sinai Medical Center, Los Angeles, California) (3). Briefly, EAT was measured from the bifurcation of the pulmonary trunk to the cardiac apex. Manual pericardial contours were drawn at 5- to 10-interval slices with assessment for slice interpolation and corrected as required. Contiguous voxels between −190 and −30 Hounsfield units were used to define and quantify EAT. EAT-TTE was performed by using the technique of Iacobellis et al. (4); EAT was considered the echo-free space in the parasternal long-axis view between the free wall of the right ventricle and the pericardium. The average value of 3 cycles at end-systole was used with linear measurement along the midline of the ultrasound beam perpendicular to the aortic annulus. Pearson correlation coefficients and linear regression with 95% prediction intervals between methods are reported. Thirty random studies were assessed for interobserver and intraobserver agreement.
EAT-CT compared with EAT-TTE revealed poor correlation (r = 0.29; p = 0.002), and poor precision was demonstrated by the broad prediction limits (Figure 1). In 28 (26%) cases, observers reported uncertainty as to placement of the linear marker for EAT-TTE, suggesting reduced confidence. When these cases were excluded, correlation was not significantly altered (r = 0.25; p = 0.01); estimated prediction limits were also not significantly altered (data not shown). Poor interobserver and intraobserver agreement was seen with EAT-TTE (intraclass correlation coefficient [ICC]: 0.39; 95% confidence interval [CI]: 0.04 to 0.65; p = 0.02; ICC: 0.56; 95% CI: 0.07 to 0.79; p = 0.001, respectively); Bland-Altman analysis demonstrated a mean bias of −0.35 mm with 95% limits of agreement from −4.5 to 3.8 mm. Dispersion was particularly evident at higher EAT thickness measurements. Conversely, excellent ICC was noted for EAT-CT (ICC: 0.99; 95% CI: 0.98 to 1; p < 0.001 for both interobserver and intraobserver) with a mean bias of 0.9 ml and 95% limits of agreement of −11.6 to 13.4 ml.
FIGURE 1.
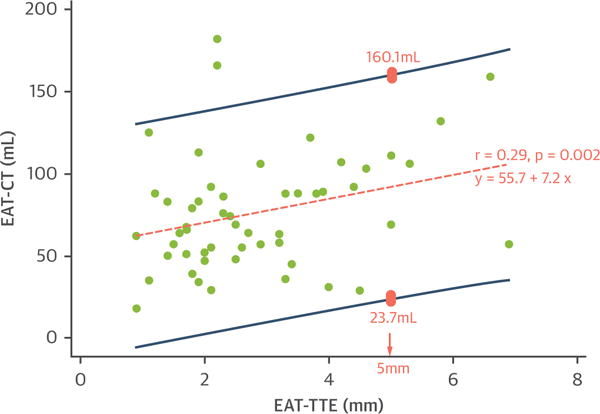
Scatter Plot Between EAT-CT and EAT-TTE
The orange dashed line represents the regression line of best fit. R value is the correlation coefficient, and the regression equation is the epicardial adipose tissue area/volume measured by using cardiac computed tomography (EAT-CT) as the outcome variable (y) and epicardial adipose tissue linear thickness according to transthoracic echocardiography (EAT-TTE) as the independent variable (x). The dark blue lines represent 95% prediction intervals. An example is illustrated by the pink boxes: when EAT-TTE is 5 mm, the predicted EAT-CT is between 23.7 and 160.1 ml, representing wide variability and suggesting poor precision.
There is significant research interest in EAT-TTE, with its proponents advocating the benefits of easy bedside assessment. However, the poor reproducibility and uncertainty of measurement require caution in drawing associative or causative relationships with EAT. The difference of approximately 8 mm in interrater EAT-TTE, and corresponding wide prediction limits for EAT-CT, may have significant implications in patient misclassification. There are no well-conducted studies comparing imaging-measured EAT versus human autopsy specimens, likely due to the extreme adherence of EAT to the underlying myocardium. However, CT scanning allows adipose tissue thresholding, optimal spatial resolution for pericardium identification, and high reproducibility regardless of the use of iodinated contrast (5).
In conclusion, EAT-CT is highly reproducible compared with EAT-TTE and could be considered as the optimal reference standard for EAT-based research.
Acknowledgments
Please note: Dr. Nerlekar is supported by a scholarship from the National Medical Health and Research Council and the National Heart Foundation. Dr. Brown is supported by an early career investigator scholarship from Monash University. Dr. Dey is supported by a National Heart, Lung, and Blood Institute grant (1R01HL133616). Dr. Wong is supported by Early Career Fellowship by National Health and Medical Research Council (Australia). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Jonathon Leipsic, MD, served as the Guest Editor for this paper.
References
- 1.Nerlekar N, Brown AJ, Muthalaly RG, et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6:e006379. doi: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nerlekar N, Ko BS, Nasis A, et al. Impact of heart rate on diagnostic accuracy of second generation 320-detector computed tomography coronary angiography. Cardiovasc Diagn Ther. 2017;7:296–304. doi: 10.21037/cdt.2017.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dey D, Suzuki Y, Suzuki S, et al. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–53. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis G, Assael F, Ribaudo MC, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 5.Marwan M, Achenbach S. Quantification of epicardial fat by computed tomography: why, when and how? J Cardiovasc Comput Tomogr. 2013;7:3–10. doi: 10.1016/j.jcct.2013.01.002. [DOI] [PubMed] [Google Scholar]


