Abstract
Purpose of review
This narrative review summarizes recent insights into the role of the CB2 receptor as potential therapeutic target in neuropathic pain and neurodegenerative conditions.
Recent findings
The cannabinoid system continues to receive attention as a therapeutic target. The cannabinoid type 2 (CB2) receptor is primarily expressed only when there is active inflammation and appears to be devoid of undesired psychotropic effects or addiction liability. The CB2 receptor has been shown to have potential as a therapeutic target in models of diseases with limited or no currently approved therapies, such as neuropathic pain and neurodegenerative conditions such as Alzheimer’s disease.
Summary
The functional involvement of CB2 receptor in neuropathic pain and other neuroinflammatory diseases highlights the potential therapeutic role of drugs acting at the CB2 receptor.
Keywords: Cannabinoid receptor type 2 (CB2), microglia, neuroinflammation
Introduction
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system, which is involved in a variety of physiological processes. The cannabinoid system continues to receive attention as a therapeutic target. Broad claims for marijuana and its derivatives are being made but these remain to be proven in carefully controlled trials and they are burdened by the side effect profile of the cannabinoid type 1 (CB1) receptor.
The cannabinoid type 2 (CB2) receptor is primarily expressed only when there is active inflammation and appears to be devoid of undesired psychotropic effects or addiction liability. The CB2 receptor has been shown to have potential as a therapeutic target in models of diseases, such as neuropathic pain and neurodegenerative conditions such as Alzheimer’s disease, where activation of the microglia and neuroinflammation are present. Significant advances have been made in the understanding of the CB2 receptor system, its role in controlling neuroinflammation, and potential therapeutic actions. These findings need to be taken into account in the discovery and evaluation of potential new drugs.
The endocannabinoid system
The endogenous cannabinoid system encompasses two cannabinoid (CB) receptors, endogenous ligands, and several enzymes required for biosynthesis and inactivation of endogenous ligands [1] (Figure 1). CB1 receptors are expressed primarily in the brain [2] and to some extent in the peripheral tissues.[3–5] CB2 receptors are identified peripherally in the circulating immune cells, the spleen [6, 7], and on macrophage-derived cells including osteocytes, osteoclasts, and hepatic Kupffer cells.[8, 9] Unlike the widespread expression of CB1 in the CNS, the expression of CB2 receptors, under normal physiological conditions, is restricted to the brainstem and the hippocampal CA2/3 pyramidal neurons.[10, 11] However, CB2 expression is highly inducible on the reactive microglia in the CNS following inflammation or injury.[12–20] Both CB1and CB2 receptors are seven transmembrane, G-protein-coupled receptors, and they share 44% overall identity. Studies performed with CB1−/− and CB2 −/− mice have indicated that certain effects of cannabinoids on tissues are mediated by neither CB1 nor CB2 [21], but currently no additional cannabinoid receptors have been definitively identified.[22] The crystal structures of CB1 (but not CB2) receptor in both active and inactive states have been reported.[23, 24]
Figure 1.
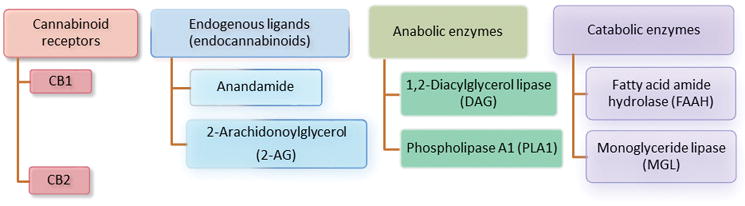
Components of the endogenous cannabinoid system. The cannabinoid (CB) receptors CB1 and CB2 belong to the G-protein-coupled receptor superfamily, coupled to Gi/o proteins and, under certain conditions, coupled to Gs. CB1 receptors are expressed mainly in the brain and CB2 are expressed mostly in the peripheral immune system and in the CNS in the hippocampal CA2/3 pyramidal neurons and glial cells. The endocannabinoid system also includes two arachidonic acid derivatives ligands (anandamide and 2-arachidonoylglycerol) [1], two enzymes responsible for synthesizing endogenous ligands (1,2-diacylglcerol lipase and phospholipase A), and two enzymes responsible for the metabolism of endogenous ligands (fatty acid amide hydrolase and monoglyceride lipase).
The endocannabinoid system also includes two arachidonic acid derivatives ligands—anandamide and 2-arachidonoylglycerol (2-AG) [1], two enzymes responsible for synthesizing endogenous ligands—1,2-diacylglycerol lipase and phospholipase A, and two enzymes responsible for the metabolism of endogenous ligands—fatty acid amide hydrolase and monoglyceride lipase.[25] The endocannabinoid 2-AG was found to act as a full agonist (whereas anandamide acts as a weak partial agonist) toward CB1 and CB2 receptors.[26] In the CNS, the release of postsynaptic endocannabinoid ligands activate the presynaptic CB1 receptors in a retrograde manner leading to inhibition of calcium channels and activation of potassium channels, resulting in inhibition of presynaptic neurotransmission release.[27–29]
The naturally-occurring cannabis family encompasses three major species (Cannabis sativa, Cannabis indica, and Cannabis ruderalis) and the cannabis plant contains more than 500 natural compounds that include more than 100 cannabinoids.[30] The full characterization of all cannabinoids is still lacking. Marijuana is composed mainly of the dried buds of Cannabis sativa. Delta-9-tetrahydrocannabinol (Δ9-THC) is the primary psychoactive cannabinoid of marijuana [31, 32] that acts as an agonist on the CB1 receptor [33] and as a weak antagonist on the CB2 receptor.[34] Cannabidiol is another major component of the cannabis that acts a non-competitive negative allosteric modulator of CB1 receptors.[35, 36] The claims that marijuana has efficacy in different medical conditions have not been substantiated.[37]
CB2 and microglia in neuroinflammatory conditions
Glial cells—including parenchymal (resident) microglia, perivascular microglia, astrocytes, and oligodendrocytes—constitute more than 70% of the total cell population in the brain and spinal cord and represent the first line of defense against inflammation and other insults.[38, 39] Microglia are the resident immune cells of the brain and play a vital role in health and disease.[16] Under normal physiological conditions (Figure 2a), microglia play a central role in the induction and maintenance of synaptic plasticity in neurons by altering the local environment and by modifying synaptic structure.[40–45] Phagocytosis of synapses by microglia contributes to synaptic development [46] and synaptic deficit in neurological disorders.[47–50] Microglial activation occurs in response to diverse CNS insults, and as a result, a transition is seen in microglial phenotype [51] from healthy anti-inflammatory to the reactive (proinflammatory) phenotype (Figure 2b). The reactive microglia express several receptors such as Toll-like receptors (TLRs)[17] and purinergic P2X4 receptors [52] with the subsequent activation of inflammatory pathway and release of several inflammatory cytokines and chemokines that eventually result in neuronal damage. Microglial activation and neuroinflammation appear to be the upstream mechanism underlying the pathogenesis of neurodegenerative diseases—including neuropathic pain, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, AIDS, and Huntington’s disease.[12, 13, 16–19, 53]
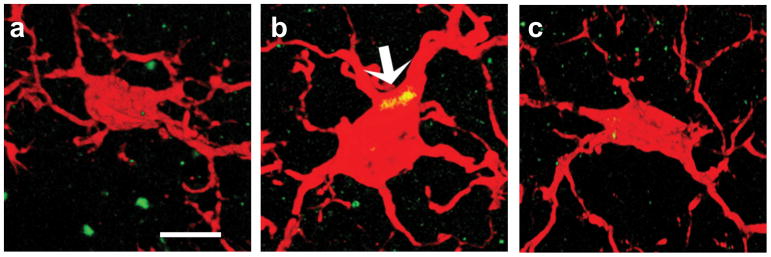
Figure 2.
Cannabinoid type 2 (CB2) receptors are expressed in reactive microglia in different neuroinflammatory and neurodegenerative conditions such as neuropathic pain and Alzheimer’s disease. The figure depicts 3D immunofluorescence confocal images of the microglial marker ionized calcium binding adaptor molecule (1 Iba1) (red) and the immunosignal of cannabinoid type 2 (CB2) receptor (green) in microglia; the colocalization of CB2 and microglia is shown in yellow. No substantial CB2 expression is seen in the healthy microglia (a), but increased expression of CB2 is seen in reactive microglia (arrow, b). Note the change from a highly branched and ramified morphology under normal physiological conditions to an amoeboid form in the presence of neuroinflammation. Treatment with a selective CB2 agonist, 1-(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl) piperidine (MDA7) restored microglial function and normal morphology (c). Scale bar = 10 μm.
Reactive microglia express CB2 mRNA in the spinal cord under neuropathic pain conditions.[54] The expression of CB2 receptors (Figure 2b) is increased in the dorsal horn in different models of neuropathic pain such as peripheral nerve injury, chemotherapy-induced neuropathic pain, and chronic post-ischemia pain (a model of complex regional pain syndrome type 1), and this expression is colocalized with the activated microglia.[17, 20, 54–57]
CB2 receptors were identified in postmortem brain tissues of patients with Alzheimer’s disease [14, 58, 59] and CB2 receptors were substantially and selectively expressed in neuritic plaque-associated microglia.[12] CB2 receptors are also upregulated in reactive microglial cells in animal models of Alzheimer’s disease, Huntington’s disease, simian immunodeficiency virus–induced encephalitis, HIV encephalitis, and multiple sclerosis.[13, 17–19, 60]
CB2 agonists modulate central neuroinflammatory conditions
In many physiologic stress settings (e.g., wound healing), a negative-feedback loop helps to reestablish homeostasis. In some inflammatory settings, activation of toll-like receptors (TLRs) in the microglia induces the release of cytokine signaling protein suppressors,[61] and tumor necrosis factor alpha (TNFα)-induced protein 8 family members [62] tend to limit inflammatory responses via modulation of TLRs. The upregulation and activation of the CB2 receptor may be part of the active process of limiting or downregulating the inflammatory process (much like tumor suppressors in cell growth or ephrin receptors in the nervous system, which can have positive or negative effects, depending on the context).[63] Activation of the CB2 receptor has been shown by different investigators to limit the acute inflammatory process.[17, 19, 20, 33, 64–69]
CB2 agonists are neuroprotective and lack psychotropic adverse effects normally seen with CB1 agonists. Activation of the CB2 receptor system results in inhibition of neuroinflammatory signaling pathways, restoration of normal microglial function (from pro-inflammatory to anti-inflammatory state). CB2 receptors modulate the extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation.[70] Both TLR2 and TLR4 are linked to the phosphorylation of ERK1/2.[17, 71] In vitro evidence indicates that the endocannabinoid anandamide acts through the mitogen-activated protein kinase (MAPK) pathway within the CNS immune system to reduce the extent of the inflammatory response and to limit neurodegenerative immune reactions.[70] We have shown that using a specific CB2 agonist, 1-(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl) piperidine (MDA7)[72] modulated the expression of the genes in paclitaxel-induced neuroinflammatory response as evidenced by relatively reduced expression of TLR2 and CB2 receptors and ERK1/2 expression (Figure 3).[73] In addition, the use of MDA7 was also associated with the adaptation of spinal glutamatergic transmission.[73]
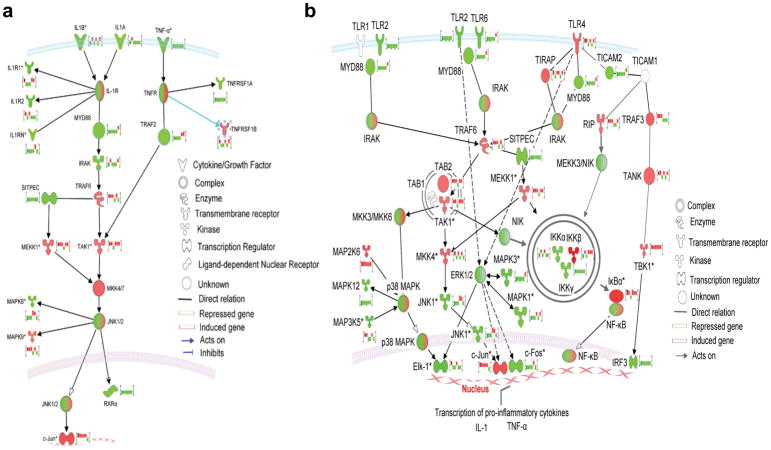
Figure 3.
(a) Modulation of interleukein-1 (IL-1) and tumor necrosis factor-alpha (TNF-α) pathways by a selective CB2 agonist, 1-(3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl) piperidine (MDA7) (generated by Ingenuity pathway analysis using comparison analysis between the 6 arrays). The brightness of node colors is proportional to the fold changes of gene expression levels. Color indicates up-regulated (red) and down-regulated (green) genes. In addition fold change expression bar charts of the six arrays have been included for each gene. In the IL-1 pathway, a complex is formed including IL-1R-associated kinases (IRAK) and the adapter protein MyD88. IRAK is rapidly phosphorylated and associates with TNF receptor-associated factor 6 (TRAF6); this association is necessary for downstream IL-1-induced translocation of signaling molecules to the nucleus, which ultimately leads to expression of genes that mediate inflammation and, frequently, tissue destruction. MDA7 acts by inhibiting IL-1 and TNF-α decreased expression of MyD88 and TNF receptor-associated factor 2 (TRAF2). (b) Signaling cascades initiated via toll-like receptor 2 (TLR2)- and TLR4-dependent activation and its modulation by MDA7. In addition fold change expression bar charts of the six arrays have been included for each gene. Engagement of TLR2 on the cell surface as a heterodimer with either TLR1 or TLR6 leads to recruitment of the adaptor protein MyD88 and interaction with TIR-domain-containing adaptor protein (TIRAP) via death-domain interactions. Phosphorylated IL-1 receptor-associated kinase (IRAK), together with TNF receptor-associated factor 6 (TRAF6), dissociates from the receptor, and then TRAF6 interacts with TAK1 binding protein 1 (TAB1)—induces autophosphorylation of the transforming growth factor β (TGFβ)-activating kinase (TAK1), and TAB2. This leads to phosphorylation of the IκBα-kinase (IKK) complex (IKKα, IKKβ, and IKKγ) and mitogen-activated protein kinases (MAPK), such as c-Jun NH2-terminal kinase (JNK), inducing the nuclear translocation of NF-κB and subsequent induction of target genes such as TNFα and ILs. The transcriptional activity of NF-κB is tightly regulated by its association with the inhibitory IκB that sequesters NF-κB in the cytosol. Beneficial effects of MDA7 include modulation of general genes that will ultimately inhibit phosphorylation of IκB proteins by the IKKs and prevention of IκB degradation. TLR4-MyD88-independent pathway activation involves signaling through the Toll-interleukin-1 receptor (TIR) adaptor TRIF (also known as TICAM1), TRIF-related adaptor molecule (TRAM; also known as TICAM2), TRAF3, receptor-interacting protein (RIP) and the transcription factor interferon regulatory factor 3 (IRF3). The green and red bar charts and shading represent the respective differential gene modulation (repression or induction, respectively) by MDA7. The numbers represent fold change. (Reproduced from Xu et al.[73] with permission).
In neuropathic pain models, treatment with CB2 agonists resulted in prevention of mechanical allodynia in different animal models (spinal nerve ligation model, paclitaxel-induced neuropathy, and chronic post-ischemic pain model of complex regional pain syndrome type I), and in animal models of Alzheimer’s disease, treatment with CB2 agonists promoted the clearance of amyloid plaques and recovery of the neuronal synaptic plasticity (Figure 2c).[17–20, 73–85]
In neuropathic pain conditions, glial activation and neuroinflammation are not restricted to the dorsal horn but are seen in several brain regions that functionally regulate the pain perception and sensitivity.[86] For example, sciatic nerve ligation induces upregulation of immature metabotropic glutamate receptor 5 and spontaneous somatic Ca2+ transients in astrocyte in the S1 sensory cortex, which contributes to the enhanced synaptic plasticity in S1 sensory cortex and mechanical allodynia in rodents.[87] Increased expression of TLR4 in glia and production of proinflammatory cytokines were noted in the prefrontal cortex in mice under chronic stress, and knockout of TLR4 or pharmacological suppression of TLR4 reduced visceral pain and prevented the development of chronic psychosocial stress-induced visceral hypersensitivity.[88] Significant activation of microglia and astrocytes was also observed in the anterior cingulate cortex (ACC) of the mice with nerve-ligation, and intracerebroventricular or/and intra-ACC injection of minocycline suppressed the phosphorylation of GluR1 subunit of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor at Ser831 and mechanical hypersensitivity in the modeled rodents.[89] A recent clinical study using integrated positron emission tomography-magnetic resonance imaging showed increased brain concentrations of the translocator protein (TSPO), a marker of glial activation, in patients with chronic low back pain.[90]
CB2 agonists modify opioid-induced tolerance and reward seeking behavior
Microglial activation seems to be involved in opioid-induced tolerance and addiction. Chronic opioid administration induces microglial activation and the release of pro-inflammatory cytokines and chemokines.[91, 92] Inhibiting microglial activation mitigated the development of tolerance, opioid-induced reward mechanism [93, 94], and restored the analgesic efficacy of opioids.[95] Systemic administration of a CB2 agonist (JWH133) attenuated both the rewarding and the psychomotor-stimulating effects of cocaine.[96]
CB2 agonists modulate peripheral neuroinflammation
Local injection of complete Freund’s adjuvant into the hindpaw of rodents increased the mRNA and protein concentrations of CB2 receptors in the skin tissue, which were primarily distributed in keratinocytes, macrophages, and T-lymphocytes in the epidermis and dermis of the inflamed skin tissue.[97] Stimulation with lipopolysaccharide enhanced the expression of CB2 receptors and the production of pro- and anti-inflammatory factors in human keratinocytes and fibroblasts. Administration of a CB2 receptor agonist JWH015 [98] or AM1241 [99, 100] reduced the concentration of major pro-inflammatory factors and increased the concentration of a major anti-inflammatory factor (TGF-β) in lipopolysaccharide-stimulated human keratinocytes and fibroblasts cells or inflamed skin tissues.
Therapeutic potential of CB2 agonists
Several CB2 agonists are described in the literature and a patent review indicated that there are several CB2 receptor modulators are in different phases of clinical development.[101] An effective molecule should be able to cross the blood brain barrier to reach the CNS to act on reactive microglia and it should be tested in the proper patient population. In this context, it is always insightful to study the earlier failures of CB2 agonists.
GlaxoSmithKline’s CB2 agonist, GW842166X, which appears to have a limited CNS permeability, was reported to be effective in the Freund’s complete adjuvant model of inflammatory pain (with an oral ED50 of 0.1 mg/kg).[102] CB2 ligands are known to have limited or no efficacy in acute pain models.[74, 103] CB2 agonists are not effective in treating acute pain in rats [74] and administration of the CB2 receptor–selective agonist HU-308 did not affect acute nociception in mice when thermal withdrawal latency was measured on a hot plate.[103] Administration of GW405833 did not affect hot plate or tail flick latency after administration of doses up to 30 mg/kg. However, 100 mg/kg of GW405833 resulted in a significant increase in both tail flick and hot plate latencies 1 h after administration.[104] The 100 mg/kg dose of GW405833 also resulted in typical CB1 effects [104], which does not support a CB2-mediated effect on acute nociception. GW842166X was subsequently found to be inferior to ibuprofen in a phase II randomized-controlled trial in patients with acute pain following third molar tooth extraction.[105] The authors discuss the possibility that plasma levels were suboptimal, although the plasma levels obtained were greater than those in preclinical studies demonstrating efficacy in the rat model.
AstraZeneca CB2 ligand, AZD1940, is a peripherally restricted CB1/CB2 receptor agonist.[106] AZD1940 has a low brain uptake at analgesic doses in both rats and primates.[107] Dose-dependent CNS-related and gastrointestinal adverse events were reported following treatment with AZD1940 in healthy male volunteers.[106] These effects were induced by the activity of AZD1940 at CB1 receptors. AZD1940 was not effective in the human capsaicin pain model[106] and did not reduce postoperative pain after lower third molar surgical removal at doses exerting subjective cannabinoid effects.[108] As stated by Rogers: “Choosing a human study group for convenience, which is kind of how osteoarthritis and third molar extraction got chosen, isn’t necessarily matching with what the preclinical results suggest may be a good patient population to study”.[109]
Conclusion
The CB2 receptor is primarily expressed only when there is active inflammation and appears to be devoid of undesired psychotropic effects or addiction liability. The CB2 receptor has been shown to have potential as a therapeutic target in models of diseases with limited or no currently approved therapies, such as neuropathic pain and neurodegenerative conditions such as Alzheimer’s disease. The challenge ahead is to identify drug candidates selective for CB2 which reaches the receptors in the CNS, and to subsequently demonstrate efficacy in clinical trials that represent the known mechanism of action of the CB2 system.
Key Points.
CB2 expression is highly inducible on the reactive microglia in the CNS following inflammation or injury.
Activation of CB2 receptor suppressed reactive microglia behavior and central neuroinflammation, and demonstrated a protective role in neuroinflammatory conditions.
Preclinical studies showed that CB2 agonists might modify opioid-induced tolerance and reward seeking behavior.
Several CB2 receptor modulators are in different phases of clinical development targeting chronic pain treatment.
Acknowledgments
None.
Financial support and sponsorship
M.N. is supported by the National Institute On Aging of the National Institutes of Health under Award Number R56AG051594. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the article for publication.
Footnotes
Conflicts of interest
MN and JFF are cofounders of NeuroTherapia™, a spin-off company created by Cleveland Clinic Innovations. MN and JFF have not received payments from NeuroTherapia™. As inventors of the technology, they and Cleveland Clinic are entitled to future royalty payments. JW and BB declare no competing interests.
References
- 1.Felder CC, Glass M. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 3.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–31. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavuoto P, McAinch AJ, Hatzinikolas G, Janovska A, Game P, Wittert GA. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochemical and biophysical research communications. 2007;364:105–10. doi: 10.1016/j.bbrc.2007.09.099. [DOI] [PubMed] [Google Scholar]
- 6.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 7.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European journal of biochemistry / FEBS. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 8.Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology (Baltimore, Md) 2011;54:1217–26. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 10.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 11**.Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron. 2016;90:795–809. doi: 10.1016/j.neuron.2016.03.034. The authors demonstrated that action potential-driven endocannabinoids (eCBs) release leads to a long-lasting membrane potential hyperpolarization, dependent on the activation of neuronal CB2Rs, in hippocampal CA3 and CA2 pyramidal cells, which modultes the neuronal activity and basic neuronal transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–41. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, et al. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–6. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of twhe cannabinoid CB2 receptor in the brain. Neuroscience letters. 2007;412:114–7. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Chung YC, Shin WH, Baek JY, Cho EJ, Baik HH, Kim SR, et al. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Experimental & molecular medicine. 2016;48:e205. doi: 10.1038/emm.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naguib M, Xu JJ, Diaz P, Brown DL, Cogdell D, Bie B, et al. Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesth Analg. 2012;114:1104–20. doi: 10.1213/ANE.0b013e31824b0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M. Activation of the CB(2) receptor system reverses amyloid-induced memory deficiency. Neurobiology of aging. 2013;34:791–804. doi: 10.1016/j.neurobiolaging.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 19**.Wu J, Hocevar M, Foss JF, Bihua Bie B, Naguib M. Activation of CB2 receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. European journal of pharmacology. 2017:811. doi: 10.1016/j.ejphar.2017.05.044. The authors demonstrated that activation of CB2 receptor suppressed the neuroinflammation, restored the neurogenesis, and rescued the hippocampal glutamatergic plasticity and cognition in the transgenic mice model of Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 20**.Xu J, Tang Y, Xie M, Bie B, Wu J, Yang H, et al. Activation of cannabinoid receptor 2 attenuates mechanical allodynia and neuroinflammatory responses in a chronic post-ischemic pain model of complex regional pain syndrome type I in rats. The European journal of neuroscience. 2016;44:3046–55. doi: 10.1111/ejn.13414. The authors demonstrated that activation of CB2 receptor suppressed the microglia activation in dorsal horn and attenuated the mechanical hypersensitivity in the rodent model of chronic post-ischemic pain. [DOI] [PubMed] [Google Scholar]
- 21.Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–75. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metabolism. 2006;3:167–75. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547:468–71. doi: 10.1038/nature23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167:750–62 e14. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn K, McKinney MK, Cravatt BF. Enzymatic Pathways That Regulate Endocannabinoid Signaling in the Nervous System. Chem Rev. 2008;108:1687–707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. The Journal of biological chemistry. 2000;275:605–12. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- 27.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3825–9. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry DJ, Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neuroscience letters. 1995;186:91–4. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RI, Nicoll RA. Endocannabinoid Signaling in the Brain. Science. 2002;296:678–82. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 30.Radwan MM, Elsohly MA, Slade D, Ahmed SA, Khan IA, Ross SA. Biologically active cannabinoids from high-potency Cannabis sativa. J Nat Prod. 2009;72:906–11. doi: 10.1021/np900067k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaoni Y, Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J Am Chem Soc. 1964;86:1646–7. [Google Scholar]
- 32.Hall W. Is cannabis use psychotogenic? Lancet. 2006;367:193–5. doi: 10.1016/S0140-6736(06)68012-4. [DOI] [PubMed] [Google Scholar]
- 33.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–4. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 34.Bayewitch M, Rhee MH, Avidor-Reiss T, Breuer A, Mechoulam R, Vogel Z. (−)-Delta9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J Biol Chem. 1996;271:9902–5. doi: 10.1074/jbc.271.17.9902. [DOI] [PubMed] [Google Scholar]
- 35.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abood ME. Allosteric Modulators: A Side Door. Journal of medicinal chemistry. 2016;59:42–3. doi: 10.1021/acs.jmedchem.5b01824. [DOI] [PubMed] [Google Scholar]
- 37.Naguib M, Foss JF. Medical Use of Marijuana: Truth in Evidence. Anesth Analg. 2015;121:1124–7. doi: 10.1213/ANE.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Sandoval EA, Horvath RJ, Deleo JA. Neuroimmune interactions and pain: Focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–34. [PMC free article] [PubMed] [Google Scholar]
- 39*.Villacampa N, Heneka MT. Microglia: You’ll Never Walk Alone! Immunity. 2018;48:195–7. doi: 10.1016/j.immuni.2018.02.009. This editorial view highlights the issues on the identification and characterization of resident and recruited leukocyte populations in healthy, aged, and diseased CNS. [DOI] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 41.Panatier A, Robitaille R. The soothing touch: microglial contact influences neuronal excitability. Developmental cell. 2012;23:1125–6. doi: 10.1016/j.devcel.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS biology. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–2. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Current opinion in neurobiology. 2013;23:1034–40. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 47.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–8. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Wake H, Moorhouse AJ, Miyamoto A, Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends in neurosciences. 2013;36:209–17. doi: 10.1016/j.tins.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016 doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016;165:921–35. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nature reviews Neurology. 2017;13:420–33. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. European journal of pharmacology. 2008;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. The European journal of neuroscience. 2003;17:2750–4. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 55.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–45. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108:722–34. doi: 10.1097/ALN.0b013e318167af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svizenska IH, Brazda V, Klusakova I, Dubovy P. Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model. J Histochem Cytochem. 2013;61:529–47. doi: 10.1369/0022155413491269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solas M, Francis PT, Franco R, Ramirez MJ. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiology of aging. 2012 doi: 10.1016/j.neurobiolaging.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Tolón RM, Núñez E, Pazos MR, Benito C, Castillo AI, Martínez-Orgado JA, et al. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Research. 2009;1283:148–54. doi: 10.1016/j.brainres.2009.05.098. [DOI] [PubMed] [Google Scholar]
- 60.Zarruk JG, Fernandez-Lopez D, Garcia-Yebenes I, Garcia-Gutierrez MS, Vivancos J, Nombela F, et al. Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke; a journal of cerebral circulation. 2012;43:211–9. doi: 10.1161/STROKEAHA.111.631044. [DOI] [PubMed] [Google Scholar]
- 61.Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–35. doi: 10.1016/j.imbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–26. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–81. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- 64.Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends in pharmacological sciences. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Romero-Sandoval A, Eisenach JC. Spinal Cannabinoid Receptor Type 2 Activation Reduces Hypersensitivity and Spinal Cord Glial Activation after Paw Incision. Anesthesiology. 2007;106:787–94. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- 66.Arevalo-Martin A, Garcia-Ovejero D, Gomez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, et al. CB(2) cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merighi S, Gessi S, Varani K, Fazzi D, Mirandola P, Borea PA. Cannabinoid CB(2) receptor attenuates morphine-induced inflammatory responses in activated microglial cells. Br J Pharmacol. 2012;166:2371–85. doi: 10.1111/j.1476-5381.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–70. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lotersztajn S, Teixeira-Clerc F, Julien B, Deveaux V, Ichigotani Y, Manin S, et al. CB2 receptors as new therapeutic targets for liver diseases. British Journal of Pharmacology. 2008;153:286–9. doi: 10.1038/sj.bjp.0707511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 71.Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Diaz P, Phatak SS, Xu J, Fronczek FR, Astruc-Diaz F, Thompson CM, et al. 2,3-Dihydro-1-benzofuran derivatives as a novel series of potent selective cannabinoid receptor 2 agonists: design, synthesis, and binding mode prediction through ligand-steered modeling. ChemMedChem. 2009;4:1615–29. doi: 10.1002/cmdc.200900226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu JJ, Diaz P, Bie B, Astruc-Diaz F, Wu J, Yang H, et al. Spinal gene expression profiling and pathways analysis of a CB2 agonist (MDA7)-targeted prevention of paclitaxel-induced neuropathy. Neuroscience. 2014;260:185–94. doi: 10.1016/j.neuroscience.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 74.Naguib M, Diaz F, Xu J, Astruc-Diaz F, Craig S, Vivas-Mejia P, et al. MDA7: A novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br J Pharmacol. 2008;155:1104–16. doi: 10.1038/bjp.2008.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–91. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–87. doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J, Bie B, Yang H, Xu JJ, Brown DL, Naguib M. Suppression of central chemokine fractalkine receptor signaling alleviates amyloid-induced memory deficiency. Neurobiology of aging. 2013;34:2843–52. doi: 10.1016/j.neurobiolaging.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 78.Anand U, Otto WR, Anand P. Sensitization of capsaicin and icilin responses in oxaliplatin treated adult rat DRG neurons. Mol Pain. 2010;6:82. doi: 10.1186/1744-8069-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, et al. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, et al. 1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol Dis. 2010;37:177–85. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 81.Kinsey SG, Mahadevan A, Zhao B, Sun H, Naidu PS, Razdan RK, et al. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–51. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, et al. Central and peripheral sites of action for CB(2) receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–40. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin-Moreno AM, Brera B, Spuch C, Carro E, Garcia-Garcia L, Delgado M, et al. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. Journal of neuroinflammation. 2012;9:8. doi: 10.1186/1742-2094-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilkerson JL, Gentry KR, Dengler EC, Wallace JA, Kerwin AA, Armijo LM, et al. Intrathecal cannabilactone CB(2)R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain. 2012;153:1091–106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aso E, Juves S, Maldonado R, Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. Journal of Alzheimer’s disease : JAD. 2013;35:847–58. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 86.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018 doi: 10.1097/ALN.0000000000002130. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126:1983–97. doi: 10.1172/JCI82859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tramullas M, Finger BC, Moloney RD, Golubeva AV, Moloney G, Dinan TG, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry. 2014;76:340–8. doi: 10.1016/j.biopsych.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 89.Miyamoto K, Kume K, Ohsawa M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J Pharmacol Sci. 2017;134:158–65. doi: 10.1016/j.jphs.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 90.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–15. doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–65. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muscoli C, Doyle T, Dagostino C, Bryant L, Chen Z, Watkins LR, et al. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J Neurosci. 2010;30:15400–8. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, et al. A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun. 2008;22:114–23. doi: 10.1016/j.bbi.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Fukagawa H, Koyama T, Kakuyama M, Fukuda K. Microglial activation involved in morphine tolerance is not mediated by toll-like receptor 4. Journal of anesthesia. 2013;27:93–7. doi: 10.1007/s00540-012-1469-4. [DOI] [PubMed] [Google Scholar]
- 95.Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, et al. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008;22:1248–56. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nature neuroscience. 2011;14:1160–6. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J, Chen L, Su T, Cao F, Meng X, Pei L, et al. Electroacupuncture increases CB2 receptor expression on keratinocytes and infiltrating inflammatory cells in inflamed skin tissues of rats. J Pain. 2010;11:1250–8. doi: 10.1016/j.jpain.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Bort A, Alvarado-Vazquez PA, Moracho-Vilrriales C, Virga KG, Gumina G, Romero-Sandoval A, et al. Effects of JWH015 in cytokine secretion in primary human keratinocytes and fibroblasts and its suitability for topical/transdermal delivery. Mol Pain. 2017;13:1744806916688220. doi: 10.1177/1744806916688220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Su TF, Zhao YQ, Zhang LH, Peng M, Wu CH, Pei L, et al. Electroacupuncture reduces the expression of proinflammatory cytokines in inflamed skin tissues through activation of cannabinoid CB2 receptors. Eur J Pain. 2012;16:624–35. doi: 10.1002/j.1532-2149.2011.00055.x. [DOI] [PubMed] [Google Scholar]
- 100.Su TF, Zhang LH, Peng M, Wu CH, Pan W, Tian B, et al. Cannabinoid CB2 receptors contribute to upregulation of beta-endorphin in inflamed skin tissues by electroacupuncture. Mol Pain. 2011;7:98. doi: 10.1186/1744-8069-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morales P, Hernandez-Folgado L, Goya P, Jagerovic N. Cannabinoid receptor 2 (CB2) agonists and antagonists: a patent update. Expert opinion on therapeutic patents. 2016;26:843–56. doi: 10.1080/13543776.2016.1193157. [DOI] [PubMed] [Google Scholar]
- 102.Giblin GMP, O’Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, et al. Discovery of 2-[(2,4-Dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide, a Selective CB2 Receptor Agonist for the Treatment of Inflammatory Pain. J Med Chem. 2007;50:2597–600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- 103.Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci USA. 1999;96:14228–33. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–72. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Ostenfeld T, Price J, Albanese M, Bullman J, Guillard F, Meyer I, et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin J Pain. 2011;27:668–76. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- 106.Kalliomaki J, Annas P, Huizar K, Clarke C, Zettergren A, Karlsten R, et al. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin Exp Pharmacol Physiol. 2013;40:212–8. doi: 10.1111/1440-1681.12051. [DOI] [PubMed] [Google Scholar]
- 107.Groblewski T, Yu XH, Lessard E. Pre-clinical pharmacological properties of novel peripherally acting CB1-CB2 agonists. 20th Annual Symposium of the International Cannabinoid Research Society; 2010; Abstract #37. [Google Scholar]
- 108.Kalliomäki J, Segerdahl M, Webster L, Reimfelt A, Huizar K, Annas P, et al. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scandinavian Journal of Pain. 2013;4:17–22. doi: 10.1016/j.sjpain.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 109.Rogers N. Cannabinoid receptor with an ‘identity crisis’ gets a second look. Nat Med. 2015;21:966–7. doi: 10.1038/nm0915-966. [DOI] [PubMed] [Google Scholar]