Abstract
Purpose
Distal radius fracture open reduction and internal fixation (ORIF) represents a significant cost burden to the healthcare system. We aimed to elucidate demographic-, injury-, and treatment-specific factors influencing surgical encounter costs for distal radius ORIF.
Methods
We retrospectively reviewed adult patients treated with isolated distal radius ORIF between 11/2014 – 10/2016 at a single tertiary academic medical center. Using our institution’s information technology value tools — which allow for comprehensive payment and cost data collection and analysis on an item-level basis — we determined relative costs (RC, or eβ) for each factor potentially influencing total direct costs (TDC) for distal radius ORIF using univariate and multivariable gamma regression analyses.
Results
Of the included 108 patients, implants and facility utilization costs were responsible for 48.3% and 37.9% of TDC, respectively. Factors associated with increased TDC include plate manufacturer (RC 1.52 for the most versus least expensive manufacturer), number of screws (RC 1.03 per screw) and distal radius plates used (RC 1.67 per additional plate), surgery setting (RC 1.32 for main hospital versus ambulatory surgery center [ASC]), treating service (RC 1.40 for trauma versus hand surgeons), and surgical time (RC 1.04 for every 10 minutes of additional surgical time). Open fracture was associated with increased costs (RC 1.55 versus closed fracture) while other estimates of fracture severity were non-significant. In the multivariable model controlling for injury-specific factors, variables including implant manufacturer, and number of distal radius plates and screws used, remained as significant drivers of TDC.
Conclusions
Substantial variations in surgical direct costs for distal radius ORIF exist, and implant choice is the predominant driver. Cost reductions may be expected through judicious use of additional plates and screws, if hospital systems utilize bargaining power to reduce implant costs, and by efficiently completing surgeries.
Clinical Relevance Statement
This study identifies modifiable factors that may lead to cost reduction for distal radius ORIF.
Keywords: Cost, Distal radius, Fracture, Open reduction internal fixation/ORIF, Payments
Introduction
Distal radius fractures are common injuries.1 Over 640,000 cases were reported in the United States adult population in 2001, and the incidence of distal radius fractures has been on the rise over the past 30–40 years.1 Likewise, the incidence of operative treatment has increased over time for these fractures,2,3 and the decision to provide treatment with open reduction and internal fixation (ORIF) over other options may be more likely amongst those with a hand surgery training background.4 In light of these trends, the growing costs associated with distal radius ORIF are of concern.4,5 ORIF has been shown to be more expensive than other treatment alternatives, with 61% of all fracture care payments attributed to the surgical encounter itself.5,6 Other estimates have suggested that 82% of the total direct costs for operatively-treated distal radius fractures are attributed to the surgical encounter.7 Over $170 million were attributed to distal radius fracture care in 2007 for the Medicare population alone, which is projected to double if ORIF utilization increases to 50% of fractures.5 With 4.3% growth in US healthcare spending in 2016 alone and a total of $3.3 trillion in expenditures,8 it is clearly imperative to derive strategies to improve the value of care and reduce unnecessary spending.9 While the costs of distal radius fracture ORIF may be higher in relation to other treatment options, it is critical to acknowledge that surgery for distal radius fractures represents a very small proportion of overall US health care expenditures, and furthermore is indicated in many cases in order to maximize patient outcomes and value.10–12
Despite prior studies raising concerns about distal radius fracture treatment costs,13 the main drivers of cost variation for the distal radius ORIF surgical encounter are not clear. Specifically, it remains unknown whether demographic-, injury-, or treatment-specific factors contribute to variations in total direct costs for distal radius ORIF. Investigation within this realm may elucidate modifiable factors that influence cost, therefore highlighting opportunities to reduce costs for distal radius ORIF – the most expensive component of distal radius fracture care. We aim to identify demographic-, injury, or treatment-specific factors that influence total direct costs for distal radius ORIF.
Methods
This IRB-approved retrospective cost analysis included adult patients (≥ 18 years of age) treated for a distal radius fracture with open reduction and internal fixation between November 2014 and October 2016. Treatment was performed at a tertiary academic medical center by four fellowship-trained orthopaedic hand or trauma surgeons at a main hospital operating room setting or at a separate orthopaedic ambulatory surgery center (ASC). Exclusion criteria included simultaneous treatment for other orthopaedic injuries (including any lower extremity injury, or other upper extremity injury), injuries to other organ systems (visceral, spine, or head injuries), patients with prior wrist surgeries, bilateral distal radius fractures, and patients undergoing treatment of a distal radius malunion, isolated percutaneous pinning, or external fixation. The presence of ipsilateral acute carpal tunnel syndrome, distal ulnar fracture, open fracture of the distal radius or ulna, or supplemental pinning in addition to ORIF with plate and screw fixation were not criteria for exclusion.
Patients were identified by an electronic procedural code search for distal radius ORIF (CPT 25607, 25608, and 25609). Patient baseline characteristics and descriptive data for each surgical encounter were collected from the electronic medical record. Chart review was performed to record data for injury-specific variables including the number of fracture parts based on CPT coding (25607, 25608, and 25609), treatment of ipsilateral distal ulna fractures (25240, 25651, 25652, 25545), irrigation and debridement for open fracture (11010, 11011, 11012) and treatment of acute carpal tunnel syndrome with carpal tunnel release (64721, 29848). Manual chart review of the operative notes rather than reliance on CPT coding alone was also performed to identify these additional procedures, as some are not mutually billable and would not be identifiable by coding alone (for example, 25606 may not be separately billable with 25607). Treatment-specific data was recorded, including surgical time, anesthesia type (general anesthesia versus regional/surgical block), implant manufacturer, number of screws and plates used, surgical setting (main operating room versus ASC), and treating surgical service (orthopedic hand versus trauma).
Our institution has developed an item-level database and set of information technology tools that facilitate collection of specific costs and payments for various health care services. This “Value Driven Outcomes” (VDO) tool prospectively allocates care costs and payments to individual patient encounters by determining costs of direct patient care, as previously described.9,14 VDO costing methods yield total direct costs incurred by the hospital system for implant and non-implant supplies used for patient care, pharmacy costs, laboratory costs, imaging costs, facility utilization direct costs (including sterile processing costs), and time-based cost allocations including procedure/operative time and the cost of staff involved in care (nursing, surgical technicians, medical assistants). Total direct cost categories, as tabulated by the VDO tool, are further described in Appendix I. As total direct costs do not include a surrogate for professional costs associated with each encounter, we added the surgeon and anesthesia payments to the total direct costs to define the ‘complete cost” of each surgical encounter. Only the costs and payments pertaining to the surgical encounter (preoperative, intraoperative, and immediate postoperative care in the post-anesthesia care unit) were considered: costs and payments related to preoperative evaluation, postoperative care, and inpatient care were not included.
Our institution does not have a two-vendor system in place, which would limit implant choices to that from only two manufacturers chosen by the hospital system based upon favorable pricing agreements. At our institution, contracts are not in place with Implantable Provider Group (IPG Inc.); a private company that partners with health plans to manage their implant and surgical costs with the goal of reducing spending. Surgeons are able to use implants from any manufacturer. Surgeons may request data regarding implant costs, however this is not routinely made available. The cost of surgical implants does not differ between ASC and main hospital settings.
Continuous variables were summarized as mean, standard deviation (SD), and range. Categorical variables were summarized as count and percentage (%). Relative cost was standardized using each individual patient’s cost divided by the average cost in the data set. Total direct costs were described as percentages. Univariate and multivariable gamma regressions with log link analyses were used to investigate factors that influenced total direct cost. Multivariable gamma regression controlled for injury-specific factors in an attempt to determine the significance of potentially modifiable treatment factors. Although our university’s value-driven outcomes tool does provide actual costs in dollars, non-normalized raw financial data cannot be publicly shared per institutional guidelines and therefore is presented in terms of relative costs (RC) to each baseline category, which correspond to regression coefficients obtained through gamma regression that were exponentiated to obtain normalized outcome variables (eβ). Exponentiated coefficients (eβ, synonymous with RC) and their 95% confidence intervals (CIs) were reported, describing relative percent change. Statistical significance was assessed at the 0.05 level, and all tests were two-sided.
Based upon the mean and standard deviation charges for distal radius ORIF presented by Farner et al. ($4633 ± $6659),2 an estimate for the coefficient of variation is 0.70. We expect a 2:1 ratio of intra-articular fracture to extra-articular fractures based upon clinical experience. With a two-sided, two-sample t-test, we would need a total sample size of 87 (58 intra-articular and 29 extra-articular fractures) to detect a 50% greater cost of intra-articular fractures relative to extra-articular fractures with 80% power at a 0.05 alpha level.
Results
We identified 171 patients treated with distal radius ORIF during the study period, and 108 met the inclusion criteria. Mean age was 51.2 years (range 20–87) and 68% of patients were female. Demographic data, injury characteristics, and payer mix are summarized in Table 1. Surgical encounter descriptive data are summarized Table 2. No patient underwent additional simultaneous percutaneous pinning of the distal radius, external fixation, or dorsal spanning plate fixation.
Table 1.
Baseline Patient Characteristics
| Factor | Value (n = 108) | ||
|---|---|---|---|
| Demographics | |||
| Age (years ± SD) | 51.2 | ± | 16.2 |
| Race | |||
| American Indian | 1 | (1%) | |
| Asian | 3 | (3%) | |
| Black and Other/Unknown | 18 | (17%) | |
| White | 86 | (80%) | |
| Sex (female) | 73 | (68%) | |
| Injury Characteristics | |||
| Acute carpal tunnel syndrome | 6 | (6%) | |
| Distal ulna fracture surgical treatmenta | 9 | (8%) | |
| Open fracture | 5 | (5%) | |
| Distal Radius Articular Parts | |||
| 1 (Extra-articular) | 33 | (31%) | |
| 2 | 29 | (27%) | |
| ≥3 | 46 | (43%) | |
| Payer Mix | |||
| Commercial | 66 | (61%) | |
| Medicare | 24 | (22%) | |
| Medicaid | 3 | (3%) | |
| Self-pay | 3 | (9%) | |
| Worker’s Compensation | 10 | (3%) | |
| Other | 2 | (2%) | |
Continuous data presented as mean ± standard deviation; categorical data presented as number of patients and (percentage).
ORIF or Darrach distal ulna resection.
Table 2.
Summary of Surgical Encounter Descriptive Data
| Factor | Value (n = 108) | ||
|---|---|---|---|
| Anesthesia Type | |||
| General | 105 | (97%) | |
| Regional | 3 | (3%) | |
| Hardware | |||
| Total number of plates | 1.0 | ± | 0.2 |
| Total number of screws | 7 | ± | 3.4 |
| Plate Manufacturer | |||
| Company A | 53 | (49%) | |
| Company B | 6 | (6%) | |
| Company C | 9 | (8%) | |
| Company D | 4 | (4%) | |
| Company E | 6 | (6%) | |
| Company F | 26 | (24%) | |
| Company G | 4 | (4%) | |
| Surgical Time (minutes)a | 78 | ± | 28.6 |
| By procedural code: | |||
| 25607 | 70.6 | ± | 19.0 |
| 25608 | 69.0 | ± | 20.0 |
| 25609 | 89.0 | ± | 35.0 |
| By treatment location: | |||
| Main OR | 77.9 | ± | 28.1 |
| ASC | 78.6 | ± | 289.0 |
| Treatment Disposition | |||
| Inpatient/23-hour observation | 2 | (2%) | |
| Outpatient | 106 | (98%) | |
| Treatment Location | |||
| Ambulatory surgery center (ASC) | 77 | (71%) | |
| Main hospital | 31 | (29%) | |
| Treating Service | |||
| Hand | 79 | (77%) | |
| Trauma | 29 | (23%) | |
Continuous data presented as mean ± standard deviation; categorical data presented as number of patients and (percentage).
Surgical time depicted as the overall mean and standard deviation, and separately by CPT code. Note that surgical time for 25609 was significantly greater than that for 25607 or 25608 based upon the Kruskal-Wallis test (p = 0.02). Surgical time at the main OR versus ASC was non-significant based upon the student’s t-test (p = 0.92).
Although institutional policies prohibit reporting of actual costs, variation in surgical encounter total direct costs was observed with a standard deviation of 28% of the average total direct cost. The range of total direct cost varied from 47% to 200% of the average total direct cost, with an interquartile range (defined as the range between the 25th and 75th percentiles, containing the middle 50% of cases) of 80% to 108%.
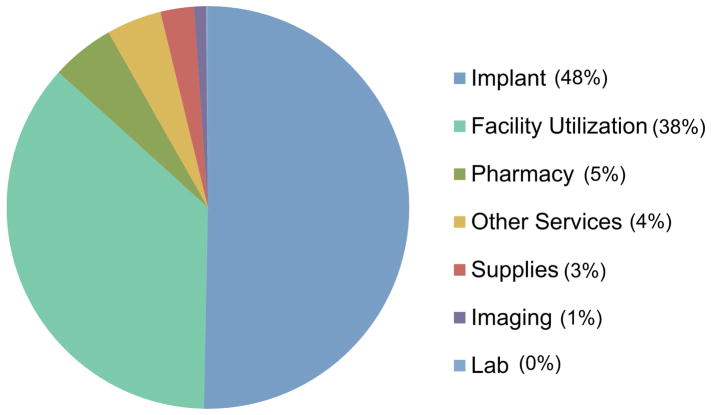
Breakdown of direct costs demonstrated that implants and facility utilization represented the majority of surgical encounter costs, with contributions of 48% and 38%, respectively (Figure 1). In the breakdown of complete cost, implants were the greatest contributor (32%), followed by the surgeon (23%), facility (23%) anesthesia (14%), and other sources (8%).
Figure 1.
Breakdown of Total Direct Costs.
Results of univariate gamma regression analyses are summarized in Tables 3–5. Of the demographic factors under study, none significantly affected surgical encounter total direct costs (Table 3). Open fracture was the only injury-specific factor under study observed to significantly affect surgical encounter total direct costs, with an increase of 55% (RC 1.55) over closed fractures (Table 4). The number of distal radius articular parts, presence of acute carpal tunnel syndrome requiring carpal tunnel release, and presence of ipsilateral distal ulna fracture deemed to require surgical treatment, did not have a significant impact on the costs. Observed distal ulna procedures included Darrach distal ulnar excision or Kirschner wire fixation; no patient received plate and screw fixation. Treatment-specific factors observed to significantly influence surgical encounter total direct costs included plate manufacturer (RC 1.52 for the most versus least expensive manufacturer), the number of distal radius plates used (RC 1.67; each additional plate increased costs by 67%), total number of screws used (RC 1.03; each additional screw increased costs by 3%), surgery setting (RC 1.32 for the main hospital versus ambulatory surgery center), and treating service (RC 1.4 for the trauma versus the hand service) (Table 5). Both the surgical time and anesthesia time significantly influenced the cost (1.04), with every 10 minutes of additional surgical or anesthesia time increasing costs by 4%. Anesthesia type was not observed to affect costs.
Table 3.
Univariate Analysis for the Effect of Demographic-Specific Factors on Total Direct Costs
| Factor | Relative Costa | P - Value | |
|---|---|---|---|
| Coefficient | 95% Confidence Interval | ||
|
| |||
| Age b | 0.99 | (0.97 • 1.01) | 0.14 |
|
| |||
| Race | - | ||
|
| |||
| White | Reference Category | - | - |
|
| |||
| American Indian | 1.01 | (0.58 • 1.75) | 0.97 |
|
| |||
| Asian | 0.86 | (0.63 • 1.18) | 0.35 |
|
| |||
| Black and Other/Unknown | 1.09 | (0.95 • 1.26) | 0.22 |
|
| |||
| Sex | |||
|
| |||
| Female | Reference Category | - | - |
|
| |||
| Male | 1.07 | (0.95 • 1.21) | 0.19 |
Relative Cost (RC): Gamma univariate regression coefficients (eβ). For example, a value of 1.6 is interpereted as a 60% increase in total direct costs as compared to the reference category. Bolded p-values were determined to be statistically-significant.
Per each additional year of age.
Table 5.
Univariate Analysis for the Effect of Treatment-Specific Factors on Total Direct Costs
| Factor | Relative Costa | P - Value | |
|---|---|---|---|
| Coefficient | 95% Confidence Interval | ||
|
| |||
| Anesthesia Type | |||
|
| |||
| General | Reference Category | - | - |
|
| |||
| Regional/Surgical Block | 0.78 | (0.57 • 1.07) | 0.12 |
|
| |||
| Number of distal radius plates | 1.67 | (1.29 • 2.15) | < 0.05 |
|
| |||
| Number of screws (locking + nonlocking) | 1.03 | (1.01 • 1.05) | < 0.05 |
|
| |||
| Plate Manufacturerb | |||
|
| |||
| Company A | Reference Category | - | - |
|
| |||
| Company B | 1.17 | (0.9 • 1.40) | 0.08 |
|
| |||
| Company C | 1.19 | (1.01 • 1.39) | < 0.05 |
|
| |||
| Company D | 1.17 | (0.95 • 1.46) | 0.14 |
|
| |||
| Company E | 1.52 | (1.28 • 1.82) | < 0.05 |
|
| |||
| Company F | 1.43 | (1.30 • 1.58) | < 0.05 |
|
| |||
| Company G | 1.20 | (0.97 • 1.49) | 0.10 |
|
| |||
| Surgery Setting | - | ||
|
| |||
| Ambulatory surgery center | Reference Category | - | - |
|
| |||
| Main hospital operating room | 1.32 | (1.2 • 1.46) | < 0.05 |
|
| |||
| Surgery Timec | 1.04 | (1.02 • 1.06) | < 0.05 |
|
| |||
| Treating Service | |||
|
| |||
| Hand | Reference Category | - | - |
|
| |||
| Trauma | 1.40 | (1.27 • 1.55) | <0.05 |
Relative Cost (RC): Gamma univariate regression coefficients (eβ). For example, a value of 1.6 is interpereted as a 60% increase in total direct costs as compared to the reference category. Bolded p-values were determined to be statistically-significant.
Per institutional policies, actual manufacterer names associated with cost data must not be disclosed.
Surgery time was measured in 10 minute increments. Value of 1.04 is interpreted as a 4% increase in cost for every 10 minutes of additional surgery time.
Table 4.
Univariate Analysis for the Effect of Injury-Specific Factors on Total Direct Costs
| Factor | Relative Costa | P - Value | |
|---|---|---|---|
| Coefficient | 95% Confidence Interval | ||
|
| |||
| Acute carpal tunnel syndrome treated with carpal tunnel release | |||
|
| |||
| No | Reference Category | - | - |
|
| |||
| Yes | 0.90 | (0.71 • 1.13) | 0.36 |
|
| |||
| Distal radius number of articular partsb | - | ||
|
| |||
| 1 | Reference Category | - | - |
|
| |||
| 2 | 0.88 | (0.77 • 1.01) | 0.07 |
|
| |||
| ≥3 | 0.94 | (0.84 • 1.06) | 0.32 |
|
| |||
| Distal ulna fracture requiring surgical treatmentc | |||
|
| |||
| No | Reference Category | - | |
|
| |||
| Yes | 1.11 | (0.91 • 1.34) | 0.28 |
|
| |||
| Open Fracture | |||
|
| |||
| No | Reference Category | - | - |
|
| |||
| Yes | 1.55 | (1.23 • 1.96) | < 0.05 |
Relative Cost (RC): Gamma univariate regression coefficients (eβ). For example, a value of 1.6 is interpereted as a 60% increase in total direct costs as compared to the reference category. Bolded p-values were determined to be statistically-significant.
Based upon CPT codes.
CPT codes include those for treatment of concomittant ulnar fractures (Darrach, or ORIF of the distal ulna or ulnar styloid).
Multivariable gamma regression analysis demonstrated continued significance of implant manufacturer, the number of screws used, and the number of distal radius plates used, while controlling for other studied injury-specific factors (Table 6). Open fracture and other injury-specific factors, surgical setting (ASC versus main OR), and treating service (hand versus trauma) were non-significant contributors to total direct costs in this model.
Table 6.
Multivariable Regression Analysis to Elucidate Modifiable Factors Affecting Total Direct Costs
| Factor | Relative Costa | 95% Confidence Interval | P - Value |
|---|---|---|---|
| Injury-Specific Factors | |||
| Acute carpal tunnel treated with release | |||
| No | Reference Category | - | - |
| Yes | 0.97 | (0.75 • 1.25) | 0.81 |
| Distal radius number of articular partsb | |||
| 1 (Extra-articular) | Reference Category | - | - |
| 2 | 0.99 | (0.90 • 1.09) | 0.78 |
| ≥3 | 1.03 | (0.95 • 1.11) | 0.54 |
| Distal ulna requiring surgical treatmentc | |||
| No | Reference Category | - | - |
| Yes | 1.12 | (0.90 • 1.38) | 0.35 |
| Open fracture | |||
| No | Reference Category | - | - |
| Yes | 1.15 | (0.93 • 1.43) | 0.19 |
| Potentially Modifiable Factors | |||
| Implant manufacturer | |||
| Company A | Reference Category | - | - |
| Company B | 1.21 | (1.05 • 1.39) | < 0.05 |
| Company D | 1.17 | (1.04 • 1.32) | < 0.05 |
| Company E | 1.45 | (1.24 • 1.69) | < 0.05 |
| Company F | 1.20 | (1.04 • 1.37) | < 0.05 |
| Company G/Cd | 1.09 | (0.92 • 1.04) | 0.35 |
| Number of distal radius platese | 1.36 | (1.14 • 1.63) | < 0.05 |
| Number of screwsf | 1.02 | (0.99 • 1.04) | < 0.05 |
| Surgery setting | |||
| Ambulatory surgery center | Reference Category | - | - |
| Main hospital operating room | 1.07 | (0.92 • 1.25) | 0.34 |
| Treating orthopaedic service | |||
| Hand | Reference Category | ||
| Trauma | 1.07 | (0.88 • 1.30) | 0.48 |
Relative Cost (RC): Gamma multivariable regression coefficients (eβ). For example, a value of 1.6 is interpereted as a 60% increase in total direct costs as compared to the reference category. Bolded p-values were determined to be statistically-significant.
Based upon CPT codes.
CPT codes include those for treatment of concomittant ulnar fractures (Darrach, or ORIF of the distal ulna or ulnar styloid).
Companies G and C were combined due to small sample size.
Each additional plate, in addition to the first one used, increased the total direct costs by 36%.
Each screw increased the total direct costs by 2%. Includes locking and nonlocking screws.
Discussion
One main finding of this study was the large magnitude of variation observed for distal radius ORIF costs, with total direct costs ranging from 47–200% relative to the average case. Implants were the largest driver of variation in surgical costs of distal radius ORIF, which represented 48% of the total direct costs for the surgical encounter and 32% of the complete cost (defined as total direct costs plus surgeon and anesthesia payments).
An additional main finding was that choice of plate manufacturer significantly affected costs, independent of injury characteristics, surgery location (main hospital versus ASC setting), and treating service (orthopaedic hand versus trauma). Use of the most expensive implant manufacturer increased surgical encounter total direct costs by 45% as compared to the least expensive. Given the paucity of literature supporting the clinical superiority of one type of distal radius plate over another, we conclude that based upon these findings from a single institution, implant choice (or implant cost) is the most important modifiable factor that may lead to cost savings for distal radius ORIF.
Congruent with this conclusion is a recent report suggesting that surgeon cost-awareness of distal radius implant options may decrease projected implant costs by 9–11%.13 This, combined with our observation that implants are the costliest component of the surgical encounter for distal radius ORIF, may influence decision-making to decrease the overall cost of distal radius fracture ORIF. Additional cost-savings may be expected if hospital systems utilize bargaining and purchasing power to reduce the cost of individual implants and share pricing data with their surgeons.
Our observation that implants account for 48% of distal radius ORIF total direct costs, and 32% of complete costs, differs from prior literature although differing definitions of the denominator makes comparing percentages difficult. In a study by Swart et al., implant costs amounted to 6.3% of the total cost for distal radius ORIF, using hospital charges and collections data.7 As opposed to the current study, charges and payment were used as a surrogate for direct costs, and the authors included costs for the entire treatment period including estimations of indirect costs such as missed work. They concluded that for operative patients, 28% of costs were indirect and 72% were direct, with 59% of total costs attributed to the surgical encounter itself. Based on provided numbers, surgical encounter direct costs (excluding anesthesia and surgeon payments, and costs related to office visits and emergency department care) amounted to $3509, and implants contributed to 27.6% of the surgical encounter ‘costs’. This differs from the 48% implant contribution observed in the current study, however differences in the definition of cost between the two studies make it difficult to interpret a direct comparison: total direct costs were used in the current study versus payment/charge data in the prior study. Previous studies have estimated that the surgical encounter is responsible for 59–82% of the total cost of care for distal radius fractures treated surgically.5,7 Although the current study was not designed to determine costs of pre-surgical and post-surgical care, these prior studies suggest that our focus on the surgical encounter itself captures the majority of the overall expense related to distal radius fracture surgical treatment.
Secondary findings include identification of additional potentially-modifiable factors that have a significant impact on the cost of distal radius ORIF. Our finding that every additional 10 minutes of surgical time increased costs by 4% highlights the importance of efficiency in the operating room, which has previously been highlighted as an important driver of cost.15 Additionally, we did not observe cost differences for surgeries performed in the main hospital setting versus ASC. While potentially appearing contradictory to prior literature supporting cost-savings for carpal tunnel surgery performed at an ASC versus a main hospital setting, we utilized total direct costs and prior work reported charges.16 We interpret these differences to mean that time-allocated costs for services at both surgical settings are similar, with substantially different charges to insurance. Further, judicious use of screws and strategic screw placement may lead to cost-savings when possible, as we observed that each additional screw increased costs by 2%.17,18 Similarly, selective use of additional distal radius plates may reduce costs based on our finding that additional plates increased costs by 36%. These conclusions are based upon multivariable regression analysis in which the number of distal radius articular parts, treatment of concomitant acute carpal tunnel syndrome or an ipsilateral distal ulna fracture, and open fracture did not significantly influence costs.
This study has several limitations. The retrospective study design and identification of patients by CPT code introduces the possibility of selection bias. The optimal method for defining medical ‘cost’ is unclear, and our focus on total direct costs, and defining “complete cost” as the total direct costs with surgeon and anesthesia payments, is only one of several potential approaches. In the hand literature, authors have defined cost in various ways with no clear consensus: Medicare payments or reimbursements,2,5 hospital charges and fees,7 and direct costs to the hospital6,9 have all been previously utilized. Other limitations include our sole focus on the surgical encounter for distal radius ORIF, and we acknowledge that identification of factors leading to cost variation in the preoperative or postoperative course may provide additional potential to reduce costs. However, our VDO tool is designed to capture medical encounter costs only at our institution, therefore expanding our analysis to the pre- and postoperative period may introduce bias resulting from missing costs for patients who had a portion of their care outside of our institution. To minimize this effect, we elected to focus on the operative encounter, as this typically accounts for the majority of cost.2 Utilizing CPT codes as a surrogate for fracture severity also poses as a limitation. Inter-rater reliability in assessing the number of articular parts is limited,19,20 surgeons may charge for a more complex CPT code even if additional fragments are minor or nondisplaced, and fractures with 3 or more parts, corresponding with CPT 25609, likely represents a wide spectrum of injury ranging from minimally-displaced intra articular fractures to those with severe comminution and displacement. For certain complex fractures, multiple surgical approaches, multiple plates, a greater number of screws, and the addition of a dorsal spanning plate or supplemental pinning may be required to achieve an adequate reduction and fixation – these factors, not captured by CPT coding, may influence the cost of surgery. By acknowledging this limitation, we advise against concluding that the cost of distal radius ORIF is independent of fracture severity. Furthermore, we acknowledge that surgeon familiarity with plating systems of choice is an important factor that may temporarily contribute to efficiency in the OR: use of less expensive but unfamiliar systems may initially counteract potential cost-savings by increasing OR time, but this would likely dissipate following the initial learning curve. Our findings that treatment of concomitant acute carpal tunnel syndrome, treatment of distal ulna or open fractures, and anesthesia type do not influence costs should be interpreted in light of the possibility of type-II error secondary to small subgroup sample sizes. It is possible that direct costs differ between main OR and ASC settings at other institutions, which may pose as an additional opportunity for cost-savings not observed in the current study. Additionally, use of CPT code to characterize the number of fracture parts may be subject to the similar limitations in regard to intra- and inter-observer reliability as for other classification systems.21–23 We remain unable to directly comment on the impact of study variables on the value of care since patient-reported outcomes were not collected.
One additional limitation that deserves specific mention pertains to the generalizability of our findings. Our results should be generalized with caution, as operating room staffing and recovery room protocols, as well as the insurance payer mix and negotiated rates for surgical implants and other supplies may differ by region. We acknowledge that implant costs, facility costs, supply costs, and other costs pertinent to distal radius ORIF surgical encounters likely differ at other institutions, as would the associated payments due to a variable payer mix across the country. Nonetheless, our main finding was that variation in implant costs was the largest contributor in driving variation in total costs for the entire surgical encounter. If variation in implant costs exists at other institutions, our conclusion would still apply that it would be beneficial for surgeons to be aware of these cost differences to inform their decisions. We acknowledge that other institutions may not achieve a 45% reduction in total direct surgical costs by using their least expensive versus most expensive plate as we observed in the current study, however cost-reductions of even a smaller magnitude for this common procedure would be expected to lead to substantial saving over time.
To conclude, lack of transparency in regard to the pricing of medical supplies and services in the United States has played a key role in rising healthcare costs.24,25 Our study has attempted to provide transparency in the context of distal radius ORIF surgical encounter costs, which vary widely. Variation is driven by implant choice, number of screws and distal radius plates used, and surgical time. Surgeon awareness of these factors that may be modifiable for certain fractures provides an opportunity to reduce costs for this common clinical entity. However, even if these cost-reducing measures are successfully implemented, it remains to be seen whether individual patients, or society at large, will financially benefit from them.
Supplementary Material
Acknowledgments
This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02 (formerly 8UL1TR000105 and UL1RR025764).
Appendix I. Breakdown of Value-Driven Outcomes database categories for total direct costs
IMAGING COST: All imaging, scans and radiology services used.
SUPPLY COST: All supplies and devices used, excluding implants.
IMPLANT COST: All supply costs related to surgical implants.
PHARMACY COST: Total cost of all medication used during the patient encounter.
LAB COST: All lab work associated with the visit, including blood work, urinalysis, hematology and all other lab or chemistry related costs.
OTHER SERVICES COST: Services that do not fall into one of the other categories. Services include Physical Therapy, Occupational Therapy, Speech Pathology, Respiratory Service, EKG, Recovery Room nursing/staff, and Other Therapeutic Services.
FACILITY UTILIZATION COST: Time and labor costs for patients stay in each unit (excluding Professional costing such as time-allocated estimates of physician costs). Cost is mapped to the individual patient level based on time spent on specific unit (surgery minutes, patient hours) or by completed visit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28:113–125. doi: 10.1016/j.hcl.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farner S, Malkani A, Lau E, Day J, Ochoa J, Ong K. Outcomes and cost of care for patients with distal radius fractures. Orthopedics. 2014;37:e866–878. doi: 10.3928/01477447-20140924-52. [DOI] [PubMed] [Google Scholar]
- 3.Mellstrand-Navarro C, Pettersson HJ, Tornqvist H, Ponzer S. The operative treatment of fractures of the distal radius is increasing: Results from a nationwide swedish study. Bone Joint J. 2014;96-B:963–969. doi: 10.1302/0301-620X.96B7.33149. [DOI] [PubMed] [Google Scholar]
- 4.Chung KC, Shauver MJ, Yin H, Kim HM, Baser O, Birkmeyer JD. Variations in the use of internal fixation for distal radial fracture in the united states medicare population. J Bone Joint Surg Am. 2011;93:2154–2162. doi: 10.2106/JBJS.J.012802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shauver MJ, Yin H, Banerjee M, Chung KC. Current and future national costs to medicare for the treatment of distal radius fracture in the elderly. J Hand Surg Am. 2011;36:1282–1287. doi: 10.1016/j.jhsa.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Karantana A, Scammell BE, Davis TR, Whynes DK. Cost-effectiveness of volar locking plate versus percutaneous fixation for distal radial fractures: Economic evaluation alongside a randomised clinical trial. Bone Joint J. 2015;97-B:1264–1270. doi: 10.1302/0301-620X.97B9.35560. [DOI] [PubMed] [Google Scholar]
- 7.Swart E, Tulipan J, Rosenwasser MP. How should the treatment costs of distal radius fractures be measured? Am J Orthop (Belle Mead NJ) 2017;46:E54–E59. [PubMed] [Google Scholar]
- 8.Centers for medicare & medicaid services; national health expenditure data. 2018 [updated 1/8/20181/31/2018]; Available from: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical.html.
- 9.Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061–1072. doi: 10.1001/jama.2016.12226. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman DM, Bindra RR, Boyer MI, et al. American academy of orthopaedic surgeons clinical practice guideline on: The treatment of distal radius fractures. J Bone Joint Surg Am. 2011;93:775–778. doi: 10.2106/JBJS.938ebo. [DOI] [PubMed] [Google Scholar]
- 11.Trumble TE, Schmitt SR, Vedder NB. Factors affecting functional outcome of displaced intra-articular distal radius fractures. J Hand Surg Am. 1994;19:325–340. doi: 10.1016/0363-5023(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins NH, Mintowt-Czyz WJ. Mal-union and dysfunction in colles’ fracture. J Hand Surg Br. 1988;13:291–293. doi: 10.1016/0266-7681_88_90090-3. [DOI] [PubMed] [Google Scholar]
- 13.Wasterlain AS, Melamed E, Bello R, Karia R, Capo JT Science of Variation G. The effect of price on surgeons’ choice of implants: A randomized controlled survey. J Hand Surg Am. 2017;42:593–601. e596. doi: 10.1016/j.jhsa.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Tashjian RZ, Belisle J, Baran S, et al. Factors influencing direct clinical costs of outpatient arthroscopic rotator cuff repair surgery. J Shoulder Elbow Surg. 2017 doi: 10.1016/j.jse.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am J Surg. 1999;177:28–32. doi: 10.1016/s0002-9610(98)00289-x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen C, Milstein A, Hernandez-Boussard T, Curtin CM. The effect of moving carpal tunnel releases out of hospitals on reducing united states health care charges. J Hand Surg Am. 2015;40:1657–1662. doi: 10.1016/j.jhsa.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehling I, Muller LP, Delinsky K, Mehler D, Burkhart KJ, Rommens PM. Number and locations of screw fixation for volar fixed-angle plating of distal radius fractures: Biomechanical study. J Hand Surg Am. 2010;35:885–891. doi: 10.1016/j.jhsa.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 18.Crosby SN, Fletcher ND, Yap ER, Lee DH. The mechanical stability of extra-articular distal radius fractures with respect to the number of screws securing the distal fragment. J Hand Surg Am. 2013;38:1097–1105. doi: 10.1016/j.jhsa.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Kural C, Sungur I, Kaya I, Ugras A, Cetinus E. Evaluation of the reliability of classification systems used for distal radius fractures. Orthopedics. 2010;33 doi: 10.3928/01477447-20100924-14. [DOI] [PubMed] [Google Scholar]
- 20.Kleinlugtenbelt YV, Groen SR, Ham SJ, et al. Classification systems for distal radius fractures. Acta Orthop. 2017;88:681–687. doi: 10.1080/17453674.2017.1338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson NJ, Asadollahi S, Parrish F, Ridgway J, Tran P, Keating JL. Reliability of radiographic measurements for acute distal radius fractures. BMC Med Imaging. 2016;16:44. doi: 10.1186/s12880-016-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen DJ, Blair WF, Steyers CM, Jr, Adams BD, el-Khouri GY, Brandser EA. Classification of distal radius fractures: An analysis of interobserver reliability and intraobserver reproducibility. J Hand Surg Am. 1996;21:574–582. doi: 10.1016/s0363-5023(96)80006-2. [DOI] [PubMed] [Google Scholar]
- 23.Kreder HJ, Hanel DP, McKee M, Jupiter J, McGillivary G, Swiontkowski MF. Consistency of ao fracture classification for the distal radius. J Bone Joint Surg Br. 1996;78:726–731. [PubMed] [Google Scholar]
- 24.Sinaiko AD, Rosenthal MB. Increased price transparency in health care--challenges and potential effects. N Engl J Med. 2011;364:891–894. doi: 10.1056/NEJMp1100041. [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt UE. The disruptive innovation of price transparency in health care. JAMA. 2013;310:1927–1928. doi: 10.1001/jama.2013.281854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.