Abstract
The promise of Δ9-tetrahydrocannabinol (THC) as a treatment for migraine depends on antinociceptive efficacy with repeated administration. Although morphine has good antinociceptive efficacy, repeated administration causes medication-overuse headache (MOH) - a condition in which the intensity/frequency of migraine increases. The present study compared the effect of repeated morphine or THC administration on the magnitude and duration of migraine-like pain induced by microinjection of allyl isothiocyanate (AITC) onto the dura mater of female rats. Acute administration of THC or morphine prevented AITC-induced depression of wheel running. This antinociception was maintained in rats treated repeatedly with THC, but not following repeated administration of morphine. Moreover, repeated morphine, but not THC administration extended the duration of AITC-induced depression of wheel running. These data demonstrate that tolerance and MOH develop rapidly to morphine administration. The lack of tolerance and MOH to THC indicates that THC may be an especially effective long-term treatment against migraine.
Keywords: headache, voluntary activity, marijuana, wheel running, morphine, cannabinoid, rat
Introduction
A major limitation of current migraine treatments is the development of medication overuse headache (MOH). MOH is a debilitating disorder characterized by an increase in the intensity and frequency of migraine attacks caused by repeated use of anti-migraine therapies (e.g., triptans, opioids, etc.) (Westergaard et al., 2016). Thus, novel therapies must be evaluated both for antinociceptive efficacy and resistance to MOH, but challenges in assessing migraine and MOH in laboratory animals has greatly limited the development of new treatments.
Repeated administration of opioids, triptans, and NSAIDs have been reported to elicit MOH in animal models of migraine (Kopruszinski et al., 2016). Unfortunately, these studies use tactile allodynia as an indirect method to assess migraine. Activation of dural afferents using the TRPA1 agonist allyl isothiocyanate (AITC) has been used to generate migraine-like pain in rats (Edelmayer et al., 2012). Our recent finding that microinjection of AITC onto the dura produces a sumatriptan-reversible depression of home cage wheel running that lasts approximately 3 h (Kandasamy et al., 2017c) indicates that this is a good model of migraine-like pain.
Survey data indicate that marijuana is a common method of self-medication for migraine pain (Baron, 2015). We have previously shown that administration of Δ9-tetrahydrocannabinol (THC), the primary ingredient in marijuana, prevents migraine-depressed wheel running in female rats (Kandasamy et al., 2018). Given that migraine patients typically take marijuana on a regular basis, marijuana may be one of the few drugs that does not produce MOH. The present experiment tested this hypothesis by comparing the effects of repeated THC or morphine administration on MOH using home cage wheel running to quantify the magnitude and duration of migraine.
Methods
Subjects
Female Sprague-Dawley rats (50–70 days old) were used because MOH is much more common in women than men. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the International Association for the Study of Pain’s Policies on the Use of Animals in Research.
Procedure
Animals were anesthetized with pentobarbital (50 mg/kg, i.p.) and implanted with a guide cannula through which allyl isothiocyanate (AITC; Sigma-Aldrich, Inc.) could be applied to the dura mater to induce migraine-like pain. Loctite® super glue and dental cement anchored the guide cannula to two screws in the skull. Following surgery, rats were housed individually in a cage with a running wheel (27.9 cm diameter; Kaytee Products, Inc) (Kandasamy et al., 2017c). Wheel revolutions were recorded 23 h/day (Multi-Varimex software, Columbus Instruments) beginning at the onset of the 12-h dark phase of the light cycle (17.00 h). Rats were allowed unrestricted access to the wheel for 23 h/day for 8 days following surgery.
Baseline wheel running was measured for 23 h on Day 8. Then, rats received repeated injections of THC (1.0 mg/kg, i.p.), morphine (3.2 mg/kg, i.p.), or vehicle beginning at 16.45 h on Day 9 and continuing twice a day on Days 10 & 11 (10.45h and 16.45h). On Day 12, the rat was injected with AITC or mineral oil onto the dura mater followed immediately by systemic injection of THC (0.1, 0.32 mg/kg, i.p.), morphine (0.32, 1.0 mg/kg, i.p.), or vehicle. All injections were completed by 16.50 h so that activity could be recorded beginning at 17.00 h. This procedure was repeated every other day (Days 14 and 16), so each rat was tested with both drug doses and vehicle in a counterbalanced order. THC, morphine, or vehicle was administered at 10.45h and 16.45h on the intervening days (Days 13 and 15) to maintain drug exposure. Rats were euthanized 48 h after the last AITC injection.
Drugs
AITC (10%) was mixed in mineral oil and injected into the periosteal space in a volume of 10 μL. THC (Sigma-Aldrich, Inc.,) was dissolved in vehicle (1:1:18; ethanol:cremophor:saline), and morphine sulfate (NIDA Supply Program) was dissolved in saline.
Statistical analysis
Hourly and daily wheel running activity was analyzed as a percent of each rat’s baseline value ( Kandasamy et al., 2017a,b). Only rats with a baseline of 400 wheel revolutions or more were included in data analysis (Kandasamy et al., 2016). Mean ± SEM wheel running levels during the 3-h period of the AITC-induced migraine were analyzed with one- and two-way ANOVAs followed by Bonferroni post-hoc analysis. Statistical significance was defined as a probability of < 0.05.
Results
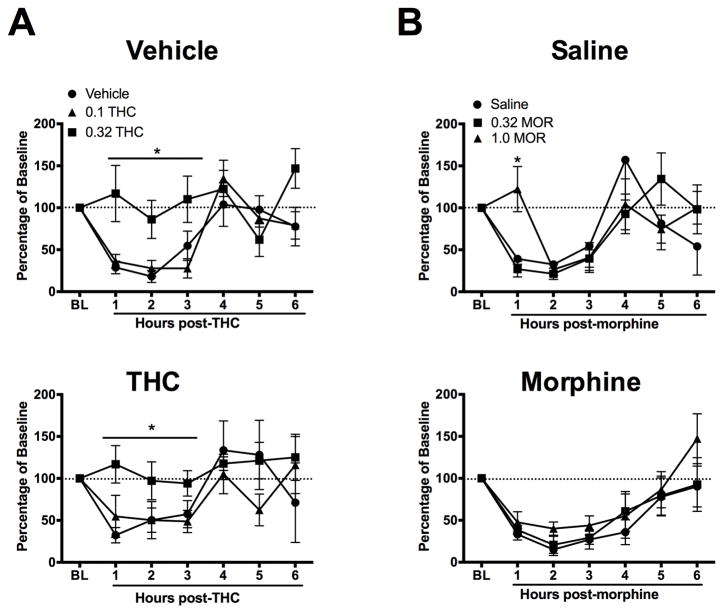
Microinjection of AITC onto the dura mater caused a reduction in wheel running that lasted for at least 3 h regardless of pretreatment (Fig. 1). Acute administration of 0.32 mg/kg of THC prevented AITC-induced depression of wheel running in both vehicle- (F(2,20) = 10.42, p < 0.001) and THC-pretreated (F(2,19) = 4.98, p < 0.02) rats, compared to rats treated with vehicle or a low dose of THC (0.1 mg/kg) (Fig. 1A).
Figure 1.
Tolerance develops to the anti-migraine effects of morphine, but not THC. Animals were pretreated with THC (1.0 mg/kg), morphine (3.2 mg/kg) or vehicle for 2.5 days. Microinjection of AITC onto the dura mater produced migraine-like pain indicated by depression of wheel running that lasted for at least 3 h in all experiments. A) Administration of 0.32 mg/kg of THC immediately after AITC administration prevented AITC-induced depression of wheel running in vehicle- (upper panel) and THC-treated animals (lower panel). Administration of a lower dose of THC (0.1 mg/kg) or vehicle did not prevent AITC-induced depression of wheel running (n = 5–7/group). B) In saline-pretreated animals, administration of 1.0 mg/kg of morphine immediately after AITC administration prevented AITC-induced depression of wheel running (upper panel). This antinociceptive effect was absent in morphine-pretreated animals as would be expected with the development of tolerance (lower panel) (n = 6–8/group).
In rats pretreated with saline, administration of 1.0 mg/kg of morphine immediately following AITC produced a transient block of AITC-induced depression of wheel running, compared to rats given saline or a low dose of morphine (0.32 mg/kg) (Fig. 1B top). Analysis of the magnitude of wheel running during the hour of morphine antinociception revealed a significant differences between morphine doses (F(2,20) = 8.98, p 0.002), driven by significantly higher levels of wheel running in rats injected with 1.0 mg/kg of morphine compared to 3.2 mg/kg of morphine or saline. This antinociceptive effect of morphine was not evident in rats pretreated with morphine for 2.5 days. Microinjection of AITC onto the dura mater caused a reduction in wheel running regardless of acute morphine administration in rats pretreated with morphine (Fig. 1B bottom). There was no significant difference in the magnitude of wheel running during the first hour following AITC administration despite morphine administration (F(2,21) = 0.68, NS).
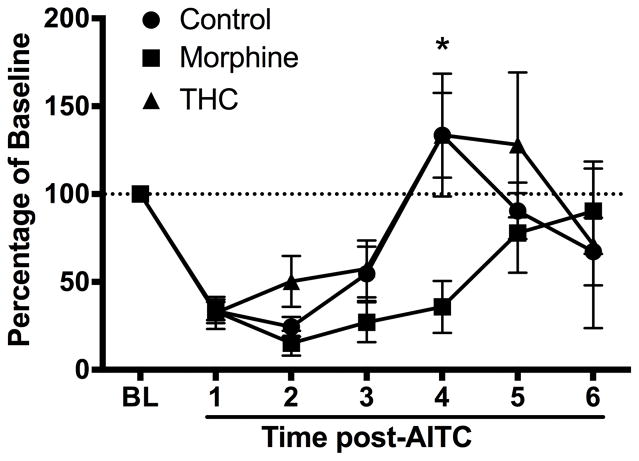
The duration of AITC-induced depression of wheel running was prolonged in animals pretreated with morphine compared to those pretreated with THC or vehicle (Fig. 2). AITC-induced depression of wheel running returned to baseline levels by Hour 4 in rats pretreated with vehicle or THC, but remained depressed in rats pretreated with morphine (F(2,29) = 3.78, p < 0.05).
Figure 2.
AITC-induced depression of wheel running is prolonged by repeated morphine administration. AITC-induced depression of wheel running lasted for 3 h in THC- (n = 8) and vehicle-treated (n = 14) rats. Repeated morphine administration (n = 8) prolonged the duration of AITC-induced depression of wheel running to 4 h.
Discussion
The present data show that administration of THC or morphine restores home cage wheel running depressed by AITC-induced migraine. THC was more effective than morphine both in the duration of antinociception and resistance to tolerance and MOH. Administration of THC restored wheel running for the entire 3-h duration of AITC-induced migraine and was as effective following the sixth injection as the first. In contrast, administration of morphine only restored wheel running for one hour following administration of AITC and this effect disappeared with repeated administration, as would be expected with the development of tolerance. In addition, repeated administration of morphine, but not THC produced MOH, as indicated by prolonging AITC-induced depression of wheel running from 3 to 4 h.
Activation of dural afferents by AITC administration produced a consistent three-hour reduction in wheel running (Kandasamy et al, 2017c). The restoration of home cage wheel running to baseline levels following administration of THC is consistent with anecdotal reports of marijuana self-medication for migraine (Rhyne et al., 2016). Marijuana may be an especially effective treatment, in that there was no evidence of tolerance or MOH with the doses or duration of administration used here. The lack of tolerance to THC contrasts with other studies examining the antinociceptive effects of THC to acute pain (Wakley et al., 2014). Whether this difference in tolerance is a result of the different pain models used or the much larger doses and extended duration of administration in the previous study is not clear (Wakley et al., 2014). The magnitude of tolerance to THC is greater with higher doses (McKinney et al., 2008). The low doses used to restore wheel running may be advantageous in reducing the development of tolerance and limiting disruptive side effects. Our finding that tolerance does not occur to administration of therapeutic doses of THC is consistent with clinical observations showing that tolerance is more likely to occur as the dose and frequency of use increases (González et al., 2005).
Our finding that administration of morphine caused a transient block of migraine pain is consistent with other rodent (Chanda et al., 2013) and human (Gallagher, 1986) data. Morphine and other opioids are used to treat severe cases of migraine, particularly when other agents are ineffective or contraindicated (Silberstein and Lipton, 1994). The problem is that tolerance and MOH develop rapidly with repeated administration of morphine. In fact, morphine is not recommended as a standard migraine treatment because of the rapid development of MOH (Tepper, 2012). Our data showing a lack of antinociception and prolonged AITC-induced depression of wheel running in rats receiving repeated morphine administration are consistent with the development of MOH. Whether this is a real model of MOH is difficult to know because spontaneous pain is difficult to assess in animals. However, the prolonged duration of migraine-like pain suggests this model may reflect MOH.
In conclusion, the present study demonstrates that both THC and morphine are effective anti-migraine treatments, but THC produces a more prolonged antinociception and one that is resistant to tolerance and MOH. These findings indicate that THC may be an especially effective long-term therapy for migraine patients.
Acknowledgments
The authors thank Shauna Schoo for technical assistance.
Funding
This investigation was supported in part by funds provided by medical and biological research by State of Washington Initiative Measure No. 171 to CTD and No. 502 and NIH grant NS095097 to MMM.
Footnotes
Declaration of conflicting interests
The authors declare no conflicts of interest.
References
- Baron EP. Comprehensive Review of Medicinal Marijuana, Cannabinoids, and Therapeutic Implications in Medicine and Headache: What a Long Strange Trip It’s Been …: Headache. The Journal of Head and Face Pain. 2015;55(6):885–916. doi: 10.1111/head.12570. [DOI] [PubMed] [Google Scholar]
- Chanda ML, et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 2013;154(8):1254–1262. doi: 10.1016/j.pain.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153(9):1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher RM. Emergency treatment of intractable migraine. Headache: The Journal of Head and Face Pain. 1986;26(2):74–75. doi: 10.1111/j.1526-4610.1986.hed2602074.x. [DOI] [PubMed] [Google Scholar]
- González S, Cebeira M, Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacology, Biochemistry and Behavior. 2005;81(2):300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. Journal of neuroscience methods. 2016;263:115–122. doi: 10.1016/j.jneumeth.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Calsbeek JJ, Morgan MM. Analysis of inflammation-induced depression of home cage wheel running in rats reveals the difference between opioid antinociception and restoration of function. Behavioural Brain Research. 2017a;317:502–507. doi: 10.1016/j.bbr.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Lee AT, Morgan MM. Depression of home cage wheel running is an objective measure of spontaneous morphine withdrawal in rats with and without persistent pain: Pharmacology. Biochemistry and Behavior. 2017b doi: 10.1016/j.pbb.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Lee AT, Morgan MM. Depression of home cage wheel running: a reliable and clinically relevant method to assess migraine pain in rats. The Journal of Headache and Pain. 2017c;18(1):S9. doi: 10.1186/s10194-017-0721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy R, Dawson CT, Craft RM, Morgan MM. Anti-migraine effect of Δ 9 -tetrahydrocannabinol in the female rat. European Journal of Pharmacology. 2018;818:212–217. doi: 10.1016/j.ejphar.2017.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopruszinski CM, Xie JY, Eyde NM, Remeniuk B, Walter S, Stratton J, Bigal M, Chichorro JG, Dodick D, Porreca F. Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents. Cephalalgia. 2016:0333102416650702. doi: 10.1177/0333102416650702. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 2008;324(2):664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyne DN, Anderson SL, Gedde M, Borgelt LM. Effects of Medical Marijuana on Migraine Headache Frequency in an Adult Population. Pharmacotherapy. 2016 doi: 10.1002/phar.1673. p. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Silberstein SD, Lipton RB. Overview of diagnosis and treatment of migraine. Neurology. 1994;44(10 Suppl 7):S6–16. [PubMed] [Google Scholar]
- Tepper SJ. Opioids should not be used in migraine. Headache: The Journal of Head and Face Pain. 2012;52(Suppl 1)(1):30–34. doi: 10.1111/j.1526-4610.2012.02140.x. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug and Alcohol Dependence. 2014;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard ML, Munksgaard SB, Bendtsen L, Jensen RH. Medication-overuse headache: a perspective review. Therapeutic advances in drug safety. 2016;7(4):147–158. doi: 10.1177/2042098616653390. [DOI] [PMC free article] [PubMed] [Google Scholar]