Abstract
Tumors often overcome the cytotoxic effects of chemotherapy through either acquired or environment-mediated drug resistance. In addition, signals from the microenvironment obfuscate the beneficial effects of chemotherapy and may facilitate progression and metastatic dissemination. Seminal mediators in chemotherapy-induced metastasis appear to be a wide range of hematopoietic, mesenchymal and immune progenitor cells, originating from the bone marrow. The actual purpose of these cells is to orchestrate the repair response to the cytotoxic damage of chemotherapy. However, these repair responses are exploited by tumor cells at every step of the metastatic cascade, ranging from tumor cell invasion, intravasation and hematogenous dissemination to extravasation and effective colonization at the metastatic site. A better understanding of the mechanistic underpinnings of chemotherapy-induced metastasis will allow us to better predict which patients are more likely to exhibit pro-metastatic responses to chemotherapy and will help develop new therapeutic strategies to neutralize chemotherapy-driven prometastatic changes.
Keywords: TMEM, cancer cell dissemination, MenaCalc, bone marrow-derived cells, mesenchymal stem cells, macrophages
1. Introduction
Current standard of cancer care for the loco-regional disease commonly includes surgery, radiotherapy and/or chemotherapy. Depending on cancer type and stage of the disease, these treatments may be curative. However, a subset of patients will develop distant metastases and face high mortality despite achieving complete control of the local disease. The conventional belief is that metastases represent growth of clinically and radiographically undetectable foci of cancer already present at the time of initial treatment (1–3). However, accumulating evidence now suggests that chemotherapy itself may under certain circumstances induce intratumoral or systemic changes, which can paradoxically exacerbate cancer cell proliferation and dissemination in certain patients (3). For example, preoperative or neoadjuvant chemotherapy (NAC) may not only select for chemoresistant tumor clones, as traditionally suggested, but it may also drive the development of novel mutant clones which directly correlate with the development of metastatic disease (4). In addition to inducing novel mutant clones, NAC may induce pro-metastatic changes in the microenvironment of the primary tumor. These pro-metastatic changes represent consequences of host-repair mechanisms in response to cytotoxic tissue damage (5), and are typically triggered by the systemic release of cytokines and chemokines, resembling those found during wound healing and inflammation. The systemic release of cytokines can also occur during post-operative or adjuvant chemotherapy and may render distant organs more prone to metastatic seeding (6, 7).
Thus, an increasing body of evidence indicates that chemotherapy in certain instances could increase the metastatic potential of cancers. Therefore, it is crucial to gain more thorough understanding of the contextual prerequisites under which chemotherapy induces or exacerbates metastasis. Consistent with the idea that treatment of tumors that induce an injury-like response can contribute to metastasis, it has been noted that increased circulating tumor cells may rise in cancer patients and preclinical animal models of cancer, as a consequence of radiotherapy, surgery and surgical biopsy, besides chemotherapy (3). However, here, we provide a comprehensive review specifically on the pro-metastatic effects of chemotherapy and not of other treatment modalities [for the later, please see an excellent review by Martin et al. (2017) (3)]. Understanding the relationship of tumor injury to metastasis will help improve treatment of metastatic disease, and help stratify cancer patients according to their potential response to chemotherapy, to achieve the highest standards in personalized medicine. In this review, we describe the key mechanisms of chemotherapy-induced metastasis and potential solutions to overcome the aforementioned unwanted pro-metastatic effects.
2. Revisiting the metastatic cascade
The metastatic cascade has been described as a sequence of events leading to the development of metastatic tumors in organs and tissues distant from the primary tumor site. The knowledge of the molecular and cellular events involved in individual steps of the metastatic cascade has expanded over the past years revealing complexity beyond what was originally thought (8, 9). This review focuses on the recent conceptual advancements on the biology of metastasis, critical for understanding how chemotherapy could paradoxically induce the progression of metastasis.
2.1. Epithelial-to-mesenchymal transition (EMT), cell invasion and migration
In most epithelial cancers, tumor cells undergo epithelial-to-mesenchymal transition (EMT), a biological program that allows the cells to gain mesenchymal phenotype and invade blood or lymphatic vessels. Hence, the evidence of EMT in many tumors has been associated with increased metastasis and worse prognosis (9–15). During EMT cancer cells typically downregulate epithelial-specific cadherin, E-Cadherin, and upregulate mesenchymal-specific cadherin, N-Cadherin (16, 17). Detailed molecular mappings of multiple EMT markers and pathways have been explored in detail to better understand how mesenchymal plasticity conveys metastatic behavior in tumor cells (18). Although EMT is a crucial hallmark of the metastatic cascade (9), the extent of EMT during metastasis is debated. For instance, an early study by Wicki et al. (2006) suggested that podoplanin-based filopodia can enhance cancer cell invasion in the absence of EMT in breast and pancreatic beta-cell cancers (19). Another study by Fischer et al. (2015) that utilized an EMT lineage-tracing system which examined the expression of a mesenchymal-specific fluorescent reporter whose expression becomes irreversible after EMT induction suggested that EMT is only partial during metastasis with the mesenchymal cells showing resistance to chemotherapy (20).
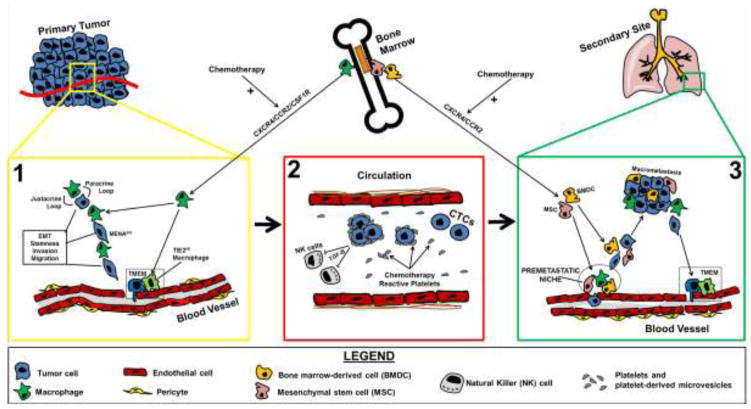
Markers of EMT and its associated tumor cell dissemination have emerged that are clinically useful in the assessment of metastatic risk in breast cancer patients. During EMT, the activity of Epithelial Splicing Regulatory Protein 1 (ESRP1) reduces the expression of MENA11a, an isoform of the actin-regulatory protein MENA that promotes cellular cohesiveness (21). The decreased level of Mena11a is frequently accompanied by concurrent increase in the expression of invasive MENA isoforms, such as MENAINV among others, which promote invasion and migration of tumor cells (22–25). This MENA expression pattern, MENA11alow and MENAINV-high, also known as MENACalc, is associated with increased cancer cell invasiveness, metastasis and poor prognosis in breast cancer patients (26–28). Mechanistically, MENAINV-high expression induces up to a 50-fold enhanced chemotactic response to EGF, HGF and IGF ligands by sequestering PTB1B away from the receptor tyrosine kinases (RTKs) (29–32). In addition, MENAINV-Hi cells have increased haptotaxis on fibronectin via interaction with integrin α5 (33), and generate mature invadopodia by enhancing the phosphorylation of cortactin in the invadopodium core structure (34). Invadopodia are actin-polymerization driven protrusions that focally degrade extracellular matrix (ECM) and are required for transendothelial migration during tumor cell dissemination (35, 36). Within the tumor microenvironment, MENAINV-Hi tumor cells migrate on collagen fibers as a stream paired with tumor associated macrophages (TAMs) towards HGF-secreting endothelial cells (31, 37–41). This pairing behavior is maintained though the EGF/CSF1 paracrine loop (Figure 1), which keeps macrophages and cancer cells in close proximity (22, 38, 42, 43). Interestingly, a recent study showed that the direct contact between cancer cells and macrophages induces MenaINV expression in cancer cells via a juxtacrine loop, (Figure 1) involving Notch-Jagged mediated signaling (44). It is plausible that macrophage-cancer cell contact during streaming is required for MENAINV expression in tumor cells in vivo. Thus, the directional streaming of MENAINV-Hi tumor cells towards the underlying vasculature may represent the major route for cancer cell dissemination and thus a prerequisite for metastasis (45).
Figure 1.
Chemotherapy-induced metastasis. A working model depicting critical molecular and cellular events of the metastatic cascade, including those in (1) primary tumor site (yellow box), (2) blood circulation (red box), and (3) secondary tumor site (green box). Illustrations of chemotherapy-induced cellular and molecular events that facilitate the metastatic cascade are shown for each compartment individually. Chemotherapy treatment induces the infiltration of a wide variety of bone marrow-derived cells (BMDCs) and mesenchymal stem cells (MSCs), mostly including proangiogenic and intratumoral macrophages, by altering the tumor chemokine network (including CXCR4/CCR2/CSF1R), thus amplifying all prometastatic pathways which involve the TMEM dissemination machinery (primary and secondary tumor sites) and the premetastatic niche formation (secondary tumor site). In addition, chemotherapy treatment may induce a platelet-mediated prometastatic response in blood circulation, as evidenced by the aggregation of platelets and platelet-derived macrovesicles around circulating tumor cells (CTCs). Cartoon abbreviations: EC, endothelial cell; M, macrophage; TC, tumor cell; TMEM, tumor microenvironment of metastasis.
2.2. Regulation of vascular permeability and intravasation by TIE2HI macrophages
The EMT and the directional streaming of MENAINV-Hi tumor cells towards blood vessels are not sufficient to cause cancer cell dissemination, since a specialized cancer cell intravasation mechanism is also required for the entry of cancer cells into the circulation (45). Intravital imaging of breast cancer in live mice has demonstrated that intravasation does not occur throughout the entirety of the cancer-associated endothelium, but instead is localized in specific microanatomical structures, known as “tumor microenvironment of metastasis” (TMEM) (Figure 1 and 2A). TMEM is composed of three cell types, a MenaHi tumor cell, a perivascular macrophage and an endothelial cell, all in direct contact with each other (46). TMEM density in the primary tumor predicts metastatic risk in breast cancer patients (45–48).
Figure 2.
TMEM in primary and secondary tumor sites. Tissue images from primary mammary tumor (A), and lung metastatic tumor (B), showing TMEM sites as visualized by triple-stain immunohistochemistry. The black boxes represent magnified inserts of the black squared areas to display TMEM structures in higher detail. EC, blue, endothelial cell; M, brown, macrophage; TC, red, MENA-expressing tumor cell. Figure from Entenberg et al. (2017) (161).
Although many macrophage subtypes may be present in perivascular regions, only macrophages expressing high levels of the angiopoietin receptor TIE2 (designated as TIE2Hi macrophages), are capable of assembling functional TMEM structures (49). It is known that tumor cells intravasating via TMEM express MenaINV, which is required for transendothelial migration and express an invasion signature that is characteristic of cell migration during embryonic development (44, 45, 50, 51).
TMEM-associated endothelial cells have not been explored with regards to their gene and protein expression profiles and characteristics essential for TMEM function. However, the role of the perivascular TIE2Hi macrophages has been recently evaluated in this context (49). It was shown that TMEM function depends on the release of vascular endothelial growth factor (VEGF) from the TIE2Hi macrophage. Indeed, the conditional knockout of the VEGF gene specifically in macrophages blocks TMEM-dependent paracellular cancer cell intravasation without affecting TMEM assembly (49).
VEGF can cause intra-tumoral endothelial cell permeability (52) via three distinct mechanisms: (a) pinocytosis paired with transcytosis, (b) endothelial fenestration, and (c) tight-junction-regulated (also known as “paracellular”) permeability (53–56). Although the tumor neovasculature generated under the control of VEGF is almost always fenestrated, tumor cells cannot cross through the fenestrae of endothelia (56). Cancer cell transendothelial migration requires paracellular permeability which involves disruption of tight junctions by relatively high concentrations of VEGF (55–58). Only endothelium around TMEM sites can achieve high enough VEGF concentrations through the function of TIE2HiVEGFHI macrophages, for the disruption of endothelial junctions and “paracellular” cancer cell intravasation as observed at TMEM (49). The proangiogenic-perivascular TIE2+ macrophages, which comprise a functional constituent of TMEM, are derived from TIE2+ monocyte progenitors from the bone marrow (59–61). TIE2 macrophage recruitment in breast and pancreatic tumors and vascular permeability at TMEM and cancer cell dissemination in breast tumors can be blocked by TIE2 inhibitor rebastinib, offering promising treatment options targeting TMEM associated vascular permeability and cancer cell dissemination (49, 62).
Monocyte infiltration could be achieved by the expression of three chemotactic receptors, the colony stimulating factor receptor (CSF1R), the C-X-C chemokine receptor type-4 (CXCR4), and the C-C chemokine receptor type-2 (CCR2), on the TIE2+ monocyte surface (60, 61, 63). Tumors and tumor-associated stromal cells often upregulate and release systemically the respective ligands for the aforementioned chemotactic receptors, namely CSF1, CXCL12 (SDF-1) and CCL2, resulting in increased monocyte and myeloid cell chemotaxis (64–66) (Figure 1). In addition, the CSF1/CSF1R axis also promotes macrophage maturation and survival in the tumor microenvironment, thus increasing the overall yield through maturation and/or macrophage repolarization (67). Moreover, the expression of TIE2 can be upregulated in both endothelial cells and macrophages under the control of hypoxia-inducible factor-1a (HIF1A) (68). Therefore, TIE2+ macrophages may be generated over time from tumor-resident macrophages undergoing hypoxic stress (68). Furthermore, TIE2 signaling can suppress apoptosis and promote survival of TIE2+ macrophages, TIE2+ endothelial cells and even TIE2+ hematopoietic stem cells in the bone marrow niche (60, 69–73). Therefore, it is possible that the perivascular TIE2+ macrophages have more prolonged lifespan than classically-activated inflammatory macrophages, due to enhanced TIE2-mediated retention in the perivascular niche. In conclusion, in many solid carcinomas the aforementioned molecular pathways may contribute to an increasing population of TIE2+ macrophages, which are known to promote angiogenesis, TMEM assembly, and TMEM-dependent cancer cell dissemination (45, 59, 60, 68, 73).
An important microanatomical element for vascular permeability and intravasation during cancer progression is the pericyte coverage of the post-capillary venules (9). Pericytes mediate antiproliferative and stabilizing paracrine signaling to the adjacent endothelium, mainly through the secretion of angiopoietin-1 (ANG1), which promotes blood vessel quiescence and basement membrane synthesis (74). The cancer-associated endothelium, and especially the neoplastic neovasculature, frequently presents with low pericyte coverage (75) (Figure 1). Although pericyte-TMEM interactions have not been described in detail, it has been noted that functional TMEM sites are depleted of pericytes (49).
2.3. Biological programs in circulating tumor cells
Once tumor cells escape the primary site, they need to establish mechanisms of survival or resistance against immunological destruction, lack of adhesion to ECM substrates, as well as physical hazards, such the increased shear stress of the blood flow (Figure 1). These are accomplished through signaling pathways that convey immune evasion, resistance to anoikis, and possibly cluster formation mediated by circulating platelets (9, 76). Only a fraction of CTCs survive, extravasate and initiate metastatic growth (77, 78) which implies that not all CTCs have tumor-initiating capability (45). In addition, it appears that stem-like properties are required for successful metastatic colonization (79, 80). Since “stemness” is triggered in specific niches within the primary tumor mostly involving juxtacrine signaling from tumor-associated macrophages (TAMs) and other myeloid-derived cells (81–83), the composition of the primary tumor microenvironment may play a pivotal role in metastatic capabilities of the primary tumor. Thus, areas of tumor enriched for tumor cell-macrophage pairing during chemotaxis and haptotaxis towards blood vessels and TMEM sites (31, 33, 37, 38), may represent niches for cancer cell education required for successful metastatic colonization.
CTCs have been extensively analyzed using high-throughput approaches, such as RNA-sequencing at the single-cell level and it has been shown that they can carry mutational and even epigenetic information from the primary tumor. However, there are certain discrepancies arising from the comparison of these profiles, making the origin of CTCs more debatable. In particular, it is not certain whether CTCs exclusively originate from the primary tumor, or also disseminate from clinically undetectable secondary sites (45). Thus, we theorize that the microenvironment of the secondary sites may also contribute to programming of tumor cells and further cancer progression (Figure 2B).
2.4. Cancer-stroma interactions in the metastatic microenvironment
While CTCs could potentially access all tissues in the body, metastatic disease usually develops in selected tissues and organs, depending on the type of cancer. Recent evidence suggests that the organotropic properties of disseminating cancer cells may be dictated by the exosomes, small membrane vesicles ranging in size from 40 to 100 nm, secreted from primary tumors (84). The exosome function has been described as a major pathway contributing to the formation of a metastasis-receptive niche (85–87). In particular, primary tumors may secrete exosomes that exert specific tropism for particular secondary sites, based on the integrin profile of the tumor-derived exosomal cargo and that of the tissue-specific stromal cells (84).
Metastasizing tumor cells frequently home to tissues in which tumor-promoting stromal cells offer a supportive microenvironment, also known as the “premetastatic niche” (88) (Figure 1). Recent evidence suggests that myeloid-derived suppressor cells (MDSCs), which have been traditionally considered components of the immunosuppresive tumor microenvironment, can promote premetastatic niche formation, as well as increase tumor angiogenesis and invasion (89). Furthermore, it seems that neutrophils may contribute to successful formation of metastatic foci. In particular, neutrophils recruited to the premetastatic niche produce a set of leukotrienes which specifically expand cancer cell subpopulations with high tumorigenic potential, such as cancer stem cells (CSCs) (90). Tissue-resident stromal cells may also contribute to premetastatic niche formation. For instance, periostin secreted locally by recruited fibroblasts facilitates metastatic initiation and colonization of breast cancer cells in the lung (91). Therefore, a variety of bone marrow-derived cells (BMDC) and tissue-resident stromal cells may contribute to the formation of micrometastatic foci in the secondary site.
The last critical step in the metastatic cascade is the transition of micrometastatic foci into clinically overt metastases; a process that involves awakening of disseminated tumor cells (DTCs) from dormancy (92, 93) (Figure 1). Dormancy represents a specialized biological program exploited by metastasizing cancer cells to convey survival advantages in the secondary tumor site, until they become capable of further expansion and colonization. Recently proposed scenarios suggest that DTCs may activate stress signals in the secondary tumor microenvironment which may help them to resume growth. DTCs may also carry specific gene signatures triggered by the hypoxic niche of the primary tumor microenvironment and these signatures may be associated with tumor progression at the secondary sites (92–94). Thus, both tumor cell intrinsic factors and the tumor microenvironment (involving both tissue-resident stromal cells and recruited BMDCs) are involved in the formation of the premetastatic niche, as well as in the regulated entry/escape of DTCs from the dormancy program and parallel initiation of the colonization step.
3. Chemotherapy-induced systemic and tissue-specific prometastatic effects
The metastatic process is controlled by a delicate balance between promoting and suppressive factors in the tumor microenvironment. The dominance of the former over the latter increases the efficiency of metastasis. A body of evidence presented over the past several years indicates that chemotherapy treatment may tilt this balance in favor of cancer cell dissemination. In the pre-operative setting, chemotherapy may lead to tissue damage and subsequent activation of host-mediated tissue repair program, which involves plethora of cytokines and chemokines (5) that can affect the metastatic susceptibility of distant organs (6, 7). If chemotherapy is given pre-operatively, significant changes occur in the composition of the primary tumor microenvironment which may favor the metastasis-promoting rather than the metastasis–suppressing components of the tumor microenvironment. Since chemotherapy treatment may, under certain circumstances, shift the balance towards favoring metastases it is important to elucidate the exact contextual prerequisites for metastasis induction and identify risk factors which could potentiate those effects in certain patient subpopulations.
3.1. Chemotherapy may provide systemic support for metastasis through the induction of pro-inflammatory circuits
It has been shown that certain chemotherapeutic drugs, such as paclitaxel, may initiate prometastatic responses in the primary tumor microenvironment by directly activating specific inflammatory signaling pathways (95). Paclitaxel structurally resembles a pattern recognition receptor called toll-like receptor-4 (TLR4), which is mainly expressed on the surface of antigen-presenting cells and responds to lipopolysaccharide (LPS), a component of the bacterial membrane (96). Macrophages with activated TLR4 pathway, mediated by either LPS or paclitaxel, quickly migrate to a site of infection, or tissue repair induced by chemotherapy and/or radiotherapy (97) to either destroy the invaders or restore homeostasis in the affected tissue (96). However, overexpression of TLR4 has been observed on tumor cells as well. Thus, TLR4 positive cancer cells can be activated by paclitaxel which can exacerbate proinflammatory tumor microenvironment. These host-initiated proinflammatory responses are frequently accompanied by increased angiogenesis and cancer cell invasion which may promote metastatic dissemination (97–101).
However, it should be noted that most of the described proangiogenic and prometastatic effects of chemotherapy are not a direct result of specific signal transduction pathways, as in the case of paclitaxel-TLR4 axis, but a more generic response to cytotoxic tissue damage, hypoxic stress and a prolonged wound healing-like process (99, 102, 103). For instance, Chang et al. (2017) (104) have shown that paclitaxel and cyclophosphamide may induce cancer cell dissemination and metastatic colonization by recruiting myeloid progenitors in both the primary and the secondary sites in a stress-inducible Atf3-dependent manner. The transcription factor Atf3, a member of the ATF/CREB family of transcription factors activated upon stress, is a master regulator of a plethora of inflammatory cytokines involved in leukocyte migration and angiogenesis (105). In both spontaneous and experimental metastasis models, Atf3 was shown to be required for cancer cell seeding and the development of distant metastasis (104), indicating that proinflammatory circuits are necessary for chemotherapy-induced metastasis.
3.2. Chemotherapy mediates the mobilization of bone marrow progenitors to primary and secondary sites to promote metastasis
It has been proposed that metastasis is regulated through an incipient host repair mechanism initiated by the mobilization of bone marrow-derived cells (BMDCs), such as hemangiocytes, endothelial progenitor cells (EPCs), TIE2+ monocytes and myeloid-derived suppressor cells (MDSCs), all known to regulate angiogenesis and blood vessel homeostasis in damaged tissues (65, 106–108). Indeed, it has been shown that chemotherapy can trigger the mobilization of various BMDCs to primary tumor site, as well as to the lung (106, 109, 110). Chemotherapy may promote the formation of the premetastatic niche, by creating a stress response and amplifying the chemotactic signals and the proinflammatory circuits to which all these BMDCs may respond. Once recruited, BMDCs can then promote metastatic dissemination by producing and systemically releasing chemokines, bioactive lipids, alarmines and growth factors (102). Depending on the adaptive characteristics and the expression profiles of the respective receptors on the tumor cell surface, the dissemination and homing of tumor cells at secondary sites can be exacerbated (102). Moreover, chemotherapy may induce metastasis by mechanisms that do not involve myeloid and/or endothelial progenitors. For instance, Roodhart et al. (2011) demonstrated that platinum analogs, such as cisplatin, may stimulate mesenchymal stem cells (MSCs) to release polyunsaturated fatty acids, which may, in turn, systemically support tumor growth, resistance to chemotherapy and metastasis in mouse models of breast, lung and colon carcinomas (111, 112). The role of MSCs in tumor progression is debatable, and depending on the context may be either tumor-promoting or tumor-suppressive (113–116). However, it has been documented that MSCs are recruited to chemotherapy-damaged tissues, where they can also exert certain prometastatic effects (111, 112, 117, 118). Since MSC are principal mediators of chemotherapy-induced metastasis in virtually all steps of the metastatic cascade (Figure 1), and are released from the bone marrow in response to proinflammatory circuitries, we will refer to them as the BMDC/MSC infiltrate.
3.3. Chemotherapy may promote EMT and increased cancer cell invasiveness
The remainder of this chapter describes chemotherapy-induced pro-metastatic changes in the sequential steps of the metastatic cascade, as described in sections 2.1 to 2.4.
As mentioned before, the acquisition of mesenchymal phenotype through EMT is considered the initial step in the metastatic cascade (11, 12, 80). Although there are studies on chemotherapy-mediated EMT suppression and/or MET induction (119, 120), several reports have also linked chemotherapy treatment with an induction of EMT in the primary tumor microenvironment. For instance, continued treatment with paclitaxel or vincristine promoted EMT and contributed to the formation of lung metastasis in mice bearing hematopoietic malignancies (121). Likewise, in breast carcinoma, paclitaxel was shown to promote the expression of EMT markers in cancer cells, including the concerted decrease of E-cadherin, increase of vimentin and nuclear localization of β-catenin, as well as induced lung metastases through a miR-21/Cyclin-dependent kinase-5 (CDK5) pathway (122). Moreover, high-dose paclitaxel treatment, which could be achievable in the clinical setting, significantly increased the formation of invadopodia in breast cancer cells in vitro (123). Chemotherapy may also affect EMT in an indirect fashion. For example, it has been reported that miRNA, miR-488, inhibits EMT in breast cancer cells (124); however chemotherapy treatment frequently suppresses miRNA-488 in an NF-kB-dependent manner which relieves miR-488 EMT inhibition and thus indirectly stimulates EMT. In particular, cancer patients who received cyclophosphamide, epirubicin plus taxotere, or epirubicine plus 5-fluororacil had significantly suppressed levels of miR-488 (124), thus indicating potential chemotherapy-mediated EMT induction. Chemotherapy-induced EMT has also been reported in non-epithelial cancers, for instance, in cisplatin-treated osteosarcomas (125). However, it still remains unclear whether the relative increase of mesenchymal-like tumor cells observed upon chemotherapy is a result of direct chemotherapy mediated EMT induction or a consequence of selection of chemoresistant cancer cells (80, 126).
Chemotherapy can also increase the proportion of invasive cancer cells. It was noted that paclitaxel treatment promotes the expression of MENAINV in the PyMT mouse model of breast carcinoma, a metastatic patient-derived xenograft (PDX) model and post-chemotherapy breast cancer tissue samples from patients (26). Since MENAINV promotes invadopodium maturation (34), the increase in MENAINV expression upon chemotherapy may be mechanistically linked to the observation that chemotherapy induces invadopodia (123). As described earlier, MENAINV sensitizes cancer cells to RTK ligand-dependent chemotaxis and ITGA5B1/FN-dependent haptotaxis (33), enhancing the migratory behavior of tumor cells. In addition, MENAINV increases tumor cell transendothelial migration at TMEM (44, 51). Thus, chemotherapy-induced MENAINV expression may be responsible for recently reported observation of chemotherapy induced increase in CTCs (26, 104). Interestingly, mice lacking both functional copies of the MENA gene (i.e. MENA−/−) developed no CTCs and DTCs, even after receiving a metastasis-exacerbating dose of neoadjuvant chemotherapy, which indicates that MENA orchestrates a cell motility/invasion program in cancer cells, irrespective of chemotherapy treatment (26). Although it is not clear how chemotherapy causes an upregulation of MENAINV expression in primary breast tumors (26), recent evidence has shown that MENAINV can be upregulated in cancer cells as a result of Notch1-mediated juxtacrine signaling upon contact of cancer cells with macrophages (44). Thus, chemotherapy-induced BMDC/MSC recruitment may be mechanistically associated with the induction of EMT and/or invasive cancer cell phenotypes (i.e. MENAINV-HI) in the primary tumor microenvironment.
3.4. Chemotherapy may affect cancer cell intravasation and dissemination
As outlined in section 2.2, the highly-invasive MENAINV cancer cells are required but are not sufficient for cancer cell dissemination, unless they utilize functional intravasation sites, called TMEM (44, 51). Accumulating evidence now demonstrates that a wide variety of chemotherapy regimens promote the mobilization of BMDCs/MSCs to the primary tumor microenvironment to repair the cytotoxic tissue damage, which in turn facilitate tumor regrowth and TMEM formation (26, 59, 61, 110, 127). In particular, in the process of eliciting this chemotherapy-driven tissue repair response, new blood vessel formation (angiogenesis) frequently takes place, and encourages residual cancer cells that survived chemotherapy to resume growth (60, 66, 68, 73, 110, 127–132). Recent experimental work by Hughes et al (2014) suggested that cancer cell death and chemotherapy-induced hypoxia/necrosis could potentially promote the expression and systemic release of chemotactic factors, such as CXCL12, which in turn signals to CXCR4+ EPCs and monocyte progenitors, naturally residing in the bone marrow to home into primary tumors (132). Indeed, cyclophosphamide treatment resulted in an influx of perivascular CXCR4+TIE2+ macrophages, which accelerated neoangiogenesis and tumor regrowth (132).
In addition, at least two different chemotherapy regimens given in the neoadjuvant setting, either paclitaxel alone or the doxorubicin-cyclophosphamide combinatorial treatment, were both capable of promoting TIE2Hi macrophage infiltration and increasing TIE2+ macrophage-associated TMEM assembly in multiple immunocompetent or immunodeficient mouse models of breast cancer (26). Chemotherapy-induced TMEM assembly was subsequently corroborated independently by another research group (104). Moreover, TMEM score increased in post-neoadjuvant breast cancer tissue samples from patients with ER+/HER2− breast cancer, who were treated with weekly paclitaxel for up to 12 weeks followed by four cycles of doxorubicin plus cyclophosphamide (26). This observation may at least in part explain why long term survival of patients who do not achieve pathologic complete response (pCR) after neoadjuvant therapy is worse than in patients who do achieve pCR (133). The most concerning observation however was that in 10 out of 20 patients neoadjuvant chemotherapy increased TMEM score over the threshold that separates low-medium risk from high risk score for developing distant metastasis (26), as determined in a retrospective case-control study which demonstrated that TMEM is prognostic for metastasis in ER+/HER2− breast cancer (47). In conclusion, chemotherapy-mobilized TIE2+ macrophages may not only elicit proangiogenic but also prometastatic effects, since the TIE2+ macrophage subpopulation is a prerequisite for function of TMEM sites.
The studies discussed above (26, 104), also documented chemotherapy-induced increase in CTCs, a result of increased TMEM assembly and function in chemotherapy-treated animal tumors. Indeed, although CTC count measured by U.S. Food and Drug Administration-approved CellSearch System is a strong prognostic factor in both primary and metastatic breast cancer in humans, there is no conclusive evidence that chemotherapy significantly reduces CTCs (134). On the contrary, several reports have indicated that CTC counts in post-chemotherapy blood samples actually increase in some patients and decrease in others, yet they all correlate with distant metastasis-free survival (135, 136). Collectively, these observations demonstrate that neoadjuvant chemotherapy may induce prometastatic changes in the primary tumor microenvironment, which may promote TMEM assembly and TMEM-dependent cancer cell dissemination. These findings indicate that chemotherapy-treated tumors do not use de novo mechanisms of cancer cell dissemination, but rather amplify the already established ones through the recruitment of BMDCs/MSCs (Figure 1).
3.5. Chemotherapy may convey prometastatic properties on circulating tumor cells
The effects of chemotherapy treatment on CTCs have been rather underexplored. It has been demonstrated that chemotherapy-mediated tissue damage may also activate proteolytic cascades, including the complement cascade, the coagulation cascade and the fibrinolytic cascade, whose primary purpose is to initiate responses in damaged endothelia, but some of their activated proteolytic cleavage products are directly or indirectly involved in the ability of CTC to form metastases (102). For example, the upregulation of urokinase plasminogen activator receptor (uPAR) (137, 138) and thrombin (139, 140), have been both linked to increased metastatic capacity. Furthermore, it has been demonstrated that chemotherapy may activate blood platelets into releasing platelet-derived microvesicles in form of small membrane fragments containing platelet-endothelium cell adhesion receptors (Figure 1), such as CD41 and CD62P (102, 141–143). These platelet-derived membrane fragments can subsequently coat the surface of CTCs, facilitating their attachment to the endothelium at the site of future metastasis (144, 145). In addition, the coating of CTCs with platelets may shield tumor cells from violent shear forces (146), as well as promote the aggregation and formation of tumor cell emboli that can be more easily entrapped and retained in small vessels (147). Finally, tumor cells within platelet-mediated aggregates are significantly protected from immunological destruction, mainly through the release of platelet-derived transforming growth factor-beta1 (TGF-β1), which inactivates NK cells through downregulation of the NK cell receptor NKG2D (148) (Figure 1). This pathway could represent one of the multiple mechanisms of NK cell evasion by cancer cells and metastatic subversion of NK cell surveillance (149).
3.6. Chemotherapy may facilitate cancer cell seeding and colonization at distant sites
Chemotherapy may inflict hypoxic damage in tissues other than the primary tumor site, thus causing the release of chemotactic factors by tissue-resident leukocytes, fibroblasts and endothelial cells, and these chemotactic factors in turn, attract various BMDCs/MSCs (150). The recruitment of BMDCs/MSCs to the secondary sites (either triggered by chemotherapy or not) initiates the formation of the premetastatic niche (88). Once homed in the premetastatic niche (Figure 1), BMDCs/MSCs may then regulate the development and progression of metastasis through paracrine interactions with the newly arrived metastasizing tumor cells. For example, Daenen et al. (2011) showed that mice treated with paclitaxel or cisplatin had significantly increased tumor cell retention in the lung vasculature with consequent metastatic colonization. This phenotype was explained by chemotherapy-induced expression of the vascular endothelial growth factor receptor 1 (VEGFR1) by the endothelial cells which enhanced endothelial-tumor cell adhesion and paracrine interactions (6). This result was obtained with different tumor types, including breast and colon carcinoma as well as melanoma cells, suggesting that creation of the prematastatic niche is a more generalized, rather than a tumor cell-dependent effect of chemotherapy (6).
Furthermore, certain chemotherapies were shown to either increase the production and release of exosomes or to alter the composition of tumor-specific exosomes, also described as chemotherapy-induced exosomes or “chemoexosomes” (151). However, the evidence of a direct effect of chemoexosomes on premetastatic niche formation is currently lacking, although these observations certainly warrant further investigations.
The final step of the metastatic cascade involves the survival of disseminated cells and micrometastatic foci in the microenvironment of the secondary site, and, following the exit from a dormancy program, the subsequent cancer cell proliferation at the secondary sites (8, 93, 152). Chemotherapy-facilitated colonization has been described in certain cancer models, following the initial interactions of tumor cells within the premetastatic niche. A critical mediator of this step was shown to be matrix metalloproteinase-9 (MMP9), which was significantly overexpressed in VEGFR1+ EPCs or in other BMDCs following chemotherapy (109, 153). Indeed, the local release of MMP9 in the metastatic niche eventually supported metastatic colonization of CTCs in an experimental metastasis mouse model, and was reversed by specific inhibition of MMP9 (109). In these studies, chemotherapy-induced MMP9 overexpression had a distinct effect on cancer cell extravasation and the formation of micrometastatic foci, which increased the overall rate of macrometastasis formation (109, 153). A different study demonstrated that inflammatory monocytes (iMs) could be recruited to the tumor microenvironment at the secondary sites through a CCL2/CCR2 chemotaxis pathway following chemotherapy (104). Recruitment of these iM promoted the local suppression of cytotoxic CD8+ T-lymphocytes in the lung, thus facilitating metastatic colonization in mouse model of either spontaneous or experimental metastasis (104). These observations collectively suggest that chemotherapy induces recruitment of BMDCs/MSCs to the premetastatic niche, which in turn, facilitate tumor cell seeding and subsequent colonization of the secondary site (Figure 1).
4. Therapeutic reversal of the chemotherapy-induced prometastatic effects
Chemotherapy increases survival in patients with a variety of localized and advanced cancers (133, 154). However, there are many patients who do not draw full benefit from chemotherapy, and according to recent findings (as described in section 3), chemotherapy may induce more aggressive disease in some patients. Therefore, new treatment modalities for preventing chemotherapy-induced metastasis as well as new markers that can predict which patients will likely develop more advanced disease due to chemotherapy are needed. It should be noted that certain biological programs, such as chemotherapy-induced MENAINV expression as described in section 3.3, are particularly attractive candidates to eliminate chemotherapy-driven metastasis. However, given the current challenges of intracellular drug delivery in vivo (155), in this section, we focus on basic principles and rationale for designing approaches based on extracellular targets.
4.1. Burning off the “catalyst” of the metastatic cascade
Various cells within the tumor microenvironment, such as leukocytes, macrophages, endothelial cells, fibroblasts as well as tumor cells release chemokines and create the so called tumor “chemokine network” (150). Chemotherapy induces cytotoxic tissue damage and hypoxia and subsequent recruitment of myeloid and/or mesenchymal cells from the bone marrow. These bone marrow-derived BMDC/MSC infiltrates modify the chemokine network of the primary tumor and shift the balance towards the prometastatic phenotype. Thus, the BMDCs/MSCs act as “catalysts” in the progression of the metastatic cascade (Figure 1). In view of this working hypothesis, various pharmacological interventions in the chemokine network could theoretically prevent the accumulation of BMDC/MSC infiltrates in the primary and secondary tumor microenvironments, thus eliminating the prometastatic effects of chemotherapy.
As already explained in section 2, chemotherapy may support the infiltration, maturation and increased retention of metastasis-promoting BMDCs/MSCs in a context-dependent manner, mainly through the induction of the CXCL12/CXCR4, CCL2/CCR2 and CSF1/CSF1R chemotactic pathways (61, 132). Therefore, pharmacological inhibition of CXCR4 paired with chemotherapy can significantly suppress primary tumor growth (156), chemotherapy-induced angiogenesis (132), and metastatic burden of chemoresistant tumors (157), as shown in preclinical models of ovarian, breast and small cell lung cancer. Therefore, the pharmacological inhibition of the chemokine receptor CXCR4 in conjunction with chemotherapy could potentially counteract the chemotherapy-exacerbated CXCR4-mediated prometastatic effects. Similarly, the selective antagonists of the chemokine receptor CCR2 (or small molecule inhibitors of CCL2) have been used quite efficiently in this context, since they are capable of disrupting M2-like macrophage recruitment, macrophage-mediated immunosuppression and metastatic efficiency in preclinical models of prostate, liver and pancreatic cancers (64, 158, 159). Finally, the specific blockade of the CSF1/CSF1R pathway can also efficiently reprogram the immunosuppressive responses of myeloid cells in the primary tumor microenvironment (67), thus reducing tumor growth and metastasis (160).
The few examples discussed above provide a proof-of-principle that the therapeutic modulation of the chemokine network in many types of solid carcinomas could pose an effective strategy for preventing chemotherapy-induced metastasis. However, the chemotactic pathways leading to recruitment of BMDCs/MSCs in the tumor microenvironment are promiscuous. In other words, there are many different types of BMDCs/MSCs that respond to a variety of chemotactic stimuli (9, 150), and therefore, the selective targeting of one such pathway may promote the selection of alternative pathways achieving similar metastatic potential through different BMDC/MSC “catalysts”. The chaotic nature of the chemokine network along with our limited knowledge behind the overall chemokine repertoire of individual human cancer types (150) make this therapeutic approach quite challenging, but worth-pursuing.
4.2. Sealing the “doorways” to cancer cells
Another approach to eliminate the chemotherapy-induced prometastatic effects would be blocking cancer cell intravasation and extravasation, the two “vulnerable” steps of the metastatic cascade with regards to the efficiency of the metastatic process (Figure 1). Indeed, since tumor cells disseminate via an intravasation mechanism involving TMEM (49) and TMEM are present in both primary tumors and secondary metastatic sites (161), the pharmacological inhibition of the TMEM doorways to seal them to tumor cell intravasation would eliminate CTCs, irrespective of the chemotherapeutic that induced prometastatic changes. In addition, since TMEM are present in both the primary tumor and its metastatic sites, TMEM inhibition would be beneficial in all stages of treatment.
The crucial BMDC involved in TMEM-dependent cancer cell dissemination is the perivascular TIE2+ macrophage, which in fact, is a proangiogenic M2-like macrophage (49, 132). TIE+ macrophages are tethered in the perivascular niche of primary tumors, because they respond to angiopoietin signals or other non-canonical ligands, such as integrins and lysyl oxidase, originating from the cancer-associated endothelium (162–166). Recently, the TIE2 kinase switch pocket inhibitor rebastinib, was shown to inhibit TIE2, and subsequently reduced tumor growth, angiogenesis and metastasis in orthotopic mouse models of metastatic mammary carcinoma and pancreatic neuroendocrine tumors (62). In particular, rebastinib inhibited TMEM function by inhibiting the angiopoietin receptor TIE2 on the TMEM macrophage and prevented VEGF-dependent vascular permeability (62). Furthermore, rebastinib significantly reduced the number of TMEM-dependent CTCs in the blood and the number of DTCs in the lungs (26), and significantly increased the overall survival of paclitaxel-treated mice even after resection of the primary tumor (62). These observations indicate that TIE2 inhibition inhibits the chemotherapy-induced prometastatic tumor microenvironment associated with TMEM (26).
Another therapeutically “vulnerable” step of the metastatic cascade is tumor cell extravasation, as it is also required for effective colonization. Previous studies have documented that tumor cell adherence and retention in the intraluminal side of blood vessels in the metastatic organs may persist for a varying period of time, which dictates tumor cell survival probability and their clearance by cytotoxic immune cells, such as NK cells (167). Therefore, the interactions of CTCs with the premetastatic niche are of utmost importance for successful seeding, and the therapeutic intervention of those interactions may pose another attractive strategy for counteracting chemotherapy-induced metastasis. For instance, Daenen et al. (2011) demonstrated that chemotherapy-recruited VEGFR1+ EPCs in the lung endothelium can significantly promote the early retention and survival of tumor cells, eventually facilitating the formation of metastasis, as already described in section 3.6. Indeed, the targeted inhibition of VEGFR1 with neutralizing antibodies, but not that of other VEGF receptors such as VEGFR2, completely eliminated the chemotherapy-mediated tumor cell retention and subsequent lung colonization (6). These observations suggest that the disruption of critical tumor-host cell interactions during cancer cell extravasation may profoundly affect the fate of metastasis in the presence of chemotherapy.
5. Conclusions and future directions
Our understanding of cancer progression has been rapidly evolving and it has moved in somewhat unexpected directions in the past several years. We have been reminded that tissues and organ systems in complex metazoan organisms operate in harmony, and that any insult, even if introduced with the intention to cure, may have complex consequences that we only now have started to unravel. As a large body of preclinical evidence indicates, cytotoxic chemotherapy in the process of destroying tumor cells activates host reparatory mechanisms that may sabotage our intentions to cure cancer. We now need to move our focus from studying cancer cells in isolation to studying cancer cells not only in the context of their immediate tumor microenvironment, but also in the context of the whole organism. As our understanding of the effect of chemotherapy on complex host repair mechanisms feedback grows, so will our efforts to develop novel therapeutic combinations. We are already witnessing the use of combined cytotoxic chemotherapy with the TMEM inhibitor rebastinib in clinical trials. In the years to come, we expect to see many more clinical trials focused on combining cytotoxic therapies with therapies targeting chemotherapy-induced pro-metastatic changes. Thus, we expect that in the future our efforts will refocus from treating cancer towards treating the cancer patient as a harmonious system.
Acknowledgments
Funding
This article is supported by grants from the NIH (CA100324 and CA150344), the SIG 1S10OD019961-01, the Gruss-Lipper Biophotonics center and its associated Integrative Imaging Program at the Albert Einstein College of Medicine.
Footnotes
Author Contributions
GSK, JSC and MHO conceived the idea and wrote the manuscript.
Competing Interests
MHO and JSC are inventors on a patent application (#96700/2505) submitted by the Albert Einstein College of Medicine that covers methods detecting and reducing chemotherapy-induced prometastatic changes in breast tumors. GSK declares no competing interests.
References
- 1.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Sanger N, Effenberger KE, Riethdorf S, Van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M, Pantel K. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011;129(10):2522–6. doi: 10.1002/ijc.25895. [DOI] [PubMed] [Google Scholar]
- 3.Martin OA, Anderson RL, Narayan K, MacManus MP. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nature reviews Clinical oncology. 2017;14(1):32–44. doi: 10.1038/nrclinonc.2016.128. [DOI] [PubMed] [Google Scholar]
- 4.Ibragimova MK, Tsyganov MM, Litviakov NV. Natural and Chemotherapy-Induced Clonal Evolution of Tumors. Biochemistry Biokhimiia. 2017;82(4):413–25. doi: 10.1134/S0006297917040022. [DOI] [PubMed] [Google Scholar]
- 5.Daenen LG, Houthuijzen JM, Cirkel GA, Roodhart JM, Shaked Y, Voest EE. Treatment-induced host-mediated mechanisms reducing the efficacy of antitumor therapies. Oncogene. 2014;33(11):1341–7. doi: 10.1038/onc.2013.94. [DOI] [PubMed] [Google Scholar]
- 6.Daenen LG, Roodhart JM, van Amersfoort M, Dehnad M, Roessingh W, Ulfman LH, Derksen PW, Voest EE. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011;71(22):6976–85. doi: 10.1158/0008-5472.CAN-11-0627. [DOI] [PubMed] [Google Scholar]
- 7.Wu YJ, Muldoon LL, Dickey DT, Lewin SJ, Varallyay CG, Neuwelt EA. Cyclophosphamide enhances human tumor growth in nude rat xenografted tumor models. Neoplasia. 2009;11(2):187–95. doi: 10.1593/neo.81352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. The Journal of clinical investigation. 2009;119(6):1417–9. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25(11):675–86. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer metastasis reviews. 2016;35(4):645–54. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer metastasis reviews. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 16.Cavallaro U, Schaffhauser B, Christofori G. Cadherins and the tumour progression: is it all in a switch? Cancer letters. 2002;176(2):123–8. doi: 10.1016/s0304-3835(01)00759-5. [DOI] [PubMed] [Google Scholar]
- 17.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nature reviews Cancer. 2004;4(2):118–32. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 18.De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130(3):481–94. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer cell. 2006;9(4):261–72. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–6. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS genetics. 2011;7(8):e1002218. doi: 10.1371/journal.pgen.1002218. Epub 2011/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, Robinson BD, Jones JG, Gertler FB, Condeelis JS, Oktay MH. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis. 2011;28(6):515–27. doi: 10.1007/s10585-011-9388-6. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Modugno F, Iapicca P, Boudreau A, Mottolese M, Terrenato I, Perracchio L, Carstens RP, Santoni A, Bissell MJ, Nistico P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc Natl Acad Sci U S A. 2012;109(47):19280–5. doi: 10.1073/pnas.1214394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oudin MJ, Hughes SK, Rohani N, Moufarrej MN, Jones JG, Condeelis JS, Lauffenburger DA, Gertler FB. Characterization of the expression of the pro-metastatic Mena(INV) isoform during breast tumor progression. Clin Exp Metastasis. 2016;33(3):249–61. doi: 10.1007/s10585-015-9775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balsamo M, Mondal C, Carmona G, McClain LM, Riquelme DN, Tadros J, Ma D, Vasile E, Condeelis JS, Lauffenburger DA, Gertler FB. The alternatively-included 11a sequence modifies the effects of Mena on actin cytoskeletal organization and cell behavior. Sci Rep. 2016;6:35298. doi: 10.1038/srep35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D’Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, Oktay MH. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Science translational medicine. 2017;9(397) doi: 10.1126/scitranslmed.aan0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forse CL, Agarwal S, Pinnaduwage D, Gertler F, Condeelis JS, Lin J, Xue X, Johung K, Mulligan AM, Rohan TE, Bull SB, Andrulis IL. Menacalc, a quantitative method of metastasis assessment, as a prognostic marker for axillary node-negative breast cancer. BMC Cancer. 2015;15:483. doi: 10.1186/s12885-015-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal S, Gertler FB, Balsamo M, Condeelis JS, Camp RL, Xue X, Lin J, Rohan TE, Rimm DL. Quantitative assessment of invasive mena isoforms (Menacalc) as an independent prognostic marker in breast cancer. Breast cancer research: BCR. 2012;14(5):R124. doi: 10.1186/bcr3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15(6):813–28. doi: 10.1016/j.devcel.2008.09.003. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes SK, Oudin MJ, Tadros J, Neil J, Del Rosario A, Joughin BA, Ritsma L, Wyckoff J, Vasile E, Eddy R, Philippar U, Lussiez A, Condeelis JS, van Rheenen J, White F, Lauffenburger DA, Gertler FB. PTP1B-dependent regulation of receptor tyrosine kinase signaling by the actin-binding protein Mena. Mol Biol Cell. 2015;26(21):3867–78. doi: 10.1091/mbc.E15-06-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung E, Xue A, Wang Y, Rougerie P, Sharma VP, Eddy R, Cox D, Condeelis J. Blood vessel endothelium-directed tumor cell streaming in breast tumors requires the HGF/C-Met signaling pathway. Oncogene. 2017;36(19):2680–92. doi: 10.1038/onc.2016.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Entenberg D, Wyckoff J, Gligorijevic B, Roussos ET, Verkhusha VV, Pollard JW, Condeelis J. Setup and use of a two-laser multiphoton microscope for multichannel intravital fluorescence imaging. Nat Protoc. 2011;6(10):1500–20. doi: 10.1038/nprot.2011.376. Epub 2011/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oudin MJ, Jonas O, Kosciuk T, Broye LC, Guido BC, Wyckoff J, Riquelme D, Lamar JM, Asokan SB, Whittaker C, Ma D, Langer R, Cima MJ, Wisinski KB, Hynes RO, Lauffenburger DA, Keely PJ, Bear JE, Gertler FB. Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression. Cancer discovery. 2016;6(5):516–31. doi: 10.1158/2159-8290.CD-15-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weidmann MD, Surve CR, Eddy RJ, Chen X, Gertler FB, Sharma VP, Condeelis JS. MenaINV dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci Rep. 2016;6:36142. doi: 10.1038/srep36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci. 2012;125(Pt 3):724–34. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eddy RJ, Weidmann MD, Sharma VP, Condeelis JS. Tumor Cell Invadopodia: Invasive Protrusions that Orchestrate Metastasis. Trends Cell Biol. 2017;27(8):595–607. doi: 10.1016/j.tcb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, Condeelis JS. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital. 2013;2(2):e25294. doi: 10.4161/intv.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roussos ET, Balsamo M, Alford SK, Wyckoff JB, Gligorijevic B, Wang Y, Pozzuto M, Stobezki R, Goswami S, Segall JE, Lauffenburger DA, Bresnick AR, Gertler FB, Condeelis JS. Mena invasive (MenaINV) promotes multicellular streaming motility and transendothelial migration in a mouse model of breast cancer. J Cell Sci. 2011;124(Pt 13):2120–31. doi: 10.1242/jcs.086231. Epub 2011/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma VP, Beaty BT, Patsialou A, Liu H, Clarke M, Cox D, Condeelis JS, Eddy RJ. Reconstitution of in vivo macrophage-tumor cell pairing and streaming motility on one-dimensional micro-patterned substrates. Intravital. 2012;1(1):77–85. doi: 10.4161/intv.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harney AS, Wang Y, Condeelis JS, Entenberg D. Extended Time-lapse Intravital Imaging of Real-time Multicellular Dynamics in the Tumor Microenvironment. Journal of visualized experiments: JoVE. 2016;(112):e54042. doi: 10.3791/54042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24(2):277–83. doi: 10.1016/j.ceb.2011.12.004. Epub 2012/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nature reviews Cancer. 2011;11(8):573–87. doi: 10.1038/nrc3078. Epub 2011/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69(24):9498–506. doi: 10.1158/0008-5472.CAN-09-1868. Epub 2009/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pignatelli J, Bravo-Cordero JJ, Roh-Johnson M, Gandhi SJ, Wang Y, Chen X, Eddy RJ, Xue A, Singer RH, Hodgson L, Oktay MH, Condeelis JS. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci Rep. 2016;6:37874. doi: 10.1038/srep37874. consultant/advisory board member for Deciphera and MetaStat. No potential conflicts of interest were disclosed by the other authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karagiannis GS, Goswami S, Jones JG, Oktay MH, Condeelis JS. Signatures of breast cancer metastasis at a glance. J Cell Sci. 2016;129(9):1751–8. doi: 10.1242/jcs.183129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, Jones JG. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(7):2433–41. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohan TE, Xue X, Lin HM, D’Alfonso TM, Ginter PS, Oktay MH, Robinson BD, Ginsberg M, Gertler FB, Glass AG, Sparano JA, Condeelis JS, Jones JG. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. Journal of the National Cancer Institute. 2014;106(8) doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sparano JA, Gray R, Oktay MH, Entenberg D, Rohan T, Xue X, Donovan M, Peterson M, AS, Hamilton DA, D’Alfonso T, Goldstein LJ, Gertler F, Davidson NE, Condeelis J, Jones J. A Novel Metastasis Biomarker (MetaSite Breast™ Score) is Associated with Distant Recurrence in Hormone Receptor-Positive. HER2-Negative Early Stage Breast Cancer NPJ Breast Cancer. 2017 doi: 10.1038/s41523-017-0043-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, Oktay MH, Pollard JW, Jones JG, Condeelis JS. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer discovery. 2015;5(9):932–43. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patsialou A, Wang Y, Lin J, Whitney K, Goswami S, Kenny PA, Condeelis JS. Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast cancer research: BCR. 2012;14(5):R139. doi: 10.1186/bcr3344. Epub 2012/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, Adler E, Cox D, Maleki S, Bresnick A, Gertler FB, Condeelis JS, Oktay MH. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Science signaling. 2014;7(353):ra112. doi: 10.1126/scisignal.2005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 53.Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6(2):83–96. [PubMed] [Google Scholar]
- 54.Lee YC. The involvement of VEGF in endothelial permeability: a target for anti-inflammatory therapy. Current opinion in investigational drugs. 2005;6(11):1124–30. [PubMed] [Google Scholar]
- 55.Stan RV, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae) Mol Biol Cell. 1997;8(4):595–605. doi: 10.1091/mbc.8.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(Pt 6):2369–79. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 57.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57(4):765–72. [PubMed] [Google Scholar]
- 58.Roberts WG, Delaat J, Nagane M, Huang S, Cavenee WK, Palade GE. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. The American journal of pathology. 1998;153(4):1239–48. doi: 10.1016/S0002-9440(10)65668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadioglu E, De Palma M. Cancer Metastasis: Perivascular Macrophages Under Watch. Cancer discovery. 2015;5(9):906–8. doi: 10.1158/2159-8290.CD-15-0819. [DOI] [PubMed] [Google Scholar]
- 60.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends in immunology. 2007;28(12):519–24. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Lewis CE, Harney AS, Pollard JW. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer cell. 2016;30(1):18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harney AS, Karagiannis GS, Pignatelli J, Smith BD, Kadioglu E, Wise SC, Hood MM, Kaufman MD, Leary CB, Lu WP, Al-Ani G, Chen X, Entenberg D, Oktay MH, Wang Y, Chun L, De Palma M, Jones JG, Flynn DL, Condeelis JS. The Selective Tie2 Inhibitor Rebastinib Blocks Recruitment and Function of Tie2(Hi) Macrophages in Breast Cancer and Pancreatic Neuroendocrine Tumors. Mol Cancer Ther. 2017;16(11):2486–501. doi: 10.1158/1535-7163.MCT-17-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawakami Y, Ii M, Matsumoto T, Kuroda R, Kuroda T, Kwon SM, Kawamoto A, Akimaru H, Mifune Y, Shoji T, Fukui T, Kurosaka M, Asahara T. SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2015;30(1):95–105. doi: 10.1002/jbmr.2318. [DOI] [PubMed] [Google Scholar]
- 64.Kalbasi A, Komar C, Tooker GM, Liu M, Lee JW, Gladney WL, Ben-Josef E, Beatty GL. Tumor-Derived CCL2 Mediates Resistance to Radiotherapy in Pancreatic Ductal Adenocarcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23(1):137–48. doi: 10.1158/1078-0432.CCR-16-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12(5):557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Di Serio C, Naldini L, De Palma M, Tozer GM, Lewis CE. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. The Journal of clinical investigation. 2011;121(5):1969–73. doi: 10.1172/JCI44562. Epub 2011/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74(18):5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67(18):8429–32. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 69.Saharinen P, Alitalo K. The yin, the yang, and the angiopoietin-1. The Journal of clinical investigation. 2011;121(6):2157–9. doi: 10.1172/JCI58196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami M. Signaling required for blood vessel maintenance: molecular basis and pathological manifestations. International journal of vascular medicine. 2012;2012:293641. doi: 10.1155/2012/293641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore KA, Lemischka IR. “Tie-ing” down the hematopoietic niche. Cell. 2004;118(2):139–40. doi: 10.1016/j.cell.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 72.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Ferrara N. Role of myeloid cells in vascular endothelial growth factor-independent tumor angiogenesis. Curr Opin Hematol. 2010;17(3):219–24. doi: 10.1097/MOH.0b013e3283386660. [DOI] [PubMed] [Google Scholar]
- 74.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 75.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature cell biology. 2005;7(9):870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Molecular oncology. 2017;11(1):40–61. doi: 10.1002/1878-0261.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koop S, MacDonald IC, Luzzi K, Schmidt EE, Morris VL, Grattan M, Khokha R, Chambers AF, Groom AC. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995;55(12):2520–3. [PubMed] [Google Scholar]
- 78.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. Journal of the National Cancer Institute. 1970;45(4):773–82. [PubMed] [Google Scholar]
- 79.Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256–60. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nature reviews Clinical oncology. 2017;14(10):611–29. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu H, Clauser KR, Tam WL, Frose J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA, Weinberg RA. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature cell biology. 2014;16(11):1105–17. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sainz B, Jr, Carron E, Vallespinos M, Machado HL. Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Mediators of inflammation. 2016;2016:9012369. doi: 10.1155/2016/9012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sica A, Porta C, Amadori A, Pasto A. Tumor-associated myeloid cells as guiding forces of cancer cell stemness. Cancer immunology, immunotherapy: CII. 2017;66(8):1025–36. doi: 10.1007/s00262-017-1997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinbichler TB, Dudas J, Riechelmann H, Skvortsova II. The role of exosomes in cancer metastasis. Seminars in cancer biology. 2017;44:170–81. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 86.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17(6):816–26. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer cell. 2016;30(6):836–48. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66(23):11089–93. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis. Journal of cellular physiology. 2017 doi: 10.1002/jcp.26075. [DOI] [PubMed] [Google Scholar]
- 90.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–7. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2011;481(7379):85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 92.Bragado P, Sosa MS, Keely P, Condeelis J, Aguirre-Ghiso JA. Microenvironments dictating tumor cell dormancy. Recent Results Cancer Res. 2012;195:25–39. doi: 10.1007/978-3-642-28160-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews Cancer. 2014;14(9):611–22. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fluegen G, Avivar-Valderas A, Wang Y, Padgen MR, Williams JK, Nobre AR, Calvo V, Cheung JF, Bravo-Cordero JJ, Entenberg D, Castracane J, Verkhusha V, Keely PJ, Condeelis J, Aguirre-Ghiso JA. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nature cell biology. 2017;19(2):120–32. doi: 10.1038/ncb3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. European journal of immunology. 2001;31(8):2448–57. doi: 10.1002/1521-4141(200108)31:8<2448::AID-IMMU2448>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 96.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5(10):987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 97.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. The EMBO journal. 2010;29(13):2242–52. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volk-Draper L, Hall K, Griggs C, Rajput S, Kohio P, DeNardo D, Ran S. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res. 2014;74(19):5421–34. doi: 10.1158/0008-5472.CAN-14-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ran S. The Role of TLR4 in Chemotherapy-Driven Metastasis. Cancer Res. 2015;75(12):2405–10. doi: 10.1158/0008-5472.CAN-14-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rajput S, Volk-Draper LD, Ran S. TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol Cancer Ther. 2013;12(8):1676–87. doi: 10.1158/1535-7163.MCT-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Volk-Draper LD, Hall KL, Wilber AC, Ran S. Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4. PLoS One. 2017;12(6):e0179257. doi: 10.1371/journal.pone.0179257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ratajczak MZ, Jadczyk T, Schneider G, Kakar SS, Kucia M. Induction of a tumor-metastasis-receptive microenvironment as an unwanted and underestimated side effect of treatment by chemotherapy or radiotherapy. Journal of ovarian research. 2013;6(1):95. doi: 10.1186/1757-2215-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang J, Erler J. Hypoxia-mediated metastasis. Advances in experimental medicine and biology. 2014;772:55–81. doi: 10.1007/978-1-4614-5915-6_3. [DOI] [PubMed] [Google Scholar]
- 104.Chang YS, Jalgaonkar SP, Middleton JD, Hai T. Stress-inducible gene Atf3 in the noncancer host cells contributes to chemotherapy-exacerbated breast cancer metastasis. Proc Natl Acad Sci U S A. 2017;114(34):E7159–E68. doi: 10.1073/pnas.1700455114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273(1):1–11. doi: 10.1016/s0378-1119(01)00551-0. [DOI] [PubMed] [Google Scholar]
- 106.Shaked Y, Voest EE. Bone marrow derived cells in tumor angiogenesis and growth: are they the good, the bad or the evil? Biochimica et biophysica acta. 2009;1796(1):1–4. doi: 10.1016/j.bbcan.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 107.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell. 2005;8(3):211–26. doi: 10.1016/j.ccr.2005.08.002. Epub 2005/09/20. [DOI] [PubMed] [Google Scholar]
- 108.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature biotechnology. 2007;25(8):911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 109.Gingis-Velitski S, Loven D, Benayoun L, Munster M, Bril R, Voloshin T, Alishekevitz D, Bertolini F, Shaked Y. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011;71(22):6986–96. doi: 10.1158/0008-5472.CAN-11-0629. [DOI] [PubMed] [Google Scholar]
- 110.Shaked Y, Henke E, Roodhart JM, Mancuso P, Langenberg MH, Colleoni M, Daenen LG, Man S, Xu P, Emmenegger U, Tang T, Zhu Z, Witte L, Strieter RM, Bertolini F, Voest EE, Benezra R, Kerbel RS. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer cell. 2008;14(3):263–73. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, Gerritsen MG, Schipper HS, Backer MJ, van Amersfoort M, Vermaat JS, Moerer P, Ishihara K, Kalkhoven E, Beijnen JH, Derksen PW, Medema RH, Martens AC, Brenkman AB, Voest EE. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer cell. 2011;20(3):370–83. doi: 10.1016/j.ccr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 112.Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. British journal of cancer. 2012;106(12):1901–6. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stagg J. Mesenchymal stem cells in cancer. Stem cell reviews. 2008;4(2):119–24. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
- 114.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Molecular cancer. 2017;16(1):31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang W. Mesenchymal stem cells in cancer: friends or foes. Cancer biology & therapy. 2008;7(2):252–4. doi: 10.4161/cbt.7.2.5580. [DOI] [PubMed] [Google Scholar]
- 116.Wong RS. Mesenchymal stem cells: angels or demons? Journal of biomedicine & biotechnology. 2011;2011:459510. doi: 10.1155/2011/459510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Skolekova S, Matuskova M, Bohac M, Toro L, Durinikova E, Tyciakova S, Demkova L, Gursky J, Kucerova L. Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell communication and signaling: CCS. 2016;14:4. doi: 10.1186/s12964-016-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nicolay NH, Lopez Perez R, Ruhle A, Trinh T, Sisombath S, Weber KJ, Ho AD, Debus J, Saffrich R, Huber PE. Mesenchymal stem cells maintain their defining stem cell characteristics after treatment with cisplatin. Sci Rep. 2016;6:20035. doi: 10.1038/srep20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hirose A, Tajima H, Ohta T, Tsukada T, Okamoto K, Nakanuma S, Sakai S, Kinoshita J, Makino I, Furukawa H, Hayashi H, Nakamura K, Oyama K, Inokuchi M, Nakagawara H, Miyashita T, Takamura H, Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Harada S. Low-dose paclitaxel inhibits the induction of epidermal-mesenchymal transition in the human cholangiocarcinoma CCKS-1 cell line. Oncology letters. 2013;6(4):915–20. doi: 10.3892/ol.2013.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park SY, Kim MJ, Park SA, Kim JS, Min KN, Kim DK, Lim W, Nam JS, Sheen YY. Combinatorial TGF-beta attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget. 2015;6(35):37526–43. doi: 10.18632/oncotarget.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang JM, Yang GY, Medina DJ, Vassil AD, Liao J, Hait WN. Treatment of multidrug resistant (MDR1) murine leukemia with P-glycoprotein substrates accelerates the course of the disease. Biochemical and biophysical research communications. 1999;266(1):167–73. doi: 10.1006/bbrc.1999.1757. [DOI] [PubMed] [Google Scholar]
- 122.Ren Y, Zhou X, Yang JJ, Liu X, Zhao XH, Wang QX, Han L, Song X, Zhu ZY, Tian WP, Zhang L, Mei M, Kang CS. AC1MMYR2 impairs high dose paclitaxel-induced tumor metastasis by targeting miR-21/CDK5 axis. Cancer letters. 2015;362(2):174–82. doi: 10.1016/j.canlet.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 123.Quintavalle M, Elia L, Price JH, Heynen-Genel S, Courtneidge SA. A cell-based high-content screening assay reveals activators and inhibitors of cancer cell invasion. Science signaling. 2011;4(183):ra49. doi: 10.1126/scisignal.2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, Wang WJ, Chen Q, Tang F, Liu XP, Xu ZD. Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell death and differentiation. 2011;18(1):16–25. doi: 10.1038/cdd.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fang S, Yu L, Mei H, Yang J, Gao T, Cheng A, Guo W, Xia K, Liu G. Cisplatin promotes mesenchymal-like characteristics in osteosarcoma through Snail. Oncology letters. 2016;12(6):5007–14. doi: 10.3892/ol.2016.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tajima H, Makino I, Ohbatake Y, Nakanuma S, Hayashi H, Nakagawara H, Miyashita T, Takamura H, Ohta T. Neoadjuvant chemotherapy for pancreatic cancer: Effects on cancer tissue and novel perspectives. Oncology letters. 2017;13(6):3975–81. doi: 10.3892/ol.2017.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roodhart JM, Langenberg MH, Daenen LG, Voest EE. Translating preclinical findings of (endothelial) progenitor cell mobilization into the clinic; from bedside to bench and back. Biochimica et biophysica acta. 2009;1796(1):41–9. doi: 10.1016/j.bbcan.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 128.Roodhart JM, He H, Daenen LG, Monvoisin A, Barber CL, van Amersfoort M, Hofmann JJ, Radtke F, Lane TF, Voest EE, Iruela-Arispe ML. Notch1 regulates angio-supportive bone marrow-derived cells in mice: relevance to chemoresistance. Blood. 2013;122(1):143–53. doi: 10.1182/blood-2012-11-459347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bourhis J, Wilson G, Wibault P, Janot F, Bosq J, Armand JP, Luboinski B, Malaise EP, Eschwege F. Rapid tumor cell proliferation after induction chemotherapy in oropharyngeal cancer. The Laryngoscope. 1994;104(4):468–72. doi: 10.1288/00005537-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 130.El Sharouni SY, Kal HB, Battermann JJ. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. British journal of cancer. 2003;89(12):2184–9. doi: 10.1038/sj.bjc.6601418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Roodhart JM, Langenberg MH, Vermaat JS, Lolkema MP, Baars A, Giles RH, Witteveen EO, Voest EE. Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia. 2010;12(1):87–94. doi: 10.1593/neo.91460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons M, Gonzalez-Angulo AM, Joyce JA, De Palma M, Pollard JW, Lewis CE. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015;75(17):3479–91. doi: 10.1158/0008-5472.CAN-14-3587. Epub 2015/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 134.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, Liu J, Pantel K. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(20):5701–10. doi: 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 135.Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, de Cremoux P, Salmon R, Vincent-Salomon A, Marty M. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14(21):7004–10. doi: 10.1158/1078-0432.CCR-08-0030. [DOI] [PubMed] [Google Scholar]
- 136.Onstenk W, Kraan J, Mostert B, Timmermans MM, Charehbili A, Smit VT, Kroep JR, Nortier JW, van de Ven S, Heijns JB, Kessels LW, van Laarhoven HW, Bos MM, van de Velde CJ, Gratama JW, Sieuwerts AM, Martens JW, Foekens JA, Sleijfer S. Improved Circulating Tumor Cell Detection by a Combined EpCAM and MCAM CellSearch Enrichment Approach in Patients with Breast Cancer Undergoing Neoadjuvant Chemotherapy. Mol Cancer Ther. 2015;14(3):821–7. doi: 10.1158/1535-7163.MCT-14-0653. [DOI] [PubMed] [Google Scholar]
- 137.D’Alessio S, Blasi F. The urokinase receptor as an entertainer of signal transduction. Frontiers in bioscience. 2009;14:4575–87. doi: 10.2741/3550. [DOI] [PubMed] [Google Scholar]
- 138.LeBeau AM, Duriseti S, Murphy ST, Pepin F, Hann B, Gray JW, VanBrocklin HF, Craik CS. Targeting uPAR with antagonistic recombinant human antibodies in aggressive breast cancer. Cancer Res. 2013;73(7):2070–81. doi: 10.1158/0008-5472.CAN-12-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104(9):2746–51. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 140.Ebrahimi S, Rahmani F, Behnam-Rassouli R, Hoseinkhani F, Parizadeh MR, Keramati MR, Khazaie M, Avan A, Hassanian SM. Proinflammatory signaling functions of thrombin in cancer. Journal of cellular physiology. 2017;232(9):2323–9. doi: 10.1002/jcp.25753. [DOI] [PubMed] [Google Scholar]
- 141.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125(7):1595–603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gunjal PM, Schneider G, Ismail AA, Kakar SS, Kucia M, Ratajczak MZ. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. Journal of ovarian research. 2015;8:20. doi: 10.1186/s13048-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schneider G, Sellers ZP, Ratajczak MZ. Induction of a Tumor-Metastasis-Receptive Microenvironment as an Unwanted Side Effect After Radio/Chemotherapy and In Vitro and In Vivo Assays to Study this Phenomenon. Methods in molecular biology. 2016;1516:347–60. doi: 10.1007/7651_2016_323. [DOI] [PubMed] [Google Scholar]
- 144.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–60. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 145.Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46(7):1199–209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 146.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood. 2000;96(5):1789–97. [PubMed] [Google Scholar]
- 147.Nierodzik ML, Plotkin A, Kajumo F, Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. The Journal of clinical investigation. 1991;87(1):229–36. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–300. [PubMed] [Google Scholar]
- 149.Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer cell. 2017;32(2):135–54. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 150.Balkwill F. Cancer and the chemokine network. Nature reviews Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 151.Bandari SK, Purushothaman A, Ramani VC, Brinkley GJ, Chandrashekar DS, Varambally S, Mobley JA, Zhang Y, Brown EE, Vlodavsky I, Sanderson RD. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix biology: journal of the International Society for Matrix Biology. 2017 doi: 10.1016/j.matbio.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shibue T, Weinberg RA. Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Seminars in cancer biology. 2011;21(2):99–106. doi: 10.1016/j.semcancer.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer cell. 2002;2(4):289–300. doi: 10.1016/s1535-6108(02)00153-8. Epub 2002/10/26. [DOI] [PubMed] [Google Scholar]
- 154.Rossi L, Stevens D, Pierga JY, Lerebours F, Reyal F, Robain M, Asselain B, Rouzier R. Impact of Adjuvant Chemotherapy on Breast Cancer Survival: A Real-World Population. PLoS One. 2015;10(7):e0132853. doi: 10.1371/journal.pone.0132853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ray M, Lee YW, Scaletti F, Yu R, Rotello VM. Intracellular delivery of proteins by nanocarriers. Nanomedicine. 2017;12(8):941–52. doi: 10.2217/nnm-2016-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reeves PM, Abbaslou MA, Kools FRW, Poznansky MC. CXCR4 blockade with AMD3100 enhances Taxol chemotherapy to limit ovarian cancer cell growth. Anti-cancer drugs. 2017;28(9):935–42. doi: 10.1097/CAD.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 157.Taromi S, Kayser G, Catusse J, von Elverfeldt D, Reichardt W, Braun F, Weber WA, Zeiser R, Burger M. CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget. 2016;7(51):85185–95. doi: 10.18632/oncotarget.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. Journal of the National Cancer Institute. 2010;102(8):522–8. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H. A Natural CCR2 Antagonist Relieves Tumor-associated Macrophage-mediated Immunosuppression to Produce a Therapeutic Effect for Liver Cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hamilton JA, Cook AD, Tak PP. Anti-colony-stimulating factor therapies for inflammatory and autoimmune diseases. Nature reviews Drug discovery. 2016;16(1):53–70. doi: 10.1038/nrd.2016.231. [DOI] [PubMed] [Google Scholar]
- 161.Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, Jones J, Baccay F, Oktay M, Condeelis J. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat Methods. 2017 doi: 10.1038/nmeth.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lewis CE, Ferrara N. Multiple effects of angiopoietin-2 blockade on tumors. Cancer cell. 2011;19(4):431–3. doi: 10.1016/j.ccr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 163.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, De Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer cell. 2011;19(4):512–26. doi: 10.1016/j.ccr.2011.02.005. Epub 2011/04/13. [DOI] [PubMed] [Google Scholar]
- 164.Mammoto T, Jiang E, Jiang A, Mammoto A. Extracellular matrix structure and tissue stiffness control postnatal lung development through the lipoprotein receptor-related protein 5/Tie2 signaling system. Am J Respir Cell Mol Biol. 2013;49(6):1009–18. doi: 10.1165/rcmb.2013-0147OC. [DOI] [PubMed] [Google Scholar]
- 165.Dalton AC, Shlamkovitch T, Papo N, Barton WA. Constitutive Association of Tie1 and Tie2 with Endothelial Integrins is Functionally Modulated by Angiopoietin-1 and Fibronectin. PLoS One. 2016;11(10):e0163732. doi: 10.1371/journal.pone.0163732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170(6):993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer discovery. 2016;6(6):630–49. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]