Abstract
Introduction
Hyporesponsiveness to recombinant human erythropoietin (rhEPO) is a major problem affecting some patients with chronic kidney disease (CKD), predominantly those on hemodialysis (HD). Daprodustat (GSK1278863) is a hypoxia-inducible factor prolyl hydroxylase inhibitor that is being investigated as a treatment for anemia of CKD.
Methods
This phase 2a, exploratory, multicenter, single-arm study assessed the ability of daprodustat to increase or maintain hemoglobin concentrations within the target range (10.0–11.5 g/dl) over 16 weeks in subjects with anemia who were on HD and who had a high erythropoietin resistance index (ERI). All included subjects met the criteria for chronic rhEPO hyporesponsiveness (i.e., an ERI based on a series of contiguous strata of patients’ hemoglobin-by−epoetin alfa for a minimum of 12 weeks). Eligible adults were on a stable HD regimen 3 to 4 times per week. Markers of iron utilization and safety were also assessed. All subjects initially received oral daprodustat 12 mg once daily.
Results
Of the 60 participants screened, 15 were enrolled, and 7 (47%) completed 16 weeks of treatment. At week 16, 2 of 7 subjects (29%) had >1 g/dl increases in hemoglobin from baseline. Daprodustat had minimal effects on markers of iron metabolism and utilization. Fourteen subjects (93%) experienced ≥1 adverse event (AE). The most common AEs included nausea, pneumonia, pleural effusion, and urinary tract infection. The majority of on-therapy AEs were mild or moderate in intensity.
Conclusion
Daprodustat increased hemoglobin concentrations within the target range in 29% of chronic rhEPO-hyporesponsive subjects. No new safety concerns were identified in this short exploratory study.
Keywords: anemia of chronic kidney disease, daprodustat, epoetin, erythropoiesis-stimulating agents, ESA hyporesponsiveness, hemodialysis
Erythropoiesis-stimulating agents (ESAs), such as the recombinant human erythropoietins (rhEPOs) epoetin alfa and darbepoetin alfa, are commonly used treatments for anemia associated with stage 3 or 4 chronic kidney disease (CKD).1, 2 Although 90% of patients with anemia of CKD respond to rhEPO in a dose-dependent manner, the remaining 10% show resistance to rhEPO and exhibit hyporesponsiveness.3, 4 Furthermore, approximately 50% of the total cost is spent on 15% of the ESA-hyporesponsive population.5 Resistance to rhEPO can be experienced as acute, short-term episodes or as longer, more chronic episodes.6 Sibbel et al. reported that 4 months of continuous rhEPO hyporesponsiveness can be used to differentiate acute from chronic rhEPO hyporesponsiveness,7 but there is no standard definition of rhEPO hyporesponsiveness.6
The dose of rhEPO is titrated based on hemoglobin (Hgb) concentrations.8 Patients who are hyporesponsive to rhEPOs may receive high doses of rhEPO because of poorer Hgb responses. Patients receiving the highest doses of rhEPO relative to Hgb response experience poorer outcomes, including an increased risk of cardiovascular events and increased rates of morbidity and mortality.9, 10 Hence, more efficient treatments are needed for patients with CKD who are rhEPO hyporesponsive.
Although rhEPO-resistant anemia persists in some HD patients even after sufficient iron supplementation, iron deficiency, either absolute or functional, is considered a main cause of rhEPO resistance.11 However, a recent case-crossover study investigating transient factors associated with developing rhEPO hyporesponsiveness in HD patients failed to identify iron deficiency as a risk factor8; rather, the transition to rhEPO hyporesponsiveness was associated with hospitalization, changes in vascular access, or worsening inflammation as demonstrated by high C-reactive protein (CRP) and low serum albumin values. The authors of the study also noted that no risk factors could be identified in about one-third of the patients. One caveat of the study was that it was not clear whether the patients experienced chronic or acute rhEPO hyporesponsiveness. In patients with adequate iron stores, concomitant inflammation or infection is among the causes recognized as leading to rhEPO resistance.11
In a randomized study, Johnson et al. compared pentoxifylline, an anti-inflammatory drug, with placebo in patients with CKD stage 4 or 5 and rhEPO-hyporesponsive anemia. Pentoxifylline showed a significant increase in Hgb concentration versus placebo.12 A majority of patients experienced serious adverse events (AEs), but there was no difference between patients receiving active treatment and those receiving placebo.
Hypoxia-inducible factor (HIF)−prolyl hydroxylase inhibitors (PHIs) are a new emerging class of investigational products to treat anemia of CKD. These molecules stimulate erythropoiesis through inhibition of HIF−prolyl hydroxylase domain enzymes.13, 14, 15, 16, 17 Daprodustat (GSK1278863) is a HIF-PHI that is currently being investigated as a treatment for anemia associated with CKD in both dialysis and nondialysis subjects.18, 19 Both preclinical and clinical data showed that daprodustat stimulates endogenous EPO production, resulting in increased erythropoiesis and elevated Hgb concentrations. These increases in Hgb are achieved with peak EPO exposures that are substantially lower than those observed with an i.v. administration of rhEPO.18, 19
The objective of this study was to explore the ability of daprodustat to increase or maintain Hgb concentration within the target range over a 16-week treatment period in rhEPO-hyporesponsive subjects. The study also assessed the effect of daprodustat on iron metabolism and utilization and inflammation, and its short-term safety and tolerability.
Methods
Study Design
This was a 16-week, single-arm, multicenter, open-label, phase 2a trial in anemic subjects receiving chronic HD (clinicaltrials.gov identifier NCT02075463). The study protocol was reviewed and approved by the ethics committees or institutional review boards of the participating centers, and the study was conducted with the ethical principles outlined in the Declaration of Helsinki (2008).
The planned sample size for this study was 20 subjects, with approximately 15 subjects completing 16 weeks of daprodustat treatment. Because of known recruitment challenges,20, 21 a dropout rate of 25% was assumed. Estimates of the anticipated Hgb efficacy if a control arm had been included were based on a historical rhEPO control arm. Estimates were defined for rhEPO chronic hyporesponsiveness using the DaVita Clinical Data Warehouse and data from a subset of subjects who matched the hyporesponder definition. A total of 2007 subjects in the DaVita database met the rhEPO chronic hyporesponder definition and Hgb inclusion criteria for the study. Of these, 926 subjects (46%) met the primary endpoint definition.
All subjects provided informed consent. The study consisted of a 4-week rhEPO run-in period followed by a 16-week daprodustat treatment period, and a 4-week follow-up period. Subjects who met the chronic rhEPO hyporesponsiveness definition 12 weeks before the run-in period could participate in the study. At the end of the rhEPO run-in period, subjects were reassessed to ensure that they met the chronic rhEPO hyporesponsiveness definition based on Hgb and rhEPO dose. Subjects meeting these criteria were switched from epoetin alfa to receive a fixed dose of daprodustat 12 mg once daily (QD), administered orally, for the first 4 weeks of treatment.
After 4 weeks of treatment with daprodustat 12 mg, the need for dose adjustment was evaluated every 4 weeks in line with a predefined dose adjustment algorithm to achieve Hgb within the target range of 10.0 to 11.5 g/dl. However, a dose decrease was permitted for a subject at week 2 if the rise in Hgb was too rapid. At week 16 of the daprodustat treatment period, subjects stopped taking daprodustat and were not to restart epoetin alfa until after the follow-up visit unless the investigator deemed that rhEPO should be started before follow-up.
A HemoCue point-of-care Hgb analyzer (HemoCue AB, Angelholm, Sweden) was used to measure Hgb concentrations. Dose adjustment decisions and assessment of whether subjects met the Hgb withdrawal criteria were based on these Hgb levels. Hgb was also measured by a central laboratory (Quest Diagnostics, Valencia, CA).
Daprodustat was supplied as film-coated tablets for oral administration containing daprodustat 1 mg, 2 mg, 5 mg, or 25 mg. Two sizes of daprodustat tablets and matching placebo were used in this study. The study sponsor and investigator were not blinded to starting dose or dose changes, but subjects were blinded to starting dose and dose changes. To maintain the blind for daprodustat dose adjustments for subjects, they were given 3 bottles of study medication on day 1 and at all subsequent dispensing visits. Subjects took 1 tablet from each bottle every day with water without regard to food.
The unblinded data from this trial were evaluated instream using Study Explorer (GlaxoSmithKline, Research Triangle Park, NC) and the Spotfire Clinical Data Visibility Tools (TIBCO Software Inc., Palo Alto, CA) at regular intervals.
Study Administration
This study was conducted at 7 centers in the United States. An internal safety review team monitored safety data during the study. An independent data monitoring committee periodically reviewed unblinded safety data.
Eligibility Criteria
Male and female subjects aged ≥18 years who were on a stable HD regimen of 3 to 4 times weekly for a minimum of 12 weeks were eligible. Single-pool Kt/Vurea ≥ 1.2 based on a historical value obtained within the prior month or an average urea reduction ratio of at least 65% was required to ensure the adequacy of dialysis. Subjects also had to meet the criteria for chronic rhEPO hyporesponsiveness in the 12 weeks before screening.7 These criteria were based on the historical i.v. epoetin alfa dose (units per session) and Hgb level (g/dl) for three 4-week periods (total of 12 weeks) before, as well as the rhEPO doses and Hgb levels at the start of the run-in period (Supplementary Data).
Use of methoxy polyethylene glycol−epoetin beta (Mircera, Hoffmann-La Roche Inc., South San Francisco, CA) or darbepoetin (Aranesp, Amgen, Thousand Oaks, CA) within 12 weeks before administration of daprodustat was an exclusion criterion. Subjects were required to be iron replete. Those with <20% transferrin saturation (TSAT), <100 ng/ml ferritin, <2.0 ng/ml folate, and vitamin B12 level at or below the lower limit of the reference range were excluded from the study. Furthermore, subjects who experienced myocardial infarction or stroke within the 8 weeks before week –4 or who had class III/IV heart failure, hypertension, thrombotic disease, inflammatory disease, hematological disease, liver disease, or major surgery before week –4 were also excluded from the study.
Oral or i.v. iron replacement therapy was allowed during the study and was managed in line with the study site’s local iron protocol.
Study Populations
The intent-to-treat (ITT) population consisted of all enrolled subjects who received at least 1 dose of daprodustat and had a baseline and at least 1 corresponding on-treatment Hgb assessment. The safety population consisted of all subjects who received at least 1 dose of daprodustat.
Endpoints
The primary endpoint was the proportion of subjects demonstrating an increase in Hgb of ≥1 g/dl (if baseline Hgb was <9.5 g/dl), or ≥0.5 g/dl (if baseline Hgb was ≥9.5 and <10.0 g/dl), or Hgb maintained within the target range (10.0−11.5 g/dl) with no drop of >0.5 g/dl (if baseline Hgb was ≥10.0 g/dl) at week 16.
Secondary endpoints included the level of Hgb over time and change from baseline at week 16, percentage of time that Hgb was within the target range (10.0−11.5 g/dl) between weeks 12 and 16, and the number and percentage of subjects with at least a 1 g/dl increase in Hgb from baseline at week 16. Additional secondary endpoints included change from baseline in markers of iron metabolism such as hepcidin, ferritin, transferrin, and %TSAT, and evaluation of indices of hematopoiesis (EPO and vascular endothelial growth factor [VEGF]).
Hgb Assessment
Blood samples for measurement of Hgb concentrations from the run-in period and throughout the treatment period were collected and measured by a central laboratory, Quest Diagnostics.
Indices of Hematopoiesis
Serum EPO and VEGF concentrations were measured using a sparse sampling approach that included predose and postdose measurements during the trial.
Erythropoietin Resistance Index
Prior rhEPO dose was defined as the average i.v. epoetin alfa dose during the 4-week run-in period. The erythropoietin resistance index (ERI) at baseline was defined as:
ERI (IU/wk per kg Hgb [g/l] = (prior rhEPO dose [IU/kg per week]) / (baseline Hgb [g/l])
Safety Assessment
Safety assessments included monitoring of AEs, serious AEs (SAEs), cardiovascular events, AEs of special interest (AESIs), AEs leading to discontinuation of randomized treatment, laboratory tests, electrocardiograms (ECGs), and physical examinations. The investigator or site staff was responsible for detecting and reporting AESIs, which included the following: (i) thrombosis and tissue ischemia secondary to excessive erythropoiesis; (ii) risk of death, myocardial infarction, venous thromboembolism, thrombosis of vascular access; (iii) cardiomyopathy; (iv) pulmonary artery hypertension; (v) increased cancer-related mortality; (vi) esophageal and gastric erosions; (vii) proliferative retinopathy, macular edema, and choroidal neovascularization; and (viii) exacerbation of rheumatoid arthritis. Pregnancy tests were performed in female subjects of childbearing potential from screening to the end of the study. For all events with a fatal outcome, death data were collected. Three measurements of systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate were recorded at all visits except weeks 13 to 15. Height, weight, ECG, pharmacokinetic (daprodustat and metabolites), hematopoiesis markers (EPO, VEGF), and hepcidin were assessed in all subjects at baseline and subsequent study visits as appropriate.
The stopping criterion for Hgb was <7.5 g/dl. Liver stopping criteria are provided in the Supplementary Data.
Statistical Analyses
The planned sample size for this single-arm exploratory study was based on feasibility. The planned statistical analyses were estimation based and were performed using SAS version 9.1.3 or higher (SAS Institute, Cary, NC). The number and percentage of subjects meeting the primary endpoint were summarized along with 95% confidence intervals for the proportion of subjects who met the primary endpoint definition. Primary efficacy analysis was based on the ITT population using an observed case dataset, comprising all nonmissing Hgb data from Quest Diagnostics (in case of missing Hgb values from Quest Diagnostics, HemoCue Hgb values from corresponding visits were used). In addition, the number and percentage of subjects meeting the primary endpoint definition were summarized using modified last observation carried forward (LOCF) (week 12 or later Hgb values were carried forward) and the average of the available Hgb values from weeks 14 to 16.
Secondary efficacy analyses were performed based on the ITT population. Secondary efficacy endpoints of percentage of time spent within, above, and below the Hgb target range of 10.0 to 11.5 g/dl while on treatment between weeks 12 and 16 were derived using the linear interpolation method of Rosendaal et al., assuming Hgb changes linearly over time between Hgb assessments at scheduled visits.22 The number and percentage of subjects within the target range at each visit including week 16 were also determined.
Secondary endpoints of observed change from baseline with at least a 1 g/dl increase in Hgb and Hgb over time were summarized. The changes from baseline in ferritin and transferrin, and percent change from baseline in hepcidin and TSAT, were summarized by visit. Similarly, percentage change and observed maximum change from baseline in EPO and VEGF were summarized by visit. Hepcidin, VEGF, TSAT, and hsCRP (high-sensitivity CRP) were log-transformed before analysis. All safety analyses were performed based on the safety population.
Because the study was terminated early, the number of subjects and events were small, and several planned endpoints were not summarized. Data are reported as the number (%) of subjects reaching Hgb stopping criteria (<7.5 g/dl). Lipid parameters, selected iron measures, and number, frequency, and timing of dose adjustments are listed.
Results
Study Population
Because of operational challenges with study recruitment (75% screen failure rate) and a high rate of withdrawals, this study was terminated after 15 subjects were enrolled. Of the 15 subjects who received daprodustat, 7 (47%) completed the 16-week treatment period and 8 (53%) were withdrawn. The reasons for withdrawal were as follows: meeting protocol-defined stopping criteria (4 subjects, 27%), AEs (3 subjects, 20%), and consent withdrawn (1 subject, 7%).
All subjects had at least 1 cardiovascular risk factor at study entry. Participant average age was 59 years, and slightly more males (53%) than females were enrolled (Table 1). The majority of subjects (80%) were diabetic. Mean baseline Hgb was 9.7 g/dl, and 9 subjects (60%) had baseline Hgb values <10 g/dl. The median prior rhEPO dose per HD session was 11,000 IU, with 9 subjects (60%) receiving a prior rhEPO dose per session >10,000 IU.
Table 1.
Demographic and baseline characteristics (ITT population)
| Characteristic | Daprodustat starting dose 12 mg (N = 15) |
|---|---|
| Age, yr | |
| Mean ± SD | 59.1 ± 12.8 |
| Median (min–max) | 61.0 (26–77) |
| Gender, n (%) | |
| Male | 8 (53) |
| Race, n (%) | |
| White | 13 (87) |
| Black or African American | 2 (13) |
| BMI, kg/m2 | |
| Mean ± SD | 31.2 ± 13.6 |
| Median (min–max) | 28.9 (16–71) |
| Diabetic, n (%) | |
| Yes | 12 (80) |
| Hgb, g/dl | |
| Mean ± SD | 9.7 ± 0.8 |
| Median (min–max) | 9.6 (9–11) |
| ≥10.0 to ≤11.5, n (%) | 6 (40) |
| <10.0, n (%) | 9 (60) |
| Prior rhEPO dose, IU/session | |
| Mean ± SD | 11,592.8 ± 2731.4 |
| Median (min–max) | 11 000 (8462–17,600) |
| >8000 to ≤10,000, n (%) | 6 (40) |
| >10 000, n (%) | 9 (60) |
| rhEPO responsiveness criteria, n (%) | |
| >8000 to ≤10,000 IU/session and Hgb ≥8.0 to ≤10.5 g/dl | 6 (40) |
| >10,000 IU/session and Hgb ≥8.0 to <11.0 g/dl | 9 (60) |
| ERI, IU/wk per kg Hgb[g/l] | |
| Mean ± SD | 4.8 ± 1.93 |
| Median (min–max) | 4.4 (2–8) |
| Parathyroid hormone, intact, ng/la | |
| Mean ± SD | 330.9 ± 304.8 |
| Median (min–max) | 195.6 (63.6–1258.3) |
| hsCRPb, ng/l | |
| Geometric mean (CV%) | 16.4 (142.2) |
| Median (min–max) | 12.2 (3.4–61.4) |
| Hepcidin, μg/l | |
| Geometric mean (CV%) | 509 (77) |
| Median (min–max) | 674 (187–1213) |
| Ferritin, μg/l | |
| Mean (SD) | 996 ± 491 |
| Median (min–max) | 928 (331–2004) |
| Transferrin, g/l | |
| Mean (SD) | 1.5 ± 0.3 |
| Median (min–max) | 1.5 (1.0–2.2) |
| TSAT, % | |
| Geometric mean (CV%) | 29 (50) |
| Median (min–max) | 26 (13–59) |
BMI, body weight index; CV, coefficient of variation; ERI, erythropoietin resistance index; Hgb, hemoglobin; hsCRP, high-sensitivity C-reactive protein; ITT, intent-to-treat; max, maximum; min, minimum; rhEPO, recombinant human erythropoietin; TSAT, transferrin saturation.
This parameter was summarized post hoc.
Only 10 patients had hsCRP values available.
Exposure and Treatment Compliance
All 15 subjects received a daprodustat starting dose of 12 mg. The mean average dose was 12.1 mg (SD, 2.9 mg) per day. Exposure to daprodustat ranged from 7 to 115 days, with a median duration of approximately 90 days. Compliance, assessed by tablet counts at each study visit, was >80% in 8 patients at all study visits, whereas compliance in 7 patients was >80% at less than 75% of all study visits.
Of the 15 subjects, 8 received i.v. iron during the study, including 3 of the 7 completers.
Efficacy Results
Hgb Response
At week 16, 2 subjects (29%) met the protocol-defined primary efficacy endpoint (Table 2). Both subjects had a baseline Hgb value of 9.3 g/dl and >1 g/dl increases in Hgb from baseline. Four subjects (40%) met the primary endpoint by use of a modified LOCF (week 12 onward) method. Of these subjects, 2 had >1.0 g/dl increases in Hgb from baseline at week 16. In the other 2 subjects, Hgb remained within the target range of 10.0 to 11.5 g/dl, which did not decrease from baseline at week 16 (Table 2).
Table 2.
Summary of subjects meeting the efficacy endpoints (ITT population)
| Category | n | n (%) | 95% CI |
|---|---|---|---|
| Week 16 | |||
| Overall subjects met primary endpoint | 7 | 2 (28.6) | 8.0 to 64.1 |
| Hgb increase ≥1g/dl with baseline Hgb ≥8.0 to <9.5 g/dl | 5 | 2 | – |
| Hgb increase ≥0.5 g/dl with baseline Hgb ≥9.5 to <10.0 g/dl | 1 | 0 | – |
| Within target range, without >0.5 g/dl drop from baseline with baseline Hgb ≥10.0 to <11.0 g/dl | 1 | 0 | – |
| Week 12 onward (last on-therapy visit) | |||
| Overall subjects met primary endpoint | 10 | 4 (40.0) | 16.8 to 68.7 |
| Hgb increase ≥1g/dl with baseline Hgb ≥8.0 to <9.5 g/dl | 5 | 2 | – |
| Hgb increase ≥0.5 g/dl with baseline Hgb ≥9.5 to <10.0 g/dl | 1 | 0 | – |
| Within target range, without >0.5 g/dl drop from baseline with baseline Hgb ≥10.0 to <11.0 g/dl | 4 | 2 | – |
CI, confidence interval; Hgb, hemoglobin; ITT, intent-to-treat.
At baseline, 6 subjects (40%) had Hgb levels within the protocol-defined target range of 10.0 to 11.5 g/dl, and 9 subjects (60%) had Hgb levels below the target range (Table 3). At week 16, 2 subjects had Hgb within the target range, and 5 subjects had Hgb below the target range. On average, between weeks 12 and 16, subjects’ Hgb was within the target range 48% of the time, below the target range 51% of the time, and above the target range <1% of the time.
Table 3.
Subjects with Hgb in target range at selected visits (ITT population)
| Category | Baselinea (n = 15) | Week 4 (n = 13) | Week 8 (n = 12) | Week 12 (n = 10) | Week 14 (n = 6) | Week 16 (n = 7) |
|---|---|---|---|---|---|---|
| Within target range,b n (%) | 6 (40) | 6 (46) | 4 (33) | 5 (50) | 3 (50) | 2 (29) |
| Above target range, n (%) | 0 | 1 (8) | 1 (8) | 1 (10) | 0 | 0 |
| Below target range, n (%) | 9 (60) | 6 (46) | 7 (58) | 4 (40) | 3 (50) | 5 (71) |
Hgb, hemoglobin; ITT, intent-to-treat.
Baseline was the average of week −4, week −2, and day 1 visits.
Hgb between 10.0 and 11.5 g/dl.
Three subjects had HemoCue Hgb values between 7.3 and 7.4 g/dl after 55 to 80 days of daprodustat treatment, and met the protocol-defined Hgb stopping criteria (<7.5 g/dl); all were withdrawn from the study. For these 3 subjects, overall compliance with their study medication was estimated to be 33%, 50%, and 100%, respectively.
One subject had Hgb above the target value between weeks 4 and 12. Hence, daprodustat was reduced to 10 mg at week 2, further reduced to 8 mg at week 4, and held at 0 mg at week 8 through the end of the study. Of note, this subject received 2 doses of epoetin alfa (11,100 IU) on day 3 and day 6, which contributed to an increase in Hgb at weeks 2 and 4.
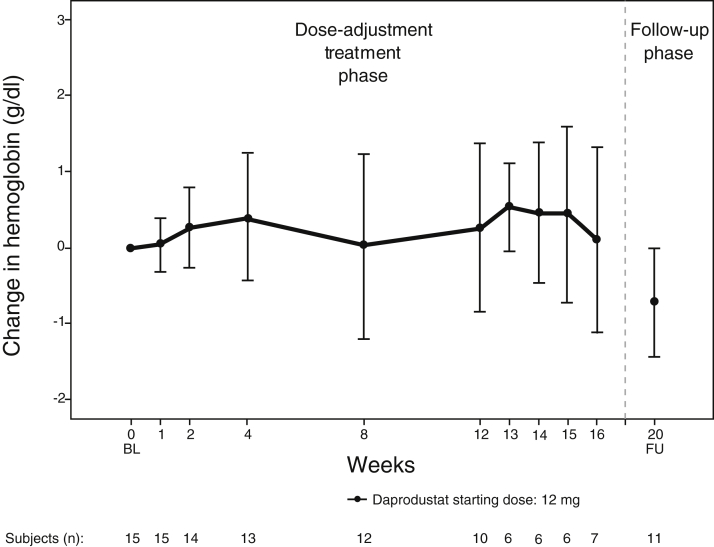
Mean Hgb throughout the daprodustat treatment period was between 9.5 and 10.2 g/dl. Mean increases in Hgb from baseline were observed at all visits during the treatment period (Figure 1), with greater increases at week 13 (0.53 g/dl) and week 14 (0.46 g/dl). Mean Hgb decreased by 0.72 g/dl at the follow-up visit. Five subjects received epoetin alfa between week 16 and the follow-up visit at the investigator’s discretion.
Figure 1.
Hemoglobin over time and change from baseline at selected visits (intent-to-treat population). BL, baseline; FU, follow-up.
hsCRP
Geometric mean hsCRP was 16 mg/l at baseline, decreased at 4 and 8 weeks, then returned to baseline at week 12. Decreases in geometric mean hsCRP from baseline were observed from weeks 4 to 12, with the greatest decrease of 47% at week 4. An increase of 8.8% was seen at week 16.
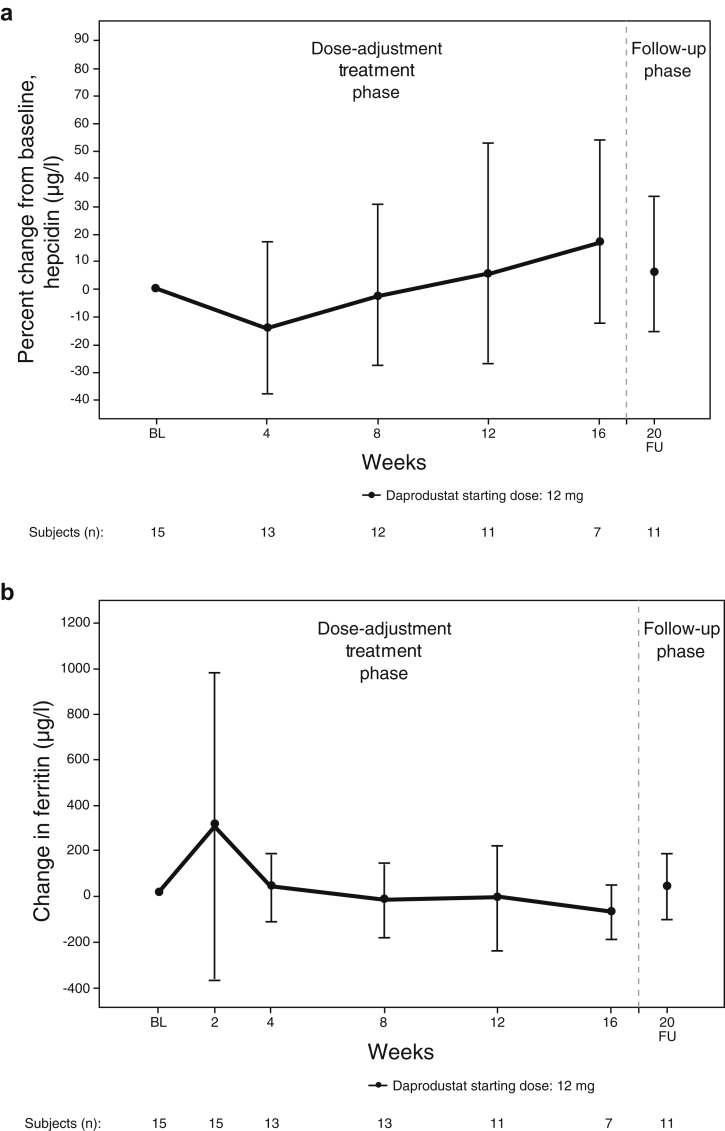
Markers of Iron Metabolism and Utilization
The mean changes from baseline in hepcidin and ferritin over time are shown in Figure 2. Geometric mean hepcidin was 509 μg/l at baseline and fluctuated between 431 and 564 μg/l throughout the daprodustat treatment and follow-up periods. Percent reductions in geometric mean change from baseline in hepcidin were observed at week 4 (14.5%) and at week 8 (2.6%). Percent change from baseline in geometric mean hepcidin increased at weeks 12, 16, and follow-up week 20 (6.4%) (Figure 2a).
Figure 2.
Change from baseline in iron-related parameters over time. (a) Hepcidin; (b) ferritin (intent-to-treat population). BL, baseline; FU, follow-up.
Mean ferritin was below baseline (mean 996 [standard deviation (SD) 491] μg/l) at weeks 8 (mean 948 [SD 377] μg/l), 12 (mean 938 [SD 401] μg/l), and 16 (mean 831 [SD 335] μg/l) (Figure 2b). Of note, 1 subject had a single available postbaseline ferritin value of 6680 μg/l at week 2. The subject received iron sucrose 100 mg i.v. 3 times per week before screening through to the week 2 visit. The subject also had a pre-existing hematoma in the left knee and developed an SAE of hematoma in the same area on day 2. Both the i.v. administration of iron and the absorption of the hematoma were likely to have contributed to a high value of ferritin at week 2, as well as at baseline (2004 μg/l).
Mean transferrin increased relative to baseline at week 2 and remained above baseline through week 16. Increases in mean transferrin from baseline were observed at all postbaseline visits, with the greatest mean increase of 0.36 g/l at week 4 (data not shown).
Geometric mean of TSAT increased at week 2 and remained higher than baseline at week 16 (data not shown). The greatest geometric mean increase was 29% at week 2.
Indices of Hematopoiesis
The maximum observed postbaseline median EPO was approximately 3.7-fold higher than the predose median baseline EPO (87 vs. 23 IU/l), with a maximum observed median increase of 51 IU/l (Table 4).
Table 4.
Maximum observed percent change from baseline in serum EPO level and plasma VEGF level (ITT population, N = 13)a
| Serum EPO level, IU/l | (N = 13) |
|---|---|
| Baselineb | |
| Mean ± SD | 35.8 ± 36.2 |
| Median (min–max) | 23.5 (6.3–137.3) |
| 25%, 75% quartiles | 18.3, 41.7 |
| Maximum observed EPO | |
| Mean ± SD | 288.9 ± 518.0 |
| Median (min–max) | 87.2 (41.4–1744.6) |
| 25%, 75% quartiles | 52.6, 134.1 |
| Maximum observed change from baseline | |
| Mean ± SD | 253.1 ± 526.7 |
| Median (min–max) | 50.9 (−58.3 to 1727.6) |
| 25%, 75% quartiles | 34.2, 115.4 |
| Plasma VEGF level, ng/l | |
| Baselineb | |
| Geometric mean (CV%) | 133.3 (34.4) |
| 95% CI | 108.9–163.1 |
| Median (min–max) | 124.2 (80.0–252.0) |
| Maximum observed VEGF | |
| Geometric mean (CV%) | 211.6 (35.1) |
| 95% CI | 172.2–259.9 |
| Median (min–max) | 200.1 (128.3–425.8) |
| Maximum observed % change from baseline | |
| Geometric mean | 58.7 |
| 95% CI | 35.6–85.8 |
| Median (min to max) | 69.0 (−9.9 to 112.5) |
CI, confidence interval; CV, coefficient of variation; EPO, erythropoietin; ITT, intent-to-treat; max, maximum; min, minimum; VEGF, vascular endothelial growth factor.
Number of subjects with baseline measurements and a maximum observed change.
Last predose value.
Relative to baseline, the geometric mean VEGF was fairly stable during the treatment period. The maximum observed postbaseline geometric mean in VEGF from baseline increased by 59% (Table 4).
Safety Results
Adverse Events
Fourteen subjects (93%) had at least 1 on-therapy AE (Supplementary Table S1). AEs that occurred in more than 1 subject were nausea (3 subjects), pneumonia (including pneumonia and pneumonia mycoplasma; 3 subjects), pleural effusion (2 subjects), and urinary tract infection (2 subjects). The majority of on-therapy AEs were assessed by the investigator to be mild or moderate in intensity.
Eight AEs in 5 subjects were assessed by the investigator as severe in intensity and included ventricular tachycardia (1 subject), lower gastrointestinal hemorrhage (1 subject), hematoma (1 subject), fatal cardiac arrest, pleural effusion, and pneumonia (all in 1 subject), and procedural hemorrhage and unstable angina (both in 1 subject). On-therapy AEs assessed by the investigator to be related to daprodustat were reported in 3 subjects and included nausea (2 subjects) and blood pressure increased (1 subject).
Serious and Other Significant Adverse Events
Nine subjects (60%) reported at least 1 on-therapy SAE (Supplementary Table S1) of which cardiac disorders were the most frequently reported. One subject (7%) had a fatal cardiac arrest while on study, which was assessed by the investigator as unrelated to daprodustat. No individual SAE was reported in more than 1 subject, and none of the SAEs were considered related to daprodustat by the investigator.
Six AEs (cardiac arrest, fatigue, gastrointestinal stoma complication, hematoma, lower gastrointestinal hemorrhage, and pain in extremity) in 5 subjects led to withdrawal from the study or permanent discontinuation of study treatment. None were considered related to daprodustat treatment. Three AESIs were identified by the investigator or site staff (see Methods): cardiac arrest, acute myocardial infarction, and arteriovenous fistula thrombosis. None were assessed as being related to daprodustat treatment.
Clinical Laboratory Evaluations
Hematology laboratory values that met the criteria for low values of potential clinical importance during the study included leukocytes in 4 subjects, platelets in 3 subjects, and lymphocytes in 2 subjects. No values of potential clinical importance were reported for liver enzymes, hematology, or clinical chemistry abnormalities.
Other Safety Evaluations
Mean baseline SBP and DBP were stable over the study period. Vital sign values of potential clinical importance were reported in 4 subjects; 2 subjects experienced predialysis SBP > 170 mm Hg (173 in 1 subject and 180 and 179 at 2 different time points in 1 subject), 1 subject experienced predialysis SBP < 85 mm Hg (79 mm Hg), and 1 subject experienced predialysis DBP < 45 mm Hg at 2 different time points (41 and 43 mm Hg). None of the subjects met the QTc withdrawal criteria during daprodustat treatment.
Discussion
The primary endpoint to assess whether daprodustat could increase or maintain Hgb concentration within the target range at week 16 was met in 2 of 7 subjects (29%) who completed the study. In 5 subjects (71%), the Hgb concentration was below the target range. Subjects included in the study were chronically rhEPO hyporesponsive based on the definition used by Sibbel et al.7 On average, Hgb concentration was within the target range 48% of the time and below the target range 51% of the time between weeks 12 and 16. The low percentage of subjects achieving the primary endpoint could be reflective of poor compliance with the study drug and a high withdrawal rate in this short-term study. Alternatively, it is possible that the relatively small increase in endogenous serum EPO levels generally achieved with HIF-PHIs, in comparison with those achieved with rhEPO and its analogues, cannot completely overcome EPO hyporesponsiveness in this subset of HD patients.
Our results based on the LOCF analysis from week 12 or later for Hgb values (Table 2) are comparable to estimates for subjects treated with rhEPO in the historical DaVita database who met the eligibility criteria for the current study. A total of 926 subjects (46%) in the historical subset (i.e., theoretically estimated rhEPO control arm) met the primary endpoint of this study, compared to 40% of subjects on daprodustat from the LOCF analysis in the current study.
Daprodustat had minimal effects on markers of iron metabolism and utilization (i.e., hepcidin, ferritin, transferrin, and TSAT) over the course of the study. Although hepcidin levels initially minimally decreased, it was surprising that hepcidin levels actually increased toward the end of the study. It is conceivable that the high level of inflammation, as determined by high CRP, could have contributed to this effect. Furthermore, efficacious daprodustat doses that increased Hgb to a target range were relatively low as compared to those in the previous GSK study.14
When compared to EPO levels observed in HD populations in previous phase 2 studies of daprodustat, both the median baseline and maximum achieved EPO levels in this study population were higher than those observed in a cohort of combined daily daprodustat doses of 4 to 12 mg (median baseline of 9 IU/l and median maximum achieved level of 36 IU/l EPO)18 and similar to EPO levels in subjects receiving daily daprodustat doses of 8 and 10 mg (median baseline 5−7 IU/ml and median maximum achieved levels of 33−89 IU/ml) in Japan.23 Geometric mean VEGF values were stable during the treatment period but lower in the current study compared with that in the previous phase 2b maintenance study in dialysis subjects.18
No new safety concerns or meaningful changes in clinical laboratory values, vital signs (including SBP or DBP), or ECG values were identified in the current study.
Limitations of this trial include the small sample population (15 subjects), single-arm design, short study period, and high drop-out rate. In addition, we were unable to investigate the effects of daprodustat on markers of iron metabolism and utilization in patients who did and did not receive supplemental iron, because the group sizes were too small to conduct meaningful subgroup analysis (8 of the 15 subjects received i.v. iron while on the study, including 3 subjects who completed the study). Future larger and longer-term studies would enable an investigation into these markers in patients who did not receive supplemental iron.
The starting dose of daprodustat 12 mg was selected based on a model-based dose-response analysis of daprodustat in rhEPO normo-responsive dialysis subjects rather than in hyporesponsive patients. Although the adaptive design of this study allowed starting-dose readjustments, no adaptation was performed, as the prespecified trigger for increasing or decreasing the starting dose was not met. The protocol allowed for daprodustat dose adjustment every 4 weeks, with the exception of titrating the dose downward at week 2 if the Hgb response was excessive. There was no algorithm change for increasing the dose at week 2. More frequent dose adjustment, perhaps allowing for earlier upward titration, may be needed for some subjects. In addition, some subjects with chronic rhEPO hyporesponsiveness may require higher starting or maintenance doses. A high withdrawal rate was observed in this short-term study, with only 7 subjects completing 16 weeks of daprodustat treatment.
In summary, this exploratory study demonstrated that daprodustat may be as effective as current rhEPO in the treatment of subjects with rhEPO hyporesponsiveness, with minimal effects on serum EPO levels. Despite that the small sample size provided limited efficacy data, this study may provide important clinical information for future studies in subjects who are rhEPO hyporesponsive, and it informs the appropriate adjustment to the daprodustat dose algorithm moving forward to improve the efficacy of daprodustat in this difficult-to-treat population. Finally, an association between lower EPO exposure and lower rates of cardiovascular events remains to be answered by the large, ongoing phase 3 cardiovascular outcomes studies.
Disclosure
BC, APS, GP, and ARC are employees of and hold stock in GlaxoSmithKline (GSK). SZ has declared no competing interests. The GSK authors contributed to the design of the study, and to the analysis and interpretation of the data; and all authors contributed to the writing and critical review of the manuscript and take responsibility for the content.
Acknowledgments
Funding for this study (NCT02075463) was provided by GlaxoSmithKline (GSK). Medical editorial support (Prachi Patil, MS, and Nancy Price, PhD) and graphic services were provided by AOI Communications, L.P., and were funded by GSK. All authors meet the authorship criteria set forth by the International Committee for Medical Journal Editors and take responsibility for the content. The authors thank the study participants and the participating study sites (Supplementary Table S2).
Footnotes
Supplementary Data.
Table S1. All on-therapy adverse events and serious adverse events (safety population).
Table S2. Principle investigators and participating study sites.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
All on-therapy adverse events and serious adverse events (safety population).
Principle investigators and participating study sites.
References
- 1.Wish J.B., Coyne D.W. Use of erythropoiesis-stimulating agents in patients with anemia of chronic kidney disease: overcoming the pharmacological and pharmacoeconomic limitations of existing therapies. Mayo Clin Proc. 2007;82:1371–1380. doi: 10.4065/82.11.1371. [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F., Del Vecchio L., Luise M.C. Current and future chemical therapies for treating anaemia in chronic kidney disease. Expert Opin Pharmacother. 2017;18:781–788. doi: 10.1080/14656566.2017.1323872. [DOI] [PubMed] [Google Scholar]
- 3.Malyszko J., Malyszko J.S., Mysliwiec M. Hyporesponsiveness to erythropoietin therapy in hemodialyzed patients: potential role of prohepcidin, hepcidin, and inflammation. Ren Fail. 2009;31:544–548. doi: 10.1080/08860220903082606. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J., Parfrey P.S., Adamson J.W., Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 5.Besarab A., Yee J. Candidate biomarkers for erythropoietin response in end-stage renal disease. Kidney Int. 2011;79:488–490. doi: 10.1038/ki.2010.479. [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson D.T., Peng Y., Arneson T.J. Comparison of methodologies to define hemodialysis patients hyporesponsive to epoetin and impact on counts and characteristics. BMC Nephrol. 2013;14:44. doi: 10.1186/1471-2369-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibbel S.P., Koro C.E., Brunelli S.M. Characterization of chronic and acute ESA hyporesponse: a retrospective cohort study of hemodialysis patients. BMC Nephrol. 2015;16:144. doi: 10.1186/s12882-015-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie I.A., Macdougall I.C., Richards S. Factors precipitating erythropoiesis-stimulating agent responsiveness in a European haemodialysis cohort: case-crossover study. Pharmacoepidemiol Drug Saf. 2015;24:414–426. doi: 10.1002/pds.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick R.D., Critchlow C.W., Fishbane S. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1077–1083. doi: 10.2215/CJN.04601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panichi V., Rosati A., Bigazzi R. Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: results from the RISCAVID study. Nephrol Dial Transplant. 2011;26:2641–2648. doi: 10.1093/ndt/gfq802. [DOI] [PubMed] [Google Scholar]
- 11.Kanbay M., Perazella M.A., Kasapoglu B. Erythropoiesis stimulatory agent-resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29:1–12. doi: 10.1159/000245041. [DOI] [PubMed] [Google Scholar]
- 12.Johnson D.W., Pascoe E.M., Badve S.V. A randomized, placebo-controlled trial of pentoxifylline on erythropoiesis-stimulating agent hyporesponsiveness in anemic patients with CKD: the Handling Erythropoietin Resistance With Oxpentifylline (HERO) trial. Am J Kidney Dis. 2015;65:49–57. doi: 10.1053/j.ajkd.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzano R., Besarab A., Sun C.H. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol. 2016;11:982–991. doi: 10.2215/CJN.06890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brigandi R.A., Johnson B., Oei C. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2a randomized trial. Am J Kidney Dis. 2016;67:861–871. doi: 10.1053/j.ajkd.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Holdstock L., Meadowcroft A.M., Maier R. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pergola P.E., Spinowitz B.S., Hartman C.S. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Meadowcroft A, Cizman B, Holdstock L, et al. Daprodustat for anemia: a 24-week, open-label, randomized, controlled trial in participants on hemodialysis [e-pub ahead of print]. Clin Kidney J. https://doi.org/10.1093/ckj/sfy014. [DOI] [PMC free article] [PubMed]
- 19.Holdstock L, Cizman B, Meadowcroft A, et al. Daprodustat for anemia: a 24-week, open-label, randomized, controlled trial in participants with chronic kidney disease. Clin Kidney J. in press. [DOI] [PMC free article] [PubMed]
- 20.Cooper A., Mikhail A., Lethbridge M.W. Pentoxifylline improves hemoglobin levels in patients with erythropoietin-resistant anemia in renal failure. J Am Soc Nephrol. 2004;15:1877–1882. doi: 10.1097/01.asn.0000131523.17045.56. [DOI] [PubMed] [Google Scholar]
- 21.Johnson D.W., Hawley C.M., Rosser B. Oxpentifylline versus placebo in the treatment of erythropoietin-resistant anaemia: a randomized controlled trial. BMC Nephrol. 2008;9:8. doi: 10.1186/1471-2369-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosendaal F.R., Cannegieter S.C., van der Meer F.J. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 23.Akizawa T., Tsubakihara Y., Nangaku M. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol. 2017;45:127–135. doi: 10.1159/000454818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All on-therapy adverse events and serious adverse events (safety population).
Principle investigators and participating study sites.