Abstract
Introduction
Local inflammation is an important regulator of vascular remodeling. We hypothesized that adipose tissue adjacent to hemodialysis arteriovenous fistulae modulates maturation.
Methods
During fistula creation, perivenous adipose was collected from 111 participants in the Hemodialysis Fistula Maturation Study. Nine adipose-associated mediators were measured. Duplex ultrasound was performed at 4 time points postoperatively from 1 day to first cannulation (10–26 weeks). Associations between logarithmically transformed biomarker levels and fistula remodeling were evaluated using mixed effects regression.
Results
Elevated interleukin (IL)-6 and monocyte chemoattractant protein (MCP)-1 were associated with a reduction in the fractional vein diameter during the early time frame of 1 day to 2 weeks (diameter change of 26.6% and 20.4% at the 25th and 75th percentile for IL-6, P = 0.01; 27.8% and 21.1% at the 25th and 75th percentile for MCP-1, P = 0.02), but not in later stages of remodeling. Local leptin levels showed a significant negative correlation with fractional venous flow increase between 2 and 6 weeks (percent flow change 31.4% and 11.3% at the 25th and 75th percentile for leptin, P = 0.03).
Conclusion
Thus, impaired fistula vein dilation and reduced capacity for flow augmentation associate with specific local adipose phenotypic signatures in a time-dependent manner. In view of adipose tissue plasticity, these findings raise the possibility of novel adipose-based strategies to facilitate fistula maturation.
Keywords: chronic kidney disease, fistula maturation, hemodialysis, inflammation, perivascular adipose tissue
Arteriovenous fistula (AVF) is recommended as the primary type of hemodialysis (HD) vascular access by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative practice guidelines due to data showing superior 5-year patency, as well as decreased rates of infectious complications and death when compared with central venous catheters and prosthetic arteriovenous grafts.1, 2 However fewer than 20% of dialysis facilities in the United States achieved the Centers for Medicare and Medicaid Services goal of 66% AVF use in prevalent HD patients. In 2012, 61% of patients initiated maintenance hemodialysis via a catheter without maturing AVF in place.3
One of the major barriers to initiation and prevalence of HD via AVF is maturation failure. The rate of early maturation failure ranges from 20% to 60% in contemporary series.4 Mechanisms underlying maturation failure are incompletely understood. It is known that considerable dilation and remodeling of both the artery and vein are required to sustain flow through an AVF.4, 5, 6, 7 Vascular remodeling is thought to rely on nitric oxide release and other endothelium-dependent and -independent mechanisms in response to changes in flow and shear stress.5, 8, 9, 10, 11 A larger preoperative vein diameter, a higher venous compliance, and an elevated AVF flow rate in the early postoperative period have been shown to predict fistula maturation.12, 13, 14, 15, 16, 17, 18, 19
Adipose tissue is contiguous with the adventitia of the inflow artery, outflow vein, and anastomosis. Long thought to function in a largely thermoregulatory and structural capacity, adipose is now increasingly recognized as a metabolically active tissue with endo-, para-, and autocrine functions.20, 21 Local adipocyte-derived signaling molecules modulate vascular smooth muscle function via endothelium-dependent and -independent mechanisms.22, 23, 24 Recently, we demonstrated in a small prospective study that levels of adipose interleukin (IL)-8, tumor necrosis factor-α, monocyte chemoattractant protein (MCP)-1, resistin, and adiponectin were inversely correlated with venous diameter change, and MCP-1 and adiponectin were inversely correlated with flow volume in human AVF at 4 to 6 weeks after access creation.25
Leveraging a subgroup of the large, well-characterized Hemodialysis Fistula Maturation (HFM) cohort,4 we sought to explore potential links between local adipose phenotype and longitudinal anatomic and physiologic characteristics of human AVF maturation.
Methods
Study Participants
The prospective, multicenter National Institute of Diabetes and Digestive and Kidney Diseases HFM Study4 includes patients from 7 distinct centers. Of these, 2 (UF Health/Malcom Randall VAMC and Boston Medical Center) agreed to participate in the study outlined herein. Patients at these 2 institutions provided their written informed consent to participate in this institutional review board–approved study protocol. Complete exclusion criteria have been detailed elsewhere.4 Most notably for the current investigation, these included (i) patients not expected to initiate hemodialysis within 3 months of fistula creation (patients already on chronic hemodialysis at the time of fistula surgery were not excluded), (ii) planned 2-stage fistula creation, and (iii) planned use of prosthetic graft or other nonautogenous conduit.
Clinical Data Collection
Demographic, clinical, and laboratory data were obtained at the time of enrollment in the HFM Study.
Ultrasound Studies
The protocol for pre- and postoperative ultrasound and vascular function studies has been described previously.4 Postoperative ultrasonography evaluating vascular diameter and flow was performed 1 day after AVF creation and repeated at 2 weeks, 6 weeks, and before first cannulation (10–26 weeks postoperatively). Venous parameters were measured at 4 locations: 2, 5, 10, and 15 cm from the arteriovenous anastomosis. All studies were read by radiologists and administered by technologists trained by the HFM Ultrasound Core, which was responsible for overseeing study quality and interoperator reliability.
Local Adipose Tissue Procurement and Protein Assay
Following 8 hours of fasting and at the time of AVF creation, trained surgeons collected 50 to 500 mg of adipose contiguous to the adventitia of the upper extremity fistula vein at the site of arteriovenous anastomosis. All samples were immediately flash frozen in liquid nitrogen then stored at −80oC until the time of analysis. Methods for protein assay from adipose tissues have been described previously.26 Briefly, proteins were isolated via serial homogenization and centrifugation, followed by quantitative protein analysis using a Luminex multiple antigen flow microparticle bead assay (Luminex Corporation, Austin, TX). Nine biologic mediators were assayed: adiponectin, IL-1β, -6, -8, leptin, MCP-1, plasminogen activator inhibitor-1, resistin, and tumor necrosis factor-α. Quantities were adjusted by the total protein concentration of each sample.
Statistical Analysis
Fractional diameter/flow changes in the outflow vein (the difference between final and initial value, divided by the initial value), were calculated in time intervals 1 day to 2 weeks, 2 to 6 weeks, and 6 weeks to first cannulation. Preliminary evaluation of the adipose mediator concentration data set revealed a non-Gaussian, right-skewed distribution, which was normalized using a logarithmic (base 10) transformation. A linear mixed-effect model was used to explore the relationship among the dependent (change in venous diameter or flow rate) and independent (adipose mediator level) variables of interest. To account for the multiple diameter measurements along the outflow vein that were obtained at multiple times following AVF creation, models included both time and location as categorical, fixed-effect parameters. Interactions among these variables and other potential covariate factors were not included in the model. Patients were modeled as a random factor. To further examine the quantitative relationship between diameter/flow changes and adipose mediator concentration, simple linear regression modeling in each time interval was used. To provide further context to the magnitude of these observed relationships, the association of clinical, demographic, and anatomic characteristics, and upper/lower protein concentration quartiles with fractional diameter or flow changes were individually assessed using analysis of variance or Student t test. Multiple linear regression modeling was performed to examine the association between fractional diameter change in the early time interval and IL-6 or MCP-1, or between fractional flow change in the intermediate time interval and leptin, adjusting 7 other factors (fistula configuration, gender, race, diabetes, smoking, renal replacement therapy, and body mass index [BMI]). Variable selection proceeded in a forward and backward stepwise fashion. Statistical analyses were performed using R, v3.1.2 (R Core Team [2014]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Baseline Patient Characteristics
In total, 111 patients from 2 of the 7 participating HFM centers underwent quantitative analysis of local AVF adipose protein levels and vascular ultrasound studies. Baseline characteristics are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics
| Variable | All patients (n = 111) |
|---|---|
| Median age (IQR) | 57 (13) |
| Sex | |
| Female (%) | 38 (34) |
| Male (%) | 73 (66) |
| Race | |
| African American (%) | 57 (51) |
| White (%) | 45 (41) |
| Other (%) | 9 (8) |
| Median BMI (IQR) | 29.6 (9.5) |
| Dialysis status | |
| Dialysis-dependent (%) | 69 (62) |
| Non–dialysis-dependent (%) | 42 (38) |
| Comorbidities | |
| Hyperlipidemia (%) | 68 (61) |
| Diabetes mellitus (%) | 74 (66) |
| Atherosclerotic vascular disease (%) | 41 (37) |
| Smoking status | |
| History of smoking | 64 (58) |
| Nonsmoker | 47 (42) |
| AVF configuration | |
| Forearm cephalic (%) | 13 (12) |
| Upper arm cephalic (%) | 54 (49) |
| Upper arm transposed (%) | 44 (39) |
AVF, arteriovenous fistula; BMI, body mass index; IQR, interquartile range.
Adipose-Associated Mediators and Postoperative Changes in Outflow Vein Diameter
We assessed the relationship between local adipose mediator levels and fractional changes in postoperative vein diameter across all time intervals using mixed effects regression modeling. The mixed effects model incorporated multiple data elements from each patient at different time intervals and locations along the outflow vein (distance from anastomosis of 2 cm, 5 cm, 10 cm, and 15 cm). The change in vein diameter served as the dependent variable, whereas logarithmically (base 10) transformed mediator levels were the independent variables. A fractional diameter change of zero represents no change in vein diameter and 1.0 represents a doubling of the vein diameter within the time interval. IL-6 and MCP-1 levels demonstrated an inverse relationship with fractional diameter change over a period ranging from day 1 to first cannulation (Table 2). For every 10-fold increase in IL-6 and MCP-1 levels, there was a mean 4.8% and 8.5% decrease in vein diameter, respectively. The fractional diameter change at the 25th and 75th percentiles of IL-6 concentration were 14.7% and 13.8%, and 17.0% and 13.6% for MCP-1 concentration, respectively. No other mediators demonstrated a statistically significant relationship with fractional diameter change (Table 2).
Table 2.
Fractional arteriovenous fistula vein diameter increase across all time intervals as a function of local adipose mediator levels
| Perivascular mediator | Slopea | Percent diameter change (at 25th–75th percentile of the mediator value) | P value |
|---|---|---|---|
| IL-6 | −0.048 | 14.7%–13.8% | 0.006 |
| MCP-1 | −0.085 | 17.0%–13.6% | 0.005 |
| IL-8 | −0.046 | 14.2%–13.4% | 0.07 |
| Adiponectin | −0.053 | 14.2%–12.7% | 0.10 |
| Leptin | −0.023 | 13.5%–12.4% | 0.19 |
| Resistin | −0.020 | 13.7%–12.7% | 0.37 |
| PAI-1 | −0.026 | 14.1%–12.7% | 0.42 |
| TNFα | −0.027 | 14.4%–12.2% | 0.51 |
| IL-1β | −0.000 | 11.3%–11.8% | 0.99 |
IL, interleukin; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; TNF, tumor necrosis factor.
Slope in relation to logarithmically transformed mediator levels.
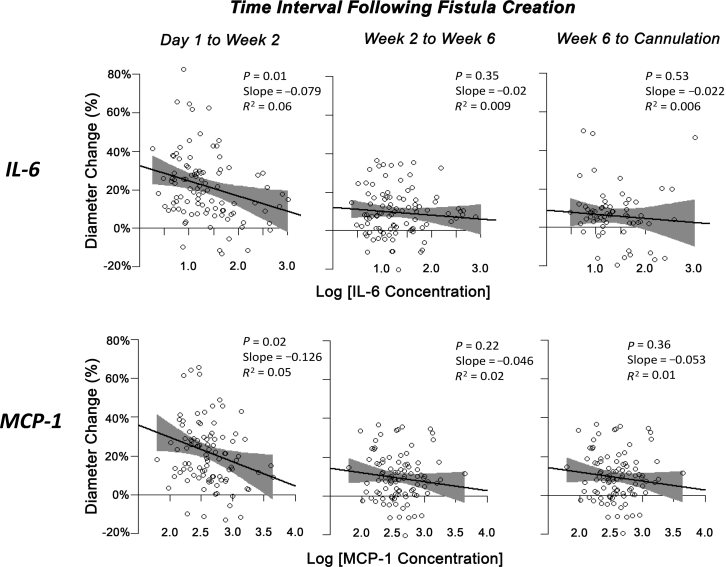
To further explore whether the interplay of local adipose IL-6 and MCP-1 levels and remodeling parameters varied during the initial weeks to months following AVF creation, we used simple linear regression modeling in each time interval. Because the diameter changes at the 4 locations along the same fistula were not significantly different, the mean of these 4 values was used. Local adipose IL-6 and MCP-1 levels were found to be highly correlated (r = 0.83, P < 0.0001), and thus were modeled individually. Both mediators were significant predictors of the fractional vein diameter change during the earliest time interval from postoperative 1 day to 2 weeks, but not at later time intervals (Figure 1), suggesting a specific biologic window for the effects of these mediators.
Figure 1.
Fractional AVF diameter change as a function of local adipose mediator levels during each time interval. A significant relationship between the fractional change in diameter and both IL-6 (P = 0.01) and MCP-1 (P = 0.02) was observed during the early phase of fistula maturation. This corresponded to a 7.9% and 12.6% decrease in fistula diameter for every 10 pg IL-6/mg tissue or 10 pg MCP-1/mg tissue increase in IL-6 and MCP-1 levels, respectively. In terms of absolute vein diameter changes, this represented a 0.45-mm and 0.30-mm difference between the 10th and 90th percentiles of IL-6 and MCP-1 concentrations, respectively. AVF, arteriovenous fistula; IL, interleukin; MCP, monocyte chemoattractant protein.
Vein Diameter Change and Demographic Characteristics, Comorbidities, and Fistula Configuration
To provide clinical context for the significant associations between adipose mediator level and outflow vein remodeling, we examined the relative influence of various clinical factors that have been previously identified or suspected to influence AVF maturation. Specifically, patient demographic characteristics, comorbidities, and differences in anatomic configuration were examined for their relationship with fractional vein diameter change for each time interval.
In the earliest time interval, fistula configuration and patients receiving dialysis were found to be significantly associated with fractional diameter change, with diameter increases of 27% and 23% in radio- and brachiocephalic AVFs versus 12% in transposed AVFs (P = 0.001, analysis of variance) and 22% versus 18% in patients with chronic kidney disease and HD-dependence, respectively (P = 0.011) (Table 3). For comparison, patients in the lowest quartile of IL-6 concentration had a vein diameter increase of 23%, whereas those in the highest quartile had a diameter increase of 11% (P = 0.0002). Similarly, patients in the lowest quartile of MCP-1 had a diameter increase of 23%, compared with 12% for those in the highest quartile (P = 0.023). Gender, race, diabetes, hyperlipidemia, smoking history, clinical manifestations of atherosclerotic disease, and BMI were not significantly associated with vein diameter change in this early time interval (Table 3).
Table 3.
Fractional arteriovenous fistula vein diameter change during the early time interval (1 day to 2 weeks), as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels
| Demographics/comorbidities | Fractional diameter change (mean ± SD) | P value |
|---|---|---|
| Fistula configuration | ||
| Radiocephalic (13) | 0.27 ± 0.22 | 0.001 |
| Brachiocephalic (50) | 0.23 ± 0.15 | |
| Basilic Transposition (37) | 0.12 ± 0.15 | |
| Gender | ||
| Male (69) | 0.18 ± 0.18 | 0.07 |
| Female (31) | 0.23 ± 0.15 | |
| Race | ||
| Non–African American (45) | 0.19 ± 0.15 | 0.81 |
| African American (54) | 0.20 ± 0.18 | |
| Diabetes | ||
| Yes (67) | 0.17 ± 0.16 | 0.12 |
| No (33) | 0.24 ± 0.18 | |
| History of atherosclerotic disease | ||
| Yes (36) | 0.17 ± 0.16 | 0.32 |
| No (64) | 0.21 ± 0.17 | |
| History of hyperlipidemia | ||
| Yes (60) | 0.19 ± 0.16 | 0.68 |
| No (40) | 0.20 ± 0.18 | |
| History of smoking | ||
| Yes (60) | 0.20 ± 0.17 | 0.44 |
| No (40) | 0.19 ± 0.17 | |
| Renal replacement therapy | ||
| Yes (61) | 0.18 ± 0.19 | 0.01 |
| No (39) | 0.22 ± 0.13 | |
| BMI | ||
| <18.5 (underweight, 3) | 0.31 ± 0.22 | 0.31 |
| ≥30 (obese, 47) | 0.20 ± 0.16 | |
| ≥18.5 and <30 (normal/overweight, 50) | 0.18 ± 0.18 | |
| IL-6 | ||
| First quartile | 0.23 ± 0.17 | 0.0002 |
| Fourth quartile | 0.11 ± 0.18 | |
| MCP-1 | ||
| First quartile | 0.23 ± 0.18 | 0.023 |
| Fourth quartile | 0.12 ± 0.13 |
BMI, body mass index; IL, interleukin; MCP, monocyte chemoattractant protein.
Numbers of patients are in parentheses.
For the intermediate (2 to 6 weeks) time interval, fistula configuration was the only variable significantly associated with diameter change; for the late (6 weeks to first cannulation) time interval, there were no variables significantly associated with diameter change (Supplementary Table S1).
The association between fractional diameter change in the early time interval and IL-6 or MCP-1 with 7 other factors included was examined. For IL-6, the final multiple linear model only included IL-6, fistula configuration, gender, diabetes, and BMI. Only fistula configuration (P = 0.028) and gender (P = 0.044) were significant; IL-6 (P = 0.166), diabetes (P = 0.082), and BMI (P = 0.125) were not significant. For MCP-1, the final multiple linear model did not have MCP-1 and included fistula configuration, gender, diabetes, and BMI. Only fistula configuration (P = 0.0007) and gender (P = 0.036) were significant; diabetes (P = 0.112) and BMI (P = 0.118) were not significant. Therefore, association between fractional diameter change in the early time interval and IL-6 or MCP-1 was no longer significant when fistula configuration and gender were considered.
Adipose-Associated Mediators and Postoperative Changes in Vein Flow
Next, we assessed the relationship between local adipose mediator levels and fractional AVF blood flow change across all time intervals using mixed effects regression modeling. The change in vein flow served as the dependent variable, whereas logarithmically transformed mediator levels were independent variables. Leptin concentration was inversely associated with fractional flow increase. For every 10-fold increase in leptin level, there was a 14.5% decrease in AVF flow. No other cytokine mediators demonstrated a significant association with fractional flow change (Table 4).
Table 4.
Fractional arteriovenous fistula flow increase across all time intervals as a function of local adipose mediator levels
| Perivascular mediator | Slopea | Percent flow change (at 25th–75th percentile of the mediator value) | P value |
|---|---|---|---|
| Leptin | −0.145 | 30%–27% | 0.03 |
| MCP-1 | −0.156 | 40%–25% | 0.18 |
| IL-6 | −0.089 | 36%–26% | 0.20 |
| PAI-1 | 0.145 | 16%–24% | 0.24 |
| Adiponectin | −0.078 | 29%–22% | 0.53 |
| Resistin | 0.031 | 25%–22% | 0.73 |
| IL-1beta | −0.053 | 26%–23% | 0.74 |
| TNF-alpha | 0.048 | 20%–22% | 0.76 |
| IL-8 | −0.008 | 31%–25% | 0.93 |
IL, interleukin; MCP, monocyte chemoattractant protein; PAI, plasminogen activator inhibitor; TNF, tumor necrosis factor.
Slope in relation to logarithmically transformed mediator levels.
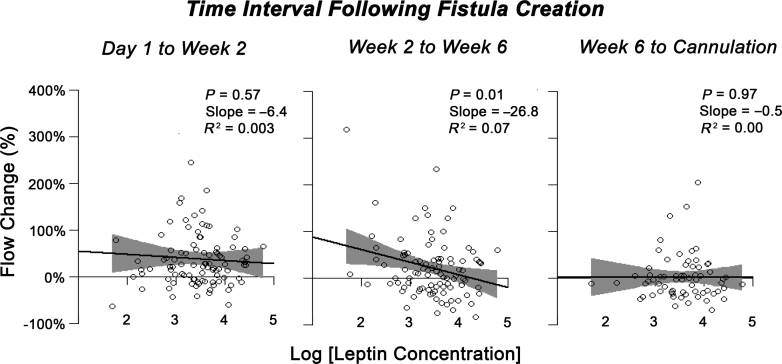
To further explore whether the interplay of local adipose leptin levels and flow change varied by time, we used simple linear regression modeling within each time interval. Leptin was a significant predictor of the increase in fractional AVF flow during the time interval from 2 to 6 weeks after AVF creation, but not at other time intervals (Figure 2).
Figure 2.
Fractional AVF flow change as a function of local adipose leptin levels during each time interval. A significant relationship between the fractional change in flow and leptin (P = 0.01) was observed during the intermediate phase of fistula maturation. This corresponded to a 26.8% decrease in flow for every 10-pg leptin/mg tissue increase in leptin levels. In terms of absolute flow changes, this represented a 132-ml/min difference between the 10th and 90th percentiles of leptin concentration.
Vein Flow Change and Demographic Characteristics, Comorbidities, and Fistula Configuration
To evaluate the relative influence of leptin versus other clinical factors, we examined the associations of AVF flow change with demographic characteristics, comorbidities, and fistula configuration. AVF configuration, sex, race, diabetes, hyperlipidemia, smoking history, clinical evidence of atherosclerotic disease, dialysis-dependence, and BMI were not significant predictors of AVF flow change at the 2- to 6-week time interval (Table 5). Note that because of the large variability in the leptin-flow relationship at the highest and lowest leptin levels, comparing only the first and fourth quartiles (as was done in Table 5) failed to replicate the previously observed significance among these variables when all data points were used. Expanding the analysis to examine the early and late time intervals also failed to demonstrate significant associations with patient demographics, comorbidities, AVF configuration, and leptin quartiles (Supplementary Table S2).
Table 5.
Fractional arteriovenous fistula flow change during the intermediate time interval (2 to 6 weeks after access creation), as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels
| Demographics/comorbidities | Fractional flow change (mean ± SD) | P value |
|---|---|---|
| Fistula configuration | ||
| Radiocephalic (13) | 0.42 ± 0.71 | 0.22 |
| Brachiocephalic (48) | 0.12 ± 0.52 | |
| Basilic Transposition (34) | 0.28 ± 0.73 | |
| Gender | ||
| Male (67) | 0.24 ± 0.65 | 0.68 |
| Female (28) | 0.17 ± 0.60 | |
| Race | ||
| Non–African American (41) | 0.21 ± 0.71 | 0.68 |
| African American (53) | 0.22 ± 0.58 | |
| Diabetes | ||
| Yes (65) | 0.17 ± 0.55 | 0.37 |
| No (30) | 0.33 ± 0.78 | |
| History of atherosclerotic disease | ||
| Yes (34) | 0.11 ± 0.55 | 0.26 |
| No (61) | 0.28 ± 0.67 | |
| History of hyperlipidemia | ||
| Yes (57) | 0.15 ± 0.58 | 0.18 |
| No (38) | 0.32 ± 0.70 | |
| History of smoking | ||
| Yes (56) | 0.19 ± 0.72 | 0.19 |
| No (39) | 0.26 ± 0.50 | |
| Renal replacement therapy | ||
| Yes (58) | 0.24 ± 0.66 | 0.72 |
| No (37) | 0.18 ± 0.60 | |
| BMI | ||
| <18.5 (underweight, 3) | 0.93 ± 0.93 | 0.14 |
| ≥30 (obese, 45) | 0.10 ± 0.54 | |
| ≥18.5 and <30 (normal/overweight, 47) | 0.28 ± 0.67 | |
| Leptin | ||
| First quartile | 0.39 ± 0.79 | 0.15 |
| Fourth quartile | 0.07 ± 0.52 |
BMI, body mass index.
Numbers of patients are in parentheses.
The association between fractional flow change in the time intermediate interval and leptin with 7 other factors included was examined. The final model of the multiple linear regression analysis included leptin only and thus is the same as shown in Figure 2. Other factors were not significant. Analysis of the current data set revealed no significant associations between adipose cytokine levels and clinical usability of the fistula (IL-6: P = 0.47; MCP-1: P = 0.64; Leptin: P = 0.10).
Discussion
In this prospective study, we evaluated the relationship between selected adipose tissue mediators, collected at the time of AVF surgery, and sonographic markers of vascular remodeling. We found that increased levels of adipose IL-6 and MCP-1 were associated with impaired expansion of the AVF vein in the earliest time interval. Similarly, increased adipose leptin level was associated with reduced capacity for flow augmentation in the 2- to 6-week post-AVF creation time period.
Although the vascular remodeling observed during AVF maturation has traditionally been ascribed to “inside-out” processes driven by hemodynamic forces,5, 6, 7, 8, 27, 28 the results of this study introduce the additional modulation of “outside-in” local factors on these vascular wall adaptations.29, 30, 31, 32 Adipose tissue lies contiguous to the arteries and veins used to construct AVF, and surrounds the arteriovenous anastomosis. The tissue likely also responds to the surgical trauma of access placement.33 Adipose depots that may be associated with proinflammatory states, such as uremia, often lie adjacent to AVF.34, 35, 36, 37 Animal studies have demonstrated that mice with a known proinflammatory adipose phenotype38 had 44% smaller vein graft lumens after arterialization due to overall negative wall remodeling.39 Circulating inflammatory mediators have been associated with AVF maturation failure and vascular access dysfunction in animal models and human studies40, 41, 42, 43; however, the role of local adipose tissue in human AVF maturation is not understood. We reported that levels of local adipose-associated mediators correlated with markers of vein remodeling at a single time point 4 to 6 weeks after access surgery.25 Here we expanded on that work by evaluating vascular wall adaptations at several time points, which extended from placement to first cannulation, and in a larger, more diverse, and better-described patient population.
Because maturation is a dynamic process occurring over 12 or more weeks,25, 44 we sought to explore whether the interplay of adipose mediators and remodeling parameters varied in the weeks to months after AVF creation. We observed an early negative effect of elevated IL-6 and MCP-1 on the degree of vein dilation. These effects were statistically significant between 1 day and 2 weeks postoperatively but not at later time intervals. Interestingly, leptin linked to flow volume adaptations in the 2- to 6-week time frame. This suggests that adipose mediators exert their impact on AVF remodeling during this early time window, which is consistent with the authors’ previous finding of a correlation between adipose mediator levels and remodeling in the first postoperative 6 weeks.25 Also interesting is the potential disconnect between mediators that are involved in the regulation of AVF geometric remodeling and flow. Flow in a fistula is dependent on a sequential series of resistances, determined by the inflow arteries, anastomosis, peripheral veins, and central veins. Although it would not be unexpected that there is a loose relationship between flow and one of these resistances (i.e., peripheral vein diameter), it would not be expected that the linkage between flow and diameter is sufficiently strong to carry through independent and separate analyses on the influence of cytokine levels on these parameters. It is intriguing to speculate that specific cytokines may have a more dominant effect on biology specific to reorganization of the wall (e.g., modulation of smooth muscle cell migration), whereas other cytokines have an influence on factors that would more directly affect fistula flow (e.g., vascular dilation).
The National Kidney Foundation Kidney Disease Outcomes Quality Initiative and the Society for Vascular Surgery clinical practice guidelines recommend radiocephalic fistula as the preferred AVF configuration based on the potential for future access options rather than on maturation outcomes.2, 45 We also explored the impact of fistula configuration on the relationship between adipose mediator levels and postoperative fistula remodeling. Interestingly, the magnitude of the effect of changes in local adipose IL-6 and MCP-1 on venous dilation were on par with differences in venous dilation between transposed upper arm basilic and simple cephalic-based AVFs. As with fistulas created in the setting of elevated IL-6 and/or MCP-1, transposed AVFs demonstrated diminished dilation.
From a clinical perspective, maturation to a durable access conduit is the most critical question. Analysis of the current data set revealed no significant associations between adipose cytokine levels and clinical usability of the fistula. In part, this speaks to the notable disconnect between physiologic and clinical maturation. Future advances in dialysis access care will need to both modulate the conduit biology to maximize outward AVF remodeling and improve processes of care to reduce the mismatch between physiologic and clinical maturation. Given these issues, the lack of a statistically significant association between cytokine levels and clinical usability is not unexpected.
Adipose phenotype has previously been shown to be altered by clinical comorbidities: adiposopathy being most commonly reported in association with metabolic syndrome and obesity.26, 46 Adiposopathy has also been reported in association with chronic kidney disease.37, 47, 48 Interestingly, we found that dialysis-dependence at the time of fistula creation had a similar effect as elevations of IL-6 and MCP-1 on vein diameter change: the subgroup of patients who were being actively dialyzed in the period of AVF creation had impaired vein dilation. In the current cohort, BMI alone did not correlate with local IL-6 or MCP-1 levels; increasing BMI was associated with increasing perivascular leptin (Supplementary Table S3), but it did not predict fractional vein diameter or flow change.
Our findings must be interpreted within the context of our study design. The demographic characteristics of participants is skewed in comparison with the broader population of HD patients in the United States, and thus the generalizability of the findings to that population is unclear. Due to the invasive nature of adipose tissue procurement, fat was collected only at the time of access creation surgery. It is certainly plausible that the adipose phenotype may be modulated by fistula creation, adding another dynamic layer to these biologic interactions. Also, the involvement of multiple surgeons for tissue harvest may have led to some variability. Although they were instructed to harvest perivenous adipose, it is possible that some subcutaneous adipose samples were included, and the phenotype of these 2 depots can differ.49 Although a dedicated ultrasound core was used, interobserver variability is inherent in the sonographic technique. Systemic inflammatory markers were not studied in this focused study of the impact of local adipose tissue; we anticipate that the current work will motivate broader examinations of interactions between systemic and local adipose inflammation and human fistula maturation. Perioperative local mediator levels were not significantly associated with ultimate access maturation; this may be in part due to the complex, multifactorial nature of fistula maturation, as well as the relatively small sample size. Most importantly, precise mechanisms cannot be derived from this observational, associative study, although the novel links discovered from this work should stimulate further investigations.
In summary, we report impaired fistula vein dilation and reduced capacity for flow augmentation in the setting of elevated local adipose-associated mediators, particularly at early time points, in transposed upper arm fistula configurations, and in patients who are dialysis-dependent at the time of AVF surgery. If further work elucidates the findings of this observational, associative study, interventions to alter baseline adipose phenotype50 in view of the plasticity of the adipose organ (such as short-term prefistula placement dietary perturbations33, 51, 52) may offer a novel strategy to enhance human fistula maturation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This research was generously supported by National Institutes of Health (NIH) 1R01HL133500, American Heart Association Grant-in-Aid 16GRNT27090006, NIH U01DK088232, NIH U01 DK082189, NIH R01DK91443, NIH T32CA009535–26, American Heart Association 12GRNT9510001 and 12GRNT1207025, the Lea Carpenter du Pont Vascular Surgery Fund, and the Carl and Ruth Shapiro Family Foundation.
Footnotes
Supplemental Methods.
Table S1. Fractional arteriovenous fistula vein diameter change across intermediate (2 to 6 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Table S2. Fractional arteriovenous fistula flow change across early (1 day to 2 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Table S3. Relationship of local adipose mediator levels and body mass index.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Fractional arteriovenous fistula vein diameter change across intermediate (2 to 6 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Fractional arteriovenous fistula flow change across early (1 day to 2 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Relationship of local adipose mediator levels and body mass index.
References
- 1.Dhingra R.K., Young E.W., Hulbert-Shearon T.E. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60:1443–1451. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 2.Vascular Access Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48:S248–S273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health NIDDK . National Institutes of Health; Bethesda, MD: 2014. United States Renal Data System, 2014 annual data report: An overview of the epidemiology of kidney disease in the United States. [Google Scholar]
- 4.Dember L.M., Imrey P.B., Beck G.J. Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis. 2014;63:104–112. doi: 10.1053/j.ajkd.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon B.S. Why don't fistulas mature? Kidney Int. 2006;70:1413–1422. doi: 10.1038/sj.ki.5001747. [DOI] [PubMed] [Google Scholar]
- 6.Roy-Chaudhury P., Spergel L.M., Besarab A. Biology of arteriovenous fistula failure. J Nephrol. 2007;20:150–163. [PubMed] [Google Scholar]
- 7.Ben Driss A., Benessiano J., Poitevin P. Arterial expansive remodeling induced by high flow rates. Am J Physiol. 1997;272:H851–H858. doi: 10.1152/ajpheart.1997.272.2.H851. [DOI] [PubMed] [Google Scholar]
- 8.Motwani J.G., Topol E.J. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 9.Nugent H.M., Groothuis A., Seifert P. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae:a safety and efficacy study in the pig. J Vasc Res. 2002;39:524–533. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 10.Widlansky M.E., Gokce N., Keaney J.F., Jr. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J., Li Y.-S., Chien S. Shear stress–initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dageforde L.A., Harms K.A., Feurer I.D. Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. J Vasc Surg. 2015;61:170–176. doi: 10.1016/j.jvs.2014.06.092. [DOI] [PubMed] [Google Scholar]
- 13.Lauvao L., Ihnat D., Goshima K. Vein diameter is the major predictor of fistula maturation. J Vasc Surg. 2009;49:1499–1504. doi: 10.1016/j.jvs.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Malovrh M. The role of sonography in the planning of arteriovenous fistulas for hemodialysis. Semin Dial. 2003;16:299–303. doi: 10.1046/j.1525-139x.2003.16069.x. [DOI] [PubMed] [Google Scholar]
- 15.Robbin M.L., Oser R.F., Allon M. Hemodialysis access graft stenosis: US detection. Radiology. 1998;208:655–661. doi: 10.1148/radiology.208.3.9722842. [DOI] [PubMed] [Google Scholar]
- 16.van der Linden J., Lameris T., van den Meiracker A. Forearm venous distensibility predicts successful arteriovenous fistula. Am J Kidney Dis. 2006;47:1013–1019. doi: 10.1053/j.ajkd.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Yerdel M.A., Kesenci M., Yazicioglu K.M. Effect of haemodynamic variables on surgically created arteriovenous fistula flow. Nephrol Dial Transplant. 1997;12:1684–1688. doi: 10.1093/ndt/12.8.1684. [DOI] [PubMed] [Google Scholar]
- 18.Lin S.L., Chen H.S., Huang C.H. Predicting the outcome of hemodialysis arteriovenous fistulae using duplex ultrasonography. J Formos Med Assoc. 1997;96:864–868. [PubMed] [Google Scholar]
- 19.Wong V., Ward R., Taylor J. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12:207–213. doi: 10.1016/s1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 20.Britton K.A., Fox C.S. Perivascular adipose tissue and vascular disease. Clin Lipidol. 2011;6:79–91. doi: 10.2217/clp.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akoumianakis I., Antoniades C. The interplay between adipose tissue and the cardiovascular system: is fat always bad? Cardiovasc Res. 2017;113:999–1008. doi: 10.1093/cvr/cvx111. [DOI] [PubMed] [Google Scholar]
- 22.Schleifenbaum J., Kohn C., Voblova N. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28:1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y.J. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–2192. doi: 10.2174/138161207781039634. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y.J., Lu C., Su L.Y. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 2007;151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauro C.R., Ding K., Xue H. Adipose phenotype predicts early human autogenous arteriovenous hemodialysis remodeling. J Vasc Surg. 2016;63:171–176.e1. doi: 10.1016/j.jvs.2014.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauro C.R., Nguyen B.T., Yu P. Inflammatory “adiposopathy” in major amputation patients. Ann Vasc Surg. 2013;27:346–352. doi: 10.1016/j.avsg.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiya A., Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- 28.Girerd X., London G., Boutouyrie P. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension. 1996;27:799–803. doi: 10.1161/01.hyp.27.3.799. [DOI] [PubMed] [Google Scholar]
- 29.Yevzlin A.S., Chan M.R., Becker Y.T. “Venopathy” at work: recasting neointimal hyperplasia in a new light. Transl Res. 2010;156:216–225. doi: 10.1016/j.trsl.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiellaro K., Taylor W.R. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pagano P.J., Gutterman D.D. The adventitia: the outs and ins of vascular disease. Cardiovasc Res. 2007;75:636–639. doi: 10.1016/j.cardiores.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Z., Yu P., Tao M. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H482–H488. doi: 10.1152/ajpheart.01372.2006. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen B., Tao M., Yu P. Preoperative diet impacts the adipose tissue response to surgical trauma. Surgery. 2013;153:584–593. doi: 10.1016/j.surg.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Police S.B., Thatcher S.E., Charnigo R. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thalmann S., Meier C.A. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Vachharajani V., Granger D.N. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho K.J., Xue H., Mauro C.R. Impact of uremia on human adipose tissue phenotype. J Surg Res. 2013;179:175–182. doi: 10.1016/j.jss.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K.A., Gu W., Lee I.A. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu P., Nguyen B.T., Tao M. Diet-induced obesity drives negative mouse vein graft wall remodeling. J Vasc Surg. 2014;59:1670–1676. doi: 10.1016/j.jvs.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juncos J.P., Grande J.P., Kang L. MCP-1 contributes to arteriovenous fistula failure. J Am Soc Nephrol. 2011;22:43–48. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Marchi S., Falleti E., Giacomello R. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol. 1996;7:1169–1177. doi: 10.1681/ASN.V781169. [DOI] [PubMed] [Google Scholar]
- 42.Liu B.C., Li L., Gao M. Microinflammation is involved in the dysfunction of arteriovenous fistula in patients with maintenance hemodialysis. Chin Med J (Engl) 2008;121:2157–2161. [PubMed] [Google Scholar]
- 43.Sung S.A., Ko G.J., Jo S.K. Interleukin-10 and tumor necrosis factor-alpha polymorphisms in vascular access failure in patients on hemodialysis: preliminary data in Korea. J Korean Med Sci. 2008;23:89–93. doi: 10.3346/jkms.2008.23.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beathard G.A., Arnold P., Jackson J. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 45.Sidawy A.N., Spergel L.M., Besarab A. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48:2S–25S. doi: 10.1016/j.jvs.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 46.Protack C.D., Jain A., Vasilas P. The influence of metabolic syndrome on hemodialysis access patency. J Vasc Surg. 2012;56:1656–1662. doi: 10.1016/j.jvs.2012.05.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roubicek T., Bartlova M., Krajickova J. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–768. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Descamps-Latscha B., Herbelin A., Nguyen A.T. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154:882–892. [PubMed] [Google Scholar]
- 49.Mauro C.R., Ilonzo G., Nguyen B.T. Attenuated adiposopathy in perivascular adipose tissue compared with subcutaneous human adipose tissue. Am J Surg. 2013;206:241–244. doi: 10.1016/j.amjsurg.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akoumianakis I., Tarun A., Antoniades C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis:identifying novel therapeutic targets. Br J Pharmacol. 2017;174:3411–3424. doi: 10.1111/bph.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell J.R., Beckman J.A., Nguyen L.L. Reducing elective vascular surgery perioperative risk with brief preoperative dietary restriction. Surgery. 2013;153:594–598. doi: 10.1016/j.surg.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hine C., Harputlugil E., Zhang Y. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fractional arteriovenous fistula vein diameter change across intermediate (2 to 6 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Fractional arteriovenous fistula flow change across early (1 day to 2 weeks) and late (6 weeks to first cannulation) time intervals, as a function of patient demographics, comorbidities, fistula configuration, and adipose mediator levels.
Relationship of local adipose mediator levels and body mass index.