Abstract
Regenerative medicine was initially focused on tissue engineering to replace damaged tissues and organs with constructs derived from cells and biomaterials. More recently, this field of inquiry has expanded into exciting areas of translational medicine modulating the body’s own endogenous processes, to prevent tissue damage in organs and to repair and regenerate these damaged tissues. This review will focus on recent insights derived from studies in which the manipulation of the innate immunologic system may diminish acute kidney injury and enhance renal repair and recovery without the progression to chronic kidney disease and renal failure. The manner in which these interventions may improve acute and chronic organ dysfunction, including the heart, brain, and lung, will also be reviewed.
Keywords: acute kidney injury, medical device, selective cytopheretic device, translational medicine
As a primer to this review, which will focus on a novel immunomodulatory therapy using a selective cytopheretic device (SCD) in a variety of studies to treat various etiologies of acute and chronic organ dysfunction, the reader is referred to recent comprehensive reviews on the classical tissue engineering approach to renal replacement function.1, 2, 3 In brief, the tissue engineering approach uses biological components (proteins, cells, tissues) as well as degradable or nondegradable biomaterials such as polymers as a support matrix or delivery vehicles to reconstruct damaged tissue in the body. In this review, a cell-processing approach will be predominantly featured that leverages the integral role of the immune system in the body’s innate repair and regenerative mechanisms, which is modulated by an extracorporeal device to avoid abnormal pathology. Immunomodulation via continuous leukocyte processing from an extracorporeal circuit with a biomimetic membrane is shown to affect neutrophils and monocytes, altering the progression of inflammatory diseases, alleviating tissue damage caused in disease states without treatment, and enhancing repair dependent upon regenerative and remodeling processes. In this regard, the immunomodulation approach in regenerative medicine is vastly different from the tissue engineering approach, where damage to tissue can be limited to avoid abnormal pathology, and normal repair processes can be augmented rather than having to engineer and recapitulate tissue structure and function.
Role of Neutrophils in Acute Tissue/Organ Injury—Kidney, Brain, Heart, and Lung
Inflammation plays a central role in the development of acute and solid organ dysfunction. It is the excessive dysregulated leukocyte inflammatory processes in many acute and chronic disease states that lead to tissue damage, which results in organ/tissue dysfunction. In an example of acute systemic inflammation, systemic inflammatory response syndrome (SIRS) evolves into multiple organ dysfunction syndrome (MODS) due to excessive inflammation promoted by both activated circulating leukocytes and activated microvascular endothelial cells of solid organs. The interaction of activated leukocytes and activated endothelium results in poor tissue perfusion, with ischemic consequences to vital organs. The interaction of activated neutrophils and endothelium also leads to increased vascular permeability with fluid leakage from the intravascular space to tissue interstitium with resulting hypovolemia, hypotension, and cardiovascular instability. In the kidney, sequestration and aggregation of neutrophils in the peritubular capillaries and infiltration into interstitial spaces of the kidney can lead to the necrosis of proximal tubule cells, promote acute kidney injury (AKI), and, if substantive, acute renal failure (ARF). In the heart, this can lead to increases in interstitial fluid and death of cardiac myocytes. In the lungs, this leads to edematous lung tissue and decreased gas exchange, which can cause hypoxia and cell death. In the brain, local tissue injury, most dramatically exemplified by intracerebral hemorrhage/hemorrhagic stroke (ICH) is aggravated by the body’s acute inflammatory response due to local tissue injury rather than systemic processes. Local tissue injury promotes tissue release of cytokines and chemokines to attract leukocytes to the area of damage and to digest and remodel injured tissue for repair and return of function. The degree of tissue damage after initial insult is potentiated by excessive inflammatory response of the circulating innate immunologic system.4, 5 Controlling these maladaptive responses may ameliorate the degree of tissue injury and dysfunction.
Role of Monocytes/Macrophages on Chronic Organ Dysfunction—Kidney, Heart, Brain, and Liver
The monocyte/macrophage component of the innate immunological system is critical in the host response to invading pathogens and tissue repair and remodeling after tissue injury.6, 7 The optimization of these processes is dependent upon a finely regulated and coordinated response of the monocyte/macrophage system. A balanced monocyte/macrophage response, both in phenotype and in timing, is necessary for optimal host defense and tissue repair.8 Monocytes are produced in the bone marrow and are continuously released into the circulation, constituting approximately 5% to 10% of the circulating leukocyte pool in humans.8 Human circulating monocytes are not a homogeneous population. Three subsets of monocytes have been identified and are based upon the expression of cell surface markers, CD14 (lipopolysaccharide [LPS] co-receptor) and CD16 (Fc γ R111). Within the population of monocytes, the majority are the classical subset with high CD14 but no CD16 expression (CD14hiCD16−), with the minority population further subdivided into the intermediate subset (CD14hiCD16+) and the nonclassical subset (CD14lowCD16++). The classical and intermediate monocytes have the ability for phagocytosis and production of inflammatory effectors, similar to Ly6chi mouse monocytes. The nonclassical monocytes have a patrolling anti-inflammatory and reparative role similar to the Ly6clow mouse monocytes.8 The monocyte/macrophage system exists in at least 2 distinct phenotypes of differentiation: pro-inflammatory and anti-inflammatory.9, 10 Upon inflammatory signals, promoted by infection or tissue injury, circulating monocytes infiltrate tissue and differentiate into the M1 (inflammatory) or M2 (anti-inflammatory, reparative) macrophage phenotype. The M1 macrophage is usually the initial responder to coordinate and accentuate the pro-inflammatory response to destroy invading pathogens and to digest cellular and tissue debris. The M2 macrophage becomes more prominent later in this process, to repair and remodel damaged tissue promoted by this vigorous inflammatory process.
Various chronic organ dysfunction disorders have been associated with chronic inflammation. Chronic heart failure (CHF), chronic kidney disease (CKD), and type 2 diabetes mellitus (T2D) have been shown to have an increase in pro-inflammatory CD14hi monocytes compared to those in normal controls.11, 12, 13, 14, 15, 16 In fact, an increase in CD14hi monocytes in these chronic disease states correlate with worse clinical outcomes.11, 12, 15, 17, 18, 19 The development of atherosclerosis in the general and CKD population, as well as in end-stage renal disease (ESRD) patients on chronic hemodialysis (HD), are associated with M1 macrophage phenotype.18, 20, 21 The circulating CD14hi monocyte subset increases as renal function declines, with higher numbers of CD14hi monocytes predicting adverse cardiovascular outcomes in ESRD patients undergoing chronic HD.12, 18, 20 Pro-inflammatory (CD14hi) monocytes are also increased in T2D and correlate with progression to diabetic nephropathy and uremia.13, 16, 17, 22 Furthermore, a higher level than normal of circulating inflammatory monocytes has been shown to result in worse clinical outcomes in both acute myocardial and brain injury.23, 24, 25 A persistent pro-inflammatory response also characterizes progressive organ dysfunction in CHF, CKD, and obesity-related T2D.11, 15, 16, 17, 18 The recognition of this relationship between the chronic pro-inflammatory monocyte/macrophage subtypes and disease progression have identified the monocyte/macrophage system as a therapeutic target for altering the clinical progression of chronic inflammatory disorders. Accordingly, a treatment that shifts the circulating monocyte pool from CD14hi to CD14low phenotype may have a clinical benefit to ameliorate the progression of various chronic inflammatory disorders.
Selective Cytopheretic Device Therapy and Acute Kidney Injury
Initial Clinical Insight and Proposed SCD Mechanism of Action
Selective cytopheretic device therapy (SCDRx) to treat inflammatory disorders originated from the clinical evaluation of a tissue-engineered renal assist device (RAD)26 containing adult human renal epithelial cells as a component of a bioartificial kidney. In the RAD phase IIa clinical study, subsets of patients were treated with a cell containing RAD or a sham (non−renal cell containing) RAD cartridge.27 The phase IIb study was a randomized, controlled, blinded, multicenter study in intensive care unit (ICU) patients with ARF secondary to AKI undergoing continuous renal replacement therapy (CRRT). The clinical study was suspended after an interim analysis due to an unanticipated high survival rate of the sham device arm. In retrospective analysis of the sham control groups, the improved survival rate was demonstrated in the presence of regional citrate anticoagulation (RCA) when compared to systemic heparin anticoagulation.28 Subjects were divided into the following 4 groups: RAD with citrate anticoagulation; sham device with citrate anticoagulation; RAD with heparin anticoagulation; and sham device with heparin anticoagulation. The 28-day survival rate in the heparin sham patient group was 50% versus 75% in the citrate sham group (n = 12 for each treatment arm), and the 90-day survival rate was 25% (heparin) versus 67% (citrate). The baseline demographics for the 2 subsets were comparable, with similar sequential organ failure assessment (SOFA) scores (13.4 ± 1.1 vs. 12.2 ± 0.9), organ failure number (4.17 ± 0.46 vs. 3.93 ± 0.36), and incidence of sepsis (58% vs. 58%) for the citrate versus heparin sham groups, respectively.28 This clinical result, although unexpected, was consistent with a potential clinical benefit of the fiber-based device without cultured renal cells (RAD sham), when used with RCA, which later became known as SDD therapy (SCDRx) (Figure 1).
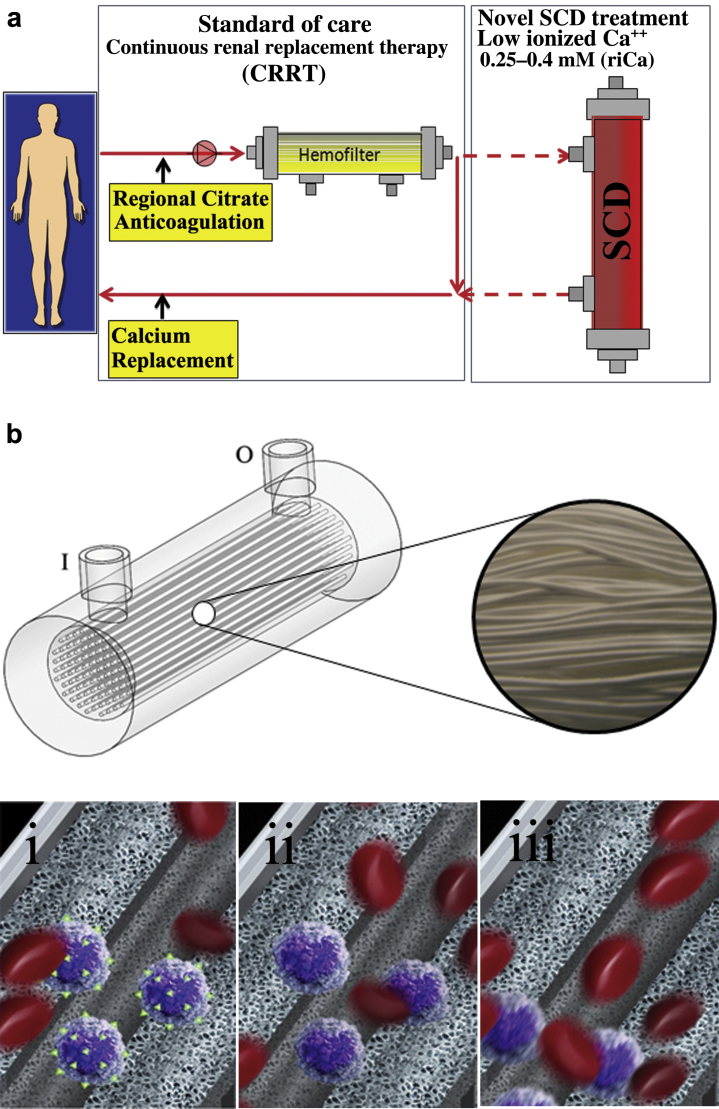
Figure 1.
Schematic representation of (a) selective cytopheretic device therapy (SCDRx) and (bi–iii) current understanding of the mechanism of action (MoA) of the SCD, which involves leukocyte (LE)/fiber interactions. (i) Binding of activated LE (purple) with mobilized surface integrins (green); (ii) “reset” LE; (iii) release of immunomodulated LE. Erythrocytes are depicted in all panels as red.
The therapeutic benefit afforded by this combination of a device and a pharmacological agent (citrate) on a systemic clinical disorder can be better understood from the following: (i) Microscopy of the sham cartridges (future SCD) after patient treatment demonstrated adherent leukocytes on the outer surface of the membranes of the cartridge along the blood flow path.27 The attached leukocytes were dominated by neutrophils and monocytes (Figure 2), which preferentially adhere, compared to other leukocytes such as lymphocytes, eosinophils, and basophils.29 The ability of leukocytes to adhere to the outer walls of the hollow fiber membranes rather than the inner walls, which is the conventional blood flow path for renal dialysis/hemofiltration applications, was due to the shear forces of blood flow. The shear stress of blood along the outer wall of the membrane was near capillary force of <1 dyne/cm2 compared to the shear stress of 100 dyne/cm2 for blood flowing along the conventional luminal surface of the hollow fiber membranes. (ii) RCA lowers the ionized calcium (iCa) in blood within the circuit to <0.4 mM, a level that inhibits the coagulation system, has an inhibitory effect on leukocyte and platelet activation,29, 30 and also affects the calcium-dependent selectin- and integrin-mediated interactions between leukocytes and the membrane.31, 32 Extravasation of neutrophils and monocytes from the systemic circulation into tissues is a highly regulated process. In a low−shear force environment like that found in capillaries or created within the SCD, neutrophils and monocytes roll along surfaces and are slowed via selectin binding followed by integrin-mediated, firm adhesion before diapedesis.31
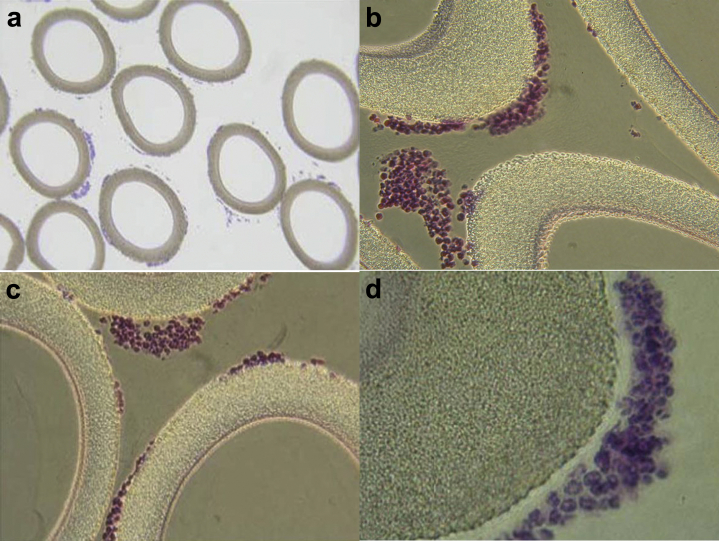
Figure 2.
Micrographs of the sham, acellular cartridges as part of the regional citrate anticoagulation arm of the Renal Assist Device (RAD) clinical trial. Patient treatment demonstrated adherent leukocytes (LE) on the outer surface of the membranes of the cartridge along the blood flow path within the extracorporeal circuit, which translated into patient benefit. This was the basis for the treatment now referred to as SCDRx. (a–d) Light micrographs stained with hematoxylin and eosin. Low-power micrograph showing adherent cells around each fiber (a, original magnification ×160). (b,c) Higher-power micrographs showing clustering of bound LE (b and c, original magnification ×400). (d) High-power micrograph displaying predominance of NE and MO in the adherent cell clusters (original magnification ×1600). MO, monocyte; NE, neutrophil.
An in vitro blood study using flow chambers to visualize leukocyte interactions with fiber materials suggests that leukocytes roll, then transiently adhere to fibers, are retained for a significant time period29 (referred to as sequestration), and are then released. Binding selectivity in the SCD is increased in the low-iCa environment, where calcium-dependent selectin rolling, integrin binding, and downstream conformational changes of attached cells are inhibited.33 Neutrophils34, 35 and monocytes36, 37 mobilize intracellular stores of CD11b, or CD11R3, the porcine analogue of human CD11b,38 to the cell surface as they become (primed) activated. Measurement of CD11b, allows for real-time measurement of systemic acute neutrophil (priming) and monocyte activation. In addition, monocyte populations are heterogeneous in their expression of CD11b,39 with CD14hiCD16− being the highest and CD14lowCD16+ being the lowest (Figure 3). The selectivity of binding of the highest-activated leukocytes has been repeatedly observed in preclinical animal models in which systemic CD11b levels decrease through the treatment course.28, 29, 40, 41 This effect was measured directly in a clinical trial by comparing the CD11b mean fluorescence intensity of the circulating cells in the peripheral blood to those directly associated with the SCD.42 These results, when taken together,28, 29, 40, 41, 42, 43 suggest an SCD mechanism of action with a simultaneous, combination effect to transiently sequester activated circulating neutrophils and monocytes, with enhanced selectivity for inflammatory leukocytes, which alters the overall activation of bound and processed leukocytes. Clinical efficacy in AKI/multiple organ dysfunction (MOD) may be due to sequestration and immunomodulation of leukocytes in the SCD, which appears to block the inflammatory sequence associated with accumulation and aggregation of leukocytes in the peritubular capillaries and to reduce infiltration into interstitial spaces, which, when unchecked, promotes kidney injury following SIRS.
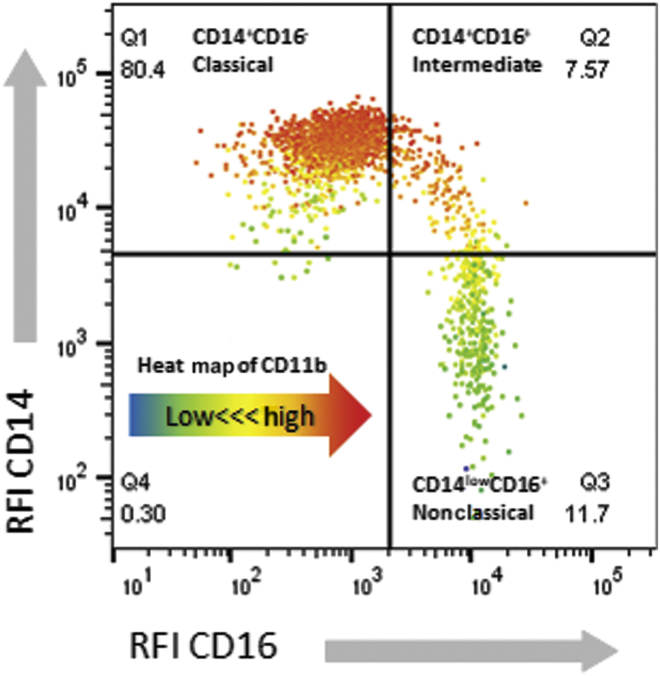
Figure 3.
Human monocytes can be classified by CD14 and CD16 expression into classical (Q1: CD14+CD16−), intermediate (Q2: CD14+CD16+), and nonclassical (Q3: CD14lowCD16+) subsets using flow-cytometric techniques. Representative cytometric analysis of systemic human blood is shown, with CD14 and CD16 expression displayed as a dot plot of relative fluorescence intensity (RFI). The intensity of CD11b expression of each event is heat mapped according to the arrow in Q4 (blue indicates lowest and red indicates highest CD11b RFI). The subsets have differential CD11b expression according to phenotype, contributing to integrin-dependent selective cytopheretic device selectivity for pro-inflammatory, classical, and intermediate MO. MO, monocytes.
SCD Clinical Treatment in AKI
SCD Phase I/II: ARF Safety, Mortality, and Device Integrity Study Performed Outside the United States
The initial clinical study of the SCD was completed at the Huashan Hospital in Shanghai, China.44 This prospective, single-arm, single-center study was designed to evaluate the safety and efficacy of SCDRx on clinical outcomes in AKI requiring CRRT in the ICU. The patients enrolled in the trial were compared with historical case-matched controls with respect to age and SOFA score. The mortality for the case-matched controls was 7 of 9 or 78%, whereas the mortality in the SCDRx group was 2 of 9 or 22% (P < 0.027). Multiple regression analysis identified treatment with SCD as the only significant variable affecting mortality among age, SOFA score, and average change in urine output over the first 7 days during or after treatment. Mean total urine output in the 9 SCDRx subjects increased from a baseline of ∼500 to >2000 ml/d by day 7 of treatment. Total white blood cell (WBC) counts also declined with SCDRx, but not to leukopenic levels. Reported serious adverse events (SAEs) were consistent with the targeted patient group and did not result in any clinical sequelae.
SCD Phase II: Pilot Prospective Multi Center US Study (Investigational Device Exemption G090189; Protocol ARF-002)
A prospective, single-arm, multicenter, US study was designed to evaluate the safety and efficacy of SCDRx on AKI requiring CRRT in the ICU. The study enrolled 35 subjects.45 The mean age was 56 ± 15 years. The average SOFA score was 11.3 ± 3.6. Death from any cause at day 60 was 31%. Renal recovery, defined as dialysis independence, was observed in all surviving subjects at day 60. The results of this pilot study indicate the potential for a substantial improvement in patient outcomes over standard-of-care therapy, which is associated with a greater than 50% 60-day mortality in the literature.45 There were no SCD-associated SAEs.
SCD Phase III: A Multicenter, Randomized, Controlled, Pivotal Study to Assess the Safety and Efficacy of an SCD in Patients With AKI (Investigational Device Exemption G090189, Protocol SCD-003)
The primary objective of this study was to determine whether CRRT+SCDRx, compared to CRRT alone, would result in a clinically relevant and statistically significant improvement in all-cause mortality through day 60.46 Secondary objectives included assessment of renal replacement therapy (RRT) dependency at day 60, mortality at day 28, number of ventilator free days (VFD) at day 28, and mortality at day 60 in the subset of patients with severe sepsis. This was a 2-arm, randomized, open-label, controlled, multicenter pivotal study that enrolled 134 patients at 21 US medical centers. The ICU AKI patients of each participating hospital were randomized to treatment undergoing CRRT or CRRT+SCDRx. Each participating clinical site used its established RCA protocol for the CRRT+SCD circuits (study arm) and for the CRRT-only (control arm). The recommended iCa (riCa) level (measured after SCD) in the CRRT and SCD circuit was specified to be between 0.25 and 0.4 mmol/l.
During the second quarter of the enrollment period, a national calcium shortage occurred in the United States from FDA-related quality manufacturing issues of the major US supplier. Due to reliance of the SCD on a narrow intracircuit iCa range for functional efficacy and the concern that patients randomized to SCDRx were not getting effective therapy, the interim analysis was performed early after enrollment of 134 patients. Enrollment was paused on 24 May 2013, to assess the clinical impact of the calcium shortage on study endpoints. The shortage of calcium infusion solutions resulted in a tendency to minimize citrate infusion rates. Accordingly, iCa levels within the blood circuit tended to be above the riCa of 0.25 to 0.40 mmol/l. Subsequently, the injectable calcium shortage resulted in 9 of the 21 open clinical sites being unable to enroll patients because of low hospital inventories of injectable calcium, contributing to the early termination of the study. Of the 134 patients in the analysis, 69 received CRRT alone and 65 received SCDRx. No significant differences were noted between the control and treatment groups in baseline characteristics. No statistically significant difference was found between the treated and control patients, with a 60-day mortality of 39% (27/69) and 36% (21/59), respectively, with 6 patients lost to follow-up. The amount of time that the patients in both the control and treatment groups were maintained in the riCa range (0.25−0.40 mmol/l), as specified in the study protocol, was substantially lower than expected due to the injectable calcium shortage. Of the 134 patients enrolled at the time of the interim analysis, 19 SCD patients and 31 control patients were maintained at riCa for greater than or equal to 90% of the therapy time. Furthermore, none of the SAE were considered device related per the principal investigator. Comparison of these subgroups of patients revealed that 60-day mortality was 16% (3/19) in the SCD group compared to 41% (11/27) in the control group (P = 0.11). Dialysis dependency showed a borderline statistically significant difference between the SCD versus control patients maintained for greater than 90% of the treatment in the protocol’s riCa target range with values of 0% (0/16) and 25% (4/16), respectively (P = 0.10). When the riCa SCD and control subgroups were compared for a composite index of 60-day mortality and dialysis dependency, the percentage in SCD subjects was 16% versus 58% in the control subjects (P < 0.01). When the riCa subpopulation was considered, a statistically significant difference was detected in several parameters: log urine output substantially increased, and WBC and neutrophil counts diminished in the SCD group versus the control group over time.
The observation that, in those patients who had the riCa level greater than 90% of the time of SCDRx, mortality improved from 41% to 16%, is clinically compelling. In addition, the observation, both in the pilot SCD-002 trial and in this SCD-003 study reported here, that no patient receiving appropriate SCDRx was dialysis dependent at day 60 is also compelling. Previous large, prospective clinical studies in AKI with MOD had a greater than 20% incidence of dialysis dependency of patients followed up for 60 or more days.47, 48 The effect of SCDRx to modulate excessive leukocyte activation most likely plays a critical role in the recovery of renal function after a substantive AKI event. The relationship of ongoing inflammation in the kidney after AKI and chronic progressive kidney disease and dialysis dependency has been demonstrated.49, 50 In this patient population, immunomodulation by SCDRx appears to positively promote kidney healing as evidenced by the lack of dialysis dependency at day 60. In addition, improvement in overall mortality may suggest improved immune balance that persists through the late SIRS process to ameliorate the compensatory anti-inflammatory response that follows the excessive systemic pro-inflammatory state in AKI and MOD.51 Furthermore, the significant decrease in WBC and neutrophil counts, as well as the improvement in urine output over time, corroborates findings in the mechanistic and pilot studies previously published.29, 44, 45, 52
SCD Phase II: Pilot Pediatric Patients With AKI
Similar to the adult AKI clinical trial, a 5-center US study of the SCD in a critically ill pediatric population (>20 kg, age up to 22 years) with AKI and MOD receiving CRRT as part of standard of care has been initiated and is ongoing under the FDA-approved investigational device exemption (IDE) G150179. Mortality rates in pediatric patients with AKI and MOD requiring CRRT have historically approached 50%.53, 54, 55 In this pilot-study clinical trial, pediatric patients have received SCDRx for up to 7 days or when CRRT is discontinued, whichever comes first. Results to date are as follows: 6 pediatric patients (3 female and 3 male) have been enrolled and have completed SCDRx. Patient age ranged from 6.5 to 17.5 years, with a Pediatric Risk of Mortality score (PRISM II) range of 2 to 14. Admission diagnoses were as follows: 1 severe rhabdomyolysis (case study presented below), 3 septic shock, 1 shigatoxin-associated hemolytic-uremic syndrome, and 1 community-acquired pneumonia. Patients received 3 (n = 2), 4 (n = 1), 6 (n = 1), or 7 (n = 2) days of SCDRx. All 6 patients survived and were off CRRT at the time of hospital discharge. No SCD-related SAEs were recorded.56
A case study of the first pediatric patient treated with SCD has been published, which describes the treatment course of an 11-year-old female patient with an uncommon reaction to anesthesia (propofol) during an elective surgery that resulted in MOD: severe AKF requiring CRRT, acute liver failure, acute respiratory failure requiring mechanical ventilation, and hematologic failure from disseminated intravascular coagulation and rhabdomyolysis with oliguria.42 After 24 hours of SCDRx, her liver injury and hematologic failure improved. After 4 days of therapy, her lung function improved, resulting in extubation. After 7 days of SCDRx, her kidney function improved, resulting ultimately in complete recovery of kidney function not requiring dialysis treatment. The patient was discharged from hospital with normal renal function.42
This technology has been tested in several different clinical trials, as summarized in Table 1, which includes trials during the original development of the RAD, in which SCDRx emerged, and in 4 trials using the SCD in adult ICU patients with AKI requiring CRRT28, 44, 45 and a pediatric trial.42 These trials have demonstrated an excellent safety profile and suggestive efficacy impact. At the 28-day survival point, MOF ICU patients treated with the SCD, on average, had a full-day decrease in ventilator dependency. A supplemental IDE is currently FDA approved for a prospective, randomized, multicenter 175 patient clinical trial with a new primary composite endpoint of 60-day mortality or dialysis dependency. The analysis clearly demonstrated clinical efficacy without safety issues, providing substantive support to move this technology to other patient populations.
Table 1.
SCDRx clinical application history
| RAD phase I/II | ARF and MOF receiving CVVH, 10 patients |
| RAD phase IIa | ARF and MOF receiving CVVH, 58 patients27 |
| RAD phase IIb | ARF and MOF receiving CVVH, Citrate Arm comparable to SCDRx, 24 patients receiving Acellular Sham, 12 with heparin, 12 with RCA28 |
| SCD phase I/II | ARF and MOF receiving CVVH, 9 patients (China)44 |
| SCD phase II | Protocol ARF-002, 35 patients45 |
| SCD phase III | Protocol SCD-003, 134 patients46 |
| SCD phase III | FDA/IDE approved, adult AKI, 175 patients |
| SCD phase II | FDA/IDE approved, pediatric AKI, 16 patients42 |
| SCD phase I/II | Safety and bioinflammatory assay ESRD study, 15 patients43 |
AKI, acute kidney injury; ARF, acute renal failure; CVVH, continuous veno-venous hemofiltration; ESRD, end-stage renal disease; FDA, US Food and Drug Administration; IDE, investigational device exemption; MOF, multiple organ failure; SCD, selective cytopheretic device; SCDRx, selective cytopheretic device therapy.
SCDRx and ESRD in HD patients
Patients with ESRD on chronic HD suffer accelerated morbidity and mortality rates due to cardiovascular disease and infections. Chronic inflammation plays a critical role in these poor outcomes. The activated monocyte has become a prime therapeutic target to modulate this inflammatory process. SCDRx was evaluated to assess its effects on the circulating monocyte pool. A pilot trial was undertaken in 15 ESRD patients on HD with C-reactive protein (CRP) levels greater than 5 mg/dl (SCD Phase I/II ESRD Safety and Bioinflammatory Assay Study, additional data added to the original submission of IDE G090189). In this study, ESRD patients were treated with one 4-hour session of SCD therapy, and an excellent safety profile was observed with no decline in leukocyte or platelet counts. The effect of SCDRx on monocyte phenotypes in these patients was determined on peripheral blood monocytes by using flow cytometry. SCDRx promoted a significant shift (P < 0.013) in monocyte phenotype, from predominantly CD14hi-expressing monocytes at baseline/pre-SCDRx to CD14low-expressing monocytes post-SCDRx.43 In a subset of patients (n = 7) presenting with T2D, this persistent decline in monocyte CD14 expression was sustained for 2 weeks after therapy.43 These results demonstrate that SCDRx has the potential to modulate the chronic pro-inflammatory state in ESRD patients.
SCDRx in Preclinical Large-Animal Models of Acute Myocardial Infarction and Chronic Heart Failure
Ischemia/reperfusion injury (IRI), characterized by a vigorous inflammatory response immediately postreperfusion via molecular signals generated by injured endothelium and cardiomyocytes, results in increased injury from leukocyte infiltration into the peri-infarct zone. This response becomes important in healing necessary to re-establish cardiac performance, but is excessive and maladaptive.
SCDRx was evaluated in a canine model of IRI, based on left circumflex coronary artery occlusion. Acute myocardial infarction was induced for 3 hours, and SCDRx was established using an extracorporeal blood circuit with RCA, 30 minutes before reperfusion and continued for up to an additional 3 hours afterward. Systemic inflammation was monitored by CD11b expression of leukocyte populations by using flow cytometry, and leukocyte infiltration was evaluated by histology. Systemic cytokine and cardiac injury marker troponin−I (cTn-I) levels were assayed. Left ventricular (LV) function, infarct sizem and edema were evaluated in treated animals (n = 3) and compared to control animals (n = 4). SCDRx resulted in a 50% reduction of infarct size (19.2 ± 2.7 vs. 10.2 ± 4.5% of LV volume respectively, P < 0.05) (Figure 4), less edema (LV wall thickening) and 10 times lower cTn-I levels.57 Leukocyte infiltration was prominent in the peri-infarct zone of controls but was negligible in the SCD group.
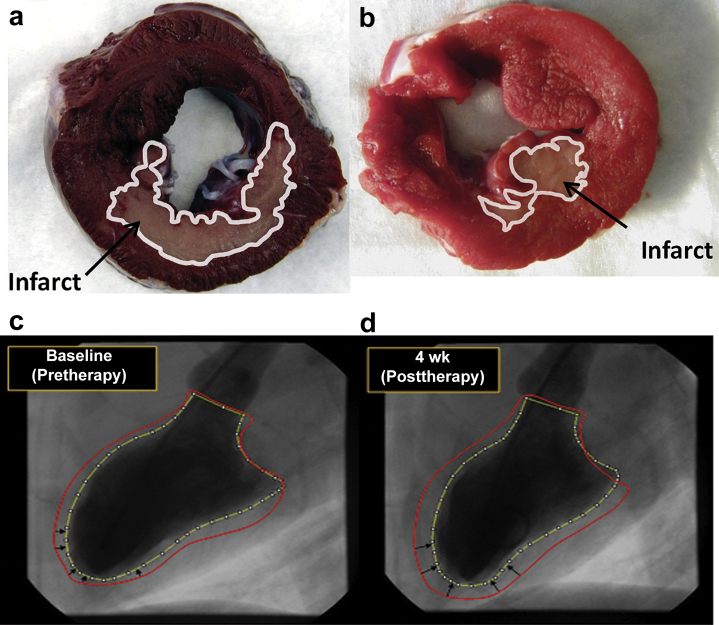
Figure 4.
(a,b) Area of the infarcted heart was evaluated using 2,3,5-triphenyltetrazolium chloride. Red indicates viable tissue; white (with corresponding outline trace) indicates irreversibly injured tissue. Uneven discoloration (darkening) of the hearts is due to residual Evans blue dye injected to identify the area at risk for infarct. Evaluation of all cross sections indicated that (b) selective cytopheretic device therapy (SCDRx) afforded a significantly reduced infarct size compared to (a) untreated controls (P < 0.05). For the chronic heart failure (CHF) model, ventriculograms of CHF canine heart: (c) baseline week 0 before SCD therapy (wk0 pre-Rx) and (d) week 4 after SCD therapy (wk4 post Rx). Red line depicts the border of the left ventricular diastolic silhouette (most relaxed state during filling); yellow line depicts the border of the left ventricular systolic image (most contracted state), demonstrating improved contractility (black arrows) of the left ventricle after SCDRx.
The SCD immunomodulatory platform therapy was also evaluated in a canine chronic heart failure (CHF) model induced by chronic trauma via multiple sequential intracoronary embolizations with microspheres.58 Dogs were administered either three 6-hour therapy sessions over 1 week (n = 7) or no treatment (n = 5). Data from an interim analysis demonstrated that LV ejection fraction (EF) increased substantially in the treatment group, from 33.6 ± 1.3 (n = 5) to 43.3 ± 2.5 (n = 5; 6−48 hours posttherapy) and 37.0 ± 0% (n = 2; 4 weeks posttherapy), reflective of 29% and 10% increases, respectively.59 In the no treatment group (n = 5), EF% did not change. This effect was not due to a decline in systemic vascular resistance, which was similar in both groups. Ventriculograms demonstrated that SCDRx converted viable but noncontracting myocardium to contracting myocardium. This benefit was maintained throughout the 4-week posttherapy follow-up period (Figure 4). Of relevance to the immunomodulatory impact of SCDRx, elevated peritoneal macrophage absolute number, which is associated with CHF,60 was lower in SCDRx dogs compared to the no-treatment dogs. In addition, the macrophage phenotype shifted from pro-inflammatory M1 seen in the no-treatment dogs to the reparative/anti-inflammatory M2 phenotype seen in SCDRx dogs (unpublished results). Modulation of peritoneal macrophages demonstrates that the SCDRx impact is not only organ specific but also affords the benefit of systemic immunomodulation. In summary, in this CHF model, SCDRx improved myocardial contractility and modulated chronic inflammation, demonstrating strong support of SCDRx as an innovative treatment approach to treat CHF.
SCDRx in Preclinical Large-Animal Models of Traumatic Brain Injury and Intracranial Hemorrhage
Inflammation has recently been recognized as a central contributor to the pathobiology of stroke and traumatic brain injury (TBI), indicating that therapies that target inflammation may provide a new approach to the treatment of the acute phase of this disease process, particularly for intracranial hemorrhage (ICH), as there are currently no interventive therapies for this subset of stroke patients. Pilot preclinical studies were initiated to determine the SCD effect on the acute inflammatory cascade in ICH.
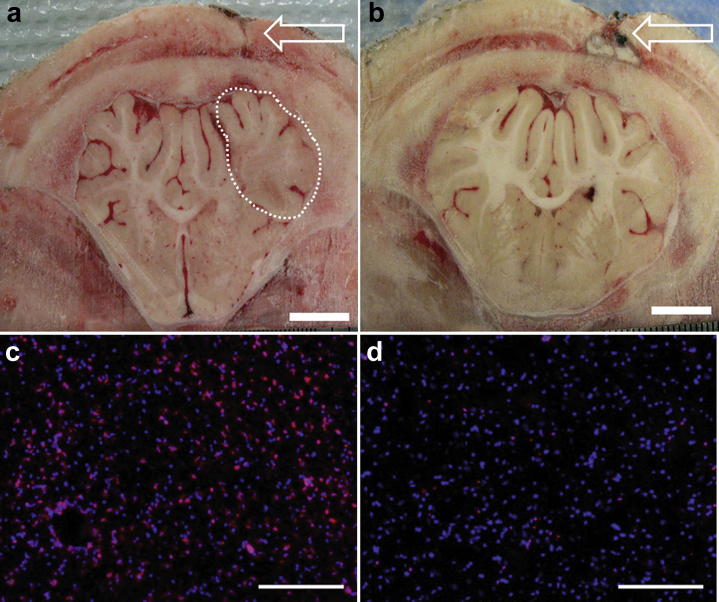
The acute effects of SCDRx were evaluated in a porcine model of ICH, for which thrombin was used as the inflammatory nidus. ICH was induced simultaneously with SCDRx by using an extracorporeal blood circuit with RCA. Therapy continued for 24 additional hours, during which systemic inflammation was monitored by assay of systemic cytokines. Platelet activation and CD11R3 expression of leukocyte populations were determined using flow cytometry. Postmortem coronal sections of frozen brain tissue were evaluated for leukocyte infiltration, and neuronal injury was evaluated by histology. SCD effects on lesion size and brain edema were evaluated in treated animals (n = 3) and compared to a contemporaneous control. SCDRx resulted in a reduction in edema (Figure 5), reduced expression of neuronal injury markers, and reduced leukocyte infiltration as evaluated in animals sacrificed at 24 hours after ictus.61 Pilot studies demonstrate that immunomodulation with the SCD represents a novel therapy that has the potential to improve outcomes associated with ICH.
Figure 5.
(a,b) Coronal brain sections are shown at the site of thrombin injection (arrows in a and b). Area of damage (demarcated by the dotted line) (a) can be identified by the lack of defined subcortical white matter due to swelling (edema) and is clearly evident in the (a) brain of the untreated control pig, but not in the (b) brain of a representative selective cytopheretic device therapy (SCDRx) animal. Bar = 1 cm. (c,d) Leukocytes (LE) normally not present in brain tissue migrate into sites of injury causing further damage. LE, identified by immunohistochemistry using a CD11R3-specific antibody (red), is more prevalent in the (c) untreated control animal, indicating that (d) SCDRx can limit damage from ICH. Nuclei of all cells are counterstained with DAPI (blue). Bar = 100 μm. SCD, selective cytopheretic device.
SCDRx in a Preclinical Large-Animal Model of Acute Lung Injury
Acute lung injury (ALI) is included within the clinical definition of acute respiratory distress syndrome (ARDS).62 ARDS affects more than 190,000 Americans annually.63 Mortality rates range from 26% to 58% and despite advances in supportive care; no pathophysiologically driven therapeutic intervention for ARDS is currently available.64 ALI results from direct (pulmonary) and indirect (extrapulmonary) injury to the lungs. Pneumonia, aspiration, pulmonary contusion, inhalation injury, and fat emboli from fractured long bones constitute direct causes of ALI, whereas indirect lung injury resulting from SIRS is observed with polytrauma requiring multiple transfusions, severe sepsis, and burns. Sepsis remains the most common cause of ARDS, with 46% of cases triggered by primary pulmonary disorders.
Aside from the use of lung-protective ventilator strategies or extracorporeal membrane oxygenation (ECMO), which are purely supportive of gas exchange while attempting to limit further lung injury, there are no pathogenesis-directed therapies for ARDS, leaving a vast unmet medical need. Patients with lung injury more commonly than not encounter more than “one hit” modulating the immunological response to injury by increasing duration and amplitude of the inflammatory response. In fact, many second “hits” occur after proinflammatory responses (e.g., SIRS) have waned, and patients manifest compensatory anti-inflammatory responses (CARS) with suppressed immunity and diminished resistance to infection. This scenario seemingly places patients at risk for manifesting clinically significant ARDS and MODS.65 Leukocytes are major contributors to the pathogenesis and progression of the inflammatory response and have been implicated in the pathogenesis of ARDS. Sequestration and infiltration in lung tissue progresses to diminish pulmonary gas exchange and to exacerbate disruption of the alveolar capillary membrane.66 Therapeutic strategies to block inflammation are expected to decrease morbidity/mortality by limiting activity and tissue accumulation of leukocytes at sites of inflammation.67, 68
Immunomodulatory effects of SCDRx during ARDS have been investigated in a pilot study using a porcine model of acid-induced ALI (Humes HD, Buffington DA, Transportable renal replacement therapy for battlefield applications DoD/TARTRC, 2011–2013 [proposal application]). In brief, pigs were anesthetized and ventilated when 0.4N HCl was delivered via a tracheal catheter. Pa:FiO2 decreased to <300 in all pigs, which were then allocated to untreated or SCDRx cohorts and followed up for 6 hours. Pulmonary vascular resistance was lower in SCDRx pigs without a concomitant decrease in systemic vascular resistance. The extent of leukocyte infiltration into the lungs at 6 hours postinjury was reduced as determined by immunohistochemical staining. Using the number of CD11R3+ events normalized for total cells identified through 4',6-diamidino-2-phenylindole (DAPI) staining, the extent of this reduction in leukocyte infiltration was quantitatively evaluated with ImageJ software (National Institutes of Health, Bethesda, MD). Leukocyte infiltration was reduced in the SCD pigs across all major lung lobes. This reduction in leukocyte infiltration into lung tissue has also been observed when assessing SCDRx in a porcine model of septic shock−associated MODS.29 Fewer neutrophils were recovered in the bronchioalveolar lavage fluid of SCDRx versus untreated ALI pigs. In the clinical setting, the concentration of neutrophils collected in bronchioalveolar lavage fluid has been shown to correlate with the severity and outcome of ARDS.69, 70 Accordingly, the significant decrease in neutrophil extravasation could possibly lead to improved long-term lung function. The impact of SCDRx on lung function has been demonstrated by an increase in ventilator-free days at 28 days during a SCD phase III clinical trial.46 Preclinical evaluation of SCDRx as a treatment for ALI in a porcine model of trauma with sepsis is currently being conducted (Humes HD, Assessment of a therapeutic device for treatment of acute lung injury using a combat-relevant porcine model. DoD/PRMRP, 2016–2019 [proposal application]).
SCDRx in a Preclinical Large-Animal Model of Type 2 Diabetes Mellitus
T2D is a complex disease whereby insulin resistance is a critical pathophysiological disorder. Obesity is associated with tissue inflammation, which is now recognized as a critical etiology of insulin resistance.71, 72, 73, 74 Circulating WBC counts, including absolute neutrophil and monocyte counts, are elevated in diabetic patients compared to nondiabetic patients.75, 76, 77 Not only do these cells of the innate immunologic system increase in absolute number, but they also exist in a persistently activated state.78, 79, 80, 81 It is clear that recruitment of circulatory monocytes to form tissue macrophages within adipose tissue is the initiating event in obesity-induced inflammation and insulin resistance.74 The internal environment of adipose tissue favors the M1 pro-inflammatory phenotype of adipocyte tissue macrophage, resulting in tissue inflammation and insulin resistance. Pro-inflammatory cytokines, produced by adipocyte tissue macrophages and other cells, have been shown to promote insulin resistance in a paracrine and endocrine fashion.82 Interventions with anti-inflammatory action therefore have beneficial effects to improve insulin sensitivity.
To evaluate the effect of SCDRx on insulin resistance in a preclinical model of T2D, an Ossabaw miniature swine model of metabolic syndrome was used. When fed an excess-calorie atherogenic diet over several months, Ossabaw swine develop at least 5 of the 6 criteria for metabolic syndrome, including primary insulin resistance, obesity with significant visceral adipose expansion, hypertriglyceridemia and increased low-density lipoprotein:high-density lipoprotein cholesterol, mild hypertension, and coronary artery disease.83, 84, 85, 86 Treatment with the SCD in this porcine model demonstrated a decline in circulating neutrophil activation parameters and monocyte counts.41 These changes were associated with improvements in insulin resistance as determined by i.v. glucose tolerance testing.41 Improvements were also reflected in lowering of homeostatic model assessment of insulin resistance (HOMA-IR) scores for up to 2 weeks after SCDRx.41
Summary
Increasingly, clinicians have recognized the critical role that the immune system plays in the response after organ injury, and the dire ramifications of excessive, dysregulated inflammation in both acute and chronic disease states, as well as the resulting impact on solid organ function. This review article has examined a number of the peer-reviewed publications, as well as work in progress, to elucidate the mechanisms of action for a novel immunomodulatory therapy, SCDRx, to treat various inflammatory disease indications. This demonstrates a growing body of clinical and preclinical support for immunomodulatory interventions.
Disclosure
HDH is a shareholder of Innovative BioTherapies Inc., and CytoPherx Inc., biotechnology spin-out companies of the University of Michigan. CJP, AJW, KAJ, and DAB are employees of Innovative BioTherapies Inc.
Acknowledgments
This work was supported by the U.S. Army Medical Research and Materiel Command, Contract W81XWH-10-2-0137, W81XWH-11-1-0615 and W81XWH-11-1-0730, National Institutes of Health by the grant HL118792, and the United States Food and Drug Administration (FDA) with FDA Grant 1R01 FD 005092, in support of the clinical trial FDA Investigational Device Exemption G150179 (ClinicalTrials.gov ID: NCT02820350). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the DoD, NIH or the FDA.
Footnotes
Definitions of Abbreviations.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
References
- 1.Buffington D.A., Westover A.J., Johnston K.A., Humes H.D. The bioartificial kidney. Transl Res. 2014;163:342–351. doi: 10.1016/j.trsl.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Pino C.J., Humes H.D. Stem cell technology for the treatment of acute and chronic renal failure. Transl Res. 2010;156:161–168. doi: 10.1016/j.trsl.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pino C.J., Yevzlin A.S., Tumlin J., Humes H.D. Cell-based strategies for the treatment of kidney dysfunction: a review. Blood Purif. 2012;34:117–123. doi: 10.1159/000341649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills C.D. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 7.Mills C.D., Kincaid K., Alt J.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 8.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon S., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barisione C., Garibaldi S., Ghigliotti G. CD14CD16 monocyte subset levels in heart failure patients. Dis Markers. 2010;28:115–124. doi: 10.3233/DMA-2010-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nockher W.A., Scherberich J.E. Expanded CD14+ CD16+ monocyte subpopulation in patients with acute and chronic infections undergoing hemodialysis. Infect Immun. 1998;66:2782–2790. doi: 10.1128/iai.66.6.2782-2790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patino R., Ibarra J., Rodriguez A. Circulating monocytes in patients with diabetes mellitus, arterial disease, and increased CD14 expression. Am J Cardiol. 2000;85:1288–1291. doi: 10.1016/s0002-9149(00)00757-8. [DOI] [PubMed] [Google Scholar]
- 14.Satoh N., Shimatsu A., Himeno A. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33:e7. doi: 10.2337/dc09-1315. [DOI] [PubMed] [Google Scholar]
- 15.Wrigley B.J., Shantsila E., Tapp L.D., Lip G.Y. CD14++CD16+ monocytes in patients with acute ischaemic heart failure. Eur J Clin Invest. 2013;43:121–130. doi: 10.1111/eci.12023. [DOI] [PubMed] [Google Scholar]
- 16.Yang M., Gan H., Shen Q. Proinflammatory CD14+CD16+ monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation. 2012;35:388–396. doi: 10.1007/s10753-011-9374-9. [DOI] [PubMed] [Google Scholar]
- 17.Fadini G.P., de Kreutzenberg S.V., Boscaro E. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia. 2013;56:1856–1866. doi: 10.1007/s00125-013-2918-9. [DOI] [PubMed] [Google Scholar]
- 18.Rogacev K.S., Seiler S., Zawada A.M. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32:84–92. doi: 10.1093/eurheartj/ehq371. [DOI] [PubMed] [Google Scholar]
- 19.Rogacev K.S., Zawada A.M., Emrich I. Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler Thromb Vasc Biol. 2014;34:2120–2127. doi: 10.1161/ATVBAHA.114.304172. [DOI] [PubMed] [Google Scholar]
- 20.Heine G.H., Ulrich C., Seibert E. CD14(++)CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–629. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 21.Woollard K.J., Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cipolletta C., Ryan K.E., Hanna E.V., Trimble E.R. Activation of peripheral blood CD14+ monocytes occurs in diabetes. Diabetes. 2005;54:2779–2786. doi: 10.2337/diabetes.54.9.2779. [DOI] [PubMed] [Google Scholar]
- 23.Tsujioka H., Imanishi T., Ikejima H. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Courties G., Moskowitz M.A., Nahrendorf M. The innate immune system after ischemic injury: lessons to be learned from the heart and brain. JAMA Neurol. 2014;71:233–236. doi: 10.1001/jamaneurol.2013.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urra X., Villamor N., Amaro S. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- 26.Humes H.D., MacKay S.M., Funke A.J., Buffington D.A. Tissue engineering of a bioartificial renal tubule assist device: in vitro transport and metabolic characteristics. Kidney Int. 1999;55:2502–2514. doi: 10.1046/j.1523-1755.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 27.Tumlin J., Wali R., Williams W. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humes H.D., Sobota J.T., Ding F., Song J.H. A selective cytopheretic inhibitory device to treat the immunological dysregulation of acute and chronic renal failure. Blood Purif. 2010;29:183–190. doi: 10.1159/000245645. [DOI] [PubMed] [Google Scholar]
- 29.Ding F., Song J.H., Jung J.Y. A biomimetic membrane device that modulates the excessive inflammatory response to sepsis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morabito S., Pistolesi V., Tritapepe L., Fiaccadori E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol. 2014;9:2173–2188. doi: 10.2215/CJN.01280214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 32.Zelensky A.N., Gready J.E. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 33.Schaff U.Y., Dixit N., Procyk E. Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow. Blood. 2010;115:657–666. doi: 10.1182/blood-2009-05-224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamblin A., Taylor M., Bernhagen J. A method of preparing blood leucocytes for flow cytometry which prevents upregulation of leucocyte integrins. J Immunol Methods. 1992;146:219–228. doi: 10.1016/0022-1759(92)90231-h. [DOI] [PubMed] [Google Scholar]
- 35.Finn A., Rebuck N. Measurement of adhesion molecule expression on neutrophils and fixation. J Immunol Methods. 1994;171:267–270. doi: 10.1016/0022-1759(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 36.Lundahl J., Hallden G., Skold C.M. Human blood monocytes, but not alveolar macrophages, reveal increased CD11b/CD18 expression and adhesion properties upon receptor-dependent activation. Eur Respir J. 1996;9:1188–1194. doi: 10.1183/09031936.96.09061188. [DOI] [PubMed] [Google Scholar]
- 37.Fontes M.L., Mathew J.P., Rinder H.M., Zelterman D., Smith B.R., Rinder C.S. Atrial fibrillation after cardiac surgery/cardiopulmonary bypass is associated with monocyte activation. Anesth Analg. 2005;101:17–23. doi: 10.1213/01.ANE.0000155260.93406.29. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez J., Alvarez B., Alonso F. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet Immunol Immunopathol. 2001;80:111–119. doi: 10.1016/s0165-2427(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 39.Wong K.L., Yeap W.H., Tai J.J. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 40.Pino C.J., Lou L., Smith P.L. A selective cytopheretic inhibitory device for use during cardiopulmonary bypass surgery. Perfusion. 2012;27:311–319. doi: 10.1177/0267659112444944. [DOI] [PubMed] [Google Scholar]
- 41.Westover A.J., Johnston K.A., Buffington D.A., Humes H.D. An immunomodulatory device improves insulin resistance in obese porcine model of metabolic syndrome. J Diabetes Res. 2016;2016:3486727. doi: 10.1155/2016/3486727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selewski D.T., Goldstein S.L., Fraser E. Immunomodulatory Device Therapy in a Pediatric Patient with Acute Kidney Injury and Multiorgan Dysfunction. Kidney International Reports. 2017 doi: 10.1016/j.ekir.2017.06.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szamosfalvi B., Westover A., Buffington D. Immunomodulatory device promotes a shift of circulating monocytes to a less inflammatory phenotype in chronic hemodialysis patients. ASAIO J. 2016;62:623–630. doi: 10.1097/MAT.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 44.Ding F., Yevzlin A.S., Xu Z.Y. The effects of a novel therapeutic device on acute kidney injury outcomes in the intensive care unit: a pilot study. ASAIO J. 2011;57:426–432. doi: 10.1097/MAT.0b013e31820a1494. [DOI] [PubMed] [Google Scholar]
- 45.Tumlin J.A., Chawla L., Tolwani A.J. The effect of the selective cytopheretic device on acute kidney injury outcomes in the intensive care unit: a multicenter pilot study. Semin Dial. 2013;26:616–623. doi: 10.1111/sdi.12032. [DOI] [PubMed] [Google Scholar]
- 46.Tumlin J.A., Galphin C.M., Tolwani A.J. A multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of a selective cytopheretic device in patients with acute kidney injury. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellomo R., Cass A., Cole L. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 48.Palevsky P.M., Zhang J.H., O'Connor T.Z. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang H.R., Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinsey G.R., Li L., Okusa M.D. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward N.S., Casserly B., Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–625. doi: 10.1016/j.ccm.2008.06.010. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayub K., Hallett M.B. Ca2+ influx shutdown during neutrophil apoptosis: importance and possible mechanism. Immunology. 2004;111:8–12. doi: 10.1111/j.1365-2567.2003.01766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Symons J.M., Chua A.N., Somers M.J. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. 2007;2:732–738. doi: 10.2215/CJN.03200906. [DOI] [PubMed] [Google Scholar]
- 54.Modem V., Thompson M., Gollhofer D. Timing of continuous renal replacement therapy and mortality in critically ill children. Crit Care Med. 2014;42:943–953. doi: 10.1097/CCM.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 55.Goldstein S.L., Somers M.J., Baum M.A. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein S., Selewski D., Asskenazi D. Multicenter evaluation of the selective cytopheteric device (SCD) in critically ill children requiring CRRT: report from the first 4 patients. J Am Soc Nephrol. 2017;28:422. [Google Scholar]
- 57.Humes HD, Buffington DA, Westover AJ, et al. Immunomodulation with a selective cytopheretic device (SCD) reduces myocardial infarct (MI) size in a canine model of ischemia-reperfusion injury (IRI). Poster presented at: American Society of Nephrology: Renal Week 2013; Atlanta, GA.
- 58.Sabbah H.N., Stein P.D., Kono T. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 59.Humes HD, Buffington DA, Westover AJ. Immunomodulatory therapy (Rx) demonstrates sustained improvement in myocardial contractility in a canine model of chronic heart failure (CHF). Poster presented at: American Society of Nephrology: Renal Week 2016; Chicago, IL.
- 60.Batista M.L., Jr., Santos R.V., Cunha L.M. Changes in the pro-inflammatory cytokine production and peritoneal macrophage function in rats with chronic heart failure. Cytokine. 2006;34:284–290. doi: 10.1016/j.cyto.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Humes HD, Buffington DA, Westover AJ, et al. Immunomodulation with a selective cytopheretic device (SCD) reduces injury in a pig model of intracranial hemorrhage (ICH). Poster presented at: American Society of Nephrology: Renal Week 2013; Atlanta, GA.
- 62.Thompson B.T., Moss M. A new definition for the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2013;34:441–447. doi: 10.1055/s-0033-1351162. [DOI] [PubMed] [Google Scholar]
- 63.American Lung Association. Lung Disease Data Report 2008. Available at: http://action.lung.org/site/DocServer/lung-disease-data-2008-report.pdf. Accessed December 21, 2017.
- 64.Modrykamien A.M., Gupta P. The acute respiratory distress syndrome. Proc [Bayl Univ Med Cent] 2015;28:163–171. doi: 10.1080/08998280.2015.11929219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mannick J.A., Rodrick M.L., Lederer J.A. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 66.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 67.Bolling K.S., Halldorsson A., Allen B.S. Prevention of the hypoxic reoxygenation injury with the use of a leukocyte-depleting filter. J Thorac Cardiovasc Surg. 1997;113:1081–1089. doi: 10.1016/S0022-5223(97)70295-2. discussion 1089–1090. [DOI] [PubMed] [Google Scholar]
- 68.Gu Y.J., de Vries A.J., Boonstra P.W., van Oeveren W. Leukocyte depletion results in improved lung function and reduced inflammatory response after cardiac surgery. J Thorac Cardiovasc Surg. 1996;112:494–500. doi: 10.1016/s0022-5223(96)70277-5. [DOI] [PubMed] [Google Scholar]
- 69.Parsons P.E., Fowler A.A., Hyers T.M., Henson P.M. Chemotactic activity in bronchoalveolar lavage fluid from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132:490–493. doi: 10.1164/arrd.1985.132.3.490. [DOI] [PubMed] [Google Scholar]
- 70.Steinberg K.P., Milberg J.A., Martin T.R. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:113–122. doi: 10.1164/ajrccm.150.1.8025736. [DOI] [PubMed] [Google Scholar]
- 71.Kahn S.E., Hull R.L., Utzschneider K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 72.Odegaard J.I., Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 74.Osborn O., Olefsky J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 75.Chung F.M., Tsai J.C., Chang D.M. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care. 2005;28:1710–1717. doi: 10.2337/diacare.28.7.1710. [DOI] [PubMed] [Google Scholar]
- 76.Shim W.S., Kim H.J., Kang E.S. The association of total and differential white blood cell count with metabolic syndrome in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;73:284–291. doi: 10.1016/j.diabres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 77.Tsai J.C., Sheu S.H., Chiu H.C. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23:111–118. doi: 10.1002/dmrr.647. [DOI] [PubMed] [Google Scholar]
- 78.Nijhuis J., Rensen S.S., Slaats Y. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity [Silver Spring] 2009;17:2014–2018. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 79.Schroder S., Palinski W., Schmid-Schonbein G.W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- 80.van Oostrom A.J., van Wijk J.P., Sijmonsma T.P. Increased expression of activation markers on monocytes and neutrophils in type 2 diabetes. Neth J Med. 2004;62:320–325. [PubMed] [Google Scholar]
- 81.Wierusz-Wysocka B., Wysocki H., Siekierka H. Evidence of polymorphonuclear neutrophils (PMN) activation in patients with insulin-dependent diabetes mellitus. J Leukoc Biol. 1987;42:519–523. doi: 10.1002/jlb.42.5.519. [DOI] [PubMed] [Google Scholar]
- 82.Lee Y.S., Li P., Huh J.Y. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dyson M.C., Alloosh M., Vuchetich J.P. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med. 2006;56:35–45. [PubMed] [Google Scholar]
- 84.Neeb Z.P., Edwards J.M., Alloosh M. Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comp Med. 2010;60:300–315. [PMC free article] [PubMed] [Google Scholar]
- 85.Spurlock M.E., Gabler N.K. The development of porcine models of obesity and the metabolic syndrome. J Nutr. 2008;138:397–402. doi: 10.1093/jn/138.2.397. [DOI] [PubMed] [Google Scholar]
- 86.Sturek M., Tune J.D., M. A . Ossabaw Island miniature swine: metabolic syndrome and cardiovascular assessment. In: Swindle M.M., Smith M.M., editors. In Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques, Third Edition. CRC Press; Boca Raton, FL: 2015. pp. 451–465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.