Abstract
The present study aims to compare the relative efficacy and safety of different interventions for IgA nephropathy (IgAN) with proteinuria more than 1 g/d by using network meta-analysis. We searched PubMed, Embase, and the Cochrane Library for studies compared the rate of clinical remission and/or end-stage renal disease (ESRD) and/or serious adverse events in IgAN patients with proteinuria (>1 g/d). The surface under the cumulative ranking area (SUCRA) was calculated to rank the interventions. A total of 21 randomized controlled trials with 1822 participants were included for the comparisons of 7 interventions. The rank of the most effective treatments to induce clinical remission was renin−angiotensin system inhibitors (RASi) plus urokinase, steroid plus tonsillectomy, and RASi plus steroid with a SUCRA of 0.912, 0.710, and 0.583, respectively. As for the prevention of ESRD or doubling of serum creatinine, RASi plus steroid (SUCRA 0.012) was the most effective, followed by RASi (SUCRA 0.282) and steroid (SUCRA 0.494), leaving mycophenolate mofetil as the least effective (SUCRA 0.644). There was no statistical difference among all interventions in the occurrence of serious adverse events. The current network meta-analysis demonstrated for the first time that RASi plus steroid is probably the best therapeutic choice, not only for reducing proteinuria but also for maintaining long-term renal protection.
Keywords: end-stage renal disease, IgA nephropathy, network meta-analysis, renin−angiotensin system, proteinuria, steroid
Ig A nephropathy (IgAN) is the most prevalent immune complex related to the cause of glomerulonephritis worldwide.1 Although the etiology and pathogenesis of IgAN are not completely understood, IgA-dominant deposition in the mesangial area has been proposed as the critical factor in the onset of IgAN.2, 3 The clinical course of IgAN is variable, and ranges from proteinuria to hematuria and even renal insufficiency. From 15% to 20% of patients with IgAN will develop end-stage renal disease (ESRD) within 10 years, and 30% to 40% within 20 years follow-up.4 Therefore, there is a need for effective treatment strategies to reduce proteinuria and to prevent a decline in kidney function. Various treatments, expected to improve long-term renal outcomes, have been applied in IgAN patients, such as use of renin−angiotensin system inhibitors (RASi), steroids, immunosuppressive agents, urokinase, and tonsillectomy, among others.1 Unfortunately, the optimal treatment of this common renal disease has not been identified.
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines 2012 regarding IgAN suggest that patients with persistent proteinuria ≥1.0 g/d despite 3 to 6 months of intensive supportive care, and an estimated glomerular filtration rate (eGFR) >50 ml/min per 1.73 m2, be treated with systemic glucocorticoids.5 However, a recent retrospective analysis including 1147 patients from the European Validation Study of the Oxford Classification of IgAN (VALIGA) cohort6 for patients with proteinuria ≥3.0 g/d demonstrated that only 4% of the individuals with supportive RASi treatment reached a level <1.0 g/d compared with 64% of those receiving corticosteroids. More recently, the Supportive versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy (STOP-IgAN)7 and the Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING)8 studies included IgAN patients with a broad range of proteinuria (>0.75 or 1.0 g/d) yielded an inconsistent outcome. Therefore, it is necessary to analyze the optimal therapeutic strategies for the population with proteinuria (>1 g/d).
Network meta-analysis (NMA) enables indirect comparison using a common comparator and combines direct and indirect comparisons to synchronously assess multiple treatments. In this approach, X versus Y is assessed by looking at X to Z and Y to Z.9, 10, 11 The present study therefore aims to compare the relative efficacy and safety of different therapies for IgAN with proteinuria >1 g/d by using NMA.
Methods
Data Sources and Search Strategy
We searched PubMed, MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and the Chinese Biomedical Database for articles to 19 August 2017, without any language restriction, with key words and Medical Subject Headings that covered the following: “IgAN” or “IgA nephropathy” or “immunoglobulin A nephropathy” or “IgA nephritis” and “RASi,” or “steroid” or “mycophenolate mofetil (MMF)” or “urokinase” or “tonsillectomy.” We also reviewed the corresponding reference list of each retrieved article to identify any relevant studies that may be neglected. We reported the meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12
Selection Criteria
In this meta-analysis, we collected all randomized controlled trials (RCTs) regarding comparison of therapeutic effects of different drugs in IgAN patients with proteinuria >1 g/d. Inclusion criteria for studies were as follows: (i) study population comprised patients with biopsy-proven IgAN; (ii) study design was RCT; (iii) subjects with proteinuria or 24-hour urinary protein excretion >1 g/d and renal function (eGFR ≥20 ml/min per 1.73 m2 or serum creatinine ≤354 μmol/l (4 mg/dl), and articles provided exact data on clinical remission and/or ESRD or doubling of serum creatinine level between patients in treatment group and control group.
In this NMA, clinical remission was defined as the disappearance of urine abnormalities, proteinuria <0.3 g/d, or a decrease of proteinuria by 50% or more. The definition of ESRD was based on a serum creatinine level >707 μmol/l or 8 mg/dl or the initiation of dialysis therapy or kidney transplantation. Serious adverse events (SAEs) were defined according to the International Conference on Harmonization of Guidelines for Clinical Safety Data Management. The definition of SAEs was based on 1 of the following conditions: all-cause mortality, serious infection, gastrointestinal hemorrhage, new diabetes, fracture or osteonecrosis, and cardiovascular events.
Criteria for exclusion were as follows: (i) studies such as systemic reviews, comments, case reports, conference abstracts, and editorials; (ii) articles that had no definitions on clinical remission or renal function; and (iii) subjects with mild or severe proteinuria, or pathology confirmed as crescent.
Included trials reported comparisons of 7 interventions (placebo, RASi, steroid, MMF, steroid + RASi, RASi + urokinase, and tonsillectomy combined with steroid pulse therapy [TSP]). Supportive and immunosuppressive interventions were classified according to the type of drugs, monotherapy or combination, regardless of dose. NMA integrates data from direct comparisons of treatments within trials and from indirect comparisons of interventions assessed against a common comparator in separate trials to compare all investigated treatments.
Data Extraction and Quality Assessment
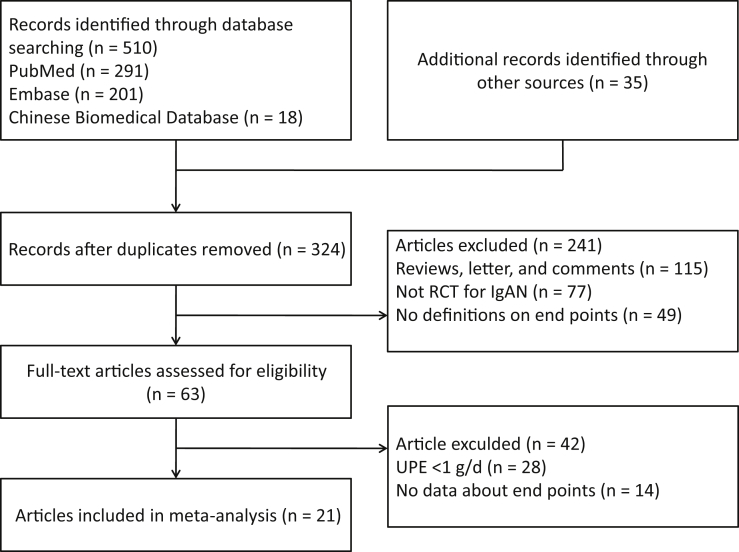
Two authors (PY and HZ) abstracted data and quality assessment independently into an electronic database. The investigators cross-checked the data and reached consensus for any discrepancies through discussion. Disagreements were settled through discussions or referral to a third author (GX). All potentially eligible citations that we had searched were examined in detail to identify studies that satisfied our criteria. Reference lists of identified trials and review articles were manually scanned to identify related research references at the same time (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of identification process for eligible articles. IgAN, IgA nepropathy; RCT, randomized controlled trial; UPE, urinary protein excretion.
The extracted data included name of first author, year of publication, kidney function, proteinuria, sample size, doses and modalities of treatment, control, follow-up duration, steroid doses and modalities of treatment, number of patients receiving control treatment/condition, and outcome of proteinuria or kidney function.
The RCT quality assessment was completed by using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) risk of bias tool including selection, performance, detection, attrition, reporting, and other bias.
Risk of Bias Assessment
Two authors (PY and HZ) independently assessed the methodological quality of included trials using a slightly adapted version of the risk of bias approach of the Cochrane Collaboration. The publication bias assessment was performed via Deek funnel plot asymmetry.
Statistical Analysis
Data were abstracted and analyzed by R software (version 3.3.2, R Foundation for Statistical Computing, China) using the “gemtc” package, STATA (version 14.0, Stata MP, StataCorp, College Station, TX), and WinBUGS (version 1.4.3, MRC Biostatistics Unit, Cambridge, UK). Odds ratios (ORs) and corresponding 95% confidence interval (CIs) were used to compare different medications with respect to various clinical outcomes. Before conducting the NMA, we conducted conventional pairwise meta-analyses for treatments that were directly compared in RCTs by R software using relative forest plots. NMA was conducted in a Bayesian random-effects model assuming a binomial likelihood and executed by R software using the “gemtc” package, which recalls JAGS in R for Markov chain Monte Carlo (MCMC) sampling. For each analysis, we generated 5000 simulations for each of the 2 sets of different initial values and discarded the first 2000 simulations as the burn-in period. The stability of the results was calculated by sensitivity analyses with discarding of each study sequentially. Convergence was checked using trace plots and the Brooks-Gelman-Rubin diagnostic.13 Ranking probabilities with respect to each clinical outcomes were obtained by using the surface under the cumulative ranking curve (SUCRA).14 Thus, larger SUCRA scores might indicate a higher probabilities of the end-point event. We also used Loop-specific inconsistency (used in STATA) and node-splitting approach (used in R with the “gemtc” package) to assess the inconsistency that is the actual differences between direct and indirect comparisons.15 Sensitivity analysis was conducted in R.
Results
Description of Included Studies
We identified 545 unique records from our searches. A total of 21 RCTs comprising 1822 participants were eligible for inclusion in our NMA; selection process details are shown in the Figure 1. Trials included were published from 2000 to 2017. Supplementary Table S1 summarizes the essential baseline characteristics of these studies. In 21 studies, 18 trials investigated the end-point event of clinical remission; 17 studies provided data on progression to ESRD and creatinine fold elevation; and 15 studies provided data on SAEs. The number of patients included in each study ranged from 32 to 262, and the follow-up for patients ranged from 3 to 120 months. Of these studies, 2 compared RASi with RASi plus antiplatelet drugs. Three trials compared placebo with steroids. Two trials compared placebo with RASi or MMF. In addition, 3 trials compared steroids with tonsillectomy with steroids pulse therapy. The overall risk of bias is shown in Supplementary Figure S1.
Exploration of Network Structure, Heterogeneity, and Consistency
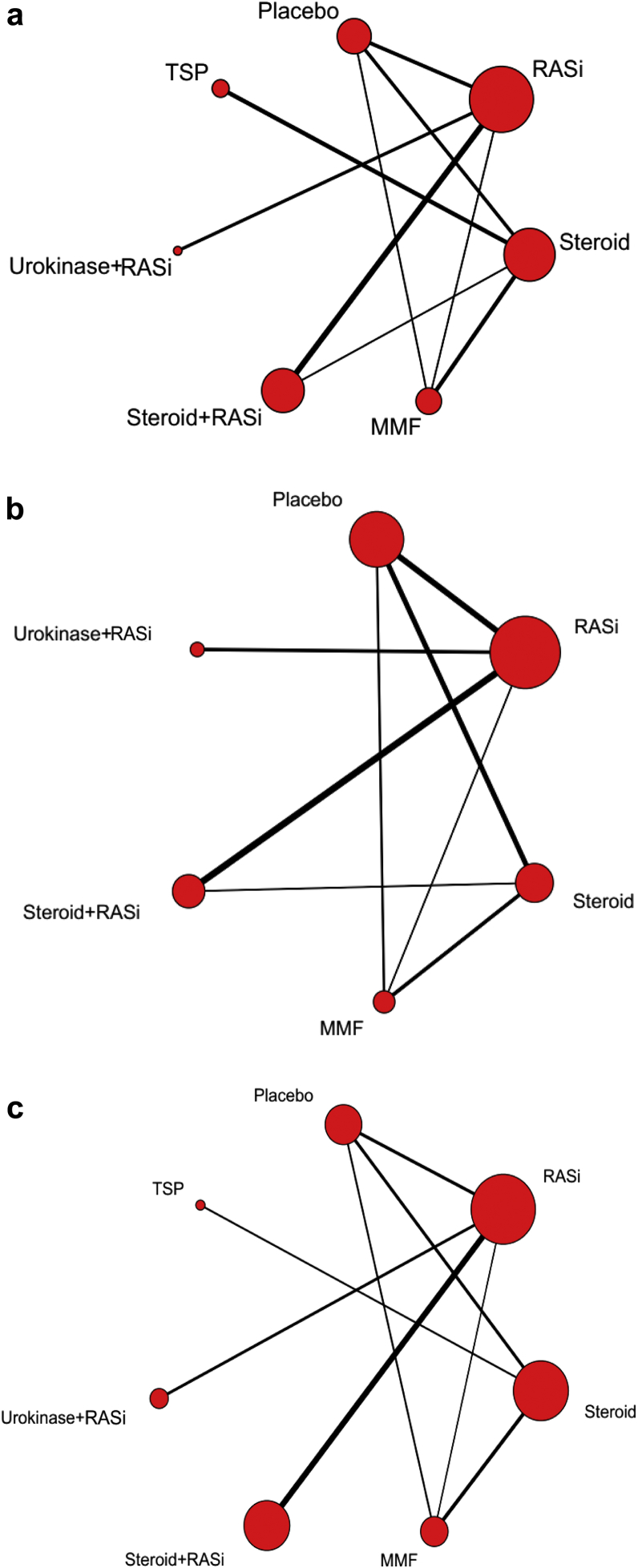
A network plot of treatment comparisons for Bayesian NMA is shown in Figure 2. There are 7 interventions for clinical remission, 6 for a doubling of serum creatinine level or ESRD, and 7 for SAEs. The size of the nodes (red circles) corresponds to the sample size of interventions. The comparisons are connected by a straight line, of which the thickness corresponds to the number of trials that assessed the comparison. As shown in the network plot, the number of interventions varied in different subjects.
Figure 2.
Network plot of treatment comparisons for Bayesian network meta-analysis. Lines represent trials comparing 2 classes of drug or drugs for (a) clinical remission, (b) end-stage renal disease and doubling of serum creatinine level, and (c) serious adverse events of IgA nephropathy. The size of the nodes (red circles) corresponds to the sample size of the interventions. Comparisons are linked with a line, of which the thickness corresponds to the number of trials that assessed the comparison. MMF, mycophenolate mofetil; RASi, renin−angiotensin system inhibitor; TSP, tonsillectomy combined with steroid pulse therapy.
First, 50,000 instances of iterations were increased to obtain satisfactory convergence, as shown in diagnostic and trace plots (Supplementary Figure S2). There was low heterogeneity in the results of disease remission, ESRD, and SAEs (Supplementary Figure S3). Pairwise and NMA estimates were similar in magnitude, and testing did not reveal evidence of inconsistency between direct and indirect treatment effects (Supplementary Figure S4). There was no sign of global inconsistency in any network.
Primary Outcomes—Clinical Remission
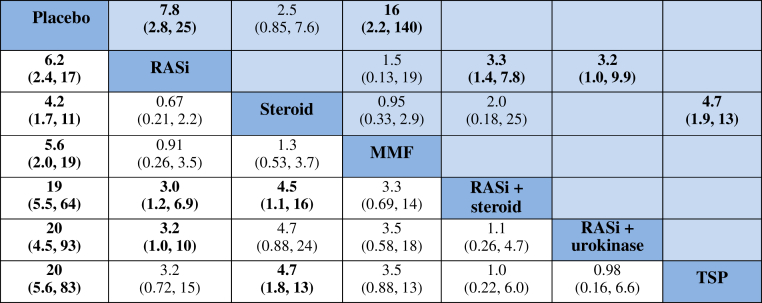
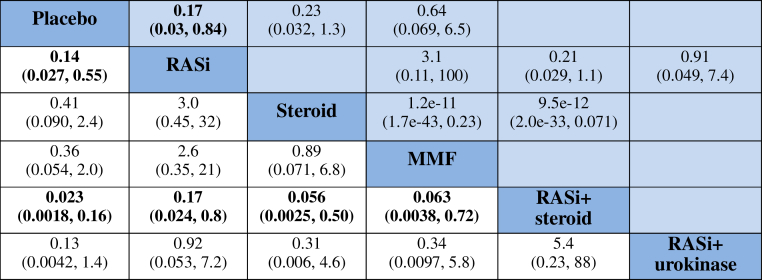
Compared with placebo, the most effective treatments to induce remission in moderate- to high-quality evidence were use of RASi alone, steroid alone, MMF alone, RASi combined with steroid, RASi combined with urokinase, or TSP, as follows: OR = 6.2, (95% CI = 2.4−17), OR = 4.2 (95% CI = 1.7−11), OR = 5.6 (95% CI = 2.0−19), OR = 19 (95% CI = 5.5−64), OR = 20 (95% CI = 4.5−64), and OR = 20 (95% CI = 5.6−83), respectively. The combination of RASi plus urokinase ranked as the best treatment to induce remission (Figure 3).
Figure 3.
Summary of results from network meta-analysis (on the lower triangle) and traditional pairwise meta-analysis (on the upper triangle) on clinical remission. On the lower triangle, the column-defining treatment is compared with the row-defining treatment, and odds ratios (ORs) of < 1 favor the column-defining treatment. On the upper triangle, the row-defining treatment is compared with the column-defining treatment, and ORs of < 1 favor the row-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Significant results are shown in boldface type. MMF, mycophenolate mofetil; RASi, renin angiotensin system inhibitor; TSP, tonsillectomy combined with steroid pulse therapy.
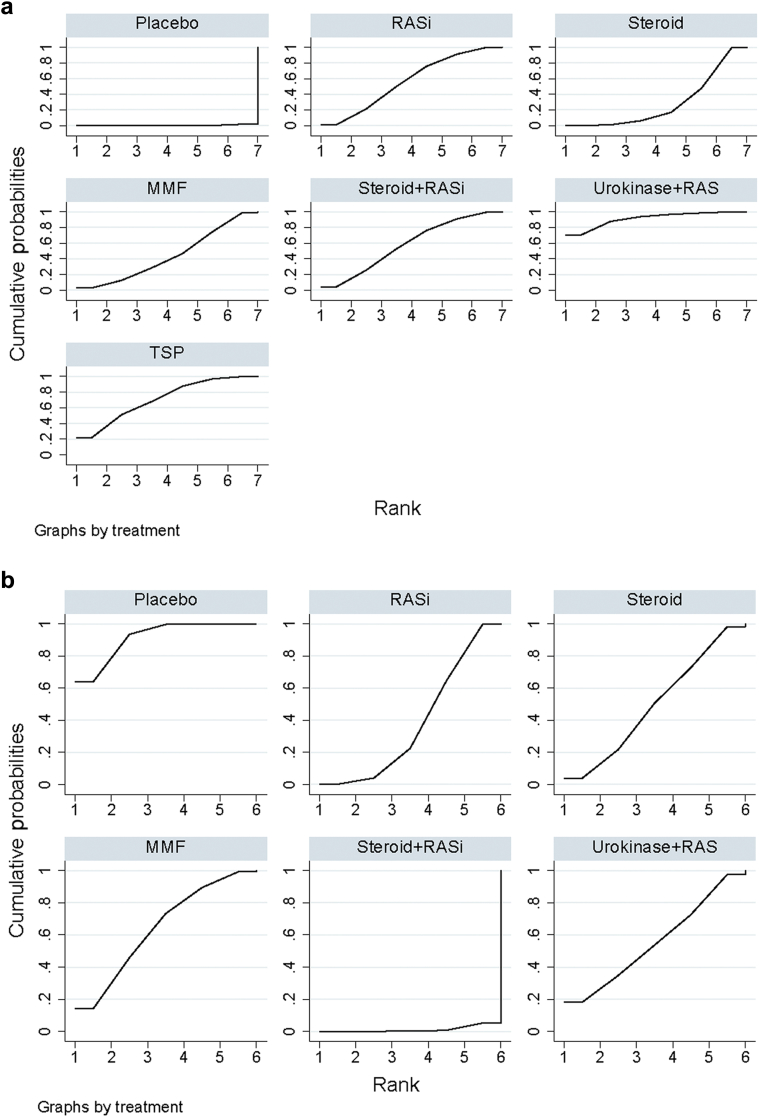
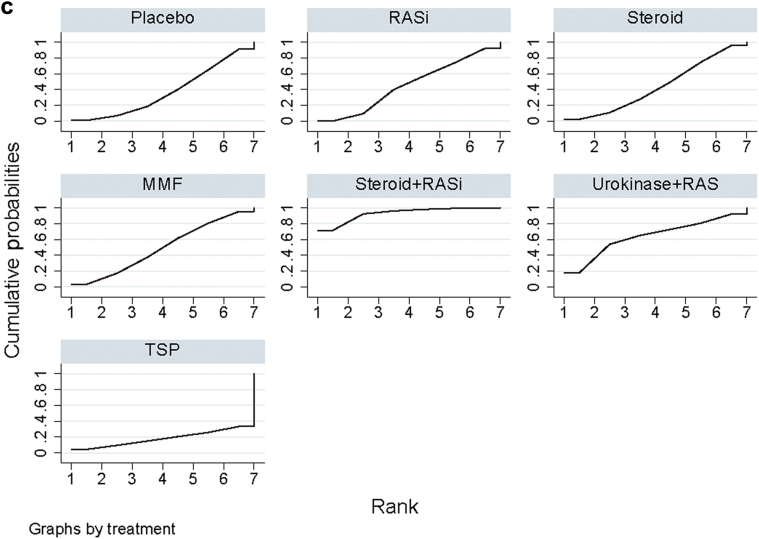
RASi plus urokinase and TSP were most likely to be ranked the best or second best (SUCRA of 0.912 and 0.710, respectively; Figure 4a and Supplementary Table S2). They were followed by RASi combined with steroid, RASi alone, and MMF (SUCRA of 0.583, 0.565, and 0.441, respectively). Placebo was ranked as the least effective treatment.
Figure 4.
Rankings of surface under the cumulative ranking area (SUCRA) for efficacy of treatments to induce end points in IgA nephropathy. (a) Clinical remission, (b) end-stage renal disease (ESRD) and doubling of serum creatinine level, and (c) serious adverse events (SAEs) of IgAN. The graphs display the distribution of probabilities of treatment, ranking from the best through the worst for each outcome. Ranking indicates the probability that the drug class is “best,” second-“best,” etc. For example, RAS inhibitors plus urokinase and steroid combined with tonsillectomy were among the best treatments for inducing disease remission, while the placebo provided the lowest probability of disease remission (worst). On the other hand, the ranking suggests that MMF posed the highest risk for incurring ESRD or creatinine doubling (worst), whereas renin−angiotensin system (RAS) inhibitors (RASi) plus steroid incurred the lowest probability of ESRD or creatinine doubling (best). MMF, mycophenolate mofetil; TSP, tonsillectomy combined with steroid pulse therapy.
Secondary Outcomes
ESRD or Doubling of Serum Creatinine
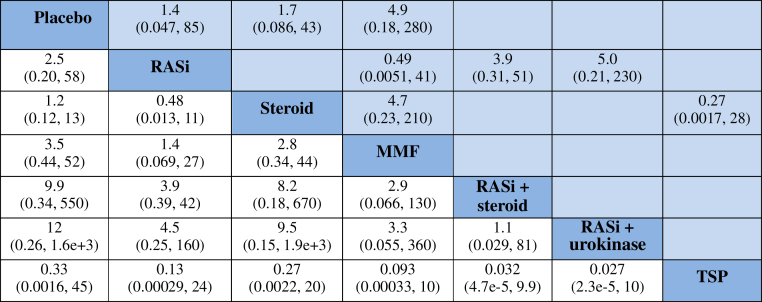
Compared with placebo, RASi plus steroid and RASi alone had lower risks for ESRD or doubling of serum creatinine level (OR = 0.023, 95% CI = 0.0018−0.16, and OR = 0.14, 95% CI = 0.027−0.55, respectively) and were ranked the first and second treatments. There was no evidence that steroid combined with tonsillectomy, or RASi combined with urokinase, had different effects on ESRD or doubling of serum creatinine level compared with RASi or with each other (Figure 5).
Figure 5.
Summary of results from network meta-analysis (on the lower triangle) and traditional pairwise meta-analysis (on the upper triangle) on end-stage renal disease and doubling of serum creatinine level. On the lower triangle, the column-defining treatment is compared with the row-defining treatment, and odds ratios (ORs) > 1 favor the column-defining treatment. On the upper triangle, the row-defining treatment is compared with the column-defining treatment, and an OR > 1 favors the row-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Significant results are shown in boldface type. Direct comparisons within 2 inconsistent loops are underlined. MMF, mycophenolate mofetil; RASi, renin−angiotensin system inhibitors.
As can be seen in Figure 4b, regarding prevention of ESRD or doubling of serum creatinine level, RASi plus steroid (SUCRA 1.2%) was the most effective treatment, followed by RASi alone (SUCRA 28.2%) and steroid alone (SUCRA 49.4%). MMF was the least effective in preventing progression to ESRD (SUCRA 64.4%).
Serious Adverse Events
For SAEs, all interventions available that were mentioned in the articles were not significant in pair-wise meta-analysis and NMA, as depicted in Figures 4c and 6, and Supplementary Figure S5. In addition, there were no statistical differences among all interventions (including steroid and RASi) in the occurrence of SAEs.
Figure 6.
Summary of results from network meta-analysis (on the lower triangle) and traditional pairwise meta-analysis (on the upper triangle) on serious adverse events. On the lower triangle, the column-defining treatment is compared with the row-defining treatment, and odds ratios (ORs) of > 1 favor the column-defining treatment. On the upper triangle, the row-defining treatment is compared with the column-defining treatment, and ORs of > 1 favor the row-defining treatment. To obtain ORs for comparisons in the opposite direction, reciprocals should be taken. Direct comparisons within 2 inconsistent loops are underlined. MMF, mycophenolate mofetil; RASi, renin−angiotensin system inhibitors; TSP, tonsillectomy combined with steroid pulse therapy.
Sensitivity Analysis and Publication Bias
A sensitivity analysis was conducted to examine the impact of studies according to the treatment effects on clinical remission, ESRD, or doubling of serum creatinine level. As there was only 1 trial with steroid plus tonsillectomy and there were only 98 patients in the trial, we eliminated it to obtain new results with no significant differences among the various interventions as before, indicating that the results for clinical remission, ESRD, or a doubling of serum creatinine level were broadly robust. Publication bias was tested by funnel plot (Supplementary Figure S5).
Discussion
With regard to efficacy and safety of therapy, this NMA indicated that use of RASi plus urokinase, steroid combined with tonsillectomy, and RASi plus steroid were superior to RASi alone for inducing remission. However, the longer-term effects of supportive or immunosuppressive treatment on risk for mortality remain uncertain, in part due to the short duration of the available studies and the comparative rarity of these events. Compared with RASi, placebo and MMF were associated with higher risks of ESRD or a doubling of serum creatinine levels. RASi plus steroid was associated with lower risk for worsening renal function, was the best treatment for maintaining disease remission, and was superior to RASi or steroid alone. For SAEs, all interventions available were not significant in pairwise meta-analysis and NMA.
The current cumulative evidence showed that angiotensin-converting enzyme inhibitor and angiotensin receptor blocker agents had statistically significant effects on reduction of proteinuria and protecting renal function in patients with IgAN.16 KDIGO guidelines recommend long-term angiotensin-converting enzyme inhibitor or angiotensin receptor blocker treatment when proteinuria is >1 g/d.5 RASi agents are fundamental in the long-term management of progressive IgAN because they stabilize systemic and renal blood pressure, then reduce proteinuria, and protect renal function.17, 18 In our study, however, the efficacy of RASi agents was less effective than RASi combined with steroid or urokinase for inducing clinical remission or renal function.
In a recent study, the efficacy of RASi combined with steroid in the treatment of patients with IgAN remains controversial. The STOP-IgAN trial7 found that the addition of immunosuppressive therapy to intensive supportive care (i.e., angiotensin-converting enzyme inhibitor use) in IgAN patients with persistent proteinuria of ≥1 g/d did not significantly improve clinical remission or eGFR, and more adverse effects were observed among the patients who received immunosuppressive therapy. Among patients with IgAN and proteinuria of ≥1 g/d, this primary outcome of the Therapeutic Evaluation of Steroids in IgA Nephropathy Global (TESTING) study8 was that oral methylprednisolone use was consistent with potential renal benefit but was associated with an increased risk of SAEs, primarily infections. The double-blind Targeted-Release Budesonide Versus Placebo in Patients With IgA Nephropathy (NEFIGAN) study19 aimed to assess the safety and efficacy of a novel targeted-release formulation of budesonide (TRF-budesonide); TRF-budesonide 16 mg/d, added to optimized RASi blockade, reduced proteinuria and the risk of future progression to ESRD in IgAN patients. TRF-budesonide could become the first specific treatment for IgAN targeting of intestinal mucosal immunity upstream of disease manifestation. Our NMA found that there were no statistical differences among all interventions (including steroid and RASi) in the occurrence of SAEs.
Urokinase apparently alters glomerular permeability and effectively reduces proteinuria by a non−blood pressure, non−RAS-related mechanism. Although the complete mechanism of urokinase effects in the kidney has not been fully identified, urokinase targets metabolic defects in the prevention and correction of thickening of the glomerular basement membrane as well as suppression of proteinuria-induced endothelial cell endothelin production.20, 21, 22, 23 A simple explanation of the efficacy of sulodexide and related compounds is that they restore the anionic heparin sulfate charges on the glomerular basement membrane. Sulodexide and related compounds may further reduce proteinuria in patients who display a partial response to RASi. As a result, urokinase could add to the therapeutic options for IgAN patients who do not respond to RASi. Bang et al. suggest that sulodexide had an additional antiproteinuric effect in IgAN patients who had already been treated with RASi.24 Our NMA shows that urokinase combined with RASi has the highest efficacy in clinical remission but does not reduce the rate of creatinine doubling and progression to ESRD. The research samples involving urokinase combined with RASi in our NMA are not very large, and they are all from Asian populations. Therefore, the results deserve further study.
Until recently, the question as to whether tonsillectomy is an effective method in the treatment of patients with IgAN has remained controversial. As we know, the galactose-deficient IgA1 (GdIgA1) has a deficiency in the O-glycan located at the hinge region of IgA1.25 GdIgA1 combined with its antibodies form a circulating immune complex, which eventually deposits in renal areas and causes kidney deterioration.26 Nakata et al. found that serum GdIgA1 levels decreased by 59% after tonsillectomy, thus indicating that the palatine tonsils are probably a major sites of GdIgA1-producing cells.27 One multicenter RCT conducted in Japan showed that tonsillectomy combined with steroid pulse therapy had no benefit effect over steroid pulse therapy alone in increasing the incidence of clinical remission. A meta-analysis of prospective and retrospective studies indicates that TSP may induce clinical remission and reduce the rates of ESRD in patients with IgAN.28
Results from several multicenter RCTs showed that MMF did not reduce proteinuria significantly in patients with IgAN who had persistent proteinuria after RASi,29, 30 but was associated with fewer SAEs in patients with IgAN with active proliferative lesions.30 Independent systematic reviews by Tan et al.31 and Xu et al.32 concluded that the evidence did not support the use of MMF in moderately advanced IgAN. Floege et al.33 offered a possible interpretation for the different outcomes observed in the Chinese patients compared with those studied in the United States and Belgium. Our study shows that MMF is associated with a higher risk of progression to ESRD and is less effective in achieving clinical remission than RASi alone in the treatment of IgAN with moderate proteinuria.
The pathogenesis of IgAN is recognized as an autoimmune renal disease that is a consequence of increased circulating levels of GdIgA1 and antiglycan autoantibodies, which is also 1 of the rationales for the use of immunosuppressive treatment in patients with IgAN.34 However, the efficacy of immunosuppressive therapy is unclear. The STOP-IgAN trial,7 which included patients with IgAN and persistent proteinuria with protein excretion >1 g/d, showed no benefit of adding immunosuppressive treatment to intensive supportive care, both in terms of change in eGFR after 3 years of follow-up and the development of ESRD. However, histologic findings were not taken into consideration in this study. In 2009, the Oxford Classification of IgAN identified 4 histopathologic features of prognostic value, namely, mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental glomerulosclerosis (S), and tubular atrophy and interstitial fibrosis (T).35 Our published meta-analysis showed that IgAN patients with serious pathological changes (M1, S1, and T1/2) were more resistant to steroid/immunosuppressive therapy than patients with slight changes (M0, S0, and T0). Patients with M1, S1, and T1/2 were resistant to steroid/immunosuppressive therapy, and E1 is a better response to steroid therapy than T1/2.36
Our study has potential limitations. First, the dosage and use of steroid are not uniform; some use methylprednisolone pulse therapy for 3 days and then half dose of steroid, and others directly sufficient oral prednisone, and there are also differences in the courses of treatment. Second, the exploration of the source of heterogeneity and bias on account of scant evidence relating to the end points needs to be further discussed. Third, except for the crescentic type, the effect of pathological types on treatment was not considered. Fourth, we did not consider the stage of renal function at the beginning of the studies. Finally, although there is evidence that heterogeneity in the network analyses is low, it is most likely that this is due to low power to detect heterogeneity because of limited data. Due to insufficient data being available, there is a need to perform larger, multicenter RCTs to obtain more robust results.
In conclusion, to our knowledge, in IgAN patients with urinary protein excretion of >1 g/d, the current NMA demonstrated for the first time that RASi plus steroid is probably the best therapeutic choice, not only for reducing proteinuria but also for maintaining long-term renal protection.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. H0517/81560132), the Supporting Project for the Foregoers of Main Disciplines of Jiangxi Province (No. 20162BCB22023), and the “5511” Innovative Drivers for Talent Teams of Jiangxi Province (No. 20165BCB18018).
Acknowledgments
Author Contributions
PY and HZ performed the meta-analysis; BX was responsible for the statistical analysis; and GX prepared the manuscript. All authors have reviewed and agreed to this information before submission.
Footnotes
Table S1. Characteristics of RCTs included in the study.
Table S2. Rankings of SUCRA for efficacy and safety of treatments to induce end points in IgAN.
Figure S1. Risk of bias of included studies. (A) Risk of bias graph. (B) Risk of bias summary.
Figure S2. Diagnostics and trace plots. (A) Diagnostic plot for clinical remission. (B) Diagnostic plot for end stage renal disease (ESRD) or doubling of serum creatinine level. (C) Diagnostic plot for severe adverse events (SAEs). (A) Trace plots for clinical remission. (B) Trace plots for ESRD or doubling of serum creatinine level. (C) Trace plots for SAEs.
Figure S3. Heterogeneity analysis on end points. (A) Heterogeneity analysis on clinical remission. (B) Heterogeneity analysis on ESRD or doubling of serum creatinine level. (C) Heterogeneity analysis on SAEs.
Figure S4. Inconsistency using node-splitting approach. (A) Inconsistency using the node-splitting approach for clinical remission. (B) Inconsistency using the node-splitting approach for ESRD or doubling of serum creatinine level. (C) Inconsistency using the node-splitting approach for SAEs.
Figure S5. Publication bias of funnel plot. (A) Funnel plot for clinical remission. (B) Funnel plot for ESRD or doubling of serum creatinine level. (C) Funnel plot for SAEs.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Characteristics of RCTs included in the study.
Rankings of SUCRA for efficacy and safety of treatments to induce end points in IgAN.
Risk of bias of included studies. (A) Risk of bias graph. (B) Risk of bias summary.
Diagnostics and trace plots. (A) Diagnostic plot for clinical remission. (B) Diagnostic plot for end stage renal disease (ESRD) or doubling of serum creatinine level. (C) Diagnostic plot for severe adverse events (SAEs). (A) Trace plots for clinical remission. (B) Trace plots for ESRD or doubling of serum creatinine level. (C) Trace plots for SAEs.
Heterogeneity analysis on end points. (A) Heterogeneity analysis on clinical remission. (B) Heterogeneity analysis on ESRD or doubling of serum creatinine level. (C) Heterogeneity analysis on SAEs.
Inconsistency using node-splitting approach. (A) Inconsistency using the node-splitting approach for clinical remission. (B) Inconsistency using the node-splitting approach for ESRD or doubling of serum creatinine level. (C) Inconsistency using the node-splitting approach for SAEs.
Publication bias of funnel plot. (A) Funnel plot for clinical remission. (B) Funnel plot for ESRD or doubling of serum creatinine level. (C) Funnel plot for SAEs.
References
- 1.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.Lin M., Du L., Brandtzaeg P., Pan-Hammarstrom Q. IgA subclass switch recombination in human mucosal and systemic immune compartments. Mucosal Immunol. 2014;7:511–520. doi: 10.1038/mi.2013.68. [DOI] [PubMed] [Google Scholar]
- 3.Roberts I.S. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10:445–454. doi: 10.1038/nrneph.2014.92. [DOI] [PubMed] [Google Scholar]
- 4.Appel G.B., Waldman M. The IgA nephropathy treatment dilemma. Kidney Int. 2006;69:1939–1944. doi: 10.1038/sj.ki.5000434. [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan J., Cattran D.C. The KDIGO practice guideline on glomerulonephritis: reading between the (guide) lines—application to the individual patient. Kidney Int. 2012;82:840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 6.Tesar V., Troyanov S., Bellur S. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA Study. J Am Soc Nephrol. 2015;26:2248–2258. doi: 10.1681/ASN.2014070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauen T., Eitner F., Fitzner C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 8.Lv J., Zhang H., Wong M.G. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318:432–442. doi: 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ades A.E., Sculpher M., Sutton A. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1–19. doi: 10.2165/00019053-200624010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Song F., Altman D.G., Glenny A.M., Deeks J.J. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G., Ades A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 13.Brooks S.P., Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 14.Salanti G., Ades A.E., Ioannidis J.P. Graphical methods and numerical summaries for presenting results from multiple treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalter D.J., Best N.G., Carlin B.P., Angelika V.D.L. Bayesian measures of model complexity and fit. J R Stat Soc. 2002;64:583–639. [Google Scholar]
- 16.Cheng J., Zhang X., Tian J. Combination therapy an ACE inhibitor and an angiotensin receptor blocker for IgA nephropathy: a meta-analysis. Int J Clin Pract. 2012;66:917–923. doi: 10.1111/j.1742-1241.2012.02970.x. [DOI] [PubMed] [Google Scholar]
- 17.Hebert L.A., Wilmer W.A., Falkenhain M.E. Renoprotection: one or many therapies? Kidney Int. 2001;59 doi: 10.1046/j.1523-1755.2001.0590041211.x. 1211–1126. [DOI] [PubMed] [Google Scholar]
- 18.Wilmer W.A., Rovin B.H., Hebert C.J. Management of glomerular proteinuria: a commentary. J Am Soc Nephrol. 2003;14:3217–3232. doi: 10.1097/01.asn.0000100145.27188.33. [DOI] [PubMed] [Google Scholar]
- 19.Fellström B.C., Barratt J., Cook H. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–2127. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
- 20.Caenazzo C., Garbisa S., Ceol M. Heparin modulates proliferation and proteoglycan biosynthesis in murine mesangial cells: molecular clues for its activity in nephropathy. Nephrol Dial Transplant. 1995;10:175–184. [PubMed] [Google Scholar]
- 21.Lewis E.J., Xu X. Abnormal glomerular permeability characteristics in diabetic nephropathy: implications for the therapeutic use of low-molecular weight heparin. Diabetes Care. 2008;31(suppl 2):S202–S207. doi: 10.2337/dc08-s251. [DOI] [PubMed] [Google Scholar]
- 22.Goldshmidt O., Zcharia E., Cohen M. Heparanase mediates cell adhesion independent of its enzymatic activity. FASEB J. 2003;17:1015–1025. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- 23.Gambaro G., Kong N.C. Glycosaminoglycan treatment in glomerulonephritis? An interesting option to investigate. J Nephrol. 2010;23:244–252. [PubMed] [Google Scholar]
- 24.Bang K., Chin H.J., Chae D.W. Anti-proteinuric effect of sulodexide in immunoglobulin A nephropathy. Yonsei Med J. 2011;52:588–594. doi: 10.3349/ymj.2011.52.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mestecky J., Raska M., Julian B.A. IgA nephropathy: molecular mechanisms of the disease. Annu Rev Pathol. 2013;8:217–240. doi: 10.1146/annurev-pathol-011110-130216. [DOI] [PubMed] [Google Scholar]
- 26.Novak J., Julian B.A., Tomana M., Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2008;28:78–87. doi: 10.1016/j.semnephrol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata J., Suzuki Y., Suzuki H. Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One. 2014;9:e89707. doi: 10.1371/journal.pone.0089707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L.L., Wang L.N., Jiang Y. Tonsillectomy for IgA nephropathy: a meta-analysis. Am J Kidney Dis. 2015;65:80–87. doi: 10.1053/j.ajkd.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Hogg R.J., Bay R.C., Jennette J.C. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis. 2015;66:783–791. doi: 10.1053/j.ajkd.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Hou J.H., Le W.B., Chen N. Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis. 2017;69:788–795. doi: 10.1053/j.ajkd.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Tan C.H.R., Loh P.T., Yang W.S., Chan C.M. Mycophenolate mofetil in the treatment of IgA nephropathy: a systematic review. Singapore Med J. 2008;49:780–785. [PubMed] [Google Scholar]
- 32.Xu G., Tu W., Jiang D., Xu C. Mycophenolate mofetil treatment for IgA nephropathy: a meta-analysis. Am J Nephrol. 2009;29:362–367. doi: 10.1159/000168483. [DOI] [PubMed] [Google Scholar]
- 33.Floege J., Eitner F. Current therapy for IgA nephropathy. J Am Soc Nephrol. 2011;22:1785–1794. doi: 10.1681/ASN.2011030221. [DOI] [PubMed] [Google Scholar]
- 34.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 35.Cattran D.C., Coppo R., Cook H.T. The Oxford Classification of IgA Nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 36.Yang P., Chen X., Zeng L. The Response of the Oxford Classification to steroid in IgA nephropathy: a systematic review and meta-analysis. Oncotarget. 2017;8:59748–59756. doi: 10.18632/oncotarget.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of RCTs included in the study.
Rankings of SUCRA for efficacy and safety of treatments to induce end points in IgAN.
Risk of bias of included studies. (A) Risk of bias graph. (B) Risk of bias summary.
Diagnostics and trace plots. (A) Diagnostic plot for clinical remission. (B) Diagnostic plot for end stage renal disease (ESRD) or doubling of serum creatinine level. (C) Diagnostic plot for severe adverse events (SAEs). (A) Trace plots for clinical remission. (B) Trace plots for ESRD or doubling of serum creatinine level. (C) Trace plots for SAEs.
Heterogeneity analysis on end points. (A) Heterogeneity analysis on clinical remission. (B) Heterogeneity analysis on ESRD or doubling of serum creatinine level. (C) Heterogeneity analysis on SAEs.
Inconsistency using node-splitting approach. (A) Inconsistency using the node-splitting approach for clinical remission. (B) Inconsistency using the node-splitting approach for ESRD or doubling of serum creatinine level. (C) Inconsistency using the node-splitting approach for SAEs.
Publication bias of funnel plot. (A) Funnel plot for clinical remission. (B) Funnel plot for ESRD or doubling of serum creatinine level. (C) Funnel plot for SAEs.