Abstract
Introduction
Rural and remote indigenous individuals have a high burden of chronic kidney disease (CKD) when compared to the general population. However, it has not been previously explored how these rates compare to urban-dwelling indigenous populations.
Methods
In a recent cross-sectional screening study, 1346 adults 18 to 80 years of age were screened for CKD and diabetes across 11 communities in rural and remote areas in Manitoba, Canada, as part of the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) program. An additional 284 Indigenous adults who resided in low-income areas in the city of Winnipeg, Manitoba, Canada were screened as part of the NorWest Mobile Diabetes and Kidney Disease Screening and Intervention Project.
Results
Our findings indicate that a gradient of CKD and diabetes prevalence exists for Indigenous individuals living in different geographic areas. Compared to urban-dwelling Indigenous individuals, rural-dwelling individuals had more than a 2-fold (2.1, 95% CI = 1.4−3.1) increase in diabetes whereas remote-dwelling individuals had a 4-fold (4.1, 95% CI = 2.8−6.0) increase, and more than a 3-fold (3.1, 95% CI = 2.2−4.5) increase in CKD prevalence.
Conclusion
Although these results highlight the relative importance of geography in determining the prevalence of diabetes and CKD in Indigenous Canadians, geography is but an important surrogate of other determinants, such as poverty and access to care.
Keywords: chronic kidney disease, Indigenous Canadians, remoteness, rurality, screening, social determinants of health
Indigenous people disproportionately suffer from higher rates of diabetes,1, 2 hypertension,3 and chronic kidney disease (CKD),4, 5 and are also at higher risk for kidney failure.6 These conditions are associated with multiple comorbidities and early mortality,7 with CKD and kidney failure representing a disproportionate economic burden for the health care system.
Although these longstanding and intergenerational health disparities between Indigenous individuals and the general population are well described,8, 9 it is in the most isolated communities that disparities are particularly prominent.10, 11 Individuals living in isolated areas are disproportionately faced with social inequalities such as poverty and poor access to services12; as such, geography may not be the primary determinant driving these observed health disparities, but rather may be a surrogate for other social determinants. However, as it is unclear how rates of diabetes, hypertension, and CKD differ by geographic area, there is a knowledge gap with regard to identifying the underlying mechanisms that contribute to higher rates of CKD and other comorbidities in Indigenous populations in Canada.
The First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) was a mobile, Indigenous-led, cross-sectional screening program that provided community-wide screening for CKD, diabetes, elevated blood pressure, and kidney failure risk prediction, as well as risk-based counseling, in rural and remote communities in Manitoba, Canada.13 An urban arm of the study was subsequently launched by an interdisciplinary clinical team from NorWest Co-op Community Health Centre, as part of the NorWest Mobile Diabetes and Kidney Disease Screening and Intervention Project, in low-income areas in the city of Winnipeg, Manitoba, Canada. The objective of the current report was to compare rates of diabetes, elevated blood pressure, and CKD among Indigenous urban, rural, and remote dwellers.
Methods
Study Population
The Canadian health care system is a complex system of policies and governing relationships between federal and provincial legislating authorities, with most people covered by universal health coverage under the Canadian Health Act, administered by the provinces. However, health coverage for Indigenous people is a complicated facet, steeped in a long historical context. Public health and preventive services are provided by the federal First Nations and Inuit Health Branch (FNIHB) to indigenous people who are registered under the Indian Act of Canada and who live on reserves and on traditional territories. Among Indigenous Canadians who have registered or obtained treaty status, roughly 44% live on more than 3100 Indigenous reserves across Canada, which are mostly located in either rural or remote locations. For Indigenous people living off-reserves, mainly in urban locations, health care services are provided by the provincial health care system, although the FNIHB provides noninsured health benefits, such as dental and vision coverage to registered Indigenous people regardless of their dwelling location.14
Manitoba is a prairie province in central Canada with a population of 1.2 million people, 17% of whom are of Indigenous ancestry. Roughly two-fifths of the Indigenous population reside in the province’s capital city, Winnipeg, the largest city in the province and home to the largest proportion of Indigenous individuals among major Canadian cities, at 11% of the municipal population. For the current study, screening occurred within rural (accessible by road) and remote (accessible only by plane or, in winter, by ice roads) communities, as well as within selected areas in the urban city of Winnipeg, including the downtown core, as well as within the North-West quadrant of the city. These urban areas are low-income areas, with median family incomes ranging from $29,696 to $61,414, far below the median income of the city ($81,880).
The FINISHED and the NorWest Co-op Community Health Centre Screening Programs
The FINISHED screening program was executed across 11 Indigenous rural and remote communities within 2 tribal councils in Manitoba, Canada. This has previously been described in detail.13 All adults 18 to 80 years of age were invited to participate in the screening program, and were recruited from community gathering centers, as well as through door-to-door recruitment. Between March 2013 and January 2015, a total of 716 individuals in 7 rural communities and 630 individuals in 4 remote communities were screened, with a compliance rate of 23%. An additional 284 individuals of Indigenous descent were screened as part of the NorWest Mobile Diabetes and Kidney Disease Screening and Intervention Project, through the Norwest Co-op Community Health Centre, an ongoing screening initiative targeting Indigenous as well as other ethnic and at-risk urban populations. Through various community-based partnerships, screening teams consisting of interdisciplinary health care professionals set up mobile clinics at 13 accessible sites in selected low-income areas in Winnipeg. Screening occurred between May 2015 and December 2016, and although promotion of the screening program was tailored for each site, overall it was targeted toward individuals who were unlikely to have a primary care provider. In both screening programs, individuals were screened regardless of whether they presented with known kidney disease risk factors, such as diabetes, elevated blood pressure, or family history of kidney disease. Mobile screening and point-of-care testing were used, which allowed for quick risk prediction, and appropriate referrals to be made to primary care and specialist services. In the urban arm of the study, partnerships with community organizations and primary care supports allowed the clinical teams to make additional referrals to a variety of counseling supports.
Ethics approval was granted by the University of Manitoba Health Research Ethics Board (HS16070), as well as from the NorWest Co-op Community Health Board of Directors, the Diabetes Integration Project Board of Directors, and the Tribal Council leaders and government leaders of the Indigenous communities in the rural and remote areas where screening occurred. Imperatively, Indigenous research principles of Ownership, Control, Access and Possession (OCAP) were stringently followed for the duration of the screening programs.
Data Collection and Study Definitions
In both screening initiatives, several data elements were collected during screening, including the following: participants’ age, sex, diastolic and systolic blood pressures, estimated glomerular filtration rate (eGFR; measured using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation), glycated hemoglobin (HbA1c), serum creatinine, and urine albumin to creatinine ratio (ACR). The Kidney Failure Risk Equation (KFRE), a validated multivariate model that predicts a patient’s risk of developing kidney failure within the next 5 years using patient age, sex, urine ACR, and eGFR, was used to rank patients into categories of no current risk, low risk, intermediate risk, and high risk (Supplementary Figure S1).15
Data Analyses
Descriptive statistics for screening results, presented by geographic area, are expressed as mean ± SD for normal categorical variables; median and interquartile ranges for nonnormal variables; and percentages for continuous variables. At the bivariate level, cross-tabulations were conducted to compare geographical groups: χ2 statistics were used for categorical variables, and either analyses of variance for normally distributed continuous variables, or the Kruskal−Wallis test for non−normally distributed variables, were used. In addition, using the Kidney Disease: Improving Global Outcomes (KDIGO) “heat map” staging system, CKD risk was visually summarized for individuals in each of the 3 geographic areas.16 At the multivariate level, logistic regressions were conducted, comparing rural and remote communities to the urban population as the reference group. Odds ratios and 95% confidence intervals are presented, with age and sex controlled for in all regression analyses.
Results
Demographic Characteristics
In total, 1872 Indigenous people were screened as part of the FINISHED and NorWest initiatives: 716 in rural (accessible by road) communities, 630 in remote (accessible by plane) communities, and 284 in low-income areas of Winnipeg. The rural and remote screening results have previously been published17; descriptive statistics for all geographic locations are presented in Table 1. Individuals screened in the urban arm were the youngest (mean age 40.6 years), and were predominantly female (63.4%). In comparison, rural-dwelling individuals had a mean age of 45.2 years, and 59.3% were female. Remote-dwelling screenees had a mean age of 44.6 years, and 62.2% were female.
Table 1.
Descriptive statistics comparing Indigenous Canadians living in urban, rural, and remote locations
| Variable | Urban Indigenous (n = 284) | Rural Indigenous (n = 716) | Remote Indigenous (n = 630) | P values |
|---|---|---|---|---|
| Age, yr | 40.6 ± 13.5 | 45.2 ± 14.4 | 44.6 ± 14.6 | <0.0001 |
| Sex, % female | 63.4% | 59.3% | 62.2% | 0.4 |
| HbA1c, % ≥6.5% | 14.1% | 28.9% | 42.1% | <0.0001 |
| eGFR, ml/min per 1.73 m2 | 106.5 ± 22.4 | 105.6 ± 20.4 | 107.2 ± 22.9 | 0.4 |
| eGFR, ml/min per 1.73 m2 (% < 60) | 2.8% | 2.8% | 4.0% | 0.4 |
| Urine ACR, mg/mmol | 1.8 (0.7−1.9) | 1.0 (0.5−1.9) | 1.9 (0.8−5.1) | <0.0001 |
| Albuminuria, % ≥ 3 mg/mmol | 13.0% | 18.0% | 35.6% | <0.0001 |
| Chronic kidney disease, eGFR < 60 or urine ACR > 3 mg/mmol | 14.8% | 19.3% | 37.5% | <0.0001 |
| Elevated blood pressure, >140 mm Hg SBP or >90 mm Hg DBP | 14.8% | 15.9% | 14.0% | 0.6 |
| Systolic BP, mm Hg | 119.4 ± 14.7 | 121.9 ± 17.1 | 121.4 ±16.3 | 0.1 |
| Diastolic BP, mm Hg | 77.8 ± 10.0 | 76.2 ± 11.0 | 74.7 ± 10.1 | 0.0001 |
| Kidney failure risk | ||||
| No risk | 73.2% | 61.6% | 47.9% | <0.0001 |
| Low risk | 25.7% | 37.1% | 49.5% | |
| Intermediate risk | 0.7% | 0.7% | 1.3% | |
| High risk | 0.4% | 0.7% | 1.3% |
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; SBP, systolic blood pressure. Summary statistics given as mean ± SD for normally distributed variables, median (interquartile range) for non−normally distributed variables, and percentages (n) for categorical variables. Some categories may not sum to 100% due to rounding. The criteria for determination of kidney failure risk are presented in Supplementary Figure S1.
Burden of Diabetes
Among the 3 geographic groups, urban-dwelling screenees had the lowest rate of diabetes, defined as HbA1c ≥ 6.5%, at 14.1%. In comparison, 28.9% of rural-dwelling individuals were diabetic, whereas 42.1% of the remote dwellers were diabetic. At the multivariate level (Table 2), rural dwellers had a 2.1 (95% confidence interval [CI] = 1.4−3.1) greater odds of having diabetes when compared to urban dwellers, whereas remote dwellers had a 4.1 (95% CI = 2.8−6.0) greater odds.
Table 2.
Adjusted odds ratios and 95% confidence intervals for diabetes, elevated blood pressure, and CKD, in Indigenous Canadians of different dwelling locations
| HbA1c (% ≥ 6.5%)a | Elevated BP (>140 mm Hg SBP or > 90 mm Hg DBP)b | CKD (eGFR < 60 or urine ACR > 3 mg/mmol)c | CKD (eGFR < 60 or urine ACR > 3 mg/mmol)c,d | |
|---|---|---|---|---|
| Urban Indigenous (reference) | — | — | — | — |
| Rural Indigenous | 2.1 (1.4−3.1) | 1.1 (0.7−1.6) | 1.2 (0.8−1.7) | 0.9 (0.6−1.4) |
| Remote Indigenous | 4.1 (2.8−6.0) | 1.2 (0.8−1.9) | 3.1 (2.2−4.5) | 2.3 (1.5−3.2) |
ACR, albumin to creatinine ratio; BP, blood pressure; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; SBP, systolic blood pressure.
Age and sex controlled for in all analyses.
Reference: aHbA1c % < 6.5%.
Low BP.
No CKD.
Controlled for age, sex, and HbA1c.
Burden of CKD
Patients screened in urban areas had the lowest burden of CKD, defined as a single measurement of elevated urine ACR or eGFR < 60 ml/min per 1.73 m2, at 14.8%. Comparatively, those screened in rural areas had a 19.3% prevalence of CKD, whereas those in remote communities had a 37.5% prevalence of CKD. Likewise, rates of elevated albuminuria increased with remoteness, with 13.0% of urban dwellers having albuminuria > 3 mg/mmol, followed by 18.0% of rural dwellers, and 35.6% of dwellers in remote communities. No significant differences were found with mean eGFR or depressed eGFR (percentage with < 60 ml/min per 1.73 m2), or with blood pressure. Furthermore, 52.1% of screenees in remote areas had at least some risk of kidney failure (Supplementary Figure S1), versus 38.5% in rural areas, and 26.8% in urban areas.
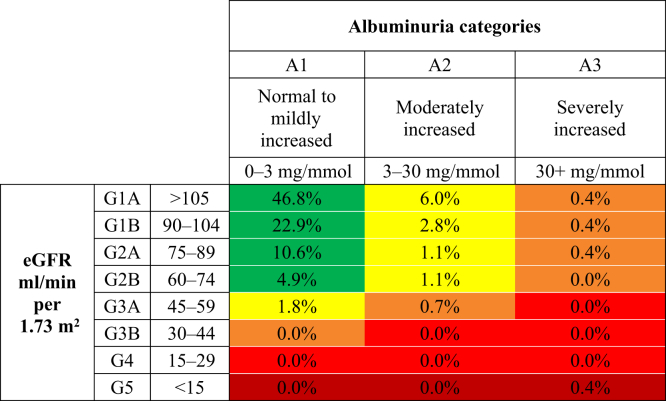
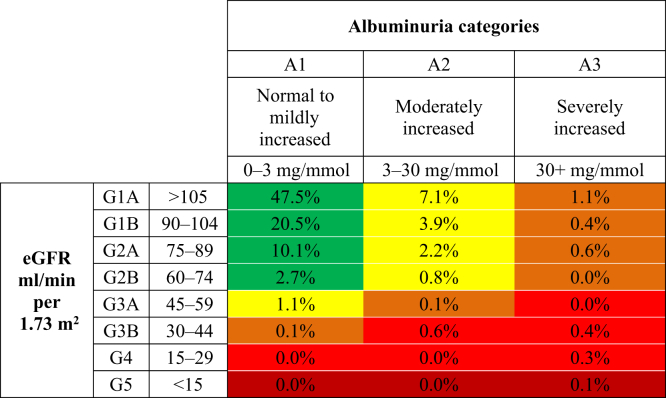
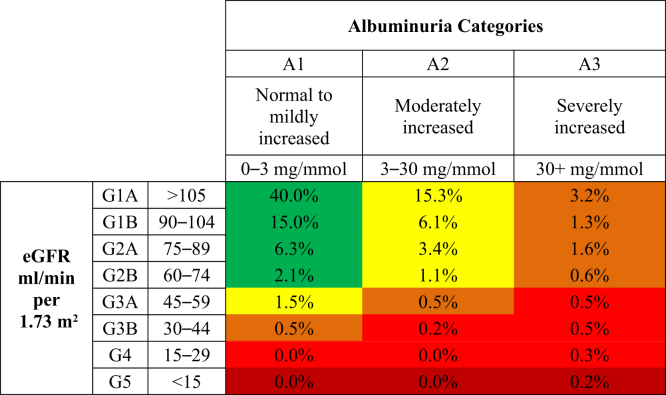
Figure 1 displays the distribution of screenees according to the KDIGO CKD progression risk staging system for urban dwellers, with 12.8% presenting at a moderately increased risk for CKD progression, followed by 1.9% high risk, and 0.4% very high risk for CKD progression. In comparison, Figure 2 shows the distribution for rural dwellers, with 15.1% marked at moderately increased risk, 2.3% high risk, and 1.4% very high risk. Finally, Figure 3 displays the distribution of CKD progression risk for the remote dwellers, with 27.4% presenting as having moderate risk, 7.7% high risk, and 1.7% very high risk.
Figure 1.
Initial risk classification of urban-dwelling Indigenous Canadian individuals, using the Kidney Disease: Improving Global Outcomes (KDIGO) classification system. eGFR, estimated glomerular filtration rate.
Figure 2.
Initial risk classification of rural-dwelling Indigenous Canadian individuals, using the Kidney Disease: Improving Global Outcomes (KDIGO) classification system. eGFR, estimated glomerular filtration rate.
Figure 3.
Initial risk classification of remote-dwelling Indigenous Canadian individuals, using the Kidney Disease: Improving Global Outcomes (KDIGO) classification system. eGFR, estimated glomerular filtration rate.
In the multivariate analyses, there were no significant differences found between CKD prevalence in urban versus rural dwellers (1.2 [95% CI = 0.8−1.7]); however, remote dwellers had a 3.1 (95% CI = 2.2−4.5) greater odds of having CKD compared to urban dwellers, a difference that attenuated to 2.3 (95% CI = 1.5−3.2) when HbA1c rates were controlled for.
Discussion
To the best of our knowledge, this is the first primary screening initiative comparing rates of diabetes, elevated blood pressure, and CKD, in Indigenous Canadians living in urban, rural, and remote locations. Compared to urban dwellers, rural dwellers presented with a 2-fold greater prevalence of diabetes, whereas remote dwellers presented with a 4-fold greater prevalence of diabetes, and a 3-fold greater prevalence of CKD. Although the gradient in rates of diabetes and CKD suggests that geography is a social determinant of health influencing the burden of diabetes and CKD in this population, there are other determinants that geography exacerbates, such as poverty and access to care, that are likely important in affecting rates of morbidities by geographic area. Furthermore, when compared to the general Canadian population, rates of CKD were increased more than 2- and 3-fold, for rural and remote dwellers, respectively,18 and rates of diabetes 2-, 4-, and 6-fold higher for urban, rural, and remote dwellers, respectively.19 These latter findings suggest that Indigenous race is a moderating factor between geography and health outcomes.
Screening in the urban arm of this study targeted all eligible Indigenous individuals, regardless of their other underlying CKD risk factors. When compared to CKD screening studies conducted in the general population, rates were significantly elevated. For example, we found that 11.62% of the urban population had moderately increased albuminuria, and 1.41% had severely increased albuminuria. The Prevention in Renal and Vascular End-Stage Disease (PREVEND) primary screening study in the Netherlands, and the National Health and Nutrition Examination Survey (NHANES) study in the United States, found microalbuminuria ranging from 7.1% to 8.2% and macroalbuminuria ranging from 0.7% to 1.3%.20, 21 Furthermore, compared to rates from a prevalence study using data representative of the Canadian population, CKD and albuminuria were also elevated.18 However, compared to screening studies of high-risk populations with underlying risk factors, such as those from the urban Kidney Early Evaluation Program (KEEP) study,22, 23, 24 rates of elevated albuminuria were significantly lower, and when compared to an at-risk African American population in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study in the United States,25 rates of moderately elevated albuminuria were also lower. However, rates in the urban population were elevated in comparison to an Australian screening study of high-risk individuals.26
Several screening initiatives have found that Indigenous individuals living in rural and remote locations are faced with a substantially higher burden of diabetes and CKD. However, previous screening studies have exclusively focused on rural and remote Indigenous populations, whereas in this current study we included urban-dwelling Indigenous individuals. Compared to other screening studies of rural and remote Indigenous populations, such as those with Canadian Indigenous populations,5, 27 as well as with Indigenous populations in the United States, such as Zuni,28 Navajo,29 and Pima Indians,30 rates in the urban arm were significantly lower. However, rates among screened FINISHED participants in the rural and remote locations were relatively similar to these other screened Indigenous populations.17
Although the urban dwellers—who, in this study, all resided in low-income neighborhoods—had reduced rates of CKD and diabetes when compared to the rural and remote dwellers, when compared to the general population, they had higher rates of these outcomes. This reflects an association between income and access to health care: individuals residing in low-income urban neighborhoods are less likely to have access to preventive health care services,31 are less likely to have a regular health care provider,32 and consequently have poorer health outcomes.33 This relationship is confounded with rurality; it is established that there is a significant divide in health status between urban and rural settings, with rural dwellers generally having poorer health overall when compared to urban dwellers.34 Rural dwellers with CKD have reduced access to primary care and nephrology specialists, and consequently, they are more likely to reach end-stage renal disease,35 and are more likely to die or be hospitalized, when compared to individuals who live closer to specialized care.36 Therefore, when taken together, these findings suggest that rurality does not unilaterally cause the health disparities that rural and remote dwellers are faced with, but rather there is an interplay among other factors, such as poverty, that aggravate the relationship between dwelling location and poor health outcomes.37
Further adding to this complex relationship, Indigenous Canadians are at a socially constructed disadvantage.38 They are more likely to live in poverty, and are more likely to live in remote areas, with poverty rates worsening as communities become more isolated.39 Indigenous Canadians are also more likely to reside in households that are faced with food insecurity,40 which is a significant risk factor for diabetes and CKD.41, 42 Although this is a significant issue that those living in low-income urban neighborhoods are faced with, Indigenous people who reside in remote and rural areas are disproportionately faced with this barrier.43, 44
However, it remains unclear as to the full extent to which geography alone is a social determinant driving these health disparities for Indigenous people in rural and remote areas, or whether geography is a surrogate exacerbating these inequalities. Indigenous Canadians living in Indigenous communities outside of urban areas are de facto served by a separately funded health system governed by separate agreements and policies that may functionally impose additional barriers, particularly with regard to accessing consultation and specialist services,45, 14 further contributing to a gradient of worsening health outcomes.46 However, it is in the most remote areas that federal jurisdiction limits access to services and resources the most severely. Nevertheless, the role of geography alone as a driver of health inequalities should not be overstated, given that Indigenous Canadians residing in urban areas are also faced with significant health inequalities. When compared to the general population, urban-dwelling Indigenous Canadians have worse health outcomes, despite having the same geographic access. This, juxtaposed against the geographic barriers to accessing health services that rural and remote dwellers are faced with, suggests that there are systemic barriers embedded within the health care system47 that make accessing health care services more difficult, despite having geographic access to them.4, 48
To improve quality of life and health outcomes, it is imperative to identify and address the underlying systemic factors that drive the higher rates of CKD and other comorbidities found in Indigenous populations of different geographic locales, allowing for upstream screening and treatment of early-stage CKD and its risk factors. Although our study is an important first step in this direction in suggesting that there are underlying mechanisms beyond geography that affect rates of diabetes and CKD, our study does not provide a better understanding of the exact mechanism(s). In particular, although it is generally accepted that a history of colonialism and its legacy of structural racism is an active precipitator of poor health outcomes in Indigenous people,49, 50, 51 more research across different disciplines, as well as a broader discourse, is needed to concretely identify the pathway that a history of colonialism has as a social determinant on Indigenous Canadians’ health.
Our findings have clinical, research, and policy implications. First, from a clinical perspective, the prevalence of diabetes and chronic kidney disease appear to be elevated in Indigenous Canadians of different dwelling locations, suggesting that primary screening programs are cost-effective in this population.52 Furthermore, more research needs to be conducted on how geography interplays with other health determinants, such as genetics, the environment, and sociodemographic factors—in particular, poverty—to affect CKD rates. Finally, from a health policy perspective, access to CKD care, as well as to primary care, needs to be improved for Indigenous individuals through health services delivery strategies that consider access barriers that Indigenous Canadians are disproportionately faced with, such as geographic and economic barriers,53 as well as through health promotion strategies. Research has shown that many individuals residing in Indigenous communities are unaware of their underlying health problems, in particular their risk of developing CKD.5
It should be acknowledged that this study has both strengths and limitations. Although other screening studies of Indigenous individuals have compared rates of diabetes, elevated blood pressure, and CKD, between rural and remote dwellers, to the best of our knowledge, this is the first study to include a cohort of Indigenous urban dwellers. The screening was conducted using a well-defined screening protocol, finding CKD rates that were comparable to other high-risk populations for whom screening is recommended.54 However, as both screening initiatives recruited individuals through convenience sampling, there is a possibility of selection bias. In the remote communities, lower screening rates were observed compared to the rural communities, likely attributed to differences in transportation and health services availability in these areas. In the urban arm of the study, although we sampled individuals from neighborhoods with a large proportion of Indigenous people, it is unknown whether this sample is reflective of other Canadian cities, and therefore participants may not be fully representative of all urban Indigenous individuals. Another limitation that is also shared by many clinical studies is that of self-selection bias; when compared to the general population, individuals who self-select to participate in screening initiatives tend to be healthier, which would bias our results by underestimating disease prevalence. Conversely, individuals who are aware of their CKD risk factors may also be more likely to participate in screening, which would overestimate prevalence rates. It is unclear as to what the net effect of these biases would be on prevalence estimates. Finally, based on a single measurement, we presumed chronicity, whereas a confirmed CKD diagnosis would require reduced eGFR or albuminaria sustained over 3 months. However, other large community screening studies also share this limitation.
Conclusion
When compared to the general population, Indigenous Canadians display a high prevalence of diabetes and chronic kidney disease. However, geography, as a surrogate exacerbating other social determinants of health, plays an important role in affecting the severity of the diseases, with a striking gradient of worsening rates from urban to rural to remote. Further research needs to be conducted to explore the underlying mechanisms responsible for the disparities in diabetes and CKD that are faced by Indigenous Canadians in different geographic areas.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding for this study was obtained through a grant from The Lawson Foundation (grant number: 2014-31). The authors gratefully acknowledge the following individuals from the NorWest Co-op Community Health Centre: Nancy Heinrichs, Executive Director; Renata Cook, Primary Care Coordinator; Sandra Marriott-Silver, Nurse Practitioner; Colin Reeve, Clinical Pharmacist; Cindy Peters, Primary Care Nurse; Kazel Ebora, Primary Care Nurse; Melissa Forester, Primary Care Nurse; and Ken Grove, Primary Care Coordinator, as well as Betty Van, Chronic Disease Management Clinician, from the Inkster/Seven Oaks My Health Team.
Footnotes
Figure S1. Criteria for determining kidney failure risk.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Criteria for determining kidney failure risk.
References
- 1.Young T.K., Reading J., Elias B., O’Neil J.D. Type 2 diabetes mellitus in Canada’s First Nations: status of an epidemic in progress. CMAJ. 2000;163:561–566. [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck R., Osgood N., Hsiang T. Epidemiology of diabetes mellitus among First Nations and non-First Nations adults. CMAJ. 2010;182:249–256. doi: 10.1503/cmaj.090846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce S.G., Riediger N.D., Zacharias J.M., Young T.K. Obesity and obesity-related comorbidities in a Canadian First Nation population. Chron Dis Can. 2010;31:27–32. [PubMed] [Google Scholar]
- 4.Gao S., Manns B.J., Culleton B.F. Prevalence of chronic kidney disease and survival among aboriginal people. J Am Soc Nephrol. 2007;18:2953–2959. doi: 10.1681/ASN.2007030360. [DOI] [PubMed] [Google Scholar]
- 5.Zacharias J.M., Young T.K., Riediger N.D. Prevalence, risk factors and awareness of albuminuria on a Canadian First Nation: a community-based screening study. BMC Public Health. 2012;12:1–8. doi: 10.1186/1471-2458-12-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel S.M., Palacios-Derflingher L., Tonelli M. Association between First Nations ethnicity and progression to kidney failure by presence and severity of albuminuria. CMAJ. 2014;186:E86–E94. doi: 10.1503/cmaj.130776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black C., Sharma P., Scotland G. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess (Rockv) 2010;14:1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- 8.Gracey M., King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75. doi: 10.1016/S0140-6736(09)60914-4. [DOI] [PubMed] [Google Scholar]
- 9.King M., Smith A., Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet. 2009;374:76–85. doi: 10.1016/S0140-6736(09)60827-8. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J.A., Vermeulen S.U., Toth E.L. Increasing incidence and prevalence of diabetes among the status of Aboriginal population in urban and rural Alberta. Can J Public Health. 2009;100:231–236. doi: 10.1007/BF03405547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newbold K.B. Problems in search of solutions: health and Canadian Aboriginals. J Community Health. 1998;23:59–74. doi: 10.1023/a:1018774921637. [DOI] [PubMed] [Google Scholar]
- 12.Richmond C.A.M., Ross N.A. The determinants of First Nation and Inuit health: a critical population health approach. Health Place. 2009;15:403–411. doi: 10.1016/j.healthplace.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Lavallee B., Chartrand C., McLeod L. Mass screening for chronic kidney disease in rural and remote Canadian First Nations people: methodology and demographic characteristics. Can J Kidney Health Dis. 2015;2:1–10. doi: 10.1186/s40697-015-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavoie JG, Gervais L, Toner J, et al. Looking for aboriginal health in legislation and policies, 1970 to 2008: the Policy Synthesis Project. Prince George, British Columbia: The National Collaborating Centre for Aboriginal Health; 2011.
- 15.Tangri N., Stevens L., Griffith J. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 16.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 17.Komenda P., Lavallee B., Ferguson T.W. The prevalence of CKD in rural Canadian Indigenous peoples: results from the First Nations Community Based Screening to Improve Kidney Health and Prevent Dialysis (FINISHED) Screen, Triage, and Treat Program. Am J Kidney Dis. 2016;68:582–590. doi: 10.1053/j.ajkd.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Arora P., Vasa P., Brenner D. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ. 2013;185:E417–E423. doi: 10.1503/cmaj.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosella L.C., Lebenbaum M., Fitzpatrick T., Zuk A., Booth G.L. Prevalence of prediabetes and undiagnosed diabetes in Canada (2007–2011) according to fasting plasma glucose and HbA1c screening criteria. Diabetes Care. 2015;38:1299–1305. doi: 10.2337/dc14-2474. [DOI] [PubMed] [Google Scholar]
- 20.Hillege H.L., Janssen W.M.T., Bak A.A.A. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 21.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S., Okada K., Yanai M. The Kidney Early Evaluation Program (KEEP) of Japan: results from the initial screening period. Kidney Int. 2010;77(suppl 116):S17–S23. doi: 10.1038/ki.2009.539. [DOI] [PubMed] [Google Scholar]
- 23.Bello A.K., Nwankwo E., El Nahas A.M. Prevention of chronic kidney disease: a global challenge. Kidney Int Suppl. 2005;68:11–17. doi: 10.1111/j.1523-1755.2005.09802.x. [DOI] [PubMed] [Google Scholar]
- 24.Obrador G.T., García-García G., Villa A.R. Prevalence of chronic kidney disease in the Kidney Early Evaluation Program (KEEP) México and comparison with KEEP US. Kidney Int. 2010;77(suppl 116):S2–S8. doi: 10.1038/ki.2009.540. [DOI] [PubMed] [Google Scholar]
- 25.Crews D.C., McClellan W.M., Shoham D.A. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis. 2012;60:779–786. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew T.H., Corso O., Ludlow M. Screening for chronic kidney disease in Australia: a pilot study in the community and workplace. Kidney Int. 2010;77(suppl 116):S9–S16. doi: 10.1038/ki.2009.538. [DOI] [PubMed] [Google Scholar]
- 27.Virani S., Strong D., Tennant M. Rationale and implementation of the SLICK project: Screening for Limb, I-eye, Cardiovascular and Kidney (SLICK) complications in individuals with type 2 diabetes in Alberta’s First Nations communities. Can J Public Health. 2006;97:241–247. doi: 10.1007/BF03405595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah V.O., Scavini M., Stidley C.A. Epidemic of diabetic and nondiabetic renal disease among the Zuni Indians: the Zuni Kidney Project. J Am Soc Nephrol. 2003;14:S1320–S1329. doi: 10.1097/01.asn.0000059920.00228.a0. [DOI] [PubMed] [Google Scholar]
- 29.Hoy W., Jim S., Warrington W. Urinary findings and renal function in adult Navajo Indians and associations with type 2 diabetes. Am J Kidney Dis. 1996;28:339–349. doi: 10.1016/s0272-6386(96)90490-4. [DOI] [PubMed] [Google Scholar]
- 30.Nelson R.G., Kunzelman C.L., Pettitt D.J. Albuminuria in type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in Pima Indians. Diabetologia. 1989;32:870–876. doi: 10.1007/BF00297452. [DOI] [PubMed] [Google Scholar]
- 31.Menec V., Sirski M., Attawar D. Does continuity of care matter in a universally insured population? Health Serv Res. 2005;40:389–400. doi: 10.1111/j.1475-6773.2005.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menec V., Roos N., Black C., Bogdanovich B. Characteristics of patients with a regular source of care. Can J Public Health. 2001;92:299–303. doi: 10.1007/BF03404965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starfield B., Shi L., Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457–502. doi: 10.1111/j.1468-0009.2005.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pong R.W., DesMeules M., Lagace C. Rural-urban disparities in health: how does Canada fare and how does Canada compare with Australia? Aust J Rural Health. 2009;17:58–64. doi: 10.1111/j.1440-1584.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- 35.Cass A., Cunningham J., Snelling P. Exploring the pathways leading from disadvantage to end-stage renal disease for Indigenous Australians. Soc Sci Medi. 2004;58:767–785. doi: 10.1016/s0277-9536(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 36.Rucker D., Hemmelgarn B.R., Lin M. Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int. 2011;79:210–217. doi: 10.1038/ki.2010.376. [DOI] [PubMed] [Google Scholar]
- 37.Smith K.B., Humphreys J.S., Wilson M.G.A. Addressing the health disadvantage of rural populations: how does epidemiological evidence inform rural health policies and research? Aust J Rural Health. 2008;16:56–66. doi: 10.1111/j.1440-1584.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 38.Czyzewski K. Colonialism as a broader social determinant of health. Int Indig Policy J. 2011;2:1–14. [Google Scholar]
- 39.Marrone S. Understanding barriers to health care: a review of disparities in health care services among indigenous populations. Int J Circumpolar Health. 2007;66:188–198. doi: 10.3402/ijch.v66i3.18254. [DOI] [PubMed] [Google Scholar]
- 40.Willows N.D., Veugelers P., Raine K., Kuhle S. Prevalence and sociodemographic risk factors related to household food security in Aboriginal peoples in Canada. Public Health Nutr. 2008;12:1150–1156. doi: 10.1017/S1368980008004345. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee T., Crews D.C., Wesson D.E. Food insecurity, CKD, and subsequent ESRD in US Adults. Am J Kidney Dis. 2017;70:38–47. doi: 10.1053/j.ajkd.2016.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seligman H.K., Jacobs E.A., Lopez A. Food insecurity and glycemic control among low-income patients with type 2 diabetes. Diabetes Care. 2012;35:233–238. doi: 10.2337/dc11-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skinner K., Hanning R.M., Tsuji L.J. Prevalence and severity of household food insecurity of First Nations people living in an on-reserve, sub-Arctic community within the Mushkegowuk Territory. Public Health Nutr. 2014;17:31–39. doi: 10.1017/S1368980013001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson S., Kamal A.G., Wiebe J. Community development to feed the family in Northern Manitoba communities: evaluating food activities based on their food sovereignty, food security, and sustainable livelihood outcomes. Can J Nonprofit Soc Econ Res. 2012;3:43–66. [Google Scholar]
- 45.Martens P.J., Martin B.D., O’Neil J.D., MacKinnon M. Diabetes and adverse outcomes in a First Nations population: associations with healthcare access, and socioeconomic and geographical factors. Can J Diabetes. 2007;31:223–232. [Google Scholar]
- 46.Martens P., Sanderson D., Jebamani L. Health services use of Manitoba First Nations people: is it related to underlying need? Can J Public Health. 2005;96:S39–S44. doi: 10.1007/BF03405315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang S.Y., Browne A.J. “Race” matters: racialization and egalitarian discourses involving Aboriginal people in the Canadian health care context. Ethn Health. 2008;13:109–127. doi: 10.1080/13557850701830307. [DOI] [PubMed] [Google Scholar]
- 48.Goodman A., Fleming K., Markwick N. “They treated me like crap and I know it was because I was Native”: the healthcare experiences of Aboriginal peoples living in Vancouver’s inner city. Soc Sci Med. 2017;178:87–94. doi: 10.1016/j.socscimed.2017.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allan B, Smylie J. First Peoples, Second Class Treatment: The Role of Racism in the Health and Well-Being of Indigenous Peoples in Canada. Toronto, ON: The Wellesley Institute; 2015.
- 50.Bourassa C., McKay-McNabb K., Hampton M. Racism, sexism, and colonialism: the impact on the heath of Aboriginal women in Canada. Can Woman Stud. 2004;24:23–30. [Google Scholar]
- 51.Wilk P., Maltby A., Cooke M. Residential schools and the effects on Indigenous health and well-being in Canada—a scoping review. Public Health Rev. 2017;38:1–23. doi: 10.1186/s40985-017-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson T.W., Tangri N., Tan Z. Screening for chronic kidney disease in Canadian indigenous peoples is cost-effective. Kidney Int. 2017;92:192–200. doi: 10.1016/j.kint.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Bailie J., Schierhout G., Laycock A. Determinants of access to chronic illness care: a mixed-methods evaluation of a national multifaceted chronic disease package for Indigenous Australians. BMJ Open. 2015;5:e008103. doi: 10.1136/bmjopen-2015-008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Komenda P., Ferguson T.W., Macdonald K. Cost-effectiveness of primary screening for CKD: a systematic review. Am J Kidney Dis. 2014;63:789–797. doi: 10.1053/j.ajkd.2013.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Criteria for determining kidney failure risk.