Abstract
Introduction
Chronic kidney disease (CKD), diabetes, and hypertension play a disproportionate role in the growing public health challenge posed by noncommunicable diseases (NCDs) in East Africa. The impact of these NCDs may pose the greatest challenge in rural areas with limited screening and treatment facilities, although precise prevalence estimates of these conditions in rural Tanzania are lacking.
Methods
The prevalence of CKD, diabetes, and hypertension, were estimated from a probability sample of adults (n = 739) residing in 2 communities within Kisarawe, a rural district of Tanzania. Following consent, participants were studied in their homes. Random point-of-care (POC) measures of glycosylated hemoglobin and blood pressure, were obtained. Serum creatinine, drawn at the POC and measured at Muhimbili National University, was used to calculate estimated glomerular filtration rate with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Results
The median age was 35 years (interquartile range 25−45 years). Overall the pooled prevalence for CKD stages III, IV, and V was 12.4% (95% confidence interval [CI] = 10.2−14.8). Surprisingly, the prevalence of CKD stage V (3.0%; 95% CI = 2.1−4.4) was high among the youngest age group (18−36 years). The prevalence estimates for prehypertension and hypertension were 38.0% (95% CI = 34.6−41.5) and 19.9% (95% CI = 17.1−22.9), respectively. The prevalence estimates for prediabetes and diabetes were 25.7% (95% CI = 22.6−29.1) and 14.8% (95% CI = 12.4−17.6), respectively.
Conclusion
Although this pilot study had a relatively small sample size, the prevalence estimates for CKD, diabetes, and hypertension were higher than we expected based on previous estimates from Tanzania. CKD was not significantly associated with diabetes or hypertension, suggesting the possibility of an alternative causality.
Keywords: diabetes mellitus, hypertension, kidney disease, prevalence, Tanzania
NCDs are the leading cause of death worldwide.1 Prominent among NCDs is the worldwide epidemic of CKD and the related risk factors of diabetes and hypertension.2 The impact of this epidemic is especially severe in low- and middle-income countries with limited health facilities for screening and treatment.2, 3, 4 Obtaining precise estimates of the prevalence of CKD, diabetes, and hypertension in areas with limited health resources is the first step in decreasing the burden imposed by these NCDs.
Relatively few studies have been conducted on the prevalence of NCDs, including CKD, diabetes, and hypertension, in East Africa.5 Stanifer et al. reported that the prevalence of CKD was higher in the Moshi urban (15.2%; 95% CI = 9.6%−23.3%) versus the Moshi rural (7.0%; 95% CI = 3.8%−12.5%) district in the Kilimanjaro Region of Tanzania.5 Janmohamed et al. reported that among a group of adult patients in a diabetes mellitus clinic of Bugando Medical Centre in Mwanza, Tanzania, the prevalence of CKD (detected by the presence of proteinuria) was 83.7%.6
Therefore, the purpose of this study was to evaluate the prevalence of CKD, diabetes mellitus (DM), and hypertension (HTN) in a random sample of participants with POC measures in rural Tanzania. The present study supplemented a National Institutes of Health (NIH)/National Institute of Mental Health (NIMH)−funded study on HIV prevention strategies (Comprehensive Triaged HIV Prevention Trial) in 2 communities in the Kisarawe District, Tanzania. We examined the prevalence of CKD, DM, and HTN in the same communities. Our overarching goal was to develop a better understanding of the prevalence of these diseases and to understand the contributing factors so that we can develop effective strategies to blunt the long-term health consequences for these areas with limited health care resources. The study was approved by the Institutional Review Boards at the Medical University of South Carolina, Charleston, South Carolina, USA, and Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Methods
The present study was designed as a supplement to a funded phase II community-based randomized controlled trial assessing HIV prevention strategies in rural Tanzania (Triage Project). The parent trial took place within 2 rural communities, with approximately 10,000 inhabitants each, in Kisarawe District, Tanzania. Data for the present study were collected during the baseline assessment of the Triage Project among a community cohort (n = 739 individuals, 372 participants from community A and 367 participants from community B). Kisarawe is a rural district located approximately 40 km from Dar es Salaam and is part of the Pwani (Coast) Region of Tanzania.
Sampling Scheme
Subjects in the community cohort were randomly sampled using a 2-stage sampling scheme. All household locations within the 2 communities were enumerated and mapped using Global Positional System (GPS) coordinates. Household locations were randomly selected from the list of all 3714 enumerated household locations in the 2 communities (1920 household locations in community A and 1794 in community B). A household was defined as family members (whether related or not related by blood) who live on the same compound and eat from the same pot. Second, all eligible household members of selected households were identified and enumerated. One participant was selected from each household containing eligible members using a random selection application programmed into a Samsung Tab 2 Tablet Computer (Samsung, Seoul, South Korea). Participants were sampled without replacement at the household level. The randomly identified participant was recruited to participate in both the parent HIV study and simultaneously contribute to our pilot NCD study. To reduce nonresponse rates, up to 3 attempts were made to contact selected households and selected participants within households. In addition, community outreach was performed prior to data collection to raise awareness about the study and to familiarize community members with study staff.
Eligible participants had to be 18 to 55 years of age, to reside in the household, and to plan on continuing to reside in the community for the next 2 years. The assessment for each participant included completing a behavioral survey with a trained research assistant and completing a biological assessment that included POC testing for HIV and other sexually transmitted diseases, as well as blood pressure (BP) measurement and POC HbA1c testing. A venous blood sample was also drawn for transport to a laboratory for measurement of serum creatinine. Behavioral survey data and biological data were captured using an electronic tablet (Samsung Tab 2) and uplinked via cellular telephone to the central data repository-data storage server. Written informed consent was provided by each participant prior to data collection. The consent document was read aloud to all participants to ensure comprehension regardless of literacy level.
Measures
Behavioral Survey
The behavioral survey was administered in Kiswahili by trained interviewers in or near the participants’ homes. The survey mostly pertained to sociodemographic information and sexual risk behavior, although participants were asked whether they had ever been diagnosed with DM, HTN, or CKD and, if so, whether they had received treatment.
Blood Pressure Measurement
Participants’ BP was measured in the household with a LifeSource UA-767PlusAC Blood Pressure Monitor (A&D Medical/LifeSource, Mississauga, ON, Canada). Blood pressure was measured according to American Heart Association (AHA) guidelines,7 with 3 measurements obtained and averaged for a final value. Hypertension was classified using American Heart Association criteria for pre-HT and HTN: BP 120−139/80−89 mm Hg; stage I HTN 140−159/90−99 mm Hg, and stage II HTN ≥160/≥ 100 mm Hg.7
HbA1c
HbA1c was measured at POC with a Bayer A1c Now kit (Bayer Healthcare AG, Leverkusen, BRG). Blood samples for the measurement of HbA1c were obtained by fingerstick. Both pre-DM (HbA1c ≥ 5.7% to ≤ 6.4%) and DM (HbA1c ≥ 6.5%) were diagnosed in accordance with American Diabetes Association criteria8 and the World Health Organization.9 To validate the HbA1c Now test in this setting, we compared the results for determination of HbA1c levels with the POC A1c Now test and a laboratory-based test (using SIEMENS DCA Analyzers; Siemens, Midrand, South Africa) among 20 randomly selected blood specimens from the Muhimbili Hospital blood bank. We analyzed results by calculating the mean difference (% A1c) between the 2 tests and used a paired t test to assess the significance of the differences between the results between the 2 tests.
Serum Creatinine
A venous blood sample was obtained and transported refrigerated (2 °C to 8 °C) to Muhimbili National University Hospital, for measurement of serum creatinine concentration. Transit time was <60 hours. After arrival at the laboratory, samples were frozen (−20 °C) until analyzed. Serum creatinine was measured by an isotope dilution mass spectrometry standardized alkaline picric acid method.10 Serum creatinine concentrations were used to calculate estimated glomerular filtration rate (eGFR) using the CKD-EPI equation.11 CKD was classified according to U.S. National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) criteria and the National Kidney Foundation Guidelines for eGFRs <60 ml/min per 1.73 m2.12, 13
Statistical Analyses
Data were analyzed with R (Vienna, Austria: Foundation for Statistical Computing, Vienna, Austria)14 and Stata v14 (StataCorp LLC, College Station, TX).15 Differences between demographic characteristics between the 2 communities were measured using a χ2 statistic for categorical variables and a t test for continuous variables. For continuous variables, the median value and interquartile range were reported. Prevalence of HTN, CKD, and DM were calculated as proportions using the number of participants screened for each disease as the total population at risk, and 95% CIs were calculated for each proportion. Prevalence estimates were weighted by the inverse of the probability of being selected at the household level to account for variations in household size and by community stratification, accounting for the 2 communities’ unequal sizes. Regarding CKD, the relationship of eGFR to age and gender was descriptively examined using locally weighted scatterplot smoothing (LOESS), and analysis of covariance was used to detect overall differences in eGFR by age and gender. The eGFR was categorized into 5 groups according to CKD stage, and multinomial logistic regression was conducted to assess the probability of group membership using age, gender, and the interaction of age and gender as independent variables. Regarding diabetes and hypertension, LOESS smoothing was used to assess the overall relationship of HbA1c and BP (systolic and diastolic) with age and gender. For HTN, ordinal logistic regression (ordered groups included: normal BP, pre-HTN, stage I HTN, and stage II HTN) was used to ascertain probability of group membership based on age, gender, and an age-by-gender interaction. For DM, ordinal logistic regression was used to assess the relationship of DM stage (normal, prediabetic, and diabetic) with age, gender, and an age-by-gender interaction. The relationship of eGFR with comorbid DM or HTN were assessed using 1-way analysis of variance, grouping by stage of DM or HTN. A Welch 2-sample t test was used to compare eGFR levels when both HTN and DM were present versus not having the 2 comorbid conditions.
Results
Demographic and medical information data acquired from the questionnaire for the NCD pilot study are shown in Table 1. Of 1237 households visited by study staff, nonresponse rates by household across communities were similar (101 of 569 in community A and 124 of 668 in community B), but community B had significantly more households containing no eligible household members (n = 147) than did community A (n = 88). Of the remaining 777 participating households with eligible members, 38 selected participants either refused to participate or could not be located, with significantly more refusals occurring in community B (n = 30) than in community A (n = 8). The overall nonresponse rate was 4.8% (38 of 776). A total of 739 participants provided data (372 in community A and 367 in community B) (Figure 1). Although 739 participants gave informed consent and contributed behavioral questionnaire information and demographic data for the study, measurements of serum creatinine were not available for 26 participants. Similarly, data for HbA1C were not available for 31 participants, and BP measurements were not available for 4 participants. The median age was 35 years (interquartile range 25−45 years) with similar distribution across the 2 communities. The sample population was 59.3% female (n = 438), which differed from 2012 census data reporting a 51% female population nationally.
Table 1.
Demographic and medical data for study population
| Characteristics | Community A (N = 372) | Community B (N = 367) | Overall (N = 739) | P value |
|---|---|---|---|---|
| Age, median (IQR) | 35 (27−45) | 36 (28−44) | 35 (25−45) | 0.87 |
| Gender | 0.20 | |||
| Male | 160 (43.0%) | 141 (38.42%) | 301 (40.7%) | |
| Female | 212 (57.0%) | 226 (61.58%) | 438 (59.3%) | |
| Ethnicity | <0.001 | |||
| Zaramo | 196 (52.7%) | 293 (79.8%) | 489 (66.2%) | |
| Other | 176 (47.3%) | 74 (20.2%) | 250 (33.8%) | |
| Occupation | 0.001 | |||
| Self-employed farmer | 264 (70.97%) | 215 (58.80%) | 479 (64.8%) | |
| Self-employed vendor | 54 (14.52%) | 91 (24.80%) | 145 (19.6%) | |
| Not employed, seeking employment | 21 (5.65%) | 18 (4.90%) | 39 (5.3%) | |
| Employed with a fixed salary | 19 (5.11%) | 15 (4.09%) | 34 (4.6%) | |
| Other or not seeking employment | 14 (3.76%) | 28 (7.63%) | 42 (5.7%) | |
| Education | 0.04 | |||
| No formal education | 81 (21.77%) | 54 (14.71%) | 135 (18.3%) | |
| Primary education | 250 (67.20%) | 271 (73.84%) | 521 (70.5%) | |
| High school and secondary education | 41 (11.02%) | 42 (11.44%) | 83 (11.2%) | |
| Monthly income (USD), median (IQR) | 13.64 (0.45−45.45) | 18.18 (4.55−45.45) | 18.18 (2.27−45.45) | 0.08 |
IQR, interquartile range; USD, U.S. dollars.
Data are numbers with percentages in parentheses unless otherwise indicated.
Figure 1.
Flowchart displaying study sampling, participant recruitment, and nonresponse rates.
Farming was the predominant occupation. Nearly all of the population were self-employed, compatible with a subsistence lifestyle that reflects the economy of the study area. Importantly, 9.6% of the population completing the questionnaire (738 responses) indicated knowledge of pre-existing HTN, and 1.6% (739 responses) had knowledge of pre-existing DM. Approximately one-half of those reporting a history of either HTN or DM also reported having been on treatment for the respective disorder. Current therapy was not questioned. Approximately 1% of those responding (737 responses) related knowledge of pre-existing kidney disease, and about one-third of those individuals reported some treatment for kidney problems. Based on the questionnaire responses, disease awareness was extremely low.
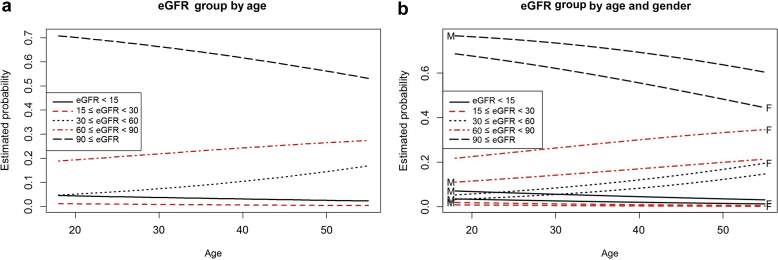
The overall estimated prevalence of CKD stages III through V was 12.4% (95% CI = 10.2−14.8) in 712 individuals for whom creatinine measurements were available (Table 2). There were 97 of 712 individuals with eGFR <60 ml/min per 1.73 m2 (Table 2). Stage III disease was the most prevalent level of CKD in this population, with 68 of 712 individuals with eGFR reported between 30 and 59 ml/min per 1.73 m2, 8.5% (95% CI = 6.8−10.6). In contrast, 5 individuals were found to have CKD stage IV, 0.8% (95% CI = 0.3−1.9) and 24 individuals CKD stage V, 3.0% (95% CI = 2.1−4.4). Estimated GFR decreased with advancing age for the population as a whole (generalized logistic regression, P ≤ 0.05) (Figure 2). This effect was more pronounced for females than for males. Furthermore, eGFR was observed to be lower with advanced age for individuals with CKD (generalized logistic regression, P ≤ 0.05). Examined by individual stage of CKD, advancing age was significantly associated with increased frequency of sCKD stage III (n = 64; P < 0.05). However, this pattern was not significant for CKD stage IV or V, perhaps because of the smaller sample sizes (n = 5 and n = 24, respectively). Moreover, there was a surprisingly high frequency of CKD stage V observed in the youngest age group. Of 24 individuals with CKD stage V, 37.5% (n = 6 of 24) were <26 years of age. Although there was no significant age-by-gender interaction, females exhibited an increased proportion of eGFR between 30 and 90 ml/min per 1.73 m2 and a decreased proportion of eGFR >90 ml/min per 1.73 m2 relative to males (P < 0.05) (Fig. 3a and b). Estimated CKD prevalence differed significantly by community. The estimated prevalence of CKD in community A was 20.8% (95 CI = 16.9−25.2) and 4.4% (95% CI = 2.8−6.9) in community B.
Table 2.
Prevalence and characteristics of chronic kidney disease in the study population
| Characteristics | n | Casesa | Prevalence estimate (95% CI)b |
|---|---|---|---|
| Overall CKD, stages III−V | 712 | 97 | 12.4 (10.2−14.8) |
| Gender | |||
| Male | 292 | 41 | 13.5 (10.2−17.7) |
| Female | 420 | 56 | 11.5 (8.9−14.7) |
| Age, yr | |||
| 18−30 | 151 | 18 | 11.7 (7.5−17.7) |
| 31−39 | 202 | 21 | 10.2 (6.7−15.3) |
| 40−49 | 212 | 30 | 13.0 (9.4−17.9) |
| ≥50 | 147 | 28 | 15.4 (10.8−21.3) |
| Community | |||
| Community A | 358 | 80 | 20.8 (16.9−25.2) |
| Community B | 354 | 17 | 4.41 (2.8−6.9) |
| Comorbidities | |||
| Hypertension | 118 | 14 | 12.3 (10.1−15.0) |
| Diabetes | 105 | 15 | 12.1 (7.5−18.8) |
| Self-reported kidney problems, n (%) | 739 | 6 | 0.8% |
| Treated for kidney problems | 6 | 2 | 33% |
CI, confidence interval; CKD, chronic kidney disease.
Cases refer to cases of CKD (stages III–V).
Weighted to account for household size and community stratification.
Figure 2.
Relationship of estimated glomerular filtration rate (eGFR) to age. Scatterplot of eGFR versus age with genders identified as female (red) and male (black). Locally weighted scatterplot smoothing (LOESS) has been fitted for each gender. Analysis of covariance confirmed that eGFR decreases with age similarly for both genders. Overall age is significant (P < 0.001), with on average a 12−eGFR unit drop per decade of life. Genders differ significantly (P < 0.001), with females on average 8.7 eGFR units lower than males. There is no significant gender-by-age interaction (P = 0.9).
Figure 3.
Relationship of (a) estimated glomerular filtration rate (eGFR) group by age and (b) eGFR group by age and gender. (a) Chronic kidney disease (CKD) stage categorized according to eGFR < 15, 15 ≤ eGFR < 30, 30 ≤ eGFR < 60, 60 ≤ eGFR < 90, and 90 ≤ eGFR. Multinomial logistic regression (unordered categories or generalized logistic regression) depicts the estimated probability of group membership for each of the 5 eGFR groupings at every age; at any given age, the estimated probabilities sum to 1. Because there are so few patients in the bottom 2 eGFR groups (those with eGFR <30), particularly for older ages, the model does not find any significant trend with age for those groups (P > 0.5 for both). Proportions between 30 and 60 and between 60 and 90 increase significantly with age, and, as a consequence, proportion >90 decreases significantly with age (P < 0.05 for all). (b) Similar to the eGFR value grouping shown in (a), the eGFR group is depicted by age and gender. Predicted estimated probability curves by gender are identified as female (red) and male (black). The gender-by-age interaction was not significant (P > 0.3), so the overall trends are similar for the genders. However, there is a significant gender effect that shows increased proportions for eGFR between 30 and 90 and decreased proportion >90 for females relative to males (that is the result of eGFR in females being slightly lower than in males).
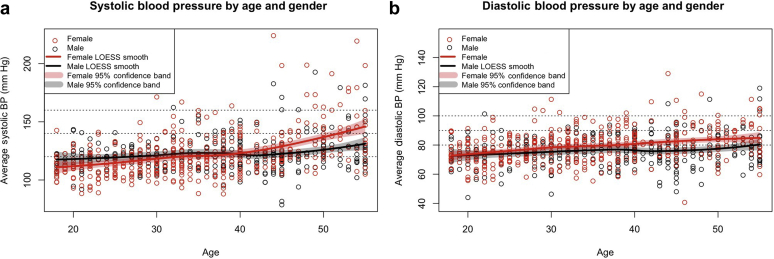
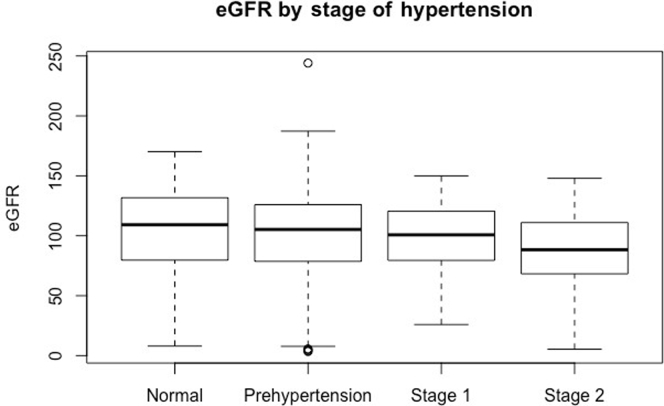
Among the 735 individuals for whom BP measurements were available, the estimated prevalence of stage I or stage II HTN was 19.9% (95% CI = 17.1−22.9) (Table 3). The pre-HTN estimated prevalence was 38.0% (95% CI = 34.6−41.5), stage I HTN was 15.6 (95% CI = 13.0−18.4), and stage II HTN was 4.3% (95% CI = 3.1−5.9). Similar to the association of increasing age with lower eGFR, logistic regression showed significantly higher systolic or diastolic BP with advancing age for the study population as a whole (P < 0.05) (Figure 4a and b). Proportional odds logistic regression analysis demonstrated significantly greater increases in BP with age in females as compared with males (P < 0.001). This was evident for both stage I and stage II HTN. Correlation of eGFR with stage of HTN failed to reveal any significant relationship (Kruskal−Wallis test, P = 0.86) (Figure 5). We also could not detect a significant relationship with analysis of covariance for the presence of HTN when the stage of HTN was correlated with the level of GFR after adjusting for the effect of age (P > 0.1).
Table 3.
Prevalence and characteristics of hypertension in the study population
| Characteristics | n | Casesa | Prevalence (95% CI)b |
|---|---|---|---|
| Overall HTN, stages I and II | 734 | 144 | 19.9 (17.1−22.9) |
| Gender | |||
| Male | 299 | 49 | 17.0 (13.1−21.7) |
| Female | 435 | 95 | 21.9 (18.2−26.1) |
| Age, yr | |||
| 18−30 | 158 | 16 | 12.3 (7.7−18.9) |
| 31−39 | 212 | 32 | 15.0 (10.8−20.5) |
| 40−49 | 215 | 44 | 23.6 (18.2−30.0) |
| ≥50 | 149 | 52 | 31.7 (25.0−39.3) |
| Community | |||
| Community A | 369 | 69 | 20.0 (16.0−24.6) |
| Community B | 365 | 76 | 19.7 (16.1−24.0) |
| Self-reported HTN, n (%) | 739 | 71 | (9.6%) |
| Treated for HTN | 71 | 38 |
CI, confidence interval; HTN, hypertension.
Cases refer to cases of hypertension (stages I and II).
Weighted to account for household size and community stratification.
Figure 4.
Relationship of hypertension to age and gender. Systolic (a) and diastolic (b) blood pressure (BP) are plotted against age, separated by gender, and identified as female (red) and male (black). Locally weighted scatterplot smoothing (LOESS) has been fitted for each gender. Although systolic and diastolic BP increases for both genders with increasing age, the effect for females is more pronounced. Proportional odds logistic regression confirmed a significant gender-by-age interaction (P < 0.001).
Figure 5.
Relationship between stage of hypertension (HTN) and estimated glomerular filtration rate (eGFR). One-way analysis of variance reveals no significant difference between individuals with normal blood pressure (BP) through stage I hypertension. However, those with stage II hypertension do exhibit significantly lower values for eGFR than individuals in the other stages (P < 0.01).
An estimated 25.7% of the population (95% CI = 22.6−29.1) exhibited prediabetic levels of HbA1c, whereas 14.8% (95% CI = 12.4−17.6) were estimated to be overtly diabetic (Table 4). Similar to the association of advancing age with increased BP and decreased levels of eGFR, levels of HbA1c increased with advancing age (Figure 6a). Proportional odds logistic regression analysis (Figure 6b) demonstrated no significant gender-by-age interaction (P > 0.05) with HbA1c, although both age (P < 0.001) and gender were significantly associated with the stage of diabetes (P < 0.002). Whereas average HbA1c levels for females were only slightly greater than those for males, females had an increased likelihood of falling in the pre-DM and DM groups at all ages (P < 0.002). Also evident from Table 4 and Figures 6a and b, the prevalence of DM increased with advancing age. When we compared results from the POC HbA1c test and the laboratory-based test among the 20 randomly selected specimens from the Muhimbili Hospital blood bank, the mean HbA1c using the POC test was 5.31% and 5.39% using the laboratory-based test. The mean difference between tests was 0.085% (SD = 0.50%). Results from the t test showed no significant difference between the 2 testing strategies (P = 0.46).
Table 4.
Prevalence and characteristics of diabetes in the study population
| Characteristics | n | Casesa | Prevalence (95% CI)b |
|---|---|---|---|
| Overall diabetes (HbA1c ≥6.5) | 707 | 105 | 14.8 (12.4−17.6) |
| Gender | |||
| Male | 289 | 38/289 | 13.1 (9.7−17.6) |
| Female | 418 | 67/418 | 16.0 (12.8−19.9) |
| Age, yr | |||
| 18−30 | 149 | 17/149 | 11.4 (7.2−17.6) |
| 31−39 | 202 | 19/202 | 9.4 (6.1−14.3) |
| 40−49 | 210 | 31/210 | 14.8 (10.6−20.2) |
| ≥50 | 146 | 38/146 | 26.0 (19.5−33.8) |
| Community | |||
| Community A | 356 | 60 | 17.4 (13.8−21.7) |
| Community B | 351 | 45 | 12.6 (9.5−16.5) |
| Self-reported diabetes n (%) | 739 | 12 | (1.6%) |
| Treated for diabetes | 12 | 6 |
CI, confidence interval.
Cases refer to cases of diabetes (HbA1c ≥ 6.5).
Weighted to account for household size and community stratification.
Figure 6.
Relationship between measured HbA1c, age, and gender. (a) Scatterplot of HbA1c versus age of patient, separated by gender (red represents female, black represents male). Locally weighted scatterplot smoothing (LOESS) smooth fits are included. (b) Estimated probabilities of diabetes (DM) stage (normal, pre-DM, DM) by age of patient, separated by gender. Calculated from proportional odds logistic regression. There was no significant gender-by-age interaction, but both age (P < 0.001) and gender (P < 0.002) were significant. The probability of being in the pre-DM or DM stage increases with age, and females are more likely to be in those stages than are males at all ages.
No significant relationship could be detected between eGFR or stage of CKD when HTN (stages I or II) and abnormal HbA1c were treated as a combined, single comorbidity (Welch 2-sample t test, P = 0.23). The extent of association of HbA1c elevation reflecting DM, elevated BP reflecting the presence of HTN, and CKD are shown in the Venn diagram in Figure 7.
Figure 7.
Venn diagram displaying the co-associations of chronic kidney disease (CKD), hypertension, and HbA1c.
Discussion
Given the increasing burden of NCDs in low- and middle-income countries, this study provides additional information regarding the prevalence of CKD, HTN, and DM among a population-based sample in rural Tanzania. We report data derived from a probability-based sample of 739 individuals from a rural environment with POD measurements obtained in the household.
We observed an overall prevalence of stages III to V CKD of 12.4%. In contrast, data reported for the National Health and Nutrition Examination Survey 1999 to 2004 by the National Center for Health Statistics indicated the prevalence of CKD stages III to V at 5.8% in the United States.16 Therefore, the prevalence of CKD (stage III and above) in the population studied in rural Tanzania appears to exceed the prevalence of CKD of the same stages in the United States. We found that the prevalence of CKD in rural Tanzania increases directly with increasing age, and that females exhibited a greater prevalence of CKD than males at all ages. Although no comparative data for age and gender for CKD are available for a more general population in Tanzania, similar trends of decreasing eGFR with age are noted in the U.S. population.17 However, there are no evident gender differences in the prevalence of CKD stages I to IV reported for National Health and Nutrition Examination Survey IV data.16
A recent systematic review and meta-analysis of CKD in sub-Saharan Africa reported the overall CKD prevalence to be 13.9% (95% CI = 12.2%−15.7%), as synthesized from 21 studies,18 which is similar to findings from this study. However, Stanifer et al., in a study conducted in northern Tanzania, found significant differences between urban and rural CKD prevalence (15.2% and 2.0%, respectively).5 Stanifer et al. identified CKD as eGFR <60 ml/min per 1.73 m2 or persistent proteinuria, and by these criteria the overall community prevalence of CKD reported was 7.0%.5 In an additional paper, Stanifer et al. reported a significant clustering of multiple NCDs, including CKD, across urban and rural neighborhoods in Tanzania, suggesting that etiologies may be driven by environmental and lifestyle factors.19 In the present study, we also found disparate prevalence of CKD based on the community of residence, although both communities were rural. More research is needed to understand what is driving the variation in CKD prevalence between neighborhoods and communities in Tanzania. Based on these observations, it is clear that CKD poses a significant health threat and health care burden in Tanzania, and more understanding of disease etiology is needed.
It was surprising that we could not identify the expected association of DM and HTN with CKD in our study. This is in striking contrast to data for the United States, where some 85% or greater of CKD is associated with DM and/or HTN.20 However, another study conducted in Tanzania by Stanifer et al. reported that more than one-half of CKD cases were not associated with the usual risk factors, including HTN and DM.5 Thus, based on these studies, it is unclear whether the causality of CKD among Tanzanian populations is related to the traditional risk factors, including HTN and DM, through some more subtle mechanism or the result of some yet-to-be-identified postinfectious, environmental, genetic, or other etiology of kidney disease. An additional report from northwestern Tanzania reported an extremely high CKD prevalence of 83.7% among diabetic patients attending a hospital-based DM clinic (CKD defined as eGFR <60 ml/min per 1.73 m2, or the presence of microalbuminuria or proteinuria).6 The prevalence of CKD in this population was likely biased by the highly selected nature of a diabetic population being evaluated or treated at a tertiary medical facility. In addition, this study did not take into account potential infectious agents and parasitic agents, such as streptococcus, schistosomiasis, Brugia Malayi (causing filarisis), and malaria, that are prevalent in the region and could be contributing to the high prevalence of CKD in this geographic area near Lake Victoria.21
Results from the present study suggest that a surprising number of young people have CKD stage V. To our knowledge, this is the first finding of high CKD prevalence biased toward this younger age group in eastern, sub-Saharan Africa. However, it should be noted that these estimates are based on limited numbers of individuals in these groups. However, if true, this trend could represent an unknown causal factor for CKD other than the usual DM and HTN risk factors prevalent in the United States and Europe.
The present study is 1 of the few to report BP measurements by age and gender for individuals in Tanzania. Overall, results from the present study are consistent with others in the region. For example, a recent study in northern Tanzania found the prevalence of hypertension to be 17.6% (95% CI = 13.9−21.9) among rural residents.22 Another study found the prevalence of hypertension in urban Tanzania to be 23.7% (95% CI = 21.3%−26.2%).23 Our observations indicate that BP significantly increased with age, and at age >55 years, the BP increase was greater for females than for males. Another study in northern Tanzania that assessed age- and gender-stratified prevalence of HTN found that HTN was significantly correlated with increasing age and was nonsignificantly higher among males as compared to females.24 Stanifer et al. reported a prevalence of HTN of 30.6% and 19.1% for urban and rural settings, respectively, in Northern Tanzania, although results were not stratified by gender or age.5 Data reported for NHANES 1999 to 2000 indicated a prevalence of stage I and stage II hypertension in 24.6% (95% CI = 22.5−26.8) in the U.S population.16 Blood pressure increased with advancing age in the United States, and, although the BP values are higher for males at younger ages, females demonstrate significantly greater increases in BP than males after age 55 years, in concert with our observations for this rural sample in Tanzania.25
The prevalence of DM in the present study was higher than in several other studies in the region. For example, a study from northwestern Tanzania and southern Uganda found that the DM prevalence ranged from 1% to 2%, as measured through random and fasting blood glucose readings.22 Another study from Tanzania found the adult DM prevalence to be 3.7%, also measured using blood glucose.23 However, a more recent study from Tanzania that also used POC HbA1c test kits found a 21.7% (95% CI = 15.2–29.8) prevalence of glucose impairment (defined as HbA1c ≥6%),26 which is comparable to the present study’s findings. It is possible that the use of POC HbA1c tests overestimated DM prevalence. A study from Uganda compared DM prevalence using 2 methods, namely, POC HbA1c testing and fasting plasma glucose, among a population-based sample of more than 700 individuals. The study found that 11.3% of the sample had DM when diagnosed using the HbA1c measure compared to only 4.8% when using fasting plasma glucose.27 HbA1c can be spuriously affected by vitamin and mineral deficiencies, illnesses, and hematologic factors. Acute or chronic blood loss results in lower HbA1c, although iron deficiency, which might be expected to be common in this largely vegetarian population, results in elevation of HbA1c.28 Although intake of carotenoids may decrease, other nutritional deficiencies such as Mg++or chromium increase HbA1c.29 In addition, hemoglobinopathies such as sickle cell could significantly affect HbA1c levels of individuals in this region.30 It is possible that these factors affected the HbA1c levels of participants, irrespective of their DM status, which could have led to an overestimation of prevalence.
The relatively high apparent prevalence for the NCDs that were examined in this pilot study was unexpected. Although similar estimates of prevalence were reported earlier for other areas, we expected them to be relatively lower in this rural, agricultural area of the country. Also unexpected to us is that the prevalence estimates for HTN, DM, and kidney disease that we observed are similar to what have been reported in high-income countries and specifically in the United States.4, 31, 32
In addition, we found no significant correlation of CKD with DM and HTN, which represent 2 of the most common traditional risk factors in the United States and Europe. The recent study on CKD in northern Tanzania conducted by Stanifer et al.5 found that approximately one-half of CKD cases were not associated with traditional DM and HTN risk. Taken together, these findings suggest likely contributions from infectious, environmental, genetic, or other yet-to-be- identified causes. Although potential infectious causes of CKD and its precursors (e.g., chronic glomerulonephritis) in this region have been acknowledged, including hepatitis C, hepatitis B, and HIV,33 no comprehensive study in sub-Saharan Africa has been undertaken to fully investigate CKD etiology. In other parts of the world, including Central America, Egypt, Sri Lanka, and India, instances of CKD of unknown etiology are increasing, specifically among males involved in agricultural activities. A recent publication postulated a role for uric acid in the pathogenesis of Mesoamerican nephropathy in Nicaragua, a potential candidate for causality of CKD in this Tanzanian population.34 A recent systematic review of CKD of unknown etiology identified some commonalities in risk factors across regions, including exposure to heavy metals and agriculture, but the review noted that risk factors were not consistent across several geographic areas.35 Evaluation of the causality of CKD of unknown etiology within sub-Saharan Africa merits further study.
The strengths of the present study include the study design and POC measures. Households were randomly selected based on random geographic mapping of a defined, statistically relevant sample of a local population for 2 different areas. An additional strength was the POC testing used to obtain all of the measurements in the household immediately after obtaining informed consent. However, the results of this study also have several limitations. CKD was assessed with a single measurement of serum creatinine concentration in a blood sample acquired at the POC but requiring transport to a distant (although clinical laboratory improvement ammendments–certified) laboratory. Although we have no evidence to suggest inaccurate measures, it is possible that sample degradation during transportation prior to serum separation altered creatinine levels.36 Chronic kidney disease was identified from estimated GFR derived from the CKD-EPI equation. The CKD-EPI equation used to estimate GFR has not been validated for this population, although efforts to evaluate the equation among various ethnicities and races, including populations from sub-Saharan Africa, have been conducted elsewhere.37, 38 A recent study from Kenya found the CKD-EPI equation to be the most accurate estimating equation when comparing results to direct measure of GFR by iohexol clearance among a small sample of HIV-infected individuals, although the authors also found that eliminating the race coefficient improved accuracy further.39 We were unable to obtain systematic observations for the presence of proteinuria or other markers of kidney disease in the present study, which did not allow estimation of the prevalence of CKD stages I and II or more robust estimates for the prevalence of CKD stages III to V. Diabetes was assessed only with HbA1c measurement at the POC; we did not measure fasting plasma glucose. Although care was taken to maintain the appropriate temperature for the HbA1c test kits prior to use and although the kits “lock-out” if exposed to extremely high temperature, this could be a variable that was difficult to control or verify. Our validation of the HbA1c POC test kits comprised a small sample size, which limited our ability to detect systematic differences between the POC and laboratory tests. It is also possible that using an HbA1c cut-off of 6.5% to diagnose diabetes is not appropriate for this population, but we used this cut-off because it is recommended by the World Health Organization and the American Diabetes Association. In addition, we were unable to collect data on height and weight. It is also possible that there is some survivor bias in this pilot study, in that patients with the most severe HTN, DM (particularly type I DM), and kidney disease may have been lost prior to the data acquisition for our study, which could explain the overrepresentation of CKD stage V seen among young people. Because we could only measure HbA1c, we could not separately identify type I from type II DM in our sample. However, advanced CKD and severe HTN were present in sufficient numbers that some early mortality related to severe disease would be expected and could have blunted the prevalence estimates that we report. Whereas HbA1c levels tend to be stable for 2 to 3 months, serum creatinine levels can vary over the course of a day, depending on nutrition, hydration, drug interactions, and presence of infections.40 The study population comprised community members 18 to 55 years of age; thus elderly patients were excluded, and estimates among this population were not obtained. Because of the age restriction within our sample and the gender imbalance, our results are not as generalizable to the larger Tanzania population, as the sample does not mirror the entire age and gender structure of the population. In addition, whereas our 2-stage sampling scheme and randomization at the household and community levels were designed to obtain a representative sample, it is possible that nonresponse bias and community differences biased our sample. Although all household locations were mapped across the 2 communities, it is also possible that more than 1 household resided at a location (e.g., a compound containing several generations of family members), which was not accounted for in our randomization.
Our findings reflect a significant burden of NCDs in a rural community in Tanzania. Furthermore, awareness of CKD, HTN, and DM was low; the capability to identify and diagnose these conditions is largely unavailable; and adequate treatment of these conditions is sorely lacking. Although outpatient medical facilities and health care workers are available in the local community health centers of Kisarawe, health care services are severely resource limited, especially for NCDs. This finding resonates throughout Tanzania, as 1 recent survey of 24 public and not-for-profit health facilities conducted across Tanzania found primary care for NCDs inadequate in most facilities.41 However, the Tanzanian government is currently working toward strengthening the diagnostic and treatment capacity for DM and HTN. Furthermore, the focus of most available health care is on treatment of acute, infectious conditions, especially febrile illnesses. The knowledge base, technology, and resources, such as pharmaceuticals, focused on diagnosis and treatment of these chronic, NCDs needs to become a health priority with stronger donor support.
In conclusion, this study identified a relatively high prevalence of NCDs including CKD, HTN, and DM among a rural population in Tanzania. Given the costs and numerous challenges involved with treating these NCDs,42 especially in the advanced stages, it is imperative that we develop low-cost, effective prevention and early treatment paradigms in low-resource settings in order to reduce the risk of renal failure and the expected high cardiovascular morbidity associated with it.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding for this project was received from the Global Health Initiative Pilot Project Award, Center for Global Health, Medical University of South Carolina, Dialysis Clinics Inc., Nashville, TN, and the National Institute of Health Award NIMH RO1MH095869.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill N.R., Fatoba S.T., Oke J.L. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney P.M., Whelton M., Reynolds K. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF Diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Practice. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Stanifer J.W., Maro V., Egger J. The epidemiology of chronic kidney disease in northern Tanzania: a population-based survey. PLoS One. 2015;10:e0124506. doi: 10.1371/journal.pone.0124506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janmohamed M.N., Kalluvya S.E., Mueller A. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol. 2013;14:1–5. doi: 10.1186/1471-2369-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering T.G., Hall J.E., Appel L.J. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . World Health Organization; Geneva: 2011. Use of Glycated Haemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. [PubMed] [Google Scholar]
- 10.Peake M., Whiting M. Measurement of serum creatinine—current status and future goals. Clin Biochem Rev. 2006;27:173–184. [PMC free article] [PubMed] [Google Scholar]
- 11.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inker L.A., Astor B.C., Fox C.H. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–S266. [PubMed] [Google Scholar]
- 14.R: A language and environment for statistical computing [computer program] Foundation for Statistical Computing; Vienna, Austria: 2016. https://www.R-project.org/ Available at: [Google Scholar]
- 15.Stata Statistical Software [computer program] StataCorp LP; College Station, TX: 2015. [Google Scholar]
- 16.Prevalence of chronic kidney disease and associated risk factors—United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56:161–165. [PubMed] [Google Scholar]
- 17.Inker L.A., Shafi T., Okparavero A. Effects of race and gender on measured GFR: the multi-ethnic study of atherosclerosis. Am J Kidney Dis. 2016;68:743–751. doi: 10.1053/j.ajkd.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Stanifer J.W., Jing B., Tolan S. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 19.Stanifer J.W., Egger J.R., Turner E.L. Neighborhood clustering of non-communicable diseases: results from a community-based study in northern Tanzania. BMC Public Health. 2016;16:226. doi: 10.1186/s12889-016-2912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.USRDS Report: Incidence, prevalence, patient characteristics, & treatment modalities. Am J Kidney Dis. 2014;63:e215–e228. [Google Scholar]
- 21.Mpondo B.C.T., Neilson E., Ernest A. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrology. 2016;17:71. doi: 10.1186/s12882-016-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavishe B., Biraro S., Baisley K. High prevalence of hypertension and of risk factors for non-communicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in northwestern Tanzania and southern Uganda. BMC Med. 2015;13:126. doi: 10.1186/s12916-015-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendriks M.E., Wit F.W., Roos M.T. Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PLoS One. 2012;7:e32638. doi: 10.1371/journal.pone.0032638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galson S.W., Staton C.A., Karia F. Epidemiology of hypertension in northern Tanzania: a community-based mixed-methods study. BMJ Open. 2017;7:e018829. doi: 10.1136/bmjopen-2017-018829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon S.S., Carroll M.D., Fryar C.D. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief. 2015;220:1–8. [PubMed] [Google Scholar]
- 26.Stanifer J.W., Cleland C.R., Makuka G.J. Prevalence, risk factors, and complications of diabetes in the Kilimanjaro region: a population-based study from Tanzania. PLoS One. 2016;11:e0164428. doi: 10.1371/journal.pone.0164428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayega R.W., Guwatudde D., Makumbi F.E. Comparison of fasting plasma glucose and haemoglobin A1c point-of-care tests in screening for diabetes and abnormal glucose regulation in a rural low income setting. Diabetes Res Clin Pract. 2014;104:112–120. doi: 10.1016/j.diabres.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Gallagher E.J., Le Roith D., Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9–17. doi: 10.1111/j.1753-0407.2009.00009.x. [DOI] [PubMed] [Google Scholar]
- 29.Nitin S. HbA1c and factors other than diabetes mellitus affecting it. Singapore Med J. 2010;51:616–622. [PubMed] [Google Scholar]
- 30.Lacy M.E., Wellenius G.A., Sumner A.E. Association of sickle cell trait with hemoglobin A1c in African Americans. JAMA. 2017;317:507–515. doi: 10.1001/jama.2016.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coresh J., Astor B.C., Greene T. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 32.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 33.Barsoum R.S. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–999. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 34.Kupferman J., Amador J.J., Lynch K.E. Characterization of Mesoamerican nephropathy in a kidney failure hotspot in Nicaragua. Am J Kidney Dis. 2016;68:716–725. doi: 10.1053/j.ajkd.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Lunyera J., Mohottige D., Isenburg M.V. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd J., Warner M.H., Kilpatrick E.S. Stability of creatinine with delayed separation of whole blood and implications for eGFR. Ann Clin Biochem. 2007;44:384–387. doi: 10.1258/000456307780945660. [DOI] [PubMed] [Google Scholar]
- 37.Stevens L.A., Claybon M.A., Schmid C.H. Evaluation of the CKD-EPI equation in multiple races and ethnicities. Kidney Int. 2011;79:555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agoons D.D., Balti E.V., Kaze F.F. Performance of three glomerular filtration rate estimation equations in a population of sub-Saharan Africans with type 2 diabetes. Diabet Med. 2016;33:1291–1298. doi: 10.1111/dme.12996. [DOI] [PubMed] [Google Scholar]
- 39.Wyatt C.M., Schwartz G.J., Owino Ong'or W. Estimating kidney function in HIV-infected adults in Kenya: comparison to a direct measure of glomerular filtration rate by iohexol clearance. PLoS One. 2013;8:e69601. doi: 10.1371/journal.pone.0069601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samra M., Abcar A.C. False estimates of elevated creatinine. Permanente J. 2012;16:51–52. doi: 10.7812/tpp/11-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peck R., Mghamba J., Vanobberghen F. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Health. 2014;2:e285–e292. doi: 10.1016/S2214-109X(14)70033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]