Abstract
Introduction
There is a need for treatment guidelines and prognostic factor identification in children with primary IgA nephropathy. We analyzed the causative effect of steroids and the applicability of the Oxford classification.
Methods
A total of 82 consecutive children (mean 10.6 years; median follow-up 3.3 years) were reviewed; 21 patients (25.6%) presented with acute kidney injury, and 6 (7.3%) with nephrotic syndrome. Renal biopsies were scored for Oxford classification and podocytopathic features in 2 groups: a group treated with steroid therapy (some in association with cyclophosphamide) and supportive care (renin angiotensin system blockade) and a group treated by supportive care alone.
Results
The 2 groups were not comparable because baseline clinical data were different. Estimated glomerular filtration rate (eGFR) in immunosupressive group significantly improved between M0 (at onset) and M6 (6 months after treatment) from 89.9 [61.2–114.5] to 110.5 [93.7–120] ml/min per 1.73 m2, P < 0.001. Proteinuria also significantly decreased from (1.6 [1–4.3] to 0.3 [0.2–0.7] g/g, P < 0.001). In the supportive care group, eGFR and proteinuria remained stable. Podocytopathic features were predictive of renal function decline by univariable (−4.9 ± 14.9 ml/min per 1.73 m2, P = 0.0079) and multivariable analysis and of poor renal prognosis to a combined event (renal function impairment more than 10% of the eGFR baseline or chronic kidney disease stage 3 at 6 months) in univariable analysis. MEST-C score failed to prove its prognostic value.
Conclusion
Immunosuppressive treatment, especially steroid therapy, seems beneficial in children with glomerular inflammation and proliferation. The Oxford classification does not appear to be entirely appropriate in predicting long-term renal prognosis for children, whereas the characteristics of podocytopathy are strongly predictive of renal prognosis.
Keywords: children, histopathology, IgA nephropathy, renal biopsy, steroid
IgA nephropathy (IgAN) is the most common primary glomerulonephritis in children and adolescents worldwide.1 IgAN includes a wide spectrum of clinical presentations, from isolated hematuria to acute nephritis, with rapid loss of renal function. In the absence of stratified recommendations, 4 prognostic factors are usually considered by clinicians contemplating therapeutic options: the presence of hypertension, proteinuria > 1 g/24 h, estimated glomerular filtration rate (eGFR), and acute and chronic histologic lesions using MEST scores (M, mesangial hypercellularity; E, endocapillary proliferation; S, focal glomerulosclerosis/adhesion; T, tubular atrophy/interstitial fibrosis). Of note, these factors are essentially derived from studies in adults.2, 3, 4
Physicians have long considered pediatric IgAN a benign disease5, 6 subject to remissions and occasionally to late relapses during adulthood.7 However, a cohort study with an extended follow-up showed that 10% to 13% of children will eventually reach end-stage renal disease (ESRD) within 10 years and 20% to 30% within 20 years, close to what has been found in adult patients.7, 8, 9 Optimizing therapy in children is therefore of primary concern but is at present an unmet need. The Kidney Disease Improving Global Outcomes guidelines recommend the use of angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers in children with proteinuria > 0.5 g per day but with a low level of evidence. There is no international consensus defining the indication for immunosuppressive regimen in more severe cases.10
Prospective studies assessing the risk-to-benefit ratio of steroids in pediatric IgAN are urgently needed, as steroids are currently central to this debate.11 To facilitate the design of such a study, we first collected and retrospectively analyzed the data from 2 major pediatric nephrology departments in Paris, France, where, despite the absence of recommendations, steroids are routinely prescribed in this setting. Here, we describe the potential causative effect of steroids on the renal outcome of 82 children with IgAN diagnosed over the past 2 decades.
Materials and Methods
Patients
This retrospective bicentric study was undertaken at 2 university hospitals in Paris (Necker Enfants Malades and Robert-Debré Hospitals) and included 82 consecutive cases of children and adolescents aged <18 years, newly diagnosed with primary IgAN following renal biopsy and recorded between 1990 and 2015. The study was approved by the ethics committee of St. Antoine Hospital in Paris, France. Diagnosis of IgAN was based on immunofluorescence microscopy findings showing mesangial deposition of IgA as the predominant or co-dominant Ig. Patients with systemic diseases, such as systemic lupus erythematosus, Henoch-Schönlein purpura, and chronic liver diseases, were not included. Four patients with minimal change disease associated with IgAN were excluded. We defined 3 categories depending on the therapy administered: (i) G1 for children receiving immunosuppressive agents (pulse steroid therapy plus cyclophosphamide, pulse steroid therapy followed by oral steroid therapy, or oral steroid therapy alone); (ii) G2 for patients treated supportively using renin angiotensin system blockade (RASB) (i.e., with angiotensin-converting enzyme inhibitors and/or angiotensin receptor blocker) or without any treatment; (iii) G3, a subgroup of G2, for children treated supportively with RASB alone.
Immunosuppression in the G1 Group
Steroids were used in patients with nephrotic syndrome, acute kidney injury (AKI) or heavy proteinuria according to each center’s practices. Pulse steroid therapy consisted of i.v. methylprednisolone 500 mg/m2 for 3 consecutive days. Oral steroid therapy consisted of prednisolone 30 mg/m2 daily (Necker Hospital) or 60 mg/m2 daily (Robert-Debré Hospital) for the first month, then 30 mg/m2 1 day out of 2 over the following 2 months (Necker Hospital) or 60 mg/m2 1 day out of 2 over the following 2 months (Robert-Debré Hospital), then 15 mg/m2 1 day out of 1 (Necker Hospital) over the subsequent 18 months or 30 mg/m2 every other day over the following 2 months and then 15 mg/m2 every other day over the subsequent 18 months (Robert-Debré Hospital). Cyclophosphamide (3 or 6 i.v. pulses of 500 mg/1.73 m2) was used in patients with persistent nephrotic syndrome despite pulse steroid and oral steroid therapy.
Clinical Data Set
The following clinical and biological parameters were collected for each patient at time of biopsy (M0) and 6 months after start of immunosuppressive treatment (M6): age, sex, weight, height, clinical presentation at disease onset, time from onset to renal biopsy, presence of macroscopic hematuria at diagnosis, history of tonsillectomy, familial IgAN history (either proven by renal biopsy or suspected because of a medical history of microscopic and/or macroscopic hematuria in a next of kin), systolic and diastolic blood pressure, serum albumin, serum IgA levels, serum creatinine (expressed in μmol/l), eGFR, urine protein-to-creatinine ratio ([g/g], used as an estimation of 24-hour protein excretion adjusted for body surface area). eGFR was calculated using the updated bedside Schwartz equation: creatinine clearance (ml/min per 1.73 m2) = K × L/serum creatinine, where L is body length (cm), and K = 41.3.12, 13 A maximum eGFR set at 120 ml/min per 1.73 m2 was selected as the accuracy of eGFR for higher values is low with the Schwartz formula in children and to avoid the disproportionate impact of small variations in creatinine on the rate of renal function.13, 14 Nephrotic range proteinuria was defined as urinary protein excretion ≥3 g/g creatinine and serum albumin <30 g/l. AKI was defined as eGFR below 70 ml/min per 1.73 m2 with proteinuria and/or hematuria. ESRD was defined as an eGFR below 15 ml/min per 1.73 m2 or the need for renal replacement therapy at diagnostic workup. Microscopic hematuria was diagnosed when more than 5 erythrocytes were seen per field of view, and macroscopic hematuria was defined as the presence of gross hematuria. We were able to extract data from the French Renal Epidemiology and Information Network Registry to establish the incidence of ESRD requiring renal replacement therapy because of IgAN.
Histopathology
In the present analysis, all biopsy samples were re-analyzed for the purpose of this study by our renal pathologists (MR and MP). Some specimens were not analyzable for all histological variables. We studied variables of the Oxford classification using different thresholds for T score only because children have few chronic lesions.15 We studied 5 variables: mesangial hypercellularity (M), scored 1 (M1) if ≥50% of glomeruli had more than 3 cells per mesangial area; focal glomerulosclerosis/adhesion (S), scored (S0) if absent or (S1) if present; podocyte hypertrophy or sclerosis at the tubular pole (tip lesion) was referred to as podocytopathies (P), scored as absent (P0) or present (P1) using the Columbia Working Group definition16; artery disease (A), scored A0 if absent or A1 if present; endocapillary hypercellularity (E), scored as E0 if absent or E1 if present; extracapillary proliferation (C), simplified from the Oxford classification, including cellular/fibrocellular, or fibrous crescent scored C0 if absent or C1 if present; tubular atrophy/interstitial fibrosis (T) was modified from the Oxford classification and scored 1 (T1) if present between 10% and 25% of the relevant biopsy core, 2 (T2) if present in ≥25% of the relevant biopsy core; interstitial infiltration (II), scored 1 (II1) if present in ≥10% of the relevant biopsy core.
Statistical Analysis
The Shapiro-Wilk test was used to test the normal distribution of quantitative variables. If normally distributed, the results were expressed as mean values and SD; otherwise, median and interquartile ranges (25th–75th percentiles) were reported. Qualitative variables were summarized as counts and percentages. Nonparametric Wilcoxon-Mann-Whitney tests were performed for the different comparisons between the 2 groups of patients with IgAN and to compare the measurements of proteinuria and eGFR between M0 and M6 in each patient group (immunosuppressive vs. supportive group). Categorical variables are summarized using proportions and compared using Fisher’s exact test for subgroup analysis or the Pearson χ2 test for the entire cohort analysis.
Study of Renal Outcome
Univariable followed by multivariable (least square regression) analysis was used to determine independent pathologic parameters in the Oxford classification as predictors of variation rate of renal function at 6 months. Univariable analysis was performed to test the association between each pathological variable finding and a combined event (renal function impairment more than 10% of the eGFR baseline at 6 months or stage 3 of chronic kidney disease at 6 months). A P < 0.05 was considered statistically significant. Data were analyzed using Prism software (GraphPad Software, San Diego, CA).
Results
Clinical Characteristics at Diagnosis
A total of 82 patients with a median age of 11.3 (7.9–13.5), 65.7% of whom were boys, were enrolled in this study at the time of renal biopsy. Median follow-up was 3.3 (1.9–5.9) years. Fifteen patients (18.2%) had a suspected or proven family history of IgAN. Median time from first symptoms to renal biopsy was 0.16 years (range from 0 to 12.25 years). History of hematuria (macroscopic or microscopic) was found in the entire cohort. Isolated hematuria was found in 17 (20.7%) children. Mean eGFR was 89.5 ± 31.76 ml/min per 1.73 m2 and median proteinuria was 1.2 (0.3–3.2) g/g (Table 1). Twenty-nine patients (35.3%) had a severe clinical presentation at the onset of the disease: AKI was noted in 21 patients (25.6%), nephrotic syndrome in 6 patients (7.3%), and ESRD in 2 patients (2.4%).
Table 1.
Baseline clinical and biological cohort characteristics at time of biopsy
| Variable | (n = 82) |
|---|---|
| Age at onset (yr) | 9.9 [6.4–13] |
| Age at diagnosis (yr) | 11.3 [7.9–13.5] |
| Time between onset and biopsy (yr) | 0.16 [0–0.66] |
| Follow-up (yr) | 3.3 [1.9–5.9] |
| Male | 54 (65.8) |
| BMI (kg/m2) | 16.94 [15.6–19.7] |
| Systolic BP (mm Hg) | 113 [107–123.75] |
| Diastolic BP (mm Hg) | 66 [60–72] |
| eGFR (ml/min per 1.73 m2) | 99.8 [67.5–120] |
| IgA (g/l) | 2.2 [1.54–3.09] |
| Serum albumin (g/l) | 35.8 [29.3–38.4] |
| Proteinuria (g/g of creatinine) | 1.2 [0.3–3.2] |
| Familial IgAN history | 15 (18.2) |
| Hematuria (microscopic or macroscopic) | 82 (100) |
| Macroscopic hematuria | 63 (76.8) |
| Initial clinical presentation | |
| • Acute kidney injury | 21 (25.6) |
| • Nephrotic syndrome | 6 (7.3) |
| • ESRD | 2 (2.4) |
| • Isolated macroscopic hematuria | 7 (8.5) |
| • Isolated microscopic hematuria | 10 (12.2) |
| • Proteinuria and microscopic hematuria | 16 (19.6) |
| • Proteinuria and macroscopic hematuria | 20 (24.4) |
| Treatments | |
| • Steroid (pulse + oral) and cyclophosphamide | 9 (11) |
| • Steroid (pulse + oral) | 23 (28) |
| • Steroid (oral) | 19 (23.2) |
| • ACE or ARBs alone | 20 (24.4) |
| • No treatment | 11 (13.4) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; ESRD, end stage of renal disease; IgAN, IgA nephropathy; systolic BP and diastolic BP, systolic and diastolic blood pressure.
For quantitative variables, values are expressed as median [interquartile ranges]. For qualitative variables, values are expressed as n (%).
Fifty-one patients (G1 group) were treated with immunosuppressive therapy consisting of a variable dosage of steroids, specific to each center protocol as detailed in the Materials and Methods section, and used in association with cyclophosphamide in only 9 cases (11%) together with supportive treatment (RASB). Thirty-one patients (constituting the RASB/Follow-up group [group G2]) received supportive treatment with RASB (20 patients [24%]) or no treatment (11 patients [13.4%]) (Table 1).
Comparison of the Clinical and Pathological Features in the Immunosuppressive (G1) and RASB/Follow-up (G2) Groups
Median age at diagnosis was 10.3 years (6.5–13.4) in G1 and 11.7 years (9.8–13.75) in G2 (P = 0.09). There was no difference regarding age at onset of disease, frequency of family history of IgAN, time between first symptoms and biopsy, tonsillectomy, eGFR, serum IgA levels, body mass index, and arterial blood pressure (Table 2). The G1 group was more likely than G2, respectively, to have a lower serum albumin level (33.3 [27.8–36.8] g/l vs. 37.7 [35.58–0.11] g/l, P < 0.001) and higher proteinuria (1.6 [1.0–4.0] g/g vs. 0.3 [0.2–1.2] g/g, P < 0.001). The G1 group was more likely than G2, respectively, to have higher mesangial, endocapillary, and extracapillary proliferation, respectively (M1, 89.8% vs. 65.5%, P = 0.016; E1, 82% vs. 53.3%, P = 0.01; C1, 62% vs. 20%, P < 0.001). As expected, few children had a tubular atrophy/interstitial fibrosis score higher than T1 (Table 2). T1, S1, and P1 were not different between the G1 and G2 groups. II1 was present in a small group of patients in the G1 group only (Table 2).
Table 2.
Comparison of clinical and biological characteristics between immunosuppressive group and supportive treatment and/or follow-up group at time of biopsy
| Variable | G1 Immunosuppressive group (n = 51) |
G2 Supportive treatment and/or follow-up group (n = 31) |
P value |
|---|---|---|---|
| Age at onset (yr) | 9.1 [6.1–13.1] | 11.6 [6.8–12.9] | 0.35 |
| Age at diagnosis (yr) | 10.3 [6.5–13.4] | 11.7 [9.8–13.75] | 0.09 |
| Time between onset and biopsy (yr) | 0.08 [0–0.33] | 0.4 [0.08–1.25] | 0.25 |
| Follow-up (yr) | 3.33 [2.1–5.58] | 3 [1.3–6.75] | 0.99 |
| Familial IgAN history | 9 (17.65%) | 6 (19.35%) | 0.9 |
| Tonsillectomy | 4 (7.8%) | 5 (19.23%) | 0.15 |
| Male | 31 (60.8%) | 23 (41%) | 0.16 |
| BMI (kg/m2) | 16.94 [15.6–19.1] | 16.94 [15.48–20.24] | 0.87 |
| Systolic BP (mm Hg) | 112 [107–123] | 114 [106.3–128.3] | 0.76 |
| Diastolic BP (mm Hg) | 64 [60–73] | 68 [59.5–72] | 0.81 |
| sCreat (μM/l) | 63 [50–103] | 61 [49–72] | 0.45 |
| eGFR (ml/min per 1.73 m2) | 89.94 [61.25–114.5] | 107.5[85.15–120] | 0.24 |
| IgA (g/l) | 2.035 [1.56–2.9] | 2.28 [1.53–3.45] | 0.5 |
| Serum albumin (g/l) | 33.3 [27.9–36.8] | 37.7 [35.4–41.6] | 0.0008 |
| Proteinuria (g/g of creatininuria) | 1.63 [1–4] | 0.3 [0.2–1.2] | <0.001 |
| RASB introduction | 48 (94.1%) | 20 (64.5%) | 0.9 |
| RASB prior immunosuppression | 8 (15.7%) | — | — |
| Pathological findings | |||
| Glomerulus count | 15 [11–20] | 15 [9–18] | 0.39 |
| • M1 | 44; 89.8%; (n = 49) | 19; 65.5%; (n = 29) | 0.016 |
| • E1 | 41; 82%; (n = 50) | 16; 53.3%; (n = 30) | 0.01 |
| • C1 | 31; 62%; (n = 50) | 6; 20%; (n = 30) | <0.001 |
| • A1 | 2; 4.26% (n = 47) | 1; 4% (n = 25) | - |
| • S1 | 30; 60%; (n = 50) | 19; 63.3%; (n = 30) | 0.8 |
| • P1 | 9; 18.7%; (n = 48) | 3; 12%; (n = 25) | 0.52 |
| • T1 | 0; 0%; (n = 48) | 1 (3.9%); (n = 26) | 0.35 |
| • II1 | 6; 12.5%; (n = 48) | 0; 0%; (n = 25) | 0.08 |
A1, presence of artery disease; BMI, body mass index; C1, presence of extracapillary proliferation; eGFR, estimated glomerular filtration rate; E1, presence of endocapillary hypercellularity; IgAN, IgA nephropathy; II1, interstitial infiltration; M1, presence of mesangial hypercellularity; P1, presence of podocytopathic features; RASB, renin angiotensin system blockade; S1, presence of segmental glomerulosclerosis or adhesion; systolic BP and diastolic BP, systolic and diastolic blood pressure; T1, presence of tubular atrophy/interstitial fibrosis
For quantitative variables, values are expressed as median [interquartile ranges]. For qualitative variables, values are expressed as n (%).
Renal Outcome in Immunosuppressive Group (G1) and RASB Group (G3)
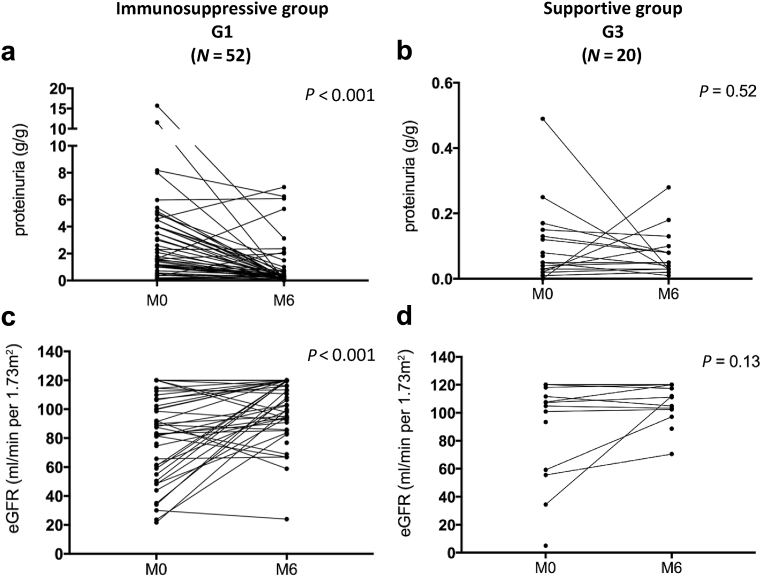
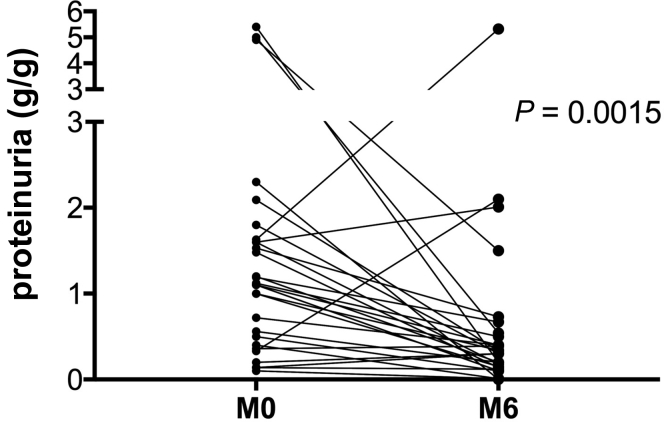
We compared the immunosuppressive group (G1) with patients receiving only RASB treatment (RASB group [G3]) to avoid potential changes (on proteinuria and eGFR) related to renin-angiotensin-aldosterone system inhibition. Clinical and histological data were not comparable at baseline between the G1 and G3 groups (data not shown). In G1, eGFR had significantly improved from 89.9 (61.2–114.5) ml/min per 1.73 m2 at time of biopsy (M0) to 110.5 (93.7–120.0) ml/min per 1.73 m2 6 months after start of immunosuppressive treatment (M6) (P < 0.001), whereas in G3 it remained stable from 111.7 (101.7–120.0) ml/min per 1.73 m2 at time of biopsy (M0) to 114.7 (102.6–120.0) ml/min per 1.73 m2 (M6) (Figure 1 and Table 3). Proteinuria was significantly reduced in G1 (from 1.6 [1–4.3] g/g to 0.3 [0.2–0.7] g/g, P < 0.001) and remained stable in G3 (Table 3 and Figure 1). Excluding the more severe forms (nephrotic syndrome and AKI) in G1, reduction of proteinuria remained highly significant (from 1.1 [0.43–1.6] g/g to 0.3 [0.13–0.5] g/g, P = 0.0015) (Figure 2). Average duration of follow-up was equivalent between the 2 groups. Data extracted from the Renal Epidemiology and Information Network Registry show that 2 patients reached ESRD: 1 patient from G1 received a renal transplant 3.5 years after diagnosis and 1 month after the start of dialysis and another had started dialysis; 2 patients from G3 were engrafted at 0.4 and 0.5 years after diagnosis, respectively. These 2 patients were identified at the point of ESRD, so any immunosuppressive treatment had been introduced at diagnosis of IgAN.
Figure 1.
Proteinuria and estimated glomerular filtration rate (eGFR) at 6 months of follow-up in the immunosuppressive group and supportive group. (a,c) Proteinuria and eGFR in immunosuppressive group at M0 and M6. (b,d) Proteinuria and eGFR in supportive group at M0 and M6. M0, at onset; M6, 6 months after treatment.
Table 3.
Proteinuria and eGFR at 6 months for immunosuppressive and RASB groups
| Variable | Group 1 Immunosuppressive group (n = 51) |
Group 3 RASB group (n = 20) |
Group 1 M0 vs. M6 P |
Group 3 M0 vs. M6 P |
||
|---|---|---|---|---|---|---|
| M0 | M6 | M0 | M6 | |||
| eGFR (ml/min per 1.73 m2) | 89.9 [61.2–114.5] | 110.5 [93.7–120] | 111.7 [101.7–120] | 114.7 [102.6–120] | <0.001 | 0.12 |
| Proteinuria (g/g creatinine) |
1.6 [1–4.3] | 0.3 [0.2–0.7] | 0.5 [0.21–1.4] | 0.5 [0.3–0.9] | <0.001 | 0.4 |
For quantitative variables, values are expressed as median [interquartile ranges]. eGFR, estimated glomerular filtration rate; M0, at onset; M6, 6 months after treatment; RASB, renin angiotensin system blockade.
Figure 2.
Proteinuria at 6 months of follow-up in the immunosuppressive group excluding nephrotic syndrome and acute kidney injury. M0, at onset; M6, 6 months after treatment.
Clinicopathological Correlation at the Time of Biopsy in the Entire Cohort
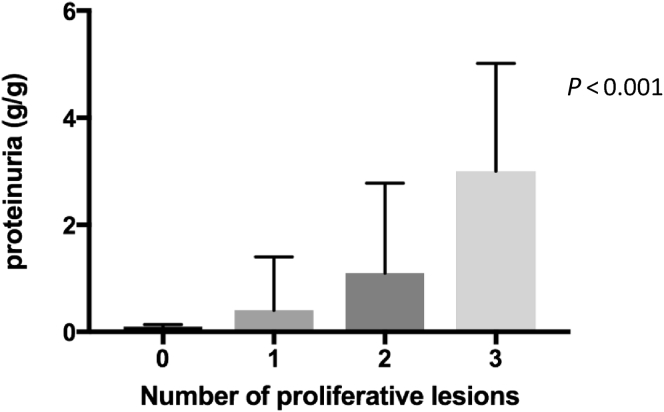
On univariable analysis, proteinuria was strongly associated with presence of mesangial proliferation (M0 vs. M1, 0.3 [0.14–1.4] vs. 1.5 [0.5–4] g/g), endocapillary proliferation (E0 vs. E1, 0.4 [0.1–1.6] vs. 1.5 [0.5–4] g/g), and extracapillary proliferation (C0 vs. C1, 0.5 [0.1–1.6] vs. 1.8 [1.1–4.5] g/g). No pathologic parameters in the Oxford classification were associated with reduced eGFR at M0 (Table 4). We observed that proteinuria reached nephrotic range when all the proliferative lesions (M1;E1;C1) were present together (Figure 3). The presence of II1 only showed a trend toward an association with a reduced eGFR (II0 vs. II1, 104.9 [78.6–120] vs. 61.6 [32.5–90.9] ml/min per 1.73 m2, P = 0.07) (Table 4). S1 and P1 were not associated with eGFR or proteinuria difference at the time of biopsy (S0 vs. S1, 1.5 [0.14–4.0] vs. 1.2 [0.3–2.4] g/g; P0 vs. P1, 1.4 [0.4–3.3] vs. 1.13 [0.2–5.4] g/g). Children with an II1 had an increase of more than 50% proteinuria but without significance (II0 vs. II1, 1.2 [0.3–3.1] vs. 3.5 [2.0–7.4] g/g, P = 0.2) (Table 4). P1 and S1, respectively, were significantly more prevalent in older children (P0 vs. P1, 10.4 [6.6–14.9] vs. 13.3 [11.8–14.9] years; S0 vs. S1, 8.8 [5.8–12.1] vs. 12.2 [9.5–13.8] years) (Table 4).
Table 4.
Implication of pathological and clinicobiological features at time of biopsy
| Variables | M0 vs. M1 | E0 vs. E1 | C0 vs. C1 | S0 vs. S1 | P0 vs. P1 | II0 vs. II1 |
|---|---|---|---|---|---|---|
| eGFR (ml/min per 1.73 m2) | 106.9 vs. 99.9 0.52 |
104.9 vs. 99.2 0.79 |
107.1 vs. 89.7 0.2 |
86.4 vs. 100.5 0.7 |
100 vs. 105.4 0.8 |
104.9 vs. 61.6 0.07 |
| Proteinuria (g/g) | 0.3 vs. 1.5 <0.001 |
0.4 vs. 1.5 <0.001 |
0.5 vs. 1.8 0.001 | 1.5 vs. 1.12 0.6 |
1.4 vs. 1.13 0.37 |
1.2 vs. 3.5 0.22 |
| Age at diagnosis (yr) | 9.3 vs. 11.7 0.1 |
11.8 vs. 10.7 0.11 |
10.4 vs. 12.7 0.15 |
8.9 vs. 12.2 0.013 |
10.4 vs. 13.3 <0.001 |
11.1 vs. 12.5 0.75 |
eGFR, estimated glomerular filtration rate.
M1 presence or M0 absence of mesangial hypercellularity; E1 presence or E0 absence of endocapillary hypercellularity; C1 presence or C0 absence of extracapillary proliferation; S1 presence or S0 absence of segmental glomerulosclerosis or adhesion; P1 presence or P0 absence podocytopathic features; II1 presence or II0 absence of interstitial infiltration. Quantitative variables are expressed as median [interquartile ranges].
Figure 3.
Association between proliferative lesions and proteinuria at time of biopsy. Proliferative lesions: M1, presence of mesangial hypercellularity; E1, presence of endocapillary hypercellularity; C1, presence of extracapillary proliferation; Kruskall-Wallis test.
Correlations Between Pathological Lesions and Short-term Renal Outcome in the Entire Cohort
We investigated the value of the histological variable to predict the rate of eGFR variation as well as renal prognosis to a combined event (chronic kidney disease stage 3 or renal function impairment more than 10% of the eGFR baseline) at M6. By univariable analysis, rate of renal function variation at M6 was significantly associated only with S1 and P1 (Tables 5 and 6). In the whole cohort, 9 of 77 patients reached the combined event at M6. Five of 12 patients with P1 reached the combined event at M6 and 8 of 49 patients with S1 reached the combined event at M6. By univariable analysis, only P1 was significantly associated with the combined event at M6. M1, E1, C1, T1, and II1 were not significantly associated with the rate of renal function variation or renal prognosis to the combined event at M6 (data not shown). Two models of multivariable analysis were designed. The first model (A) was designed to show whether the biopsy findings predicted a short-term (M6) rate of renal function variation independently of the initial assessment. It considered S1 and P1, in addition to the initial presentation (eGFR, proteinuria, and age at diagnosis). The second model (B) was designed to show whether the biopsy findings predicted short-term rate of renal function variation independently of the initial assessment even when clinical follow-up data and immunosuppressive treatment were taken into account. It considered the first model (A) in addition to proteinuria at M6 and immunosuppressive treatment. Linear least squares fitting for rate of renal function variation at M6 correlated only with podocytopathic features and failed to attain significance for S1 (Tables 5 and 6).
Table 5.
Correlations between segmental glomerulosclerosis or adhesion and renal outcome at 6 months in the whole cohort: univariable and multivariable analysis
| Rate of renal function variation |
Risk of combined event: CKD stage 3 or 10% decline in eGFR n = 9 |
|||
|---|---|---|---|---|
| Univariable rate of renal function variation (ml/min per 1.73 m2) | Multivariablea (Linear least-squares fitting) |
Univariable odds ratio (95% CI) | ||
| Model A n = 68 β (SD) |
Model B n = 66 β (SD) |
|||
| S0 (n = 27) | 23.6 ± 31 | −9.9 (4.5) P = 0.029 |
−7.1 (4.8) P = 0.14 |
n = 8 −1.62 (−4.57 to 0.15) P = 0.14 |
| S1 (n = 49) | 8.3 ± 21.4 P = 0.015 |
|||
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; S1 presence or S0 absence of segmental glomerulosclerosis or adhesion.
Model A: Multivariable with 2 pathological features + initial eGFR + initial proteinuria + age at diagnosis; Model B: Multivariable with 2 pathological features + initial eGFR + initial proteinuria + age at diagnosis, follow-up proteinuria and immunosuppressive treatment.
Table 6.
Correlations between podocytopathic features and renal outcome at 6 months in the whole cohort: univariable and multivariable analysis
| Rate of renal function variation |
Risk of combined event: CKD stage 3 or 10% decline in eGFR n = 9 |
|||
|---|---|---|---|---|
| Univariable rate of renal function variation (ml/min per 1.73 m2) | Multivariablea (Linear least-squares fitting) |
Univariable odds ratio (95% CI) | ||
| Model A n = 62 β (SD) |
Model B n = 60 β (SD) |
|||
| P0 (n = 57) |
17.2 ± 27 | −20.7 (6.9) P < 0.001 |
−18.2 (6.1) P = 0.004 |
n = 5 −2.6 (−4.3 to −0.96) P = 0.002 |
| P1 (n = 12) |
−4.9 ± 14.9 P = 0.0079 |
|||
CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; P1 presence or P0 absence podocytopathic features.
Model A: Multivariable with 2 pathological features + initial eGFR + initial proteinuria + age at diagnosis; Model B: Multivariable with 2 pathological features + initial eGFR + initial proteinuria + age at diagnosis, follow-up proteinuria and immunosuppressive treatment.
Discussion
There are no recommendations for the treatment of children with IgAN. Our study results were not based on Kidney Disease Improving Global Outcomes recommendations, and no common indication was defined before the start of the study. Because children have a long life expectancy, pediatricians are prone to prescribe steroids in addition to RASB when proteinuria is >0.5 g/l, eGFR deteriorates <70 ml/min, or when a biopsy sample shows glomerular inflammation.17 However, the management of patients differs from one center to another and from what is recommended in adult patients. Here, the Kidney Disease Improving Global Outcomes guidelines recommend steroids in the case of persistent proteinuria >1 g per day despite 3 to 6 months of optimized supportive care by RASB with GFR >50 ml/min per 1.73 m2.18 These guidelines were, however, challenged by the results of a recent prospective and randomized controlled trial showing that in adult patients with IgAN at high risk of progression (i.e., with a proteinuria >0.7 g per day) immunosuppressive therapy (steroids ± cyclophosphamide) did not significantly improve outcome.19 In the absence of histological assessment of the gravity of the disease, we have recently suggested that the interpretation of these results should be treated with caution.11 In our retrospective study, pediatricians used steroids from the outset. Less than 15% of children treated with steroids had received prior RASB treatment. However, in a randomized controlled trial, Coppo et al.20 showed that RASB treatment is effective in young patients with mild proteinuria (1–3 g/d); 40.6% reached proteinuria remission in the angiotensin-converting enzyme inhibitors group versus 8.8% in the placebo group. RASB use is strongly supported by the literature on IgAN because it not only improves prognostic factors, such as hypertension and proteinuria, but also significantly reduces glomerular inflammation. Moreover, physicians should consider the potential for spontaneous remission, especially when mesangial proliferation is absent.10 One limit of our study is that it is retrospective. However, children from the G1 group (treated at the least with steroids because of a severe phenotype) had favorable outcomes in the short term, with improvement of eGFR, reduction of proteinuria, and very few incident cases of ESRD (although it must be acknowledged that the follow-up might be insufficient because most cases of ESRD occur between 10 and 20 years after diagnosis). Previous case reports and uncontrolled studies with a small number of patients have shown efficacy of steroid therapy in children with IgAN.21, 22, 23 Niaudet et al.24 reported favorable efficacy with pulse steroid therapy in the setting of a rapidly progressive glomerulonephritis: none of the 12 children progressed to ESRD and 9 showed improvement in histologic lesions on subsequent biopsy. Other smaller pediatric studies have, however, reported different findings.25, 26, 27 Oral steroid therapy has occasionally been reported to provide a benefit for patients with proteinuria >0.3 g/g and with no eGFR deterioration.22, 23 Recently, the VALIGA study of children younger than 16 years used a propensity score and showed that steroid therapy significantly reduced the risk of progression to ESRD.28 Steroids are the most common anti-inflammatory and immunosuppressive drugs used in the treatment of both primary and secondary glomerulonephritis. Steroid therapy is relatively safe and our data, combined with those from others, support their efficacy.11 Nevertheless, RASB treatment should be encouraged, especially in association with steroid therapy. There is a strong case for the use of RASBs in the treatment of IgAN, not only because they improve prognostic factors, such as hypertension and proteinuria, but because they also may inhibit the long series of potentially negative angiotensin II effects, and achieve diminution of histological inflammation.29 We believe that prospective multicenter studies in larger pediatric cohorts should be designed to confirm that the steroid therapy's benefit/risk ratio is favorable, especially because the STOP-IgA trial cast doubt on the use of steroids in adults with intermediate risk.19
Adult patients with E1 and C1 at diagnosis not treated with immunosuppressive therapy have significantly more eGFR deterioration30 than patients receiving immunosuppressive treatment.3 Pediatricians use pulse steroids to treat children with both E1 and C1, although, again, there is no official recommendation. Our study shows that the decision to initiate steroid treatment is guided by clinical and pathologic parameters. In the VALIGA study, which covers a population of young adults, histological lesions did not predict the response to steroid use.28 However, young children have a very different histological presentation from adults, with significantly greater glomerular inflammation and proliferation and fewer chronic lesions. Our cohort had considerably more severe initial histology lesions than those reported by others in the literature.17, 21, 22, 31, 32
The Oxford classification is commonly used to evaluate the severity of IgAN and the risk of progression to ESRD. But the original Oxford study included only a limited number of children (56 cases).3 At the time of biopsy in our cohort, cross-sectional correlations between pathological findings and proteinuria for both mesangial, endocapillary, and extracapillary proliferation were present, comparable to the findings from the Oxford study. However, unlike the initial Oxford study, any correlations were found between eGFR and, in particular, S1 and T1. Furthermore, by analyzing both the short- and long-term outcomes, MEST-C classification was not predictive of renal prognosis. P1 was the only pathologic parameter predictive of the renal function decline, independent of the clinical data at the time of renal biopsy and the immunosuppressive treatment. As P1 was superimposed on S1 for all patients, it is possible that the prognostic value of the S1 was underestimated on multivariable analysis. The prognostic role of these features has already been established in an adult cohort by El Karoui et al.,33 but for the first time they can be associated with an impaired prognosis in children. Bellur et al.34 showed that P1 was associated with better renal survival in patients treated by immunosuppressive therapy. Our finding did not confirm the conclusions of Bellur et al.34 In our cohort, 4 of 5 patients with P1 reached the combined event while receiving immunosuppressive treatment. Nevertheless, interpretation of our results is limited by the small number of patients with P1 in our cohort. Presence of podocytopathic attributes may reflect specific lesions in IgAN of unknown origin beyond a final state to nephron loss.35 Gd-IgA1 may be involved by inhibiting podocyte nephrin expression.36, 37, 38 Persistence of podocyte injury leads to irreversible cellular damage, with podocyte cell death or the detachment of podocytes from the glomerular basement membrane, and ultimately glomerulosclerosis and kidney failure. P1 should clearly be interpreted differently from S1 as reported in the MEST score. Our results confirm that podocytopathic features should be considered as part of the Oxford classification, as it has been suggested by Bellur et al.34 We have not found significant correlation regarding S1, probably because IgAN is mostly diagnosed at an early stage of the disease in children who have no nephronic reduction. Our results differ from those published by the working group of the International IgA Nephropathy Network and the Renal Pathology Society, which exclude rapidly progressive cases from their study.39 In our cohort, many children had severe histological presentation with AKI and had been treated with steroids. Given this, the rate of renal function decline can be difficult to interpret in terms of the prognostic value of the pathological findings, especially for lesions of glomerular inflammation and proliferation. All these findings do not support the applicability of the Oxford classification to define long-term renal prognosis in young children, but instead suggest steroid sensitivity if M, E, and C lesions are present.
In our cohort, 9 patients were treated with steroids and cyclophosphamide. Niaudet et al.24 and Jiang et al.40 reported on the use of cyclophosphamide in children with crescentic IgAN. Jiang et al.40 showed that the combination of cyclophosphamide and steroids stabilized renal function, and significantly reduced hematuria, proteinuria, mesangial IgA deposition, and severity of histologic lesions. Kidney Disease Improving Global Outcomes recommendations have few data to support an evidence-based treatment by cyclophosphamide for rapidly progressive disease. The pathophysiological relevance of this potent immunosuppressive drug is, at best, uncertain.
Our observational study has several limitations. It is retrospective and observational. The lack of power due to the small sample size in our cohort makes it difficult to conclude about null findings. Steroid therapy can make it difficult to interpret the prognostic value of pathologic parameters. We used different definitions for T and C lesions compared with the Oxford classification. In our study, we have shown that immunosuppressive treatment in our cohort may have contributed to reduced proteinuria and improved eGFR. Nevertheless, baseline clinical data and pathologic parameters were not comparable between the G1 and G3 group. To assess renal progression, the missing data in the long-term follow-up led us to use the composite endpoint of 10% decline of renal function at 6 months or chronic kidney disease stage 3 at 6 months, which is not widely used as a consistent and stable marker of decline in GFR when the observation time is rather limited.
Conclusion
Immunosuppressive treatment using the Paris protocol may have contributed to reduced proteinuria and improved eGFR over a short time in children with severe primary IgAN. According to our results, IgAN presents as acute glomerulonephritis in children, unlike in adults where in most cases it presents as chronic glomerulonephritis. It therefore appears crucial to incorporate histological lesions in the inclusion criteria for future randomized studies. Despite its limitations, this retrospective study will help in the design of future randomized controlled trials, which are urgently needed to establish evidence-based recommendations for childhood IgAN. In addition, the Oxford classification, in its present form, seems appropriate to define steroid sensitivity status but not long-term renal prognosis in children. This interpretation must take into account the putative impact of immunosuppression treatment in our cohort. Podocytopathic features seem to be the best short-term predictive factor of renal prognosis in this population.
Disclosure
All the authors declared no competing interests.
References
- 1.Coppo R., Gianoglio B., Porcellini M.G., Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant. 1998;13:293–297. doi: 10.1093/oxfordjournals.ndt.a027821. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez E., Zamora I., Ballarín J.A. Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol. 2012;23:1753–1760. doi: 10.1681/ASN.2012010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattran D.C., Coppo R., Cook H.T. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 4.Kamei K., Harada R., Hamada R. Proteinuria during follow-up period and long-term renal survival of childhood IgA nephropathy. PLoS One. 2016;11:e0150885. doi: 10.1371/journal.pone.0150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCoy R.C., Abramowsky C.R., Tisher C.C. IgA nephropathy. Am J Pathol. 1974;76:123–144. [PMC free article] [PubMed] [Google Scholar]
- 6.Kusumoto Y., Takebayashi S., Taguchi T. Long-term prognosis and prognostic indices of IgA nephropathy in juvenile and in adult Japanese. Clin Nephrol. 1987;28:118–124. [PubMed] [Google Scholar]
- 7.Wyatt R.J., Kritchevsky S.B., Woodford S.Y. IgA nephropathy: long-term prognosis for pediatric patients. J Pediatr. 1995;127:913–919. doi: 10.1016/s0022-3476(95)70027-7. [DOI] [PubMed] [Google Scholar]
- 8.Hastings M.C., Delos Santos N.M., Wyatt R.J. Renal survival in pediatric patients with IgA nephropathy. Pediatr Nephrol. 2007;22:317–318. doi: 10.1007/s00467-006-0303-3. [DOI] [PubMed] [Google Scholar]
- 9.Ronkainen J., Ala-Houhala M., Autio-Harmainen H. Long-term outcome 19 years after childhood IgA nephritis: a retrospective cohort study. Pediatr Nephrol. 2006;21:1266–1273. doi: 10.1007/s00467-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 10.Shima Y., Nakanishi K., Hama T. Spontaneous remission in children with IgA nephropathy. Pediatr Nephrol. 2013;28:71–76. doi: 10.1007/s00467-012-2294-6. [DOI] [PubMed] [Google Scholar]
- 11.Robert T., Cambier A., Hertig A. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2016;374:991. doi: 10.1056/NEJMc1600141. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz G.J., Muñoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pottel H. Measuring and estimating glomerular filtration rate in children. Pediatr Nephrol. 2017;32:249–263. doi: 10.1007/s00467-016-3373-x. [DOI] [PubMed] [Google Scholar]
- 14.Miller W.G. Estimating equations for glomerular filtration rate in children: laboratory considerations. Clin Chem. 2009;55:402–403. doi: 10.1373/clinchem.2008.122218. [DOI] [PubMed] [Google Scholar]
- 15.Roberts I.S., Cook H.T., Troyanov S. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 16.D'Agati V.D., Fogo A.B., Bruijn J.A., Jennette J.C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa N., Ito H., Sakai T. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. J Am Soc Nephrol. 1999;10:101–109. doi: 10.1681/ASN.V101101. [DOI] [PubMed] [Google Scholar]
- 18.Abstract. Kidney Int Suppl (2011) 2012;2:142. [Google Scholar]
- 19.Rauen T., Eitner F., Fitzner C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 20.Coppo R., Peruzzi L., Amore A. IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol. 2007;18:1880–1888. doi: 10.1681/ASN.2006040347. [DOI] [PubMed] [Google Scholar]
- 21.Waldo F.B., Wyatt R.J., Kelly D.R. Treatment of IgA nephropathy in children: efficacy of alternate-day oral prednisone. Pediatr Nephrol. 1993;7:529–532. doi: 10.1007/BF00852535. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y., Hiki Y., Kokubo T. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–242. doi: 10.1159/000188848. [DOI] [PubMed] [Google Scholar]
- 23.Shibano T., Takagi N., Maekawa K. Epidemiological survey and clinical investigation of pediatric IgA nephropathy. Clin Exp Nephrol. 2016;20:111–117. doi: 10.1007/s10157-015-1129-8. [DOI] [PubMed] [Google Scholar]
- 24.Niaudet P., Murcia I., Beaufils H. Primary IgA nephropathies in children: prognosis and treatment. Adv Nephrol Necker Hosp. 1993;22:121–140. [PubMed] [Google Scholar]
- 25.Roccatello D., Ferro M., Coppo R. Report on intensive treatment of extracapillary glomerulonephritis with focus on crescentic IgA nephropathy. Nephrol Dial Transplant. 1995;10:2054–2059. [PubMed] [Google Scholar]
- 26.Welch T.R., McAdams A.J., Berry A. Rapidly progressive IgA nephropathy. Am J Dis Child. 1988;142:789–793. doi: 10.1001/archpedi.1988.02150070103038. [DOI] [PubMed] [Google Scholar]
- 27.Itami N., Akutsu Y., Kusunoki Y. Does methylprednisolone pulse therapy deteriorate the course of rapidly progressive IgA nephropathy? Am J Dis Child. 1989;143:441–442. doi: 10.1001/archpedi.1989.02150160063007. [DOI] [PubMed] [Google Scholar]
- 28.Tesar V., Troyanov S., Bellur S. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. J Am Soc Nephrol. 2015;26:2248–2258. doi: 10.1681/ASN.2014070697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppo R., Amore A., Gianoglio B. Angiotensin II local hyperreactivity in the progression of IgA nephropathy. Am J Kidney Dis. 1993;21:593–602. doi: 10.1016/s0272-6386(12)80031-x. [DOI] [PubMed] [Google Scholar]
- 30.Shi S.F., Wang S.X., Jiang L. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6:2175–2184. doi: 10.2215/CJN.11521210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppo R., Lofaro D., Camilla R.R. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. 2017;32:139–150. doi: 10.1007/s00467-016-3469-3. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa N., Honda M., Iijima K. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol. 2006;1:511–517. doi: 10.2215/CJN.01120905. [DOI] [PubMed] [Google Scholar]
- 33.El Karoui K., Hill G.S., Karras A. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 2011;79:643–654. doi: 10.1038/ki.2010.460. [DOI] [PubMed] [Google Scholar]
- 34.Bellur S.S., Troyanov S., Cook H.T., Roberts I.S., Working Group of International IgA Nephropathy Network and Renal Pathology Society Immunostaining findings in IgA nephropathy: correlation with histology and clinical outcome in the Oxford classification patient cohort. Nephrol Dial Transplant. 2011;26:2533–2536. doi: 10.1093/ndt/gfq812. [DOI] [PubMed] [Google Scholar]
- 35.Kuppe C., Gröne H.J., Ostendorf T. Common histological patterns in glomerular epithelial cells in secondary focal segmental glomerulosclerosis. Kidney Int. 2015;88:990–998. doi: 10.1038/ki.2015.116. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Liu X., Ye Z. Mesangial medium with IgA1 from IgA nephropathy inhibits nephrin expression in mouse podocytes. Eur J Clin Invest. 2009;39:561–567. doi: 10.1111/j.1365-2362.2009.02135.x. [DOI] [PubMed] [Google Scholar]
- 37.Ye Z.C., Wang C., Tang Y. Serum IgA1 from patients with IgA nephropathy up-regulates integrin-linked kinase synthesis and inhibits adhesive capacity in podocytes through indirect pathways. Clin Invest Med. 2009;32:E20–E27. doi: 10.25011/cim.v32i1.5083. [DOI] [PubMed] [Google Scholar]
- 38.Coppo R., Fonsato V., Balegno S. Aberrantly glycosylated IgA1 induces mesangial cells to produce platelet-activating factor that mediates nephrin loss in cultured podocytes. Kidney Int. 2010;77:417–427. doi: 10.1038/ki.2009.473. [DOI] [PubMed] [Google Scholar]
- 39.Coppo R., Troyanov S., Camilla R. The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int. 2010;77:921–927. doi: 10.1038/ki.2010.43. [DOI] [PubMed] [Google Scholar]
- 40.Jiang X.Y., Mo Y., Sun L.Z. Efficacy of methylprednisolone, cyclophosphamide in pediatric IgA nephropathy assessed by renal biopsy. Clin Nephrol. 2009;71:625–631. doi: 10.5414/cnp71625. [DOI] [PubMed] [Google Scholar]