Abstract
Introduction
Acute kidney injury associated with near-drowning (ND-AKI) has rarely been reported and its incidence among survivors is unknown. A patient with AKI and urine biomarkers indicating tubular injury led us to assess the occurrence and clinical characteristics of ND-AKI and to evaluate possible causative mechanisms.
Methods
We evaluated medical records of patients rescued from near-drowning in the Mediterranean Sea and treated in a tertiary-level medical center during 2000 to 2017.
Results
Ninety-five patients with the diagnosis of near-drowning in seawater were treated. Forty-two of these patients (43%) developed ND-AKI and 17 (18%) were classified as AKI Kidney Disease: Improving Global Outcomes stages 2 to 3. ND-AKI was associated with the need for resuscitation and mechanical ventilation, with the calculated seawater volume ingestion (extrapolated from rising plasma sodium) and with the degree of acidemia, lactemia, and ventilatory failure. This series and 28 additional published cases of ND-AKI in the literature showed an overall male predisposition.
Conclusion
AKI is a common complication of near-drowning and is associated with increased in-hospital mortality. Data analysis suggests a predominant role of hypoxic tubular injury due to systemic hypoxemia in ND-AKI, combined with intense sympathetic activity (reflected by tachyarrhythmias, hyperglycemia, and relative hypokalemia) and increased oxygen expenditure for intensified distal tubular sodium transport. Androgen-related reduced renal vasodilatory capacity may explain male gender predominance.
Keywords: acute kidney injury, gender, hypoxia, rhabdomyolysis
In the summer of 2016, we treated a patient recovering from near-drowning who developed acute kidney injury (AKI) despite swift rescue, stable hemodynamics, and adequate oxygenation throughout the hospitalization course. This unusual incidence led us to review and analyze previously reported cases of AKI following near-drowning (ND-AKI) and to perform a retrospective analysis of patients admitted with near-drowning to a tertiary-level referral hospital in Northern Israel, located near Haifa's popular beaches on the Mediterranean Sea. The aim of this study was to evaluate the incidence of AKI among survivors following near-drowning and to identify plausible mechanisms and factors predisposing to this complication. Specifically, we looked for indices suggesting hypoxic injury, likely the outcome of reduced oxygen delivery, coupled with enhanced oxygen consumption for tubular transport.
Methods
A retrospective analysis was performed using the electronic medical record database of the Rambam Health Care Campus. Medical records of all hospitalized patients diagnosed as near-drowning at discharge during the period of January 1, 2000, to August 31, 2017, were retrieved and analyzed with approval by the institutional ethical committee. Diagnosis of near-drowning was confirmed by review of the patient history, follow-up, and discharge notes. Patients were diagnosed with ND-AKI if within the first 72 hours of their presentation the difference between highest and lowest creatinine (either ratio or absolute difference) at that period fulfilled the Kidney Disease: Improving Global Outcomes definition and staging system of AKI,1 which was also used for staging of AKI severity of our patients. Patients were divided into 3 groups. The first group had no AKI. The second group was patients diagnosed with AKI stage 1, defined as a creatinine increase of at least 0.3 mg/dl or a proportional change of creatinine of 1.5 to 1.9 from baseline. The third group was patients diagnosed with AKI stage 2 to 3 (stage 2 was defined as the proportional increase in creatinine of 2.0 to 2.9 from baseline and AKI stage 3 as an increase in creatinine of at least 4 mg/dl, or a proportional change of creatinine of 3 of more from baseline).
Comorbidity data were acquired from patients’ clinical notes and list of diagnoses. Patients were considered to have chronic kidney disease if it was mentioned either in the clinical notes or diagnosis list or if a previous baseline estimated glomerular filtration rate was available and lower than 60 ml/min per 1.73 m2. Vital signs, blood gases, chemistry, and complete blood count obtained on admission were used for data assessment, as well as follow-up creatinine values during the first 120 hours following admission. Creatine phosphokinase values used for analysis were the highest values measured in the week following initial presentation. Chest radiograph interpretation was taken from the text description in the patient record. For analysis purposes, all images interpreted as edema, congestion, or diffuse or bilateral consolidations were defined as pulmonary congestion, in contrast to the images interpreted as localized consolidation. An estimation of seawater ingested and/or aspirated was performed using the Adrogue formula for changes in plasma sodium2 corrected for changes in glucose concentration,3 taking into account a Mediterranean Sea water salinity of 3.8%, with an average estimated weight of 70 kg used for all patients.
Urine levels of neutrophil gelatinase-associated lipocalin and kidney injury molecule 1 were determined in our index patient with commercially available enzyme-linked immunosorbent assay kits (Rapid ELISA Kit-037; Bio Porto Diagnostics, Gentofte, Denmark, and Wuhan EIAab Science, Wuhan, People's Republic of China, respectively).
Previous cases of ND-AKI reported in the English literature were traced using MEDLINE and Google Scholar. A search for an association between kidney injury and near-drowning was performed using the following keywords: renal failure, renal injury, kidney injury, immersion, drowning. Altogether, 11 such publications were traced and analyzed, including 1 series of 15 patients.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14
Statistical Analysis
Data analysis was performed using R version 3.4.0 (The R Foundation). Continuous variables are presented as means and SDs. Group comparisons were performed with 1-way analysis of variance test, and between- and within-group variance of repeated creatinine measurements were assessed using 2-way analysis of variance. Tukey test was applied for post hoc comparisons. Categorical variables are presented as proportions and were analyzed using χ2 test. To identify possible baseline predictors of AKI, linear regression analysis was performed using the difference between highest and lowest creatinine values for each patient as the dependent variable. All independent variables were analyzed using backward stepwise model selection by minimum Akaike Information Criterion to select variables for regression analysis. Variables that were missing values for more than 20% of the patients were excluded from analysis. Patients who had missing values for more than 20% of the independent variables were excluded. Missing values of continuous variables were imputed with mean values and missing values of categorical variables were imputed with most frequent value. Statistical significance was set at P < 0.05.
Results
Index Case
A 31-year-old previously healthy man had been submerged in seawater for approximately 4 minutes before rescue and was found to be stuporous at the scene suffering from respiratory distress, a heart rate of 230 beats per minute, and an oxygen saturation of 65% on room air. Midazolam and ketamine were administered for sedation during his swift transport to the hospital and, after failed attempts of endotracheal intubation, he was oxygenated using bag mask ventilation. On arrival, wide-complex tachycardia was detected but resolved spontaneously. His blood pressure on admission was 123/76 mm Hg and his rectal temperature was 36.6°C. He remained hypoxic with copious clear respiratory secretions. He underwent intubation and sedation and was placed in a critical care facility. As shown in Table 1, laboratory evaluation revealed severe mixed respiratory and metabolic (lactic) acidosis. Potassium levels were unexpectedly low (3.3 mmol/l). Chest radiograph performed on arrival showed pulmonary edema that cleared within 10 hours. The patient remained hemodynamically stable and well-oxygenated with permissive hypercapnia. By 24 hours, he was weaned from ventilation and by 72 hours, he was fully recovered and ambulatory and was discharged, with clinically unremarkable outpatient follow-up.
Table 1.
Laboratory evaluation of the index case
| On arrival | 48 h | 72 h (discharge) | 9 mo (follow-up) | |
|---|---|---|---|---|
| Blood gases | ||||
| pH | 7.12 | 7.32 | 7.41 | |
| pO2, mm Hg | 62 | 46 | 48 | |
| pCO2, mm Hg | 52 | 48 | 41 | |
| HCO3, mmol/l | 16.9 | 24 | 26 | |
| Lactate, mmol/l | 13.6 | 2.9 | 1.5 | |
| Blood chemistry | ||||
| Sodium, mmol/l | 143 | 144 | 141 | 139 |
| Potassium, mmol/l | 3.3 | 3.9 | 4.7 | 4.3 |
| Chloride, mmol/l | 108 | 109 | 106 | 105 |
| Phosphate, mg/dl | 6.2 | 3.9 | 3.7 | 3.0 |
| Glucose, mg/dl | 121 | 118 | 84 | 78 |
| Creatinine, mg/dl | 1.09 | 2.22 | 1.67 | 0.78 |
| Blood urea nitrogen, mg/dl | 15 | 27 | 24 | 18 |
| Creatine kinase, IU/l | ND | 339 | 1445 | |
| Myoglobin, μg/l | ND | ND | 569 | |
| Uric acid, mg/dl | 6.9 | 5.0 | 3.8 | |
| AST, U/l | ND | 40 | 52 | |
| ALT, U/l | ND | 63 | 43 | |
| Urine chemistry | ||||
| Osmolarity, mOsmol/kg | 363 | |||
| Sodium, mmol/l | 69 | |||
| Potassium, mmol/l | 11.8 | |||
| Protein, mg/dl | 80 | 7 | ||
| Myoglobin, μg/l | 7 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ND, not determined.
However, it is noteworthy that nonoliguric AKI developed with plasma creatinine normal on admission (1.09 mg/dl) gradually rising to 2.22 mg/dl by 48 hours, with partial recovery on discharge. A subsequent full recovery was confirmed 9 months later (Table 1). Myoglobinuria was established with a modest increase in creatine phosphokinase and myoglobin and a positive reaction for blood in urine dipstick with unremarkable urine sediment. Evaluation of AKI biomarkers in the urine at 72 hours revealed increased levels of both neutrophil gelatinase-associated lipocalin, 150 ± 10 ng/mg creatinine, and kidney injury molecule 1, 14 ± 2 ng/mg creatinine, with reference normal values in our laboratory of 20 ± 5 and 1.0 ± 0.1 ng/mg creatinine, respectively.15
Study Group
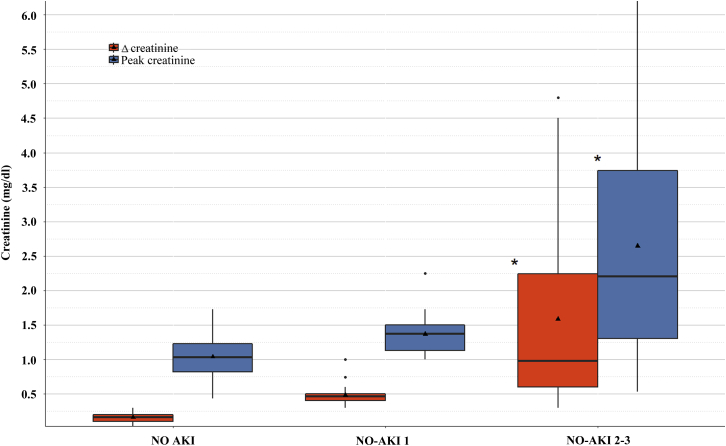
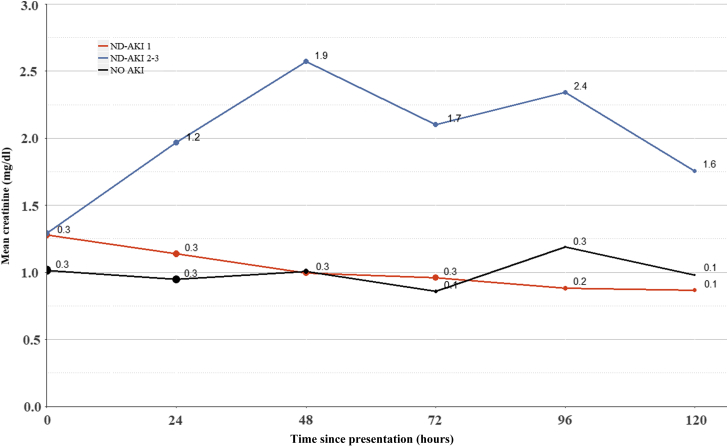
Our medical record search revealed 95 patients who were admitted in Rambam Health-Care Campus between January 1, 2000, and August 31, 2017, and diagnosed as near-drowning. Forty-two of these patients (43.1%) developed AKI and 17 of them (18%) were classified as AKI stages 2 or 3. As shown in Figure 1, a graded increment was noted in peak creatinine and in the magnitude of change in serum creatinine over 72 hours from admission, reaching 2.6 ± 1.8 and 1.6 ± 1.8 mg/dl, respectively, in the ND-AKI 2–3 group. Interestingly, although average creatinine reached a maximum by 48 hours in the ND-AKI 2–3 group, with a gradual decline over 120 hours, the ND-AKI 1 group often had maximal values on arrival that usually normalized by 72 hours (Figure 2). Twelve patients (12.6%) out of the entire cohort died within 90 days from hospitalization, none directly due to renal impairment, with a trend for increased mortality in the ND-AKI 2–3. Only 1 patient included in this group required dialysis, but this occurred during a protracted hospitalization course complicated with sepsis. A comparison among the features of patients with ND-AKI 1, patients with ND-AKI 2–3, and the group of patients without AKI is summarized in Table 2. There were no statistically significant differences between the defined groups regarding age, gender, or major background comorbidities. There was a trend for reduced incidence of coronary artery disease, hypertension, and diabetes among patients with ND-AKI 2–3 patients, none of whom were on chronic treatment with renin-angiotensin-aldosterone blockade. Advanced ND-AKI stage was associated with the need for resuscitation, invasive mechanical ventilation, and placement in an intensive care unit. Interestingly, there was a higher proportion of patients presenting with new-onset atrial fibrillation in the AKI groups. Graded changes in blood gas parameters on admission correlated with increasing AKI stage, including a declining pH and increasing pCO2 and lactate levels. Similarly, glucose and sodium levels at presentation also were significantly higher with increased AKI severity. The progressive increment in sodium levels among groups led us to estimate the differences in calculated seawater consumption for each group. Indeed, ND-AKI severity was found to be associated with the estimated ingested seawater volumes. Remarkably, lymphocyte counts also were directly associated with ND-AKI.
Figure 1.
Comparisons of peak and Δ plasma creatinine between the specified groups. The boxplot demonstrates the range, median (solid line inside each box), and mean (triangle) of the highest plasma creatinine values in each group (blue boxes) and the difference between the highest and lowest creatinine values (Δ creatinine, red boxes). * P < 0.001 versus the 2 other groups (analysis of variance with Tukey post hoc test for multiple comparisons). Δ creatinine comparison between NO AKI and ND-AKI 1 falls short of statistical significance (P = 0.06). AKI, acute kidney injury; ND-AKI, acute kidney injury associated with near-drowning.
Figure 2.
Changes in plasma creatinine along the hospitalization course. Compared are the 3 study groups, NO AKI, AKI 1, and AKI 2–3, monitored during the first 120 hours following admission. Although plasma creatinine remained stable in the NO-AKI group, in general it was higher on admission in the AKI-1 group and declined subsequently, as opposed to the average values in the AKI-2–3 group that peaked on the second/third day and partially recovered later on. The symbol size reflects the number of observations, decreasing with time in all groups. Numbers adjacent to each time point represent SDs. Two-way analysis of variance reveals a P < 0.001 for both between-group and within-group variance. The test was restricted to the first 72 hours only, due to missing values of discharged patients at later time points. Creatinine values over time were significantly higher in the ND-AKI 2–3 group as compared with the 2 other categories (P < 0.001, Tukey post hoc test). A trend for higher cumulative creatinine values over time in the ND-AKI 1 group as compared with the NO-AKI group did not reach statistical significance (P = 0.12), but, as illustrated in Table 2, it was significantly higher on admission. AKI, acute kidney injury; ND-AKI, acute kidney injury associated with near-drowning.
Table 2.
Demographic, clinical, and laboratory features on admission in patients without and with AKI stages 1 and 2–3
| NO AKI, n = 53 | ND-AKI 1, n = 25 | ND-AKI 2–3, n = 17 | P | |
|---|---|---|---|---|
| Demographic | ||||
| Male gender (%) | 28/53 (52.8) | 15/25 (60) | 13/17 (76.5) | 0.22 |
| Age, mean ± SD (range) | 46.7 ± 23.0 (18–93) | 53.4±23.4 (20–88) | 49±18.7 (23–80) | 0.47 |
| Comorbidities | ||||
| Chronic kidney disease (%) | 4/52 (7.7) | 1/25 (4) | 1/17 (5.9) | 0.82 |
| Heart failure (%) | 1/53 (1.9) | 0/25 | 1/17 (5.9) | 0.42 |
| Diabetes (%) | 8/53 (15.1) | 6/25 (24) | 1/17 (5.9) | 0.28 |
| Hypertension (%) | 12/53 (22.6) | 10/25 (40) | 3/17 (17.6) | 0.18 |
| Coronary heart disease (%) | 5/53 (9.4) | 5/25 (20) | 0/17 (0) | 0.11 |
| Medications | ||||
| Diuretics (%) | 6/53 (11.3) | 4/25 (16.7) | 1/17 (5.9) | 0.57 |
| RAAS inhibitors (%) | 6/53 (11.3) | 6/25 (24) | 0/17 (0) | 0.05 |
| Clinical parameters at presentation | ||||
| On-site resuscitation (%) | 8/53 (15.4) | 8/25 (32) | 12/17 (70.6) | < 0.001 |
| Invasive ventilation (%) | 11/53 (20.8) | 13/25 (52) | 15/17 (88.2) | < 0.001 |
| Intensive care unit (%) | 14/53 (26.4) | 15/25 (60) | 13/17 (76.5) | < 0.001 |
| Temperature | 36.4 ± 0.9 | 36.2 ± 1.1 | 36 ± 1.7 | 0.47 |
| Heart rate, bpm | 93.3 ± 20.6 | 103.7 ± 28.5 | 108.1 ± 24.2 | 0.04 |
| Systolic blood pressure, mm Hg | 129.7 ± 26.3 | 126.8 ± 28.6 | 127.9 ± 25.9 | 0.91 |
| Diastolic blood pressure, mm Hg | 76.2 ± 15 | 75 ± 21.3 | 76.2 ± 16.2 | 0.96 |
| Atrial fibrillation on rescue | 2/35 (5.7) | 3/17 (17.6) | 4/11 (36.4) | 0.04 |
| pH | 7.2 ± 0.2 (n = 52) | 7.1 ± 0.2 (n = 25) | 7 ± 0.2 (n = 17) | < 0.001 |
| pCO2, mm Hg | 44.3 ± 16.3 (n = 52) | 52.2 ± 16.8 (n = 25) | 65.1 ± 21.7 (n = 17) | < 0.001 |
| HCO3, mmol/l | 19.2 ± 5.6 (n = 52) | 17 ± 6.3 (n = 25) | 15.1 ± 5.6 (n = 17) | 0.03 |
| Lactate, mmol/l | 7.7 ± 5.8 (n = 51) | 9.9 ± 5.2 (n = 25) | 14.8 ± 7 (n = 17) | < 0.001 |
| Glucose, mg/dl | 170.9 ± 63.4 (n = 52) | 222.3 ± 82.8 (n = 25) | 244.1 ± 98.2 (n = 17) | < 0.001 |
| Sodium, mmol/l | 143.7 ± 4.9 (n = 52) | 144.2 ± 5.7 (n = 25) | 149.9 ± 6.8 (n = 17) | < 0.001 |
| Potassium, mmol/l | 4.1 ± 0.6 (n = 52) | 4.1 ± 0.4 (n = 25) | 3.8 ± 0.6 (n = 17) | 0.15 |
| Chloride, mmol/l | 110.2 ± 15.1 (n = 50) | 110 ± 6.7 (n = 24) | 110.4 ± 6.5 (n = 15) | 0.99 |
| Hemoglobin, g/dl | 14.3 ± 1.7 (n = 53) | 14.1 ± 1.8 (n = 25) | 14.1 ± 1.6 (n = 17) | 0.86 |
| White blood cells, K/μl | 12.6 ± 4.5 (n = 53) | 15.3 ± 6.5 (n = 25) | 14.5 ± 4.6 (n = 17) | 0.08 |
| Platelets, K/μl | 259 ± 124.8 (n = 53) | 242.6 ± 81.2 (n = 25) | 240.2 ± 57 (n = 17) | 0.73 |
| Neutrophils, K/μl | 8.4 ± 4.4 (n = 48) | 9 ± 5.1 (n = 25) | 7.3 ± 4.8 (n = 15) | 0.55 |
| Lymphocytes, K/μl | 3.2 ± 2.1 (n = 47) | 5 ± 2.6 (n = 25) | 5 ± 2 (n = 15) | 0.002 |
| Creatinine, mg/dl | 1 ± 0.3 (n = 53) | 1.3 ± 0.3 (n = 25) | 1.3 ± 0.4 (n = 17) | < 0.001 |
| Blood urea nitrogen, mg/dl | 15.4 ± 5.6 (n = 48) | 17.8 ± 7.5 (n = 25) | 16.1 ± 6.6 (n = 17) | 0.3 |
| Albumin, g/dl | 3.7 ± 0.4 (n = 38) | 3.3 ± 0.5 (n = 21) | 3.2 ± 0.6 (n = 17) | 0.001 |
| Creatine kinase, IU/μl | 231 ± 163 (n = 22) | 1184 ± 1667 (n = 18) | 1988 ± 2824 (n = 13) | 0.01 |
| Calculated ingested sea water, ml | 418 ± 405 (n = 52) | 556 ± 516 (n = 25) | 1038 ± 626 (n = 17) | < 0.001 |
| Congestion on chest radiograph (%) | 29/50 (58) | 13/24 (54.2) | 10/17 (58.8) | 0.94 |
| Outcomes | ||||
| Length of stay, d | 3.3 ± 1.8 (n = 49) | 8.5 ± 7.4 (n = 22) | 25.1 ± 31.6 (n = 12) | < 0.001 |
| 90-day mortality (%) | 4 (7.5) | 3 (12) | 5 (29.4) | 0.06 |
AKI, acute kidney injury; bpm, beats per minute; ND-AKI, near-drowning acute kidney injury; RAAS, renin-angiotensin-aldosterone system.
Multiple regression analysis was performed with the exclusion of 1 patient and the variables albumin and creatine phosphokinase, due to a high proportion of missing values. Six independent baseline variables were found to be linearly associated with the change in creatinine. The variables, their regression coefficients, and P values are shown in Table 3. Older age and male sex were strongly associated with changes in creatinine. Higher lactate and pCO2 values were found as significant predictors for increasing changes in creatinine. Interestingly, sodium level was found to be a predictor of increasing changes in creatinine, although the regression coefficient was not statistically significant (P = 0.08).
Table 3.
Multiple regression analysis with the change in plasma creatinine as the dependent variable
| Independent variable | βa | P |
|---|---|---|
| Age, yr | 0.01 | 0.04 |
| pCO2 | 0.01 | 0.01 |
| Lactate | 0.05 | <0.001 |
| Sodium | 0.03 | 0.08 |
| Sex, male | 0.38 | 0.02 |
| Coronary heart disease, true | −0.61 | 0.03 |
β represents the change in the difference between highest and lowest creatinine value as a result of a change of 1 unit of the continuous independent variable or the presence of the categorical variable.
Twenty-eight previous reports of patients with ND-AKI were traced and are outlined in Table 4.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 The largest series, by Spicer et al.,8 compared 15 patients developing AKI with 15 rescued patients without AKI. In these series, AKI was noted in approximately 50% of rescued patients, most of them young, with an average age of 32.5 ± 11.1 years. Renal impairment was mild and reversible in approximately half of them, and the rest developed full-blown AKI. Two of these 28 patients died, and an additional 9 individuals required renal replacement therapy before full recovery.
Table 4.
Literature review: acute renal failure following near-drowning
| Authors | Year | Study type | n | Age, yr | Gender | Saltwater | Proposed mechanism | Outcome |
|---|---|---|---|---|---|---|---|---|
| Grausz H et al.4 | 1971 | CR | 2 | 39, 26 | M | Y | Hypoxemia, hypoperfusion | Full recovery |
| Neale TJ et al.5 | 1984 | CR | 2 | 32, 22 | M | Y | Rhabdomyolysis (traumatic), acute tubular necrosis | RRT before full recovery |
| Agar JW6 | 1994 | CR | 2 | 22, 45 | M | Y | Rhabdomyolysis | RRT before full recovery and recovery |
| Yoshitomi Y et al.7 | 1998 | CR | 1 | 27 | M | N | Hypoxemia, acute tubular necrosis | RRT before full recovery |
| Spicer ST et al.8 | 1999 | RCC | 15 | 33 ± 11 | 14/15 M | Y | Hypoxemia, hypoperfusion, rhabdomyolysis | Two died, 13 - fully recovered (2 underwent RRT before full recovery) |
| Bonnor R et al.9 | 1999 | CR | 1 | 36 | M | Y | Hypothermia, rhabdomyolysis | RRT before full recovery |
| Lester JL10 | 2002 | CR | 1 | 28 | M | Y | Rhabdomyolysis | No follow-up |
| Hegde SN and Anupama YJ11 | 2003 | CR | 1 | 21 | M | N | Rhabdomyolysis | No follow-up |
| Hottelart C et al.12 | 2004 | CR | 1 | 51 | M | Y | Hypothermia | RRT before full recovery |
| Seong EY et al.13 | 2012 | CR | 1 | 21 | M | N | Acute tubular necrosis | Full recovery |
| Amir A and Lee YL14 | 2013 | CR | 1 | 20 | M | N | Hypoxemia, hypoperfusion | No follow-up |
CR, case report; F, female; M, male; RCC, retrospective case control; RRT, renal replacement therapy; Y, yes.
Discussion
Our case series of ND-AKI, which is the largest reported to date, enables the evaluation of its incidence, as cohort assessment included all hospitalized patients subjected to near-drowning who arrived at a single institute in a region where seawater sports represent the dominant recreational activity for both genders at all age groups throughout long summers. As shown in Table 2, our findings illustrate that ND-AKI is common, occurring in 43% of all rescued near-drowning hospitalized patients. Advanced AKI was noted in 18% of near-drowning patients and possibly contributed to its 29% mortality rate, often in association with multiorgan failure. However, none of our patients required renal replacement therapy at the early convalescence phase. This is in contrast with the series of reported cases so far, in which renal impairment has often been substantial and necessitates renal replacement therapy in one-third of patients. This discrepancy likely reflects publication bias. Altogether, our series combined with collected reports suggest that renal impairment is an often underdetected common complication of near-drowning and immersion injuries.
The pathogenesis of ND-AKI is conceivably multifactorial and primarily includes rhabdomyolysis, systemic inflammatory response associated with multiorgan failure, and hypoxic renal injury related to anoxic insult with reduced renal blood flow.
Indices of rhabdomyolysis were detected in most patients subjected to near-drowning, as illustrated by our index case (Table 1), possibly related to muscle hypoxia and in part to strenuous exercise in the struggle to survive. In some reports, it might have played a central role.6, 9, 11 Nevertheless, although creatine kinase values were significantly associated with the propensity to develop AKI (Table 2), muscle injury parameters were usually mild and not indicative of extensive muscle damage.
Multiorgan failure, which is often associated with advanced AKI, was likely associated with transient shock and a systemic inflammatory response that could have participated in the development of ND-AKI. This possibility is suggested by the elevated lymphocyte count (Table 2), which was also noted by Spicer et al.8; however, with the lack of related data regarding cytokine expression, the importance of this mechanism remains speculative.
By contrast, our findings may emphasize the role of renal hypoxia in the pathogenesis of ND-AKI. Renal hypoxia likely resulted from poor oxygenation during submersion, with subsequent respiratory failure due to faulty pulmonary gas-exchange. Circulatory failure possibly played a role in renal hypoxic injury in a few pulseless patients on rescue, yet in all cases blood pressure assessed on admission was maintained. In view of our findings, we propose an additional factor leading to renal hypoperfusion and hypoxia, namely profound renal vasoconstriction. This hypothesis is based on indices of intense sympathetic activity, reflected by the very common occurrence of hyperglycemia and tachyarrhythmias in the AKI groups, and especially by the frequent finding of low-normal plasma potassium, despite profound acidosis and rhabdomyolysis, as clearly illustrated in Tables 1 and 2 and in the detailed history of our index case.
Intense sympathetic activity may indeed lead to AKI, as occasionally encountered with pheochromocytoma or following cocaine or amphetamine intoxication.16, 17, 18, 19 In fact, a comparable situation has been reproduced in dogs by intrarenal infusion of norepinephrine, leading to renal vasoconstriction and hypoxic AKI associated with ATP depletion and tubular necrosis.20 In line with this assumption, blocking the sympathetic nervous system was shown to improve renal blood flow in rats with prerenal failure and to ameliorate experimental ischemic AKI.21, 22, 23 Renal vasoconstrictors other than catecholamines also might be involved in an amplification loop of renal hypoxic insult in the drowning patient, such as stress-related release of angiotensin II or hypoxia-driven enhanced renal expression of endothelin.21, 24, 25
Interestingly, ND-AKI inversely correlated with plasma potassium levels, likely reflecting intense sympathetic activity, and correlated with the rise in plasma sodium and with the calculated volume of saltwater absorbed via the respiratory or gastrointestinal routes. The latter associations probably reflect the degree of drowning severity, respiratory compromise, and associated related distress response. Moreover, a plausible causal association of AKI severity with intense sympathetic activity is suggested by its direct association with the degree of acidosis, lactemia, and CO2 retention, by the propensity to tachyarrhythmias and by the need for resuscitation, invasive respiration, and placement in an intensive care unit. Our findings are in line with the report of Spicer et al.,8 showing an association between ND-AKI with the degree of metabolic acidosis and the propensity to develop atrial fibrillation.
A greater proportion of men was noted among patients with severe ND-AKI, in line with an outstanding male predominance for ND-AKI (97% of patients) found in our literature review. This is only partially explained by a male predominance of 67.2% among nonfatal near-drowning cases as reported by the Centers for Disease Control and Prevention.26 Combining these historic and current data leads to an overall predisposition for men to develop ND-AKI, 55 of 93 men as compared with 15 of 45 among women (P = 0.006, Fisher exact test). Interestingly, male gender was found to specifically predispose to hypoxic AKI,27, 28 with androgen-related reduced renal vasodilatory capacity, as compared with women.29, 30 Male predominance thus potentially supports our proposed hypothesis, with a more pronounced renal vasoconstriction in response to stress among men that leads to an intensified and protracted hypoxic insult. A plausible additional role of a larger muscle mass among male patients with a more pronounced rhabdomyolysis is not supported by our data (creatine kinase values of 1102 ± 1949 IU/μl vs. 760 ± 1520 IU/μl in men and women, respectively).
Renal parenchymal pO2 is governed by both oxygen supply and demand. In addition to reduced oxygen delivery, renal medullary hypoxia may be intensified by enhanced distal tubular transport and oxygen consumption.31 Therefore, an additional mechanism that may aggravate renal hypoxia following near-drowning, specifically in seawater, is an abrupt sodium and osmotic load, enhancing the release of natriuretic peptides and inducing solute diuresis. Indeed, a consequent increase in distal sodium delivery and transport activity in medullary thick ascending limbs were shown to increase regional oxygen consumption for tubular transport and may further augment renal medullary hypoxic injury.31, 32, 33, 34, 35
To conclude, our study suffers the deficiencies of a retrospective analysis with an incomplete database, as some clinical and laboratory variables were not available for all patients. We could not validate the pre-insult baseline kidney function in many patients or the precise prehospital sequence of events, including the cause, course, and pattern of resuscitation. Also, data on the extent of the anoxic period was often imprecise or not detailed enough, barring addition to the database and analysis. Yet our records provide a glimpse at various physiological parameters on arrival of near-drowning patients that may enlighten our understanding of this disorder. Our findings indicate that hypoxic tubular injury is likely a principal pathogenic mechanism in the development of renal impairment, conceivably resulting from systemic hypoxemia, renal vasoconstriction, and tissue hypoperfusion combined with enhanced oxygen consumption for tubular transport. Additional studies are required to validate these observations. With this hypothesis in mind, future interventions to be evaluated may include the administration of modalities promoting renal vasodilation and inhibiting tubular transport in combination with the restoration of systemic oxygenation.
Disclosure
All the authors declared no competing interests.
Acknowledgment
This study was supported by the Israeli Science Foundation (grant no. 2383/17).
References
- 1.Kellum J.A., Lameire N., Aspelin P. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1. [Google Scholar]
- 2.Adrogué H.J., Madias N.E. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 3.Hillier T.A., Abbott R.D., Barrett E.J. Hyponatremia: evaluating the correction factor for hyperglycemia. Am J Med. 1999;106:399–403. doi: 10.1016/s0002-9343(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 4.Grausz H., Amend W.J., Earley L.E. Acute renal failure complicating submersion in sea water. JAMA. 1971;217:207–209. [PubMed] [Google Scholar]
- 5.Neale T.J., Dewar J.M., Parr R. Acute renal failure following near drowning in salt water. N Z Med J. 1984;97:319–322. [PubMed] [Google Scholar]
- 6.Agar J.W. Rhabdomyolysis and acute renal failure after near-drowning in cold salt water. Med J Aust. 1994;161:686–687. [PubMed] [Google Scholar]
- 7.Yoshitomi Y., Kojima S., Ogi M. Acute renal failure in accidental hypothermia of cold water immersion. Am J Kidney Dis. 1998;31:856–859. doi: 10.1016/s0272-6386(98)70057-5. [DOI] [PubMed] [Google Scholar]
- 8.Spicer S.T., Quinn D., Nyi Nyi N.N. Acute renal impairment after immersion and near-drowning. J Am Soc Nephrol. 1999;10:382–386. doi: 10.1681/ASN.V102382. [DOI] [PubMed] [Google Scholar]
- 9.Bonnor R., Siddiqui M., Ahuja T.S. Rhabdomyolysis associated with near-drowning. Am J Med Sci. 1999;318:201–202. doi: 10.1097/00000441-199909000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Lester J.L. Rhabdomyolysis: a late complication of near-drowning. J Emerg Nurs. 2002;28:280–283. doi: 10.1067/men.2002.126235. [DOI] [PubMed] [Google Scholar]
- 11.Hegde S.N., Anupama Y.J. Acute renal failure secondary to rhabdomyolysis following near-drowning in sea water. J Assoc Physicians India. 2003;51:512–513. [PubMed] [Google Scholar]
- 12.Hottelart C., Diaconita M., Champtiaux B. When the kidney catches a cold: an unusual cause of acute renal failure. Nephrol Dial Transplant. 2004;19:2421–2422. doi: 10.1093/ndt/gfh401. [DOI] [PubMed] [Google Scholar]
- 13.Seong E.Y., Rhee H., Lee N. A case of severe acute kidney injury by near-drowning. J Korean Med Sci. 2012;27:218–220. doi: 10.3346/jkms.2012.27.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir A., Lee Y.L. A case of acute kidney injury by near-drowning. Malays Fam Physician. 2013;8:34–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Lahoud Y., Hussein O., Shalabi A. Effects of phosphodiesterase-5 inhibitor on ischemic kidney injury during nephron sparing surgery: quantitative assessment by NGAL and KIM-1. World J Urol. 2015;33:2053–2062. doi: 10.1007/s00345-015-1579-3. [DOI] [PubMed] [Google Scholar]
- 16.Leblanc M. Cocaine-induced acute renal failure without rhabdomyolysis. Ann Intern Med. 1994;121:721. doi: 10.7326/0003-4819-121-9-199411010-00020. [DOI] [PubMed] [Google Scholar]
- 17.Craver M.L.A.L. Cocaine-induced acute renal failure without rhabdomyolysis. Nephrol Dial Transpl. 1999;14:2971. doi: 10.1093/ndt/14.12.2970. [DOI] [PubMed] [Google Scholar]
- 18.Rivero M., Karlic A., Navaneethan S.D. Possible cocaine-induced acute renal failure without rhabdomyolysis. J Nephrol. 2006;19:108–110. [PubMed] [Google Scholar]
- 19.Golshevsky J.R., Karel K., Teale G. Phaeochromocytoma causing acute pulmonary oedema during emergency caesarean section. Anaesth Intensive Care. 2007;35:423–427. doi: 10.1177/0310057X0703500319. [DOI] [PubMed] [Google Scholar]
- 20.Sinsteden T.D., O’Neil T.J., Hill S. The role of high-energy phosphate in norepinephrine-induced acute renal failure in the dog. Circ Res. 1986;59:93–104. doi: 10.1161/01.res.59.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Kon V., Yared A., Ichikawa I. Role of renal sympathetic nerves in mediating hypoperfusion of renal cortical microcirculation in experimental congestive heart failure and acute extracellular fluid volume depletion. J Clin Invest. 1985;76:1913–1920. doi: 10.1172/JCI112187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujii T., Kurata H., Takaoka M. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol. 2003;481:241–248. doi: 10.1016/j.ejphar.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Salman I.M., Ameer O.Z., Sattar M.A. Role of the renal sympathetic nervous system in mediating renal ischaemic injury-induced reductions in renal haemodynamic and excretory functions. Pathology. 2010;42:259–266. doi: 10.3109/00313021003631304. [DOI] [PubMed] [Google Scholar]
- 24.Khamaisi M., Toukan H., Axelrod J.H. Endothelin-converting enzyme is a plausible target gene for hypoxia-inducible factor. Kidney Int. 2015;87:761–770. doi: 10.1038/ki.2014.362. [DOI] [PubMed] [Google Scholar]
- 25.Robinette J.B., Conger J.D. Angiotensin and thromboxane in the enhanced renal adrenergic nerve sensitivity of acute renal failure. J Clin Invest. 1990;86:1532–1539. doi: 10.1172/JCI114872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Nonfatal and fatal drownings in recreational water settings—United States, 2001–2002. MMWR Morb Mortal Wkly Rep. 2004;53:447–452. [PubMed] [Google Scholar]
- 27.Wei Q., Wang M.H., Dong Z. Differential gender differences in ischemic and nephrotoxic acute renal failure. Am J Nephrol. 2005;25:491–499. doi: 10.1159/000088171. [DOI] [PubMed] [Google Scholar]
- 28.Hutchens M.P., Dunlap J., Hurn P.D. Renal ischemia: does sex matter? Anesth Analg. 2008;107:239–249. doi: 10.1213/ane.0b013e318178ca42. [DOI] [PubMed] [Google Scholar]
- 29.Wangensteen R., Moreno J.M., Sainz J. Gender difference in the role of endothelium-derived relaxing factors modulating renal vascular reactivity. Eur J Pharmacol. 2004;486:281–288. doi: 10.1016/j.ejphar.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Ajayi A.A., Fidelis P. The effect of flutamide on systemic and renal hemodynamics in Zucker diabetic rats: paradoxic renal vasodilator response to endothelin-1 and TXA2 receptor activation in female sex. J Cardiovasc Pharmacol. 2006;48:191–198. doi: 10.1097/01.fjc.0000246941.84607.11. [DOI] [PubMed] [Google Scholar]
- 31.Brezis M., Rosen S. Hypoxia of the renal medulla—its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 32.Washington J.A., Holland J.M. Urine oxygen tension: effects of osmotic and saline diuresis and of ethacrynic acid. Am J Physiol. 1966;210:243–250. doi: 10.1152/ajplegacy.1966.210.2.243. [DOI] [PubMed] [Google Scholar]
- 33.Brezis M., Agmon Y., Epstein F.H. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 34.Brezis M., Heyman S.N., Epstein F.H. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol. 1994;267:F1063–F1068. doi: 10.1152/ajprenal.1994.267.6.F1063. [DOI] [PubMed] [Google Scholar]
- 35.Redfors B., Swärd K., Sellgren J. Effects of mannitol alone and mannitol plus furosemide on renal oxygen consumption, blood flow and glomerular filtration after cardiac surgery. Intensive Care Med. 2009;35:115–122. doi: 10.1007/s00134-008-1206-5. [DOI] [PubMed] [Google Scholar]