Abstract
We report 2 cases of apixaban use as prophylaxis against thromboembolism in the nephrotic syndrome (NS), and review the existing literature on direct-acting oral anticoagulant (DOAC) use in this scenario. Our cases appear to be the first reported use of apixaban as prophylaxis against thromboembolism in NS. We report our systematic review of the existing literature on direct-acting oral anticoagulant (DOAC) use in NS, and discuss theoretical issues relevant to their therapeutic use in this clinical scenario. We searched electronic databases such as OVID, EMBASE, PubMed, and CENTRAL, DARE. The search to identify studies and the application of inclusion and exclusion criteria was performed in duplicate independently. We identified 1 pilot randomized study, 3 case reports, and 3 conference proceedings abstracts relating to DOAC use in NS. These reports all pertain to the treatment of clinically evident thrombosis in NS with rivaroxaban, edoxaban, and dabigatran rather than prophylaxis against thrombosis. Although the existing literature on DOAC use in NS is limited, initial preliminary experience appears promising.
Keywords: anticoagulation, DOAC, nephrotic, thrombosis
Herein we report of 2 cases of anticoagulation with apixaban to prevent thrombosis in severe nephrotic syndrome (NS).
Case 1
A 28-year-old woman presented with sudden-onset bilateral edema, severe hypoalbuminemia, and proteinuria with preserved estimated glomerular filtration rate (eGFR). Laboratory investigations are presented in Table 1. Kidney biopsy demonstrated normal appearances on light microscopy, with diffuse effacement of podocyte foot processes on electron microscopy, consistent with a diagnosis of minimal change nephropathy. Corticosteroids were commenced at 1 mg/kg along with furosemide, and amiloride. The patient’s response to corticosteroids was initially suboptimal, with ongoing severe NS. After kidney biopsy, she was commenced on oral apixaban 5 mg BD. Over 8 weeks of follow-up with NS, she did not have any thrombotic episodes, although she had 1 episode of minor epistaxis on day 3 of apixaban use. This settled after withholding 1 dose and stopping nasal cannula oxygen supplementation. She subsequently achieved complete remission after 8 weeks, at which point apixaban was discontinued.
Table 1.
Patient characteristics for two cases of nephrotic syndrome treated with apixaban as thromboprophylaxis
| Characteristic | Case 1 | Case 2 |
|---|---|---|
| Age, yr | 28 | 49 |
| Sex | Female | Female |
| Serum albumin | <15 g/l | 17 g/dl |
| Urinary protein excretion | 12 g/24 h | 10 g/24 h |
| Serum cholesterol | 19.3 mmol/l | 12.6 mmol/l |
| Serum creatinine | 57 μmol/l | 253 μmol/l |
| Hemoglobin | 15.4 g/dl | 14.9 g/dl |
| PT | 14.3 s | 12.9 s |
| INR | 1.08 | 0.94 |
| aPTT | 56.2 s | 30.3 s |
| Kidney biopsy diagnosis | Minimal change nephropathy | Membranous nephropathy |
| Additional thrombosis risk | No | DVT during pregnancy 15 years prior |
| Smoking | No | No |
| DOAC used | Apixaban 5 mg BD | Apixaban 5 mg BD |
aPTT, activated partial thromboplastin time; BD, twice daily; DOAC, direct-acting oral anticoagulant; DVT, deep vein thrombosis; INR, international normalized ratio; PT, prothrombin time.
Case 2
A 49-year-old woman presented with sudden-onset edema and was diagnosed with NS. Initial 24-hour urine protein excretion was 10 g, serum albumin was 17 g/dl, and serum cholesterol was 12.6 mmol/l (Table 1). Renal biopsy showed membranous nephropathy likely to be secondary to etanercept therapy prescribed for seronegative inflammatory arthritis. Serum anti-PLA2R antibody was weakly positive. She had experienced deep vein thrombosis (DVT) during pregnancy 15 years prior. Given the severity of NS and the fact that the glomerular pathology was membranous nephropathy, she was treated with apixaban prophylactically.1 She did not develop thrombosis while being treated with apixaban. Upon withdrawal of etanercept she achieved complete and sustained remission within 3 months and was able to discontinue anticoagulation.
Introduction
The cases of severe, sudden-onset NS described above led to a consideration of the potential risks and benefits of anticoagulation. Despite effective treatment regimens for inducing remission in the various forms of NS, there remains a proportion of patients resistant to remission induction who are at ongoing risk for thrombosis.2 The exact pathophysiology of the thrombotic risk remains incompletely characterized.2 Leakage of high−molecular-weight proteins in addition to albumin leads to a hypercoaguable state involving abnormal platelet activation and an imbalance in procoagulant and anticoagulant cascades.3, 4 Urinary losses of antithrombin III and plasminogen may contribute to impaired fibrinolysis.4, 5 Other cascade factors may also be increased synchronous with generalized compensatory hepatic protein synthesis. Serum d-dimer, for example, a marker of fibrin turnover, appears to be mildly raised in NS, independent of glomerular filtration rate (GFR) and in the absence of clinical evidence of thrombosis.6 However, whether this represents increased hepatic fibrin synthesis or subclinical low-grade thrombosis is unclear.7 Abnormalities in other components such as antithrombin III, plasminogen, thromboxane, von Willebrand factor, protein C and S, and platelet regulation have also been reported.8 In addition, compared to the inactive bound form, the active free form of protein S, a regulatory protein, may be disproportionately lost in the urine.8 Therefore, even when levels appear normal, they may comprise mainly the inactive form of the protein, which may also be the case for other regulatory proteins. To add to the complexity of the situation, Factor X levels can vary from normal to increased or even decreased in NS,2 and antithrombin III is not invariably reduced.9 In addition, loss of certain regulatory components can occasionally even result in hemorrhage in NS.10
Although the issue of anticoagulation in NS has constituted a longstanding conundrum,11 it is now perhaps even more complicated in the advent of additional therapeutic choices such as direct-acting thrombin and Factor Xa inhibitors.
Search Methods for Literature Review
We approached this search using systematic review methodology, looking for literature pertaining to DOAC use in NS, both for prophylaxis against thrombosis and for the treatment of thrombosis. Databases that we searched were as follows: MEDLINE/PubMed (up to 2017), Embase (up to 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane database, Web of Science, and Ovid through the Royal College of Surgeons in Ireland on 27 November 2017. This included articles in press and electronic publications (Epub) ahead of print. The Medline at Ovid search included Evidence-Based Medicine (EBM) Reviews, which includes the following: Cochrane Database of Systematic Reviews (DSR), American College of Physicians (ACP) Journal Club, Database of Abstracts of Reviews of Effects (DARE), Cochrane Methodology Register (CMR), Health Technology Assessments (HTA), and NHS Economic Evaluation Database (NHSEED). We did not limit the earliest date; however, we did limit our search to articles in English or English translation. We also conducted online resource searches through Google for full-text searches, and reference lists of nephrology textbooks, review articles, and relevant studies.
The publication status and date of publication of all studies were included. In terms of unpublished literature, we searched the publications from the main nephrology conferences proceedings for randomized trials, systematic reviews, and meta-analyses of direct-acting oral anticoagulants (DOACs)/novel oral anticoagulants (NOACs)/target-specific oral anticoagulants (TOACs) in NS, including the American Society of Nephrology Kidney Week abstract books for years 2010 to 2017. Embase searches also identified case report abstracts from conference proceedings from specialties other than nephrology (Table 2).
Table 2.
Summary of the literature to date, which consists only of case reports
| Authors and reference | Anticoagulant used | Case details | Clinical course summary |
|---|---|---|---|
| Dupree and Reddy16 | Rivaroxaban | 18-yr-old female | Patient was nephrotic with renal vein thrombosis and was readmitted with PE on warfarin within therapeutic INR range (2.0−3.0). She was then transitioned to rivaroxaban and successfully treated for at least 6 months at the time of writing. |
| Shimada et al.15 | Edoxaban | 39-yr-old male; serum creatinine 0.83 mg/dl, 5.7 g proteinuria, serum albumin 26 g/dl, no kidney biopsy | Patient presented with pulmonary emboli on CT, and PE. Serum anti-thrombin III protein C and S were normal. Patient was initially treated with heparin and warfarin; 20 days later, he presented with new symptoms and new PE on CT; INR was 2.28 at the time. He commenced edoxaban, which treated the renal vein thrombosis and pulmonary emboli successfully. Approximately 9 months later was switched to aspirin 100 mg. |
| Sasaki et al.13 | Dabigatran | 35-yr-old Japanese male; ∼7.5 g proteinuria, serum albumin 18 g/dl, creatinine 0.96 mg/dl, membranous nephropathy | Patient presented with hemiparesis and cerebral infarct. Left carotid thrombus was detected, without atherosclerosis. He had a history of smoking. Initially he was treated with heparin and warfarin, but developed hepatitis from warfarin and so DOAC was contemplated. Dabigatran was used at a lower dose of 110 mg BD to reduce bleeding risk. |
| Conference proceedings | |||
| Basu et al.17 | Rivaroxaban | 21-yr-old female with presumed lupus nephritis, no biopsy | Patient had a pulmonary embolism 2 yr prior and developed inferior vena cava thrombus after 6 months of warfarin therapy. She was pregnant and so was switched to heparin. After pregnancy, she was started on rivaroxaban; 2 months later, she developed splenic infarcts while taking rivaroxaban. She was actively nephrotic at the time, with presumed lupus nephritis, and kidney biopsy was judged to be an excessive risk. No details were given about further treatment. The authors postulate that raised coagulation factor levels in NS might make anticoagulants ineffective. However, this is a complicated case, and the thrombotic risk of active systemic lupus may be different than in other forms of NS. |
| Han et al.19 | Rivaroxaban | 60-yr-old male; membranous nephropathy | Patient initially presented with PE and was discharged on rivaroxaban, prescribed 15 mg BD for 3 wk, followed by 20 mg OD. After 5 mo, he presented with new-onset renal vein thrombosis and pulmonary emboli and was switched to warfarin and the Ponticelli regimen. |
| Kamran et al.18 | Rivaroxaban | 34-yr-old male; membranous nephropathy | PE and myocardial infarction (NSTEMI) discharged on rivaroxaban but discontinued it after 1 wk; 2 mo later, he presented with left ventricular thrombus and bilateral renal infarcts. |
BD, twice daily; CT, computed tomography; DOAC, direct-acting oral anticoagulant; INR, international normalized ratio; NS, nephrotic syndrome; NSTEMI, non−ST-elevation myocardial infarction; OD, once daily; PE, pulmonary emboli;
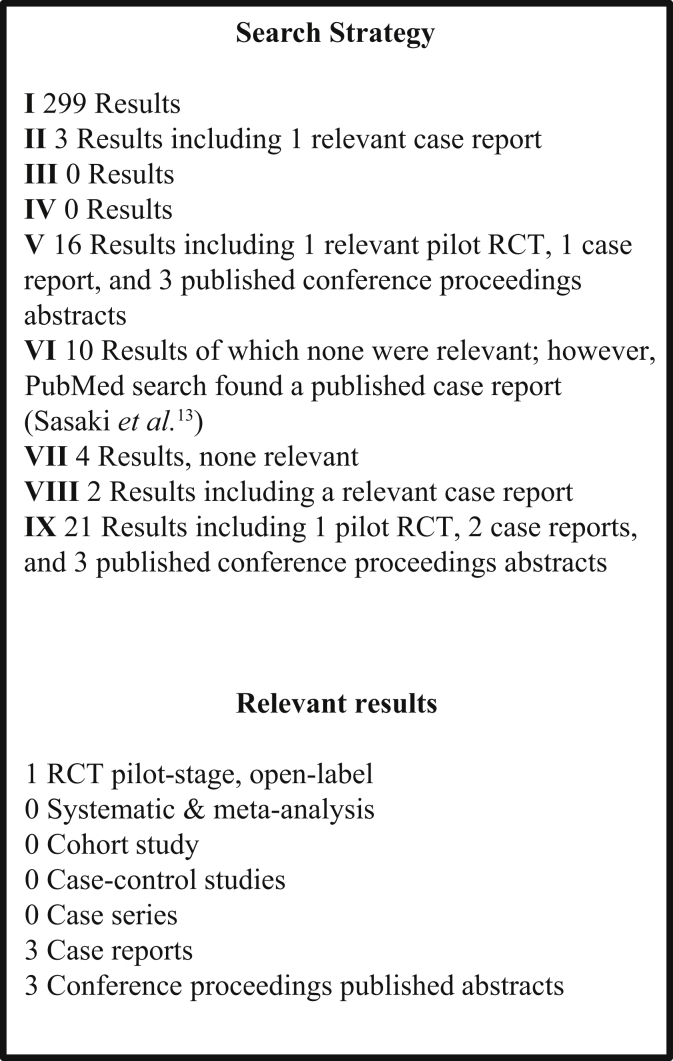
Search terms included (Embase) the following (Figure 1): (i) “nephrotic” AND “anti-coagulation”: 299 results; (ii) “nephrotic” AND “NOAC”: 3 results, of which there was 1 relevant case report; (iii) “nephrotic” and “TOAC”: no results; (iv) “nephrotic” AND “DOAC”: no results; (v) “nephrotic” AND “rivaroxaban”: 16 results, of which relevant findings included 1 pilot randomized study, 1 case report, and 3 abstracts from conference proceedings; (vi) “nephrotic” AND “dabigatran”: 10 results, of which none were relevant, but the same search in PubMed identified the case report by Sasaki et al.13; (vii) “nephrotic” AND “apixaban”: 4 results, of which none were relevant; (viii) “nephrotic” AND “edoxaban”: 2 results, including 1 relevant case report; and (ix) “rivaroxaban” OR “apixaban” OR “edoxaban” OR “dabigatran” AND “nephrotic.”
Figure 1.
Preferred Reporting Items for Systematic Reviews (PRISMA) diagram summary of the search results. RCT, randomized controlled trial.
Following detailed searching, 2 reviewers (D.S. and T.Mc.) simultaneously and independently conducted a title and abstract screen and subsequent full text screen on identified studies (Figure 1).
Search Results
Figure 1 provides a Preferred Reporting Items for Systematic Reviews (PRISMA) diagram for the results of the literature search. For the search strategy combination number 9 (ix), Embase yielded 21 results, including 1 small randomized controlled trial,12 2 case reports, and 3 conference proceedings published abstracts, 2 case reports of anticoagulant-related nephropathy in IgA nephritis, and 1 narrative review on hypercoagulability in NS, and another on prophylaxis of thromboembolism in NS. The remaining articles related to thromboprophylaxis in general (not specific to NS). Using the same search terms in PubMed yieled an additional case report of dabigatran use for carotid artery thrombosis in NS by Sasaki et al.13 and a case series of pulmonary emboli by Chaudesaygues et al.14 The results of these searches were assessed for suitability.
We found only 1 randomized trial and 3 published case reports with relevance to DOAC use in NS.13, 15, 16 We found 3 additional cases published as abstracts in conference proceedings17, 18, 19 (Table 2). All of these reports related to the use of DOACs for the treatment of clinically evident thrombosis rather than prophylaxis against thrombosis. We did not find any relevant prospective or retrospective cohort studies, systematic reviews, meta-analysis on the use of DOACs/novel oral anticoagulants in NS. The existing case reports describe the use of rivaroxaban, edoxaban, and dabigatran for the treatment of clinically evident thrombosis in NS. The majority of these cases were in patients with membranous nephropathy (Table 2). Our two cases represent the fourth published report of DOAC use in NS and appears to be the first reported case of apixaban use in NS and the first to describe DOAC use for prophylaxis against thrombosis rather than for treatment of established clinically evident thrombosis.
Individual Results
Randomized Pilot Study
We found 1 pilot study from China, by Zhang et al.,12 which investigated the use of rivaroxaban 30 mg/d in comparison to Dalteparin (low−molecular- weight heparin) in the treatment of venous thrombosis in patients with NS and low serum ATIII levels.12 This was a pilot-stage, open-label, randomized trial with an active control and 8 adult patients with NS in each arm, with AT3 level activity <70% and venous thrombosis confirmed on imaging followed up over 4 weeks. Patients with poor kidney function, recent bleeding, or surgery were excluded. The premise of the study was that, since rivaroxaban is an oral factor Xa inhibitor, it would be more useful in patients with low ATIII due to NS, and may have a lower risk of heparin resistance, with the added advantage of being a fixed-dose regimen without a need for laboratory monitoring.12 The primary outcome was a >90% decrease in thrombus volume over 4 weeks and was similar in both groups at study end. AT3 levels increased in both groups over time, likely related to the achievement of remission and reduction in proteinuria. There were no major bleeding episodes in either group, although there was 1 submucosal bleed in the rivaroxaban group.
This study is too small to yield comparative efficacy and safety data, but it is nonetheless the best available evidence in terms of clinical research hierarchy. In this small pilot-stage, prospective study with defined and planned ascertainment of primary outcome, rivaroxaban appeared to be effective in the treatment of thrombosis in NS. In addition, heparin appeared to be effective despite low AT3 levels, although AT3 levels rose in both groups throughout the study.12 Limitations include the small sample size, open-label design, and lack of blinding of outcome ascertainment.
Case Reports and Conference Proceedings Identified by Literature Search
Table 2 provides a summary of the case reports and conference abstracts identified.
Discussion
Thrombosis Risk in NS
The literature regarding the elevated thrombotic risk in NS is well established and consistent,2 and thromboembolic disease is thought to occur in approximately 26% of cases of adult NS overall.2 However, although the thrombotic risk is congruous across different samples and studies, the absolute risk estimates for thrombosis appear to vary by study cohort, both overall and by primary form of glomerulonephritis.20, 21 Risk also appears to vary by duration of NS, age, etiological glomerular condition (membranous nephropathy being highest risk), personal thrombosis history, degree of proteinuria, central venous catheterization, and coexisting maliganancy.2, 21, 22 Thrombosis appears to be highest risk in the first 6 months of diagnosis or presentation; however, it may occur at any stage.23
A recent large retrospective cohort study focused on a prevalence sample of U.S. veterans that further characterized the absolute and relative risk of thrombosis in NS based on serum albumin.24 A total of 7037 patients met International Classification of Diseases, Ninth Revision (ICD-9) coding for NS, with a mean age of 57 (±11) years. As in other studies, the relationship between thrombotic risk and serum albumin appeared to be inversely linear. In total, there were 158 events over 8.1 years of follow-up, equating to an absolute risk of 8.5/1000 patient-years in patients with albumin <2.5 g/dl, with a hazard ratio of 2.79 (95% confidence interval = 1.45−5.37).1, 24 In this study, incident thrombosis was defined as an ICD-9 code for renal vein thrombosis, deep venous thrombosis, or pulmonary embolism. Therefore these risk estimates do not take account of arterial thrombosis and may not be generalizable to all nephrotic patients, as the cohort was 96% male, with only 26% (n = 1810) having a definite primary glomerulopathy, of which 52% consisted of diabetic nephropathy. This absolute risk contrasts with that in a previous study in patients with membranous nephropathy from the Toronto Glomerulonephritis Registry (N = 898), which found a 7.2% risk over follow-up, mostly within 2 years of presentation.25
As a result of the low frequency of this clinical scenario, there are no large randomized controlled trials proving the efficacy of prophylaxis for thrombosis in NS; however, the available evidence in the form of case reports, case series, and retrospective cohort studies appears to suggest that anticoagulation does reduce risk.3, 26, 27 Perhaps the biggest challenge in this area is that, without an accurate definition of absolute thrombosis risk, individual level prediction is hampered, which makes it difficult to counterbalance the risk of bleeding from prophylactic anticoagulation.11 Although clinically evident thrombotic events may be infrequent overall, morbidity and mortality from events such as cavernous sinus, renal vein thrombosis, pulmonary embolization, and arterial thrombosis are substantial. In practice, many clinicians rely on the degree of hypoalbuminemia to decide on prophylactic anticoagulation; a threshold of <2.5g/dl is commonly used for patients with membranous glomerulopathy.11, 28 A number of risk calculators attempt to provide aids in risk prediction; however, these models are naturally based on relatively small sample sizes overall.
Since the literature suggests heterogeneous thrombotic risk by different forms of glomerulonephritis, with membranous nephropathy being highest,2 whether DOACs will be equally effective in each of these conditions is also unclear, as we do not have large prospective cohort studies looking at Factor Xa and thrombin levels stratified by primary form of glomerulopathy.
Direct-Acting Oral Anticoagulants
There is little published data on the use of DOACs such as thrombin and factor Xa inhibitors in NS. However, there are many potential benefits of their application in this setting, including oral administration, rapid direct onset of action, proven efficacy in reducing thrombotic events in other settings such as nonvalvular atrial fibrillation and DVT, and relative ease of interruption for procedures, reportedly lower bleeding risk and, for certain DOACs, no need for bridging with heparin. Whether these agents might have equivalent, superior, or inferior efficacy compared with traditional agents such as heparin and warfarin in NS, and whether DOACs are safe in this scenario, is not yet known.16 In addition, whether DOACs will protect against arterial events in addition to venous thrombosis in NS is unclear.
Arterial thrombosis appears to be less common than venous forms in NS, but does appear to be elevated nonetheless.29, 30 Whether DOACs have the ability to prevent stroke and myocardial infarction in NS as they do in patients with stable atherosclerotic vascular disease without NS is not yet known.31 However, case reports of DOAC use in arterial thrombosis in NS are emerging (Table 2).13
The risk of arterial events in NS appears to be more variable than venous thrombosis,5 and are thought to be related to platelet aggregation, which could be related to urinary loss of inhibitory substances (such as plasminogen) and compounded by age, diabetes, previous arterial events, and corticosteroid use.32 A recent review of the literature on arterial thrombosis, particularly stroke in NS, raises the possibility that arterial events in NS have a different inciting pathophysiology from that of venous events.5 Traditional risk factors for arterial events, particularly smoking, appear to be important.5
It is unclear whether the risk of arterial events in membranous nephropathy are prevented by anticoagulation with warfarin, and therefore it is also unclear whether concomitant use of aspirin with DOACs would be needed in this scenario. Some investigators advocate the use of aspirin for primary prevention of arterial thrombotic events in patients with membranous glomerulonephritis, but acknowledge that the estimates of the absolute risk of arterial events appear to be heterogeneous.32
Although it is tempting to contemplate how DOACs may simplify thromboembolism prophylaxis in NS and might obviate the need for concern about heparin resistance due to antithrombin urinary losses, these novel agents may also present new challenges.2 Factor X levels may be normal, increased, or decreased in NS.2 Whether this means that the efficacy and bleeding risk of oral Factor Xa inhibitors will vary depending on baseline levels and whether factor Xa levels should be used to guide therapy in NS is currently unknown. Amyloidosis can also occasionally be associated with an acquired factor X deficiency,33 and so perhaps DOAC application should depend on the specific glomerular pathology. Use of these agents in NS likely requires further investigation before widespread adoption.
Bleeding Risk
Randomized controlled trial evidence from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial suggests that use of DOACs for standard indications in comparison to warfarin is associated with a lower risk of bleeding.34 The rate of major bleeding was 2.13% in the apixaban group compared to 3.09% in the warfarin group.35 However, whether the reduced rate of bleeding is transferrable to the nephrotic population is unknown. Because patients with reduced kidney function (as defined by creatinine >221 μmol/l or creatinine clearance <25 ml/min) were excluded from trials such as ARISTOTLE, the bleeding risk in reduced GFR is as yet incompletely defined.34, 35
GFR in NS
Although reduced estimated glomerular filtration rate (eGFR) in itself is associated with increased risk of venous thromboembolism independent of degree of proteinuria,36 reduced GFR is also a risk factor for hemorrhage with DOACs. Furthermore, GFR estimating equations may not be reliable in NS.37 Clinicians should be aware of studies suggesting that serum creatinine may not be a reliable marker of eGFR in NS because of increased tubular secretion of creatinine, which may lead to overestimation of actual GFR.37, 38 This fact has relevance for DOAC prescribing practices, especially given that the need for ongoing anticoagulation for poorly controlled NS is likely to be coupled with a requirement for diuretic use and renin−angiotensin−aldosterone axis (RAAS) blockade, which may further reduce GFR. Whether the hemo-concentration associated with diuretic use augments this thrombotic risk is also not clear.
Any decline in kidney function might place the patient at increased risk for bleeding and may either necessitate discontinuing DOAC therapy or switching anticoagulants to prevent major bleeding. One theoretical issue is whether the thrombotic risk in NS is augmented by supervening oligoanuric acute kidney injury. Because thrombosis risk is thought to be mediated through the urinary losses of regulatory proteins, it is possible that the development of oligoanuric acute kidney injury reduces thrombosis risk, but this has not been formally studied. Platelet inhibition from uremia could also possibly interfere with platelet aggregation in this setting.
The topic of DOAC use in patients with reduced GFR is a broader issue. A recent Cochrane Systematic Review assessed the efficacy of and safety of DOACs (apixaban, dabigatran, and edoxaban) in comparison to dose-adjusted warfarin for the prevention of stroke and systemic embolic events in nonvalvular atrial fibrillation in CKD.39 DOACs appeared to be as effective as warfarin for preventing stroke and systemic embolization without an increase in major bleeding episodes in CKD.39 This review included 5 randomized controlled trials; however, unfortunately, as patients with more advanced CKD were excluded from the trials, the results apply only to CKD stage 3 or milder.39 In addition, many of the randomized trials of DOAC relied on the Cockcroft−Gault equation to estimate eGFR, a method of GFR estimation that is now thought to be suboptimal in comparison to more contemporary equations.40 The Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation (RENAL-AF) trial hopefully will help to inform questions regarding the safety and efficiency of DOAC use in advanced and end-stage kidney disease.41
General Pharmacology of DOACs
Rivaroxaban is an oral, direct-acting Factor Xa inhibitor that is highly protein bound (90%−95%) in vitro in humans, serum albumin being the main binding component.42 Because of this, it is not likely to be dialyzable and has a volume of distribution of ∼50 L (0.62 L/kg).42 Metabolic degradation accounts for two-thirds of elimination, with 50% of this done renally and the other 50% hepatobiliary; the remaining one-third is eliminated by renal excretion as unchanged drug by renal secretion.43 Therefore, impairment of renal function leads to an increased area under the curve of rivaroxaban, and percent inhibition of Factor Xa activity.44 Prolongation of the prothrombin time is more pronounced in moderate to severe CKD.44
Apixaban has slightly less protein binding (∼86%) and was likewise approved by the U.S. Food and Drug Administration and the European Commission for the prevention of thromboembolic complications for hip and knee replacements, treatment of DVT, and the prevention of stroke and embolization in atrial fibrillation.45 When added to anti-platelet therapy in acute coronary syndrome to prevent recurrence, it appeared to reduce ischemic events, but also increased the bleeding risk46 and was not superior to enoxaparin for thromboprophylaxis in hospitalized, medically ill patients.47 Hepatobiliary elimination accounts for 75% with 25% being renally excreted.
Apixaban and rivaroxaban are metabolized by CYP3A4 and are substrates of P-glycoprotein; hence, medications that affect these pathways may interact with these medications if coadministered.45 Recent in vitro evidence suggests that indoxyl sulphate, which rises as renal function declines, results in an upregulation of P-glycoprotein expression, leading to increased hepatic clearance of drugs that are substrates for this glycoprotein, such as cyclosporin.48 Among 109 heart and kidney transplant recipients in this study, those with higher indoxyl sulphate levels required higher cyclosporin doses.48 This raises the possibility that the metabolism and elimination of apixaban and rivaroxaban might also be increased at reduced GFRs.
The relative contribution from renal clearance of unchanged bioavailable drug is approximately 85% for dabigatran, 27% for apixaban, 50% for edoxaban, and 33% for rivaroxaban.49 The remaining 66% of rivaroxaban is metabolized by the liver, and the metabolites are excreted 50% by the kidney and 50% by the colon.50 For apixaban, the remaining 73% is excreted through the hepatobiliary route.50 Finally, edoxaban relies on metabolism and elimination via biliary and intestinal excretion for clearance of the remaining 50%.50 Elimination half-lives are approximately 12 to 14 hours for dabigatran, 8 to 15 hours for apixaban, 9 to 11 hours for edoxaban, and 5 to 13 hours for rivaroxaban.49 These elimination half-lives appear to increase with age. In addition, dabigatran and edoxaban require a lead-in period of bridging with heparin of at least 5 days for venous thrombosis.49
Thus, the low reliance on renal clearance and quick onset of action may favor the use of apixaban and rivaroxaban over other DOACs in patients with NS. The slightly lower reliance of apixaban on renal clearance and the lower protein binding than that of rivaroxaban formed the basis of our choice in our reported cases.
DOACs are of proven benefit in scenarios such as thromboprophylaxis in nonvalvular atrial fibrillation, DVT prophylaxis in patients undergoing hip and knee replacement.45 However, whether the pharmacokinetics and pharmacodynamics of these agents are altered in NS is unknown. Some data suggest that thrombin may directly injure podocytes,51 so perhaps thrombin inhibitors could have an independent beneficial effect, but whether these agents will be lost in the urine is not described. In general it is thought that for drugs that are highly protein bound, urinary loss of albumin in NS could lead to higher free drug levels, which in the case of DOACs could potentially lead to toxicity.52 However it is also possible that high protein binding could lead to urinary losses of the drug. Basu et al., in their conference abstract, suggest that high protein binding of rivaroxaban and subsequent urinary loss in NS may have led to treatment failure in their reported case.17 However, this case was presumed to be a case of systemic lupus erythematosus, with splenic infarcts in addition to pulmonary emboli and NS likely due to lupus nephritis. Perhaps there are thrombotic issues specific to systemic lupus erythematosus that contributed to treatment failure in this case, rather than urinary losses of rivaroxaban.
Drug Interactions
Although the expanding range of available agents for anticoagulation is encouraging, the elucidation of optimal dosing, cost, and monitoring compared to established anticoagulation in NS merits consideration by the nephrology community. Because calcineurin inhibitors (CNIs) and DOACs share certain characteristics such as metabolism by cytochrome P450 enzymes (CYP) and P-glycoprotein binding, a potential interaction needs to be considered in the setting of calcineurin inhibitor use in NS and also in the scenario of DOAC use after kidney transplantation.53 One study of 39 solid organ transplant recipients found that apixaban, and particularly rivaroxaban, can cause a limited increase in CNI trough levels.53 Interactions with other immunosuppressants such as mycophenolate, cyclophosphamide, and rituximab are less likely than with CNIs; however, it may take time for unexpected interactions to emerge in addition to expected interactions.54 Interactions between DOACs and CNIs are also of relevance to the treatment of NS outside the transplantation context.
DOAC Reversal
For dabigatran, unlike other DOACs, a specific monoclonal antibody idarucizumab, has been developed and licensed for reversal of anticoagulation in bleeding episodes. In addition, dabigatran may be removed to some extent by hemodialysis.55 The U.S. Food and Drug Administration has given a fast-track status to andexanet alfa, an investigational reversal agent that competitively binds to and reverses direct and indirect Factor Xa inhibitors.56 Preliminary assessment of this agent appears to be promising both at reducing Factor Xa inhibition and also at reducing bleeding.56 To our knowledge, however, patients with kidney disease have not been specifically included in these early-stage studies.57 Until specific agents are available, the nonspecific management of acute bleeding with DOACs includes prothrombin complex concentrates, recombinant factor VI, and activated prothrombin complex concentrates.55 As might be expected by their pharmacological properties, the direct Factor Xa inhibitors do not appear to be substantially removed with hemodialysis.35 Whether hemodiafiltration would be more effective at removal has not been formally assessed to date. The American College of Cardiology Task Force has produced a decision pathway on the management of bleeding in patients on oral anticoagulants.58
In the case of lupus nephritis−induced NS, one needs to be cognizant of additional risks of thrombosis such as antiphospholipid syndrome and arterial thrombotic episodes associated with active systemic lupus and thrombotic microangiopathy. Whether DOACs are suitable for these scenarios is not yet fully established.
Interrupting Anticoagulation for Kidney Biopsy or Dialysis Access
Timing of interruption depends on the thrombosis and bleeding risk for the individual patient, the elimination half-life (which is influenced by renal and hepatic function), and the level of risk of the procedure. Renal biopsies are considered high risk for bleeding in terms of stratification of bleeding risk of invasive procedures.50 Neuraxial anesthesia is also considered high risk, and the American Society of Regional Anesthesia recommend an interval of 5 half lives for complete elimination before medium- to high-risk procedures.50 This corresponds to 4 to 5 days for dabigatran, and 3 days for rivaroxaban and apixaban.50 Depending on thrombosis risk, bridging with heparin may also be required. Recommendations on restarting of DOACs are variable. Dubois et al. comprehensively summarize the data on stopping and restarting DOACs for procedures.50
Conclusion
The existing published data on the use of DOACs in NS is limited and consists of 1 pilot phase randomized study, 3 published case reports, and 3 published conference proceeding abstracts. All of these reports describe the use of DOACs for the treatment of clinically evident thrombosis in NS rather than prophylaxis against thrombosis. We report the successful use of apixaban as thromboprophylaxis in 2 cases of NS.
Although ideally an adequately powered, randomized controlled trial of DOAC use in NS ought to be performed with comparator groups consisting of heparin and warfarin, the low frequency of this disorder at a population level make such a study difficult to conduct. Hopefully the prospective cohorts being assimilated by the NEPTUNE group will make trials such as these possible in future, or at the very least will help to inform this scenario further with better quality observational data.59 As an initial priority, the nephrology community requires an improved characterization of the specific pharmacokinetic and pharmacodynamic intricacies of DOACs use in the hypoalbuminemic and proteinuric state of NS.
There are a number of limitations to our review. The existing literature as it stands on DOAC use in NS is extremely limited and as such it is inappropriate to make firm conclusions or recommendations at present. This is also a limitation of this review, since it is dependent on the available literature. It is possible that our search may not have identified all relevant studies, particularly those not yet published or published in a language other than English. Furthermore, in the case of published abstracts from conference proceedings, the available details were naturally limited. Much remains to be further elucidated before the adoption of widespread use of DOACs in this context. It is unclear whether the reduced bleeding rates attributed to DOAC use in other contexts apply to the NS scenario. Similarly, DOAC toxicity in NS has not been comprehensively characterized. Finally, the implications of possible drug loss in the urine of patients with NS need to be defined, and its relationship to treatment failure, target Factor Xa levels, and indeed DOAC toxicity in this context.
Once the decision to anticoagulate in NS has been made, the choice between DOACs and warfarin ought to be individualized based on specific patient factors such as kidney function, and risks of thrombosis and bleeding. Although the use of these agents in NS likely requires further study before widespread adoption, the experience to date, although limited, appears promising. DOACs may at least provide alternatives in cases of treatment failure with heparin and warfarin. Until such time as the literature is robust enough to inform us further, it should probably not be assumed that DOACs have the same therapeutic efficacy in all forms of NS, particularly in the case of systemic lupus erythematosus and the antiphospholipid syndrome.60
Clinicians need to be cognizant of the deficiencies in the literature. Many uncertainties remain, such as whether dosing and monitoring should be based on or augmented by serum Factor Xa levels, and whether high protein binding leads to urinary losses of active agent. While awaiting further, larger studies of DOAC use in NS, it would seem prudent to monitor patients carefully, both for bleeding and for treatment failure. Clinicians ought also to be cognizant of the possible limitations of eGFR estimation in NS, particularly for patients on large doses of diuretics and renin−angiotensin axis blockade, where GFR may be more labile.
Disclosure
All the authors declared no competing interests.
References
- 1.University of North Carolina School of Medicine Kidney Center. UNC Kidney Center. Available at: https://unckidneycenter.org/.
- 2.Kerlin B.A., Ayoob R., Smoyer W.E. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513–520. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbano B., Gigante A., Amoroso A. Thrombosis in nephrotic syndrome. Semin Thromb Hemost. 2013;39:469–476. doi: 10.1055/s-0033-1343887. [DOI] [PubMed] [Google Scholar]
- 4.Singhal R., Brimble K.S. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118:397–407. doi: 10.1016/j.thromres.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Roy C.D.Y., Sabbagh R., Roy D., Cardinal H., Bollee G. Ischemic stroke of possible embolic etiology associated with nephrotic syndrome. Kidney Int Rep. 2017;2:988–994. doi: 10.1016/j.ekir.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sexton D.J., Clarkson M.R., Mazur M.J. Serum D-dimer concentrations in nephrotic syndrome track with albuminuria, not estimated glomerular filtration rate. Am J Nephrol. 2012;36:554–560. doi: 10.1159/000345475. [DOI] [PubMed] [Google Scholar]
- 7.Orth S.R., Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338:1202–1211. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 8.Mirrakhimov E., Ali A.M., Barbaryan A. Primary nephrotic syndrome in adults as a risk factor for pulmonary embolism: an up-to-date review of the literature. Int J Nephrol. 2014;2014:916760. doi: 10.1155/2014/916760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rydzewski A., Mysliwiec M., Soszka J. Concentration of three thrombin inhibitors in the nephrotic syndrome in adults. Nephron. 1986;42:200–203. doi: 10.1159/000183667. [DOI] [PubMed] [Google Scholar]
- 10.Epstein O., Bevan G., Siddiqui N. Factor VII deficiency associated with nephrotic syndrome. Br Med J. 1976;2:1361. doi: 10.1136/bmj.2.6048.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glassock R.J. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18:2221–2225. doi: 10.1681/ASN.2006111300. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L., Zhang H., Zhang J., Tian H., Liang J., Liu Z. Rivaroxaban for the treatment of venous thromboembolism in patients with nephrotic syndrome and low AT-III: a pilot study. Exp Ther Med. 2018;15:739–744. doi: 10.3892/etm.2017.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki Y., Raita Y., Uehara G. Carotid thromboembolism associated with nephrotic syndrome treated with dabigatran. Case Rep Nephrol Urol. 2014;4:42–52. doi: 10.1159/000362162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudesaygues E., Grasse M., Marchand L. [Nephrotic syndrome revealed by pulmonary embolism: about four cases] Ann Cardiol Angeiol [Paris] 2014;63:385–388. doi: 10.1016/j.ancard.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Shimada Y., Nagaba Y., Nagaba H. Edoxaban was effective for treating renal vein thrombosis in a patient with nephrotic syndrome. Intern Med. 2017;56:2307–2310. doi: 10.2169/internalmedicine.8742-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupree L.H., Reddy P. Use of rivaroxaban in a patient with history of nephrotic syndrome and hypercoagulability. Ann Pharmacother. 2014;48:1655–1658. doi: 10.1177/1060028014549349. [DOI] [PubMed] [Google Scholar]
- 17.Basu A.J.S., Patel D., Bodapati G., Venkatappa N., Bhattacharya P. Failure of anticoagulation in a case of nephrotic syndrome with recurrent thromboembolism. Chest. 2015;148:983A. [Google Scholar]
- 18.Kamran H.F.E., Khalil Q., Bates J., Morse M. Venous and arterial thromboses in nephrotic syndrome: where only warfarin has walked. J Gen Intern Med. 2016;31 [Google Scholar]
- 19.Han T.H.C., Thet Z. Warfarin vs new oral anticoagulant in primary adult nephrotic syndrome associated venous thormboembolism. Nephrology. 2017;22:64. [Google Scholar]
- 20.Rankin A.J., McQuarrie E.P., Fox J.G. Venous thromboembolism in primary nephrotic syndrome—is the risk high enough to justify prophylactic anticoagulation? Nephron. 2017;135:39–45. doi: 10.1159/000448628. [DOI] [PubMed] [Google Scholar]
- 21.Barbour S.J., Greenwald A., Djurdjev O. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 22.Kerlin B.A., Haworth K., Smoyer W.E. Venous thromboembolism in pediatric nephrotic syndrome. Pediatr Nephrol. 2014;29:989–997. doi: 10.1007/s00467-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoodi B.K., ten Kate M.K., Waanders F. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117:224–230. doi: 10.1161/CIRCULATIONAHA.107.716951. [DOI] [PubMed] [Google Scholar]
- 24.Gyamlani G., Molnar M.Z., Lu J.L. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant. 2017;32:157–164. doi: 10.1093/ndt/gfw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lionaki S., Derebail V.K., Hogan S.L. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medjeral-Thomas N., Ziaj S., Condon M. Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin J Am Soc Nephrol. 2014;9:478–483. doi: 10.2215/CJN.07190713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus K.J., Hynicka L.M. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann Pharmacother. 2013;47:725–734. doi: 10.1345/aph.1R530. [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2012;2:139–274. [Google Scholar]
- 29.Zhao Y., Su W., Liu S. Acute myocardial infarction in a young girl with nephrotic syndrome: a case report and literature review. Can J Cardiol. 2017;33:950.e915–950.e950. doi: 10.1016/j.cjca.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Tachamo N., Dhital R., Timilsina B. Popliteal arterial thrombosis in nephrotic syndrome: a case report. J Community Hosp Intern Med Perspect. 2017;7:34–36. doi: 10.1080/20009666.2017.1286814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eikelboom J.W., Connolly S.J., Bosch J. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 32.Hofstra J.M., Wetzels J.F.M. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Kidney Int. 2016;89:981–983. doi: 10.1016/j.kint.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Thompson C.A., Kyle R., Gertz M. Systemic AL amyloidosis with acquired factor X deficiency: a study of perioperative bleeding risk and treatment outcomes in 60 patients. Am J Hematol. 2010;85:171–173. doi: 10.1002/ajh.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granger C.B., Alexander J.H., McMurray J.J. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 35.Mavrakanas T.A., Samer C.F., Nessim S.J. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–2248. doi: 10.1681/ASN.2016090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmoodi B.K., Gansevoort R.T., Ness I.A. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964–1971. doi: 10.1161/CIRCULATIONAHA.112.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofstra J.M., Willems J.L., Wetzels J.F. Estimated glomerular filtration rate in the nephrotic syndrome. Nephrol Dial Transplant. 2011;26:550–556. doi: 10.1093/ndt/gfq443. [DOI] [PubMed] [Google Scholar]
- 38.Sexton D.J., Kinsella S.M., Eustace J.A. Serum phosphate varies with degree of proteinuria in nephrotic syndrome and is associated with elevated pulse wave velocity. J Nephrol. 2013;26:540–548. doi: 10.5301/jn.5000186. [DOI] [PubMed] [Google Scholar]
- 39.Kimachi M., Furukawa T.A., Kimachi K. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017;11:CD011373. doi: 10.1002/14651858.CD011373.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harel Z., Sood M.M., Perl J. Comparison of novel oral anticoagulants versus vitamin K antagonists in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:183–192. doi: 10.1097/MNH.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Health. National Library of Medicine. Available at: https://clinicaltrials.gov/.
- 42.Mueck W., Stampfuss J., Kubitza D. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16. doi: 10.1007/s40262-013-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueck W., Kubitza D., Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–466. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubitza D., Becka M., Mueck W. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70:703–712. doi: 10.1111/j.1365-2125.2010.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhanwra S., Ahluwalia K. The new Factor Xa inhibitor: apixaban. J Pharmacol Pharmacother. 2014;5:12–14. doi: 10.4103/0976-500X.124409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.APPRAISE Steering Committee and Investigators. Alexander J.H., Becker R.C., Bhatt D.L. Apixaban, an oral, direct, selective Factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 47.Goldhaber S.Z., Leizorovicz A., Kakkar A.K. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 48.Santana Machado T., Poitevin S., Paul P. Indoxyl sulfate upregulates liver p-glycoprotein expression and activity through aryl hydrocarbon receptor signaling. J Am Soc Nephrol. 2018;29:906–918. doi: 10.1681/ASN.2017030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turpie A.G.G., Purdham D., Ciaccia A. Nonvitamin K antagonist oral anticoagulant use in patients with renal impairment. Ther Adv Cardiovasc Dis. 2017;11:243–256. doi: 10.1177/1753944717714921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubois V., Dincq A.S., Douxfils J. Perioperative management of patients on direct oral anticoagulants. Thromb J. 2017;15:14. doi: 10.1186/s12959-017-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma R., Waller A.P., Agrawal S. Thrombin-induced podocyte injury is protease-activated receptor dependent. J Am Soc Nephrol. 2017;28:2618–2630. doi: 10.1681/ASN.2016070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard D.B. Extrarenal complications of the nephrotic syndrome. Kidney Int. 1988;33:1184–1202. doi: 10.1038/ki.1988.129. [DOI] [PubMed] [Google Scholar]
- 53.Vanhove T., Spriet I., Annert P. Effect of the direct oral anticoagulants rivaroxaban and apixaban on the disposition of calcineurin inhibitors in transplant recipients. Ther Drug Monit. 2017;39:77–82. doi: 10.1097/FTD.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 54.Chang S.H., Chou I.J., Yeh Y.H. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almegren M. Reversal of direct oral anticoagulants. Vasc Health Risk Manag. 2017;13:287–292. doi: 10.2147/VHRM.S138890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaatz S., Bhansali H., Gibbs J. Reversing factor Xa inhibitors—clinical utility of andexanet alfa. J Blood Med. 2017;8:141–149. doi: 10.2147/JBM.S121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Institute of Health, U.S. National Library of Medicine. Available at: https://clinicaltrials.gov/ct2/results?cond=andexanet+alfa&term=&cntry1=&state1=&recrs=.
- 58.Tomaselli G.F., Mahaffey K.W., Cuker A. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:3042–3067. doi: 10.1016/j.jacc.2017.09.1085. [DOI] [PubMed] [Google Scholar]
- 59.NEPTUNE. Nephrotic Syndrome Study Network. Available at: https://www.rarediseasesnetwork.org/cms/NEPTUNE.
- 60.Erkan D., Aguiar C.L., Andrade D. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev. 2014;13:685–696. doi: 10.1016/j.autrev.2014.01.053. [DOI] [PubMed] [Google Scholar]