Abstract
Oviposition by lepidopteran herbivores on Nicotiana attenuata primes plant defence responses that are induced by the feeding larvae. While oviposition by both the generalist Spodoptera exigua and the specialist Manduca sexta primes the production of defensive phenylpropanoids, their larvae are differentially affected. We investigate here the impact of prior oviposition on the transcriptome and phytohormone levels of plants that were later attacked by larvae to find regulatory signals of this priming. In a full-factorial design, we evaluated the effects of oviposition and herbivory by both species. Oviposition alone had only subtle effects at the transcriptional level. Laval feeding alone induced species-specific plant responses. Larvae of the generalist regulated phytohormones and gene expression stronger than larvae of the specialist. A day after larvae started to feed, we detected no significant alterations of the plant’s response to larval feeding due to prior oviposition by conspecific moths. Yet, oviposition by each of the species profoundly influenced the plant’s transcriptional and phytohormonal response to feeding larvae of the other species. Remarkably, the species-specific plant responses to larval feeding shifted towards the response normally elicited by larvae of the ovipositing species. Thus, plants may already recognise an insect’s identity upon its oviposition.
Introduction
Plants evolved a multitude of adaptations to resist herbivorous insects. In turn, insects evolved adaptations to find and colonise suitable host plants. Plants possess diverse defence traits which are often inducible upon herbivory1. Insects evolved traits to avoid, resist, or even manipulate a plant’s defence response2. The closer the evolutionary relationship between plants and insect herbivores, the more sophisticated and specialised defence and counter-defence can be geared to each other. The degree of such coevolution is driven by the insect’s impact on plant fitness and its host plant range. Specialised herbivores with narrow host plant ranges can often tolerate, detoxify or even co-opt plant defences for their own defence3. In several plants, generalist and specialist insects elicit different responses4–6, which may be adaptive for the plant or the insect7. Plants may render their defence responses most efficient against different insects that are differentially susceptible, and adapted insects may evolve traits to manipulate plant defence.
Inducible plant defences allow plants to limit their investments of resources into defence to circumstances in which these pay off, i.e. herbivores are present. Plants can even integrate environmental cues that predict upcoming herbivory to accelerate or enhance their induced defences which is termed as priming8,9. For example volatiles of herbivore-attacked plants can prime herbivory-induced defence in adjacent plants10. The egg-deposition of herbivorous insects on a host plant can also prime plant defence against the feeding larvae as has been shown for the wild tobacco Nicotiana attenuata11,12. Also in tomato, moth oviposition primes the wound-induced expression of defence-related parameters13. Priming by insect oviposition is likely more common because several plants possess an increased resistance to insect larvae if previously exposed to its eggs14–17. Yet, the larvae may also be affected by defence responses that are directly activated upon insect oviposition. Plant responses to insect oviposition include the formation of neoplasms, necrosis, egg-crushing tissues, ovicidal substances or volatiles that attract egg predators and parasitoids18–24.
Several plant responses to oviposition as well as the effects on plant resistance to subsequent larval attack can be specific to the insect species. For example, Brassica nigra plants are more resistant to feeding larvae in response to a specialist’s but not a generalist’s moth oviposition25, suggesting a differential plant response to oviposition by both insects. Yet, N. attenuata plants are more resistant to larvae of only the generalist Spodoptera exigua but not the specialist Manduca sexta in response to oviposition by either of the two species12. Oviposition by both moth species equally primes the feeding-induced plant production of phenylpropanoid-polyamine conjugates (PPCs), which reduces performance of S. exigua larvae feeding on oviposited plants11. This suggests a differential susceptibility of the larvae but no species-specific plant response to oviposition, though N. attenuata may show other, still undiscovered, responses that may differ for the two moth species.
Plant responses to herbivory are multifaceted, which is reflected in a large reprogramming of a plant’s transcriptome and various shifts in its primary and secondary metabolism26. This reprogramming is regulated by a network of signalling pathways in which the phytohormone jasmonic acid (JA) plays a central role27. Differential activation of these signalling pathways in response to different elicitors, e.g. damage- and herbivore associated molecular patterns, allows species-specific responses to different herbivores28–30. For example, larval feeding of M. sexta and S. exigua elicits distinct transcriptional profiles in N. attenuata4, likely due to differential phytohormone induction patterns that result from different ratios of elicitors in the larval oral secretions of both species31.
The phytohormonal and transcriptional plant responses to insect oviposition are less examined than those to insect feeding but an involvement of salicylic acid (SA) and JA signalling pathways has been inferred32. Arabidopsis thaliana leaves oviposited by Pieris brassicae accumulate high levels of SA33 and SA-responsive transcripts34. Consistent with the role of SA as a key regulator of plant responses to phytopathogens, the transcriptional profile in response to P. brassicae oviposition is more similar to that of plants harbouring a bacterial infection than to plants fed by P. brassicae larvae34. The SA signal is also detected in systemic leaves of oviposited plants that thereby even acquire resistance (SAR) to bacterial pathogens35. JA may also play a role in plant responses to oviposition as indicated by an increased hatching rate of spider mite eggs on JA-deficient tomato mutants36. Moreover, JA-biosynthesis genes are induced upon oviposition that involves wounding of plant tissue37,38 and plant responses to such oviposition can be mimicked with JA treatments39,40.
Very few studies examined the influence of an earlier oviposition on plant signalling in response to the feeding larvae. Transcriptome analyses revealed no significant effects of P. brassicae oviposition on A. thaliana’s response to larval feeding41 or very few B. nigra genes that were altered in larval attacked plants due to a pre-treatment with P. brassicae egg extract42. In tomato, the increase of wound-induced expression of a defensive protease inhibitor (PI) gene in response to a moth’s oviposition coincides with a stronger JA-burst13. Yet, in N. attenuata, the priming effect of moth oviposition on the induction of PI activity and PPC production in response to larval feeding could not be explained by increased induction of JA11,43. Overall, our current knowledge on the signalling of oviposition-induced responses is anecdotal, especially regarding the question how oviposition affects plant responses to subsequently feeding larvae.
Here we explore N. attenuata’s signalling response to oviposition and herbivory by two of its major herbivores. Specifically, we examine the plant for i) systemic imprints of oviposition by M. sexta and S. exigua, ii) how the moth’s oviposition affects the feeding-induced plant response to its larvae, and iii) whether these effects of oviposition on plant responses to feeding are specific for the insect species. In a full-factorial design (Fig. 1), we explore the effects of larval feeding and prior oviposition by S. exigua and M. sexta on N. attenuata’s transcriptional and phytohormonal profiles in leaves that were systemic to oviposition but local for the larval attack.
Figure 1.
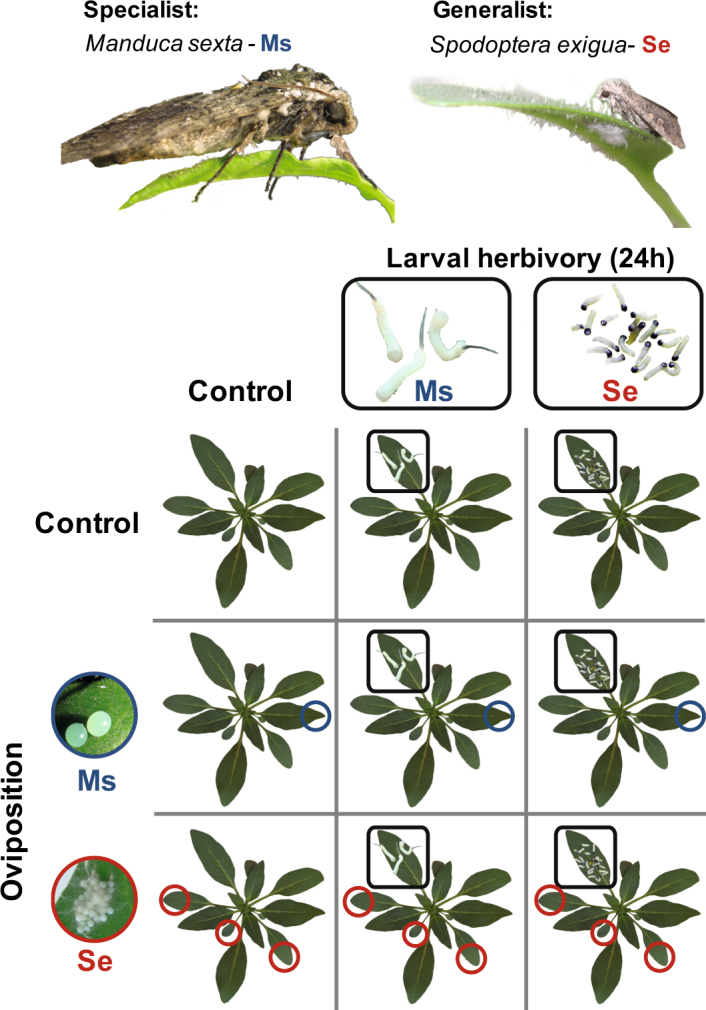
Experimental design. A 3 × 3 full-factorial priming experiment was conducted with N. attenuata (12 plants per treatment) to assess the effects of oviposition and larval feeding by the specialist herbivore M. sexta (Ms, blue) and the generalist herbivore S. exigua (Se, red). Shortly before hatching, the eggs were removed, which was 4 days after oviposition on a standardised leaf position in case of M. sexta (blue circles) but after 3 days in case of S. exigua that only oviposited on elder leaves (red circles). Neonate larvae were applied at the next day to a standardised leaf position (black square), which was harvested from all plants after the larvae fed for 24 hours.
Results
Plant responses to oviposition in leaves systemic to the oviposition site
We evaluated the direct effects of oviposition on plants without larval feeding. These leaves were harvested at the same time and from the same leaf position as the leaves of feeding-induced plants and were therefore systemic to the oviposited leaf (see methods). We found no differences in the phytohormone profiles between oviposited and non-oviposited control plants. Levels of JA and JA-Ile generally varied around the limit of quantification (ca. 7 and 3 ng/g FW, respectively) and are therefore not displayed. Levels of SA and ABA did not differ between plants oviposited by S. exigua or M. sexta and control plants (see Supplementary Fig. S1). However, the transcriptome profiles of M. sexta-oviposited plants (EmsL0) separated from those of control plants (E0L0) in a principal component analysis (PCA) along the second principal component (Fig. 2a). In contrast, transcriptome profiles of S. exigua-oviposited plants (EseL0) grouped within those of control plants. Oviposition by M. sexta, but not by S. exigua, significantly altered the expression of 59 genes, several of which are related to ethylene and auxin signalling as well as regulators of transcription (Supplementary Table S1).
Figure 2.
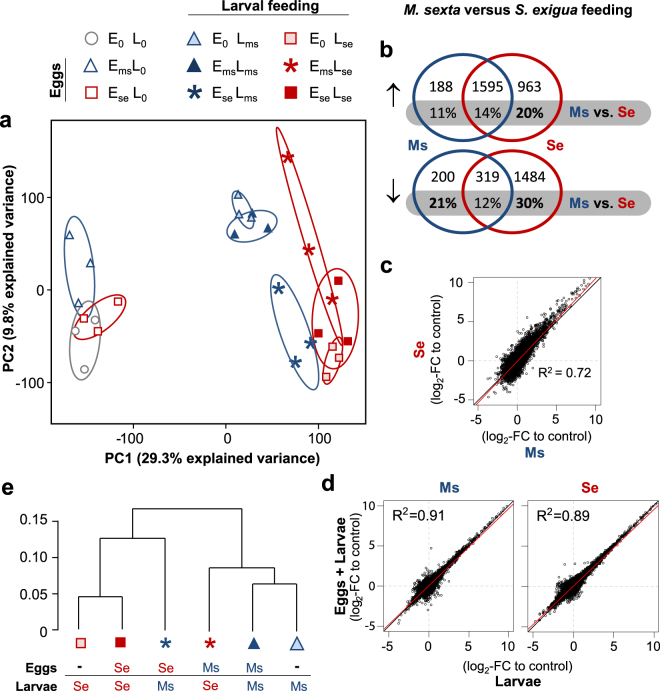
Species-specific effects of oviposition and larval herbivory on N. attenuata’s transcriptional regulation. (a) Principle component analysis (PCA) of transcript profiles of N. attenuata in response to either S. exigua (Se) or M. sexta (Ms) oviposition and/or larval feeding. The legend above presents all symbols used to depict all treatments in the 3 × 3 factorial design and treatment replicates are outlined by 68% confidence intervals. (b) Venn diagram comparing the numbers of genes that were regulated in the microarray analysis by either S. exigua or M. sexta larval feeding (relative to control plants) and those significantly different between the two (at P < 0.05 after correction for false discovery rate and a log2-fold change (FC) > 1). (c) Scatter plots of the log2-FC in gene expression in response to larval feeding by M. sexta and S. exigua. (d) Scatter plots of the log2-FC in gene expression in response to larval feeding on plants oviposited and non-oviposited by conspecific moths (left: M. sexta, right: S. exigua). (e) Hierarchical clustering of the log2FC in gene expression (relative to control plants) of all feeding-induced treatments.
N. attenuata responds species-specific to larval feeding
Altogether, transcriptomes of plants attacked by larvae clearly separated from the non-attacked plants along the first principal component in a PCA (Fig. 2a). Along the second principal component, explaining about 10% of the variation, S. exigua-fed plants (E0Lse; filled squares) clearly separated from M. sexta-fed plants (E0Lms; filled triangles).
Herbivory by any of the two species significantly altered the expression of 4749 genes (11% of all genes on the array) relative to control plants (E0L0). Whereas feeding by S. exigua altered the expression of almost 92% (4361) of these genes, M. sexta feeding regulated only about half (2302) of them (Fig. 2b). As a consequence, the feeding-induced plant responses to both species overlapped just for 40% (1914). Of all feeding-inducible transcripts, only about 8% (388) were specific to M. sexta but almost 52% (2447) were unique for feeding S. exigua. In a direct comparison between S. exigua-fed (E0Lse) and M. sexta-fed (E0Lms) plants, 20% of the feeding-induced genes differed significantly. The transcriptional changes (log2-fold changes; log2-FC) relative to untreated controls correlated with an R2 of 0.72 between plants fed by larvae of the two different species (Fig. 2c), which is about 20% lower than correlations between plants fed by larvae of the same species (either or not previously oviposited by conspecifics; see below and Fig. 2d).
The difference in the transcriptional response to the two herbivore species was accompanied by a different induction of phytohormones. Feeding by both species increased levels of JA, JA-Ile, SA and ABA. In S. exigua-fed plants, levels of SA and the ratio of JA-Ile to JA were about twofold higher compared to M. sexta-fed plants (see outer bars in Fig. 3), while ABA was altered similarly by both species (Supplementary Fig. S1).
Figure 3.
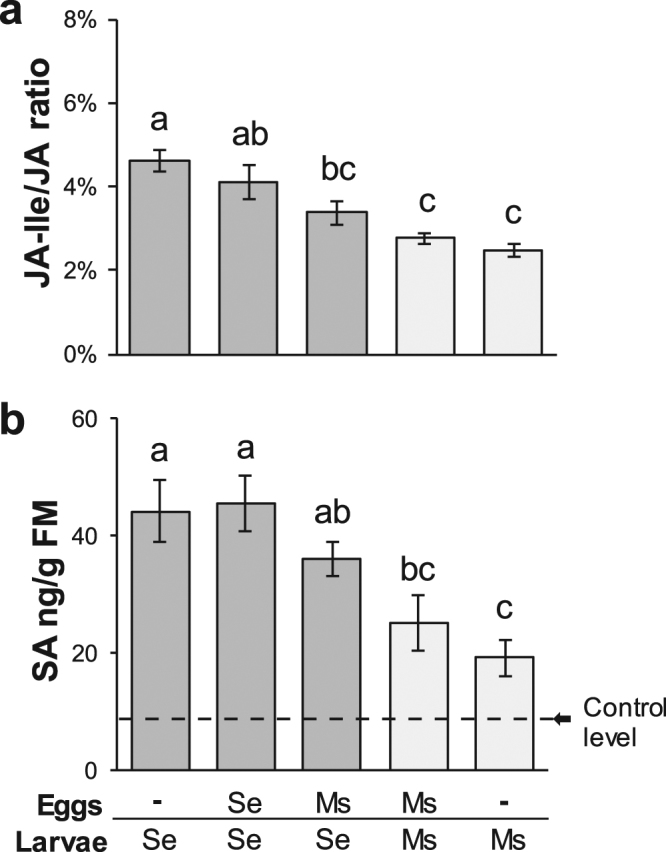
Species-specific effects of larval herbivory on N. attenuata’s phytohormone levels. Mean ± SEM (N = 8–10) of (a) the ratio of the jasmonic acid (JA) to its conjugate with isoleucine (JA-Ile) and (b) salicylic acid (SA) content in N. attenuata leaves fed by S. exigua (Se) or M. sexta (Ms) for 24 hours. The plants were previously either untreated or oviposited at a systemic leaf. An arrow marks the SA level of unattacked control plants. Letters indicate significant differences at P < 0.05 according to Kruskal Wallis and pairwise Wilcoxon rank sum test (in case of the JA-Ile/JA ratio) or ANOVA followed by paired t-test (in case of SA).
Effects of oviposition on N. attenuata’s response to herbivory by conspecific larvae
Oviposition by neither M. sexta nor S. exigua altered any of the assessed phytohormone accumulations in response to herbivory by conspecific larvae (Fig. 3, see Supplementary Fig. S1 and Supplementary Table S2). For each of the two species, non-oviposited and previously oviposited plants shared about 75% of the transcriptional regulation in response to feeding by conspecific larvae (relative to control plants; see Supplementary Fig. S2).
To test whether the previous oviposition changed the plant’s transcriptional response to the subsequent feeding by conspecific larvae, we evaluated the effects of previous oviposition and the larval feeding in two-factorial analyses (including E0L0/EmsL0/E0Lms/EmsLms in case of M. sexta and E0L0/EseL0/E0Lse/EseLse plants in case of S. exigua), but we found no interactions between oviposition and larval feeding for neither of the two species. Similarly, we found no significant differences in direct comparisons of gene expression in plants only exposed to larval feeding and plants that were oviposited prior to larval herbivory by the same species (E0Lms versus EmsLms and E0Lse versus EseLse).
In accordance with that, the feeding-induced transcriptional changes (log2-FC) relative to control plants correlated very strongly for previously oviposited and non-oviposited plants (Fig. 2d). Also in the PCA, M. sexta-fed plants with a prior oviposition by conspecifics (EmsLms) cluster closely together with plants only fed by M. sexta (E0Lms) and similarly do S. exigua-fed plants with a prior oviposition by conspecifics (EseLse) and plants only fed by S. exigua (E0Lse) cluster together (Fig. 2a).
Oviposition shapes the species-specific response of N. attenuata to larval feeding
We investigated furthermore, whether the plant response to feeding larvae is altered in plants previously oviposited by heterospecific moths. In the PCA, transcript profiles of the EseLms cross-species treatment (blue stars in Fig. 2a) clearly separated from the other two M. sexta-fed plant treatments (blue filled triangles) and aggregated with the S. exigua-fed treatments (red filled squares). Similarly, transcript profiles of the other EmsLse cross-species treatment (red stars) shifted towards plants fed by M. sexta. This pattern was further supported by a clustering analysis of the six feeding induced treatments, in which EseLms plants clustered together with S. exigua-fed plants that were either oviposited by conspecific moths or not (Fig. 2e). Vice versa, EmsLse clustered with M. sexta-fed plants.
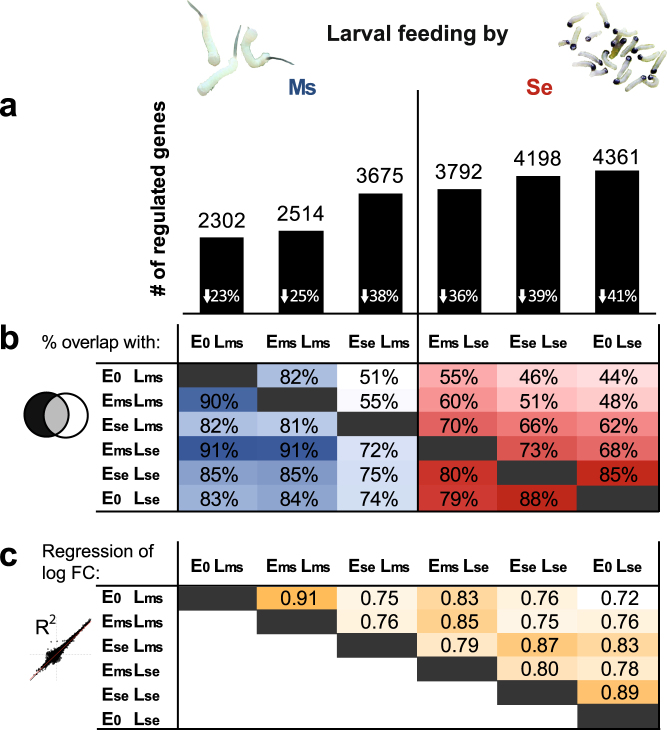
The numbers of genes regulated by the cross-species treatments fall in-between those regulated in plants that only experienced one of the species (Fig. 4a). For example, the number of genes regulated in EseLms plants increased by more than 1000 compared to the other two M. sexta-fed plant treatments (E0Lms, EmsLms). As a consequence, EseLms plants shared only 51% of its regulated genes with E0Lms plants, while these overlapped with 82% of the genes regulated in EmsLms (Fig. 4b). Instead, genes regulated in EseLms plants overlap with 62% of the genes regulated in E0Lse plants, which is considerably more than the 44–48% that the other M. sexta-fed plants overlapped with the E0Lse plants.
Figure 4.
Oviposition by the other species shifted the transcriptional response to feeding by M. sexta and S. exigua larvae in-between the species-specific plant responses to their herbivory. (a) Bars represent the numbers of genes (given above) differentially regulated relative to control plants (FDR-adjusted P < 0.05, log2-fold change >1) in all feeding-induced plant treatments. The white numbers in the bars display the proportion of down-regulated genes. The plants had experienced egg deposition (E) by M. sexta (Ms), S. exigua (Se), or none before they were fed by neonate larvae (L) of either of the two species for 24 hours. (b) Matrix of the proportions of gene sets similarly regulated by the different feeding-induced treatments: For each treatment designated on top of a column, the proportion of genes that are also regulated by another treatment (designated in front of a row) is given. (c) Matrix of the regression coefficients of pairwise correlations between log2-FC in gene expression (compared to control plants) among all feeding-induced treatments.
Similarly, plants of the S. exigua-fed cross-species treatment (EmsLse) had about 500 genes less regulated than the other two S. exigua-fed plant treatments. While, E0Lse and EseLse plants overlapped in 85–88% of their regulated genes, EmsLse plants overlapped only with 68% of the genes regulated in E0Lse plants. Conversely, the proportion of M. sexta-responsive genes (E0Lms) that are also altered in response to S. exigua feeding increased from 83% (E0Lse) to 91% (EmsLse) when M. sexta oviposition preceded S. exigua feeding. Thus, the comparisons of the regulated gene sets support that the plant response in cross-species treatments is intermediate to the species-specific responses to larval feeding.
Finally, the shift in the species-specific response to feeding was also reflected in pairwise regression analyses of the log2-FC relative to untreated controls (Fig. 4c, see Supplementary Fig. S3). The strong correlations between E0Lse and EseLse as well as between E0Lms and EmsLms with an R2 around 0.9 were not found between the cross-species treatments (EmsLse and EseLms) and the E0Lms and E0Lse treatments. Instead, the expression pattern of EmsLse plants correlated similarly with E0Lms and E0Lse (R2 of 0.82 and 0.83) and EseLms plants correlated even slightly stronger with E0Lse than with E0Lms plants.
Lastly, the cross-species treatment for which phytohormones were analysed (EmsLse) showed a phytohormonal induction pattern in-between of E0Lms and E0Lse plants (Fig. 3a,b). The intermediate levels of SA and JA-Ile/JA in EmsLse plants did not significantly differ from both treatments with M. sexta feeding and JA-Ile/JA levels in EmsLse plants even differed significantly from E0Lse plants.
Validation of microarray analysis by real-time qPCR of candidate genes
We quantified transcripts in 9-10 biological replicates for a set of genes that we selected based on their regulation in the microarray analysis or that are known to be key regulators in different plant signalling pathways. We selected (i) genes that were significantly regulated by the oviposition alone (Supplementary Table S3) or that indicated an altered regulation after larval feeding due to prior oviposition by the same species (Fig. 5), (ii) signalling-related genes that may reflect the species-specific response to the larval feeding of both species (Fig. 6), and (iii) genes that signify the shift of the species-specific feeding-induced response by the other species’ oviposition (Fig. 7). Overall, we validated the expression patterns determined by the microarray analysis for all 17 genes by real-time qPCR (Supplementary Figs S4 and S5).
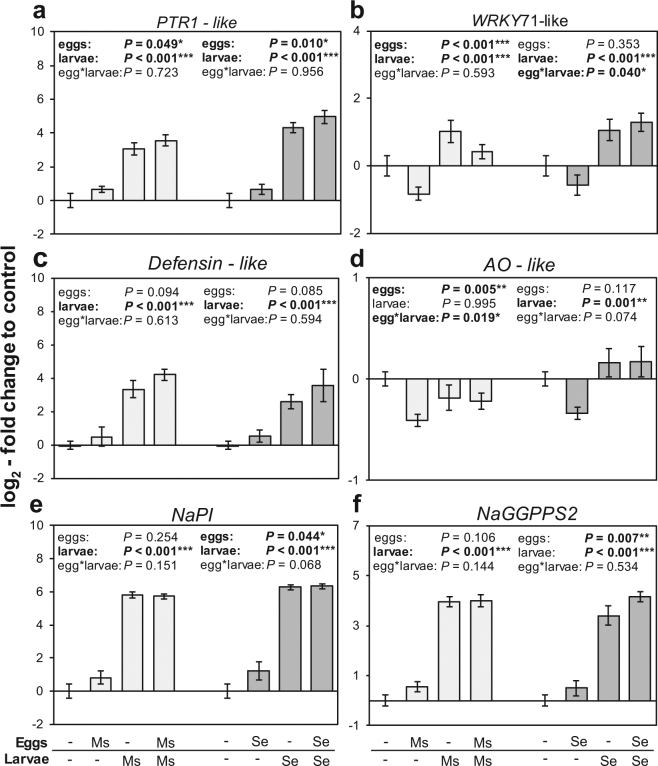
Figure 5.
Effects of oviposition and feeding on expression of N. attenuata genes. Transcript accumulation (mean ± SEM, N = 9–10) of (a) PTR1 homolog (oligo peptide transporter: Na_454_15271), (b) WRKY transcription factor (WRKY 71-like: Na_454_21846), (c) a defensin-like protein (Na_454_03111), (d) L-aspartate oxidase (AO: Na_454_00281), (e) trypsin protease inhibitor NaPI (Na_454_02256) and (f) geranylgeranyl diphosphate synthase NaGGPPS2 (Na_454_01146) in N. attenuata plants in response to the oviposition by and later the feeding larvae of either S. exigua or M. sexta. Transcript accumulation relative to untreated plants was determined by real-time qPCR normalized to β-actin and EF1α as reference genes. Asterisks indicate significant differences at P < 0.05/0.005/0.001 according to linear mixed-effects models with oviposition and larvae of either S. exigua or M. sexta as fixed factors and replicate blocks as random factor.
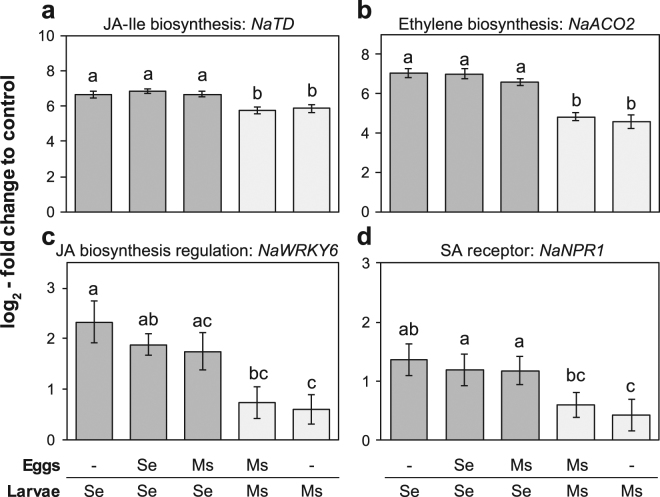
Figure 6.
N. attenuata signalling genes differentially induced by larvae of M. sexta and S. exigua. Transcript accumulation (mean ± SEM) of selected genes known to be key regulators in different plant signalling pathways: (a) threonine deaminase (NaTD: Na_454_03285), (b) aminocyclo-propanecarboxylate oxidase (NaACO2: Na_454_06634), (c) transcription factor WRKY6 (Na_454_14052), (d) SA receptor (NaNPR1: Na_454_12995) in plants fed by either S. exigua (Se, dark bars) or M. sexta (Ms, light bars) larvae. The plants were previously oviposited by either of the two species or not (−). Transcript accumulation relative to untreated plants was determined by real-time qPCR and normalized to β-actin and EF1α as reference genes. Letters indicate significant differences at P < 0.05 according to linear mixed-effects models with the different herbivory treatments as fixed factors and replicate blocks as random factor followed by a Tukey post-hoc test (N = 9–10).
Figure 7.
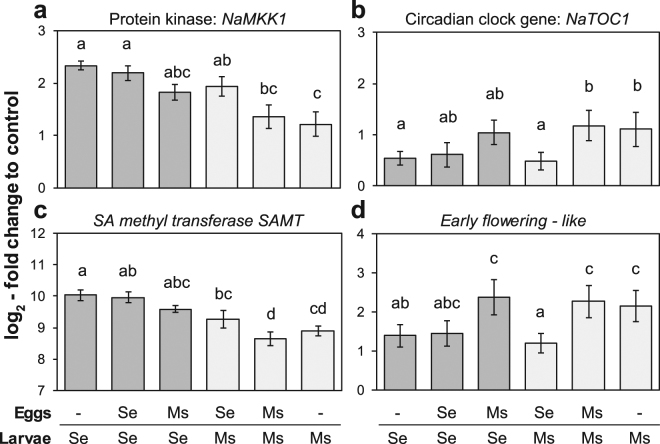
N. attenuata genes shifted by oviposition in their species-specific feeding-induced expression. In plants fed by either S. exigua (Se, dark bars) or M. sexta (Ms, light bars) larvae, transcript accumulation (mean ± SEM) of (a) the mitogen-activated protein kinase kinase (NaMKK1: Na_454_10210), (b) a SA methyl transferase (SAMT: Na_454_08320), (c) the circadian clock gene timing of CAB expression (NaTOC1: Na_454_09895), and (d) an early flowering-like gene (Na_454_09170) was determined by real-time qPCR and normalized to EF1α as reference gene and expressed relative to untreated control plants. Plants were previously oviposited by either of the two species or not (−) and letters indicate significant differences at P < 0.05 according to linear mixed-effects models with the different herbivory treatments as fixed factors and replicate block as random factor followed by a Tukey post-hoc test (N = 9–10).
Our analyses verified the up-regulation of the oligo peptide transporter PTR1-like in response to the oviposition by both species whereas up-regulation of a defensin-like protein by M. sexta oviposition was only indicated by trend (Fig. 5). Plants with M. sexta oviposition had significantly reduced transcript levels of a predicted WRKY71 transcription factor and an L-aspartate oxidase gene (AO). While plants with S. exigua oviposition had increased transcript levels of the trypsin protease inhibitor gene NaPI and the geranylgeranyl diphosphate synthase gene NaGGPPS2.
Transcripts of PTR1 and defensin also increased in response to larval feeding by both species, which tended to be stronger when the plants were previously oviposited by conspecifics. Though not statistically significant, this could point towards additive effects of oviposition and feeding. The increased transcript accumulation of the NaPI gene in response to S. exigua oviposition did also not result in increased NaPI transcripts after larval feeding compared to only feeding-damaged plants (Fig. 5e). In contrast, dual comparisons between feeding-induced plants revealed significantly increased transcripts of NaGGPPS2 in S. exigua-oviposited compared to only feeding-induced plants (Fig. 5f). Transcript levels of the WRKY71-like transcription factor and the AO gene were similar in feeding-induced plants whether the plants were oviposited or not. But since these genes were down-regulated in only-oviposited plants, they show a significant interaction between oviposition and larval feeding by S. exigua and M. sexta respectively (Fig. 5b,d). In summary, 24 hours after onset of larval feeding, we could still determine some genes affected by the previous oviposition per se but we could not pin down the effect of prior oviposition by conspecific moths on feeding-induced gene expression.
We additionally analysed a set of well characterised N. attenuata genes that play a role in different plant signalling pathways related to herbivory. These genes are involved in the regulation of the JA pathway (NaLOX3, NaTD, NaWRKY6), biosynthesis of ethylene (NaACO2) and of SA signalling (NaNPR1). All of these genes were significantly induced by larval feeding but not by oviposition and oviposition did not affect the level of induction after feeding (Fig. 6). They all showed a higher transcript accumulation in response to S. exigua than to M. sexta, except for NaLOX3, which was not differently regulated between the two species (Supplementary Fig. S4).
As oviposition shifted the species-specific transcriptomic imprint of the larval feeding, we also verified such a transcriptional shift for a selected set of genes. Three of them, the mitogen-activated protein kinase kinase NaMKK1, the SA methyl transferase SAMT and the 1-aminocyclopropane-1-carboxylic acid oxidase NaACO2 were stronger upregulated in response to S. exigua feeding than in response to M. sexta feeding but showed an intermediate expression to feeding by both larvae when they were previously oviposited by the other species (Fig. 7, Supplementary Fig. S5, Supplementary Table S4). Three other genes, the circadian clock gene timing of CAB expression NaTOC1, an early flowering-like gene and a gene homologous to Jumonji C-domain-containing proteins, JmjC30-like showed a higher expression to M. sexta than to S. exigua feeding, which was switched when the plant was previously oviposited by the other species.
Discussion
We examined whether the oviposition per se left imprints in the plant’s phytohomonal and transcriptional regulation in a systemic leaf. In line with previous studies11,12, we found no differences in phytohormone levels between oviposited and control plants. The transcript profile in plants oviposited by S. exigua did also not differ from that of control plants, but our microarray analysis revealed an imprint of M. sexta oviposition on N. attenuata’s transcriptome. Even a day after the end of the egg incubation time, expression of some genes was significantly altered and many of them were annotated to signalling and regulatory functions (e.g. transcription factors, hormone biosynthesis, see Supplementary Table S1). Moreover, about half of these genes were also altered in response to M. sexta feeding (see Supplementary Fig. S2). This is consistent with the idea that also subtle changes in gene regulation can be biologically relevant44.
We identified several genes responsive to oviposition by both M. sexta and S. exigua in our better replicated qPCR analysis (Fig. 5). Among them are genes involved in plant defence, for example NaGGPPS and NaPI45,46. These were induced by S. exigua- but not by M. sexta-oviposition. Interestingly, PI activity in response to larval feeding was also increased in S. exigua- but not in M. sexta-oviposited plants11,12. However, compared to the effect of larval feeding, oviposition only slightly induced NaPI expression and whether that could explain the priming of feeding-induced PI activity remains an open question.
Generally, it is not surprising that one day after egg-removal, systemic N. attenuata leaves did not display the large extent of changes in gene expression as described for the local leaves of A. thaliana 24–72 hours after oviposition by Pieris moths34. Even in local A. thaliana leaves, no transcriptional imprint was detected anymore, one day after egg-removal41. Yet, both plant species differ in their physiological response to insect oviposition. Other than N. attenuata, A. thaliana responds to oviposition with HR-like symptoms34 which can result in SAR against phytopathogenic bacteria33,35. Whereas oviposition by the specialist P. brassicae induces HR-like necrosis in many brassicaceous plants17,21, oviposition by the generalist Mamestra brassicae does not, nor does the latter affect larval performance25. However, in N. attenuata, oviposition by both a specialist and a generalist moth species prime feeding-induced defences and lower the performance of S. exigua larvae11,12. In line with that, all defence-related candidate genes that were affected by oviposition in our qPCR analysis showed similar tendencies for the oviposition by both moth species even if the effect was only significant for one of the two species.
As expected, N. attenuata exhibited diverging responses to the feeding larvae of both species. Larvae of S. exigua induced more SA than those of M. sexta, just as had been previously described31. Contrasting this earlier study31, M. sexta feeding also induced some SA and S. exigua-fed plants showed no lower JA induction but a greater JA-Ile/JA ratio than M. sexta-fed plants, which may even suggest a higher conversion of JA to the biologically active conjugate. However, in line with our results, a recent study47 also describes SA induction after M. sexta feeding and an equal JA-elicitation for N. attenuata’s response to M. sexta and Spodoptera littoralis. Consistent with our phytohormone analysis, transcripts of the Ile-biosynthesis gene NaTD as well as the SA-receptor NaNPR1 were differentially activated by larval feeding of both species, whereas that of the JA-biosynthesis gene NaLOX3 was not (Fig. 6, Supplementary Fig. S4). Thus, our results support that SA signalling plays a role in tuning N. attenuata’s species-specific response to larval feeding, but this tuning is not necessarily linked to JA-SA antagonism as had been suggested earlier31.
The distinguished species-specific response to the feeding larvae was reflected in the number of regulated genes as well as in PCA, regression and cluster analyses of the plant’s transcriptome (Figs 2–4). Our results match well with those of a study that pioneered N. attenuata’s species-specific transcriptional response to different herbivores4 using cDNA microarrays displaying only 240 genes with a bias for herbivory-related genes. Like this earlier study4, our unbiased transcriptome microarray, showed that a large fraction of feeding-responsive genes was specifically regulated by feeding of the generalist S. exigua and this fraction was greater among down- than among up-regulated genes. In line with this, the shared transcriptional response to two generalist herbivores Heliothis virescens and S. exigua differentiating from the response to M. sexta is also dominated by down-regulated genes4. Differential plant responses to herbivores of the same feeding guild but different diet breadth are also apparent in other plant-insect interactions, which could be either adaptive for the plant or the result of host manipulation by insects5–7,26. Presumably, generalist herbivores are more likely to evolve mechanisms to suppress plant defences than specialists as they have to cope with diverse plant defences7. However, S. exigua feeding altered more than 2000 genes more than M. sexta feeding. Although, this was more pronounced for down-regulated genes, defence-related genes and signalling pathways were often stronger induced in response to the generalist (Figs 2–7) and the majority of genes down-regulated after herbivory are usually related to primary plant metabolism48. N. attenuata effectively defends itself against S. exigua11,46,49, whereas M. sexta is relatively tolerant to many of its direct defences47,50. Thus, it is more likely that the differential plant response is driven either by the specialist herbivore or the plant itself.
The distinct response of N. attenuata to both herbivores is likely due to the divergent composition of elicitors in their oral secretions31. The receptors recognizing these elicitors have not been identified so far but they presumably activate mitogen-activated protein (MAP) kinases, usually three in a cascade, that mediate the transduction of the signal. We found that one of the MAP kinases (NaMKK1) that are involved in the regulation of N. attenuata’s defence response to herbivory51, reflected the species-specific expression pattern (Fig. 7). Even though NaMKK1 is not required for feeding-induced JA and ethylene production, it fine-tunes N. attenuata’s defence response52 and thus it may also be involved in adjusting the plant’s response to the attacking herbivore species.
To examine plant responses that may be associated with N. attenuata’s oviposition-primed defence response to larval feeding11, we compared only feeding-induced plants with plants that had been additionally oviposited by conspecific moths. Yet, we could not determine how insect oviposition on N. attenuata alters feeding-induced plant defence metabolites and the performance or immune state of S. exigua and M. sexta larvae, respectively11,12. The transcriptional responses between only feeding-induced plants and plants with prior oviposition by conspecifics correlated to 90%, which only emphasizes the high reproducibility of the herbivory treatments within our microarray analysis. However, despite their biological relevance, subtle changes in only few genes can be easily overlooked due to the large number of variables that are analysed with a very limited number of replicates44. For example, pre-treatments with phytopathogenic bacteria or egg extract that both have a negative impact on larval performance of P. brassicae, show just very subtle effects on the transcriptional response of B. nigra to larval feeding42.
That we could not determine signalling components which could explain these effects may be due to one or a combination of the following reasons: The regulation of the oviposition-priming of plant defence may (i) occur at different levels, e.g. post-transcriptionally, (ii) involve only few key components at an effect-size that is too small to be revealed in a low-replicated multi-variate approach, and (iii) occur at other time points after the onset of larval feeding. Priming of feeding-induced responses may result in an earlier, faster, stronger, more sensitive or even qualitatively different response on the molecular level9. In order to minimize variation between plant-replicates, that may be driven by random differences in the feeding behaviour of the larvae on a shorter time scale, we examined a time point at which the feeding-induced response on the transcriptional and phytohormonal level is well settled. However, thereby we may have missed the time point at which the plant defence priming by oviposition is regulated, for instance if it would cause an earlier or faster plant response just upon the larvae started feeding.
Opposite to prior oviposition by conspecifics, our results revealed that oviposition by one species altered the species-specific feeding response to larvae of the other species. In both cross-species treatments, the plant’s response to feeding larvae became intermediate in those characteristics that were specific for the larval feeding of both species. This was consistently apparent in all analyses of N. attenuata’s transcriptional response (PCA, cluster and regression analyses as well as the numbers and overlaps of significantly regulated genes) as well as in the phytohormone data for SA and JA-Ile/JA (Figs 2–4).
The oviposition-dependent shift of the species-specific gene expression in response to larval feeding was further confirmed by qPCR on six selected genes. Half of them were higher expressed in response to S. exigua than to M. sexta and showed an intermediate expression in the cross-species treatments (Fig. 7, Supplementary Fig. S5). These genes (ACO, SAMT, NaMKK1) are related to signalling pathways that mediate plant defence. Again, as NaMKK1 is fine-tuning N. attenuata’s defence response52, it may also play a role in the oviposition-mediated shift of the plant response to larval feeding. Three circadian clock genes were stronger expressed after M. sexta- than after S. exigua-feeding but showed the reversed pattern in the cross-species treatments. Two of them were also induced by M. sexta oviposition at levels comparable to feeding (Supplementary Table S1). Previous studies suggested that diurnal regulation plays a role in orchestrating plant anti-herbivore defences53,54 and this may be the case for the oviposition-mediated responses we found in N. attenuata as well.
Attack by multiple herbivores modifies plant responses in a non-additive manner and consequently can strongly affect the outcome of plant-insect interactions55. Our study shows that such interactions between different insects can begin before actual herbivory occurs. That insect oviposition alters plant resistance to the ovipositing species is well-established today56 while the notion that also other biotic stressors can be affected is just emerging12,35.
Our study revealed that insect oviposition can shape the species-specificity of a plant’s response to feeding larvae. What are possible explanations of this surprising phenomenon? On the one hand, N. attenuata may prepare its feeding-induced response to defend most effectively against the herbivore that announced itself by its oviposition. On the other hand, herbivores may start to manipulate their host plant right upon oviposition. The latter scenario may be more likely for the specialist, yet the shift of the species-specific plant response was more pronounced in response to the generalist’s oviposition. Despite its wide host plant range, S. exigua is a frequent herbivore in the native habitat of N. attenuata49 and as such it may exert considerable selection pressure on the plant. If the plant would benefit from rendering its response specific to the insect species, the effect that oviposition can determine this species-specificity connotes a novel mechanism of defence priming.
Methods
Plants and insects
Seeds of a Nicotiana attenuata Torr. ex Watson inbred line (15x) from a field collection were sterilized and smoke-germinated on agar plates and seedlings were transferred to potting soil and grown in 1.5 L pots in a greenhouse (24 °C (±10):15 °C; 16:8 h L:D) as described earlier11. Manduca sexta L. and Spodoptera exigua Hübner were cultured in a climate chamber (24 °C; 16:8 L:D). Insect cultures and the wheat germ based artificial diets of the larvae were described earlier57.
Experimental procedure
We assigned 5-week-old early elongated experimental plants of equal size and elongation state to one of nine treatments for each of 12 biological replicate blocks (Fig. 1). In a 3 × 3 full-factorial experimental design, we examined the plant responses to oviposition and larval feeding by M. sexta and S. exigua and the interactive effects between them. The setup of the oviposition and larval feeding treatments were previously described in detail58. In brief, we exposed the second youngest source leaf (2 positions below the sink-source transition leaf) through slots in the side of a flight cage to M. sexta oviposition. As S. exigua does not accept this setup for oviposition, we exposed whole plants to oviposition by S. exigua for 12 h. M. sexta-oviposited plants received 2.2 ± 0.4 (mean ± SEM) and S. exigua-oviposited 37.7 ± 5.6 eggs (dispersed on one to four elder leaves). Within the range of the natural egg incubation time, 3 and 4 days after oviposition by S. exigua and M. sexta respectively, we removed the eggs of all plants without damaging the leaf surface either with a soft brush or a featherweight forceps.
Within 12 h after egg-removal, we applied either three M. sexta or 20 S. exigua neonates per plant to a standardised leaf position relative to the sink-source transition leaf. It was the next younger leaf above the M. sexta-oviposited leaf and the corresponding leaf position for control or S. exigua-oviposited plants. Due to the size difference of both species, these numbers of larvae inflict comparable amounts of damage. Larvae were kept on the leaf in vented clip cages and plants without herbivory treatments received empty clip cages at corresponding leaf positions. After 24 hours of larval feeding, the standardised leaf (either exposed to larvae or empty clip cages) was harvested from all plants, flash-frozen in liquid nitrogen and stored at −80 °C. Aliquots of the leaf material powdered using mortar and pestle under liquid nitrogen were weighed into separate tubes.
Extraction and analysis of phytohormones
As previously described in more detail59, we extracted about 150 mg of the samples in 1 mL ethyl acetate spiked with deuterated internal standards of JA, JA-Ile, SA, ABA and re-eluted the dried extracts in 400 µL 70% methanol with 0.1% formic acid before analysis by UPLC-ESI-MS/MS (Synapt G2-S HDMS; Waters®, Milford, Massachusetts, USA). After separation on a C18-column (Acquinity UPLC BEH-C18) with water and methanol (both containing 0.1% formic acid) as eluents in gradient mode (250 μL min−1), phytohormones were quantified (negative ionization mode, parent/daughter ion selections for JA: 209/59, JA-Ile: 322/130, SA: 137/93, ABA: 263/153, D6-JA: 215/59, D6-JA-Ile: 328/130, D4-SA: 141/97, D6-ABA: 269/159) according to the peak areas of the respective fragment ions relative to that of the internal standards using MassLynxTM (4.1, Waters).
RNA extraction
We extracted total RNA from 100 mg leaf powder of all individual plants (including those of the EseLms treatment) with TRIzol® Reagent before the precipitated RNA (using 1.2 M sodium chloride and 0.8 M sodium citrate) was DNase digested using TURBO DNA-free™ (both AmbionTM, Thermo Fisher Scientific, Waltham, MA USA; http://www.thermofisher.com) according to the manufacturer’s instructions. After a clean-up of the RNA using NucleoSpin® RNA (Macherey-Nagel, Düren, Germany; http://www.mn-net.com/), we adjusted the RNA to 200 ng/µL based on photometric concentration measurements (μDrop™ Plate; Multiskan™ GO Microplate Spectrophotometer, Thermo Scientific).
Microarray analysis
RNA of 3-4 plant replicates per treatment was pooled at equal proportions resulting in 3 biological replicates for each treatment on the microarray (performed by Oaklabs, http://www.oak-labs.com/). All samples had a RNA integrity index between 7.7 and 8.0 as determined with a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA, http://www.agilent.com). Fluorescent cRNA was generated using the Low Input QuickAmp Labeling Kit (Agilent Technologies) and oligo-dT primers following the manufacturer’s protocol. Of the cyanine 3-CTP-labelled cRNA, 600 ng were hybridised using the Agilent Gene Expression Hybridisation Kit (Agilent Technologies) following the manufacturer’s protocol on the microarray at 65 °C for 17 h. After the microarray was washed twice, the fluorescence signals on microarrays were detected by the SureScan Microarray Scanner (Agilent Technologies) at a resolution of 3 micron per pixel. The 8 × 60 K microarray harboured the same 60-mer oligonucleotide probes as the Agilent 4 × 44 K microarray with the GEO accession no. GPL1352760 that was designed based on the N. attenuata transcriptome (BioProject PRJNA223344 at http://www.ncbi.nlm.nih.gov/). Although all RNA samples were processed at once, a first hybridisation on three arrays (24 samples) included only one cross-species treatment. The EmsLse was chosen because oviposition by M. sexta affects larval performance of S. exigua but not vice versa12. The analysis revealed that, opposite to the effect of prior oviposition of the same species that later fed on the plants, prior exposure to M. sexta oviposition shifted the response to larval feeding by S. exigua. Therefore, a second analysis including the EseLms treatment was performed.
Real-time qPCR analysis
To verify microarray results and to test the effects of oviposition and larval feeding on potentially regulated genes with a higher statistical power, we performed real-time qPCR on 9-10 individual plants of each treatment. The verification of the qPCR data was initially performed for 11 genes on the same 8 treatments used in the first microarray hybridisations and was then extended for 6 genes to all feeding-induced treatments including both cross-species treatments and control plants. We synthesized cDNA from the total RNA with the Reverse Transcriptase Core kit (Eurogentec, Seraing, Belgium, http://www.eurogentec.com) according to the manufacturer’s instructions. For each gene, 1 µL cDNA was used in 10 µL reactions of SYBR®Green I-based real-time PCR using a qPCR kit without ROX (Eurogentec) on a StratageneTM Mx3005P® instrument (Agilent Technologies). Real-time PCR was performed with gene-specific primers (see Supplementary Table S5) in triplicates with all treatments of a replicate on one 96 well plate. We inspected melting curves and used LinRegPCR (http://LinRegPCR.HFRC.nl) to determine amplification efficiencies and the relative quantities (RQ) of each gene61. The RQ of the genes of interest was normalised to the averaged RQ of two reference genes β-actin and EF1α (NRQ). Transcript accumulations were analysed on the log2-normalized NRQs and depicted relative to the mean of the control treatment.
Data analysis
All statistics were performed in R version 3.2.3 with the packages lme4, multcomp, psych and stats (http://www.r-project.org). Phytohormone and qPCR data were checked for normality and variance homogeneity (QQ-plots and Bartlett’s test) before their statistical evaluation. To test for differences in phytohormone levels between oviposited and control plants and for differences among the five groups of plants exposed to larval feeding, we used either ANOVA followed by paired t-tests or Kruskal Wallis test followed by pairwise Wilcoxon rank sum tests. To evaluate the effect of the factors oviposition and larval feeding as well as their interaction within the 2 × 2 full-factorial priming experiments for each species separately we used linear mixed-effects models (LMMs) and included the replicate blocks as random factor. We also analysed transcript accumulations determined by qPCR with LMMs to evaluate differences between control and oviposited plants as well as those between plants of the different larval feeding treatments.
We used the limma package62 to analyse the microarray data. To reduce background noise, a detection limit was set at the 10% quantile of fluorescence signal intensity of each array. All structural spots, as well as oligos that were not above this detection limit in any of the arrays, were removed from further analysis. Subsequently, the expression levels were background-corrected using the normexp method63 and normalized between the arrays using quantile normalisation64. Principal component analysis was performed on normalised fluorescence signals using prcomp and visualised using ggplot265. We performed hierarchical clustering using hcust and analysed gene expression using lmFit. Genes were assumed to be significantly regulated when they differed between treatments by a log2-fold change of at least 1 and at P-values of below 0.05 after correction for false discovery rate according to Benjamini-Hochberg. Genes of interest were functionally annotated according to NCBI nucleotide blast and blastx of the corresponding p454 sequences (BioProject PRJNA223344).
Accession codes
The microarray data are available at NCBI Gene Expression Omnibus under accession GSE116273.
Electronic supplementary material
Acknowledgements
We thank Lara Friebe and Julia Felderhoff for help with the experiments and the plant and insect cultures, Vivien Firtzlaff for help with the initial microarray analysis, Martina Schad and Jim Kallarackal from Oaklabs for great support on the microarray hybridisations and Andreas Springer for excellent service with the UPLC-ESI-MS/MS. We are grateful for the financial support of the German Research Foundation (DFG; project B2 within the Collaborative Research Centre ‘SFB973’) and the stipend of the German Federal Environmental Foundation (DBU) for Michele Bandoly.
Author Contributions
M.B., S.D. and A.S. designed this study. M.B., S.D. and S.S. conducted the experimental and laboratory work. S.D., M.B. and T.L. analysed the results. S.D., M.B. and A.S. wrote the first draft of the manuscript that was edited and revised by all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Sylvia Drok and Michele Bandoly contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28233-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schaller, A. Induced plant resistance to herbivory (Springer, 2008).
- 2.Karban R, Agrawal AA. Herbivore offense. Annu. Rev. Ecol. Syst. 2002;33:641–664. doi: 10.1146/annurev.ecolsys.33.010802.150443. [DOI] [Google Scholar]
- 3.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. Toxic cardenolides: Chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 2012;194:28–45. doi: 10.1111/j.1469-8137.2011.04049.x. [DOI] [PubMed] [Google Scholar]
- 4.Voelckel C, Baldwin IT. Generalist and specialist lepidopteran larvae elicit different transcriptional responses in Nicotiana attenuata, which correlate with larval FAC profiles. Ecol. Lett. 2004;7:770–775. doi: 10.1111/j.1461-0248.2004.00633.x. [DOI] [Google Scholar]
- 5.Vogel Hea. Different transcript patterns in response to specialist and generalist herbivores in the wild Arabidopsis relative Boechera divaricarpa. PLoS. ONE. 2007;2:e1081. doi: 10.1371/journal.pone.0001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong N, Wang C-Z. Larval feeding induced defensive responses in tobacco: comparison of two sibling species of Helicoverpa with different diet breadths. Planta. 2007;226:215–224. doi: 10.1007/s00425-006-0459-x. [DOI] [PubMed] [Google Scholar]
- 7.Ali JG, Agrawal AA. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012;17:1360–1385. doi: 10.1016/j.tplants.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Conrath U, Beckers GJM, Langenbach CJG, Jaskiewicz MR. Priming for enhanced defense. Annu. Rev. Phytopath. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 9.Hilker M, et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016;91:1118–1133. doi: 10.1111/brv.12215. [DOI] [PubMed] [Google Scholar]
- 10.Heil M, Kost C. Priming of indirect defences. Ecol. Lett. 2006;9:813–817. doi: 10.1111/j.1461-0248.2006.00932.x. [DOI] [PubMed] [Google Scholar]
- 11.Bandoly M, Hilker M, Steppuhn A. Oviposition by Spodoptera exigua on Nicotiana attenuata primes induced plant defense against larval herbivory. Plant J. 2015;83:661–672. doi: 10.1111/tpj.12918. [DOI] [PubMed] [Google Scholar]
- 12.Bandoly M, Grichnik R, Hilker M, Steppuhn A. Priming of anti-herbivore defence in Nicotiana attenuata by insect oviposition: Herbivore specific effects. Plant Cell Environ. 2016;39:848–859. doi: 10.1111/pce.12677. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Tooker JF, Luthe DS, De Moraes CM, Felton GW. Insect eggs can enhance wound response in plants: A study system of tomato Solanum lycopersicum L. and Helicoverpa zea Boddie. PLoS. ONE. 2012;7:e37420. doi: 10.1371/journal.pone.0037420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austel N, Eilers EJ, Meiners T, Hilker M. Elm leaves “warned” by insect egg deposition reduce survival of hatching larvae by a shift in their quantitative leaf metabolite pattern. Plant Cell Environ. 2016;39:366–376. doi: 10.1111/pce.12619. [DOI] [PubMed] [Google Scholar]
- 15.Beyaert I, et al. Can insect egg deposition ‘warn’ a plant of future feeding damage by herbivorous larvae? Proc. R. Soc. B. 2012;279:101–108. doi: 10.1098/rspb.2011.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geiselhardt S, et al. Egg laying of cabbage white butterfly (Pieris brassicae) on Arabidopsis thaliana affects subsequent performance of the larvae. PLoS. ONE. 2013;8:e59661. doi: 10.1371/journal.pone.0059661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pashalidou FG, Fatouros NE, van Loon JJA, Dicke M, Gols R. Plant-mediated effects of butterfly egg deposition on subsequent caterpillar and pupal development, across different species of wild Brassicaceae. Ecol. Entomol. 2015;40:444–450. doi: 10.1111/een.12208. [DOI] [Google Scholar]
- 18.Bittner N, Trauer-Kizilelma U, Hilker M. Early plant defence against insect attack: involvement of reactive oxygen species in plant responses to insect egg deposition. Planta. 2017;245:993–1007. doi: 10.1007/s00425-017-2654-3. [DOI] [PubMed] [Google Scholar]
- 19.Desurmont GA, Weston PA. Aggregative oviposition of a phytophagous beetle overcomes egg-crushing plant defences. Ecol. Entomol. 2011;36:335–343. doi: 10.1111/j.1365-2311.2011.01277.x. [DOI] [Google Scholar]
- 20.Doss RP, et al. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA. 2000;97:6218–6223. doi: 10.1073/pnas.110054697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fatouros, N. E. et al. Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proc. R. Soc. B281, 10.1098/rspb.2014.1254 (2014). [DOI] [PMC free article] [PubMed]
- 22.Geuss D, Stelzer S, Lortzing T, Steppuhn A. Solanum dulcamara’s response to eggs of an insect herbivore comprises ovicidal hydrogen peroxide production. Plant Cell Environ. 2017;40:2663–2677. doi: 10.1111/pce.13015. [DOI] [PubMed] [Google Scholar]
- 23.Petzold-Maxwell J, Wong S, Arellano C, Gould F. Host plant direct defence against eggs of its specialist herbivore. Heliothis subflexa. Ecol. Entomol. 2011;36:700–708. doi: 10.1111/j.1365-2311.2011.01315.x. [DOI] [Google Scholar]
- 24.Seino Y, Suzuki Y, Sogawa K. An ovicidal substance produced by rice plants in response to oviposition by the whitebacked planthopper, Sogatella furcifera (HORVATH) (Homoptera: Delphacidae) Appl. Entomol. Zool. 1996;31:467–473. doi: 10.1303/aez.31.467. [DOI] [Google Scholar]
- 25.Pashalidou FG, Lucas-Barbosa D, van Loon JJA, Dicke M, Fatouros NE. Phenotypic plasticity of plant response to herbivore eggs: effects on resistance to caterpillars and plant development. Ecology. 2013;94:702–713. doi: 10.1890/12-1561.1. [DOI] [PubMed] [Google Scholar]
- 26.Appel HM, et al. Transcriptional responses of Arabidopsis thaliana to chewing and sucking insect herbivores. Frontiers in Plant Science. 2014;5:565. doi: 10.3389/fpls.2014.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu. Rev. Cell Develop. Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 28.Bonaventure G. Perception of insect feeding by plants. Plant Biol. 2012;14:872–880. doi: 10.1111/j.1438-8677.2012.00650.x. [DOI] [PubMed] [Google Scholar]
- 29.Heil M. Damaged-self recognition in plant herbivore defence. Trends Plant Sci. 2009;14:356–363. doi: 10.1016/j.tplants.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, III, Teal PEA. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA. 2009;106:653–657. doi: 10.1073/pnas.0811861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diezel C, von Dahl CC, Gaquerel E, Baldwin IT. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009;150:1576–1586. doi: 10.1104/pp.109.139550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reymond P. Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta. 2013;238:247–258. doi: 10.1007/s00425-013-1908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruessow F, Gouhier-Darimont C, Buchala A, Metraux JP, Reymond P. Insect eggs suppress plant defence against chewing herbivores. Plant J. 2010;62:876–885. doi: 10.1111/j.1365-313X.2010.04200.x. [DOI] [PubMed] [Google Scholar]
- 34.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in. Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilfiker O, et al. Insect eggs induce a systemic acquired resistance in Arabidopsis. Plant J. 2014;80:1085–1094. doi: 10.1111/tpj.12707. [DOI] [PubMed] [Google Scholar]
- 36.Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 2004;135:2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchel, K. et al. An elm EST database for identifying leaf beetle egg-induced defense genes. BMC Genomics13, 10.1186/1471-2164-13-242 (2012). [DOI] [PMC free article] [PubMed]
- 38.De Puysseleyr V, Hofte M, De Clercq P. Ovipositing Orius laevigatus increase tomato resistance against Frankliniella occidentalis feeding by inducing the wound response. Arthropod Plant Interact. 2011;5:71–80. doi: 10.1007/s11829-010-9117-0. [DOI] [Google Scholar]
- 39.Hilker M, Kobs C, Varama M, Schrank K. Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J. Exp. Biol. 2002;205:455–461. doi: 10.1242/jeb.205.4.455. [DOI] [PubMed] [Google Scholar]
- 40.Meiners T, Westerhaus C, Hilker M. Specificity of chemical cues used by a specialist egg parasitoid during host location. Entomol. Exp. Appl. 2000;95:151–159. doi: 10.1046/j.1570-7458.2000.00653.x. [DOI] [Google Scholar]
- 41.Firtzlaff V, Oberländer J, Geiselhardt S, Hilker M, Kunze R. Pre-exposure of Arabidopsis to the abiotic or biotic environmental stimuli ‘chilling’ or ‘insect eggs’ exhibits different transcriptomic responses to herbivory. Sci. Rep. 2016;6:28544. doi: 10.1038/srep28544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnet C, et al. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against a chewing herbivore in Brassica nigra. BMC Plant Biol. 2017;17:127. doi: 10.1186/s12870-017-1074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur H, Heinzel N, Schottner M, Baldwin IT, Galis I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 2010;152:1731–1747. doi: 10.1104/pp.109.151738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez DH, Szymanski J, Erban A, Udvardi MK, Kopka J. Mining for robust transcriptional and metabolic responses to long-term salt stress: a case study on the model legume Lotus japonicus. Plant Cell Environ. 2010;33:468–480. doi: 10.1111/j.1365-3040.2009.02047.x. [DOI] [PubMed] [Google Scholar]
- 45.Jassbi AR, Gase K, Hettenhausen C, Schmidt A, Baldwin IT. Silencing geranylgeranyl diphosphate synthase in Nicotiana attenuata dramatically impairs resistance to tobacco hornworm. Plant Physiol. 2008;146:974–986. doi: 10.1104/pp.107.108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steppuhn A, Baldwin IT. Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol. Lett. 2007;10:499–511. doi: 10.1111/j.1461-0248.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 47.Xu S, Zhou W, Pottinger S, Baldwin IT. Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol. 2015;15:2. doi: 10.1186/s12870-014-0406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lortzing T, et al. Transcriptomic responses of Solanum dulcamara to natural and simulated herbivory. Mol. Ecol. Resour. 2017;17:e196–e211. doi: 10.1111/1755-0998.12687. [DOI] [PubMed] [Google Scholar]
- 49.Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS. Biol. 2004;2:1074–1080. doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wink M, Theile V. Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae) Chemoecology. 2002;12:29–46. doi: 10.1007/s00049-002-8324-2. [DOI] [Google Scholar]
- 51.Hettenhausen C, Schuman MC, Wu JQ. MAPK signaling: A key element in plant defense response to insects. Insect Sci. 2015;22:157–164. doi: 10.1111/1744-7917.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heinrich M, Baldwin IT, Wu JQ. Two mitogen-activated protein kinase kinases, MKK1 and MEK2, are involved in wounding- and specialist lepidopteran herbivore Manduca sexta-induced responses in Nicotiana attenuata. J. Exp. Bot. 2011;62:4355–4365. doi: 10.1093/jxb/err162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. USA. 2012;109:4674–4677. doi: 10.1073/pnas.1116368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SG, Yon F, Gaquerel E, Gulati J, Baldwin IT. Tissue specific diurnal rhythms of metabolites and their regulation during herbivore attack in a native tobacco. Nicotiana attenuata. PLoS. ONE. 2011;6:e26214. doi: 10.1371/journal.pone.0026214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stam JM, et al. Plant Interactions with Multiple Insect Herbivores: From Community to Genes. Annu. Rev. Plant Biol. 2014;65:689–713. doi: 10.1146/annurev-arplant-050213-035937. [DOI] [PubMed] [Google Scholar]
- 56.Hilker M, Fatouros NE. Plant responses to insect egg deposition. Annu. Rev. Entomol. 2015;60:493–515. doi: 10.1146/annurev-ento-010814-020620. [DOI] [PubMed] [Google Scholar]
- 57.Trauer U, Hilker M. Parental legacy in insects: variation of transgenerational immune priming during offspring development. PLoS. ONE. 2013;8:e63392. doi: 10.1371/journal.pone.0063392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bandoly M, Steppuhn A. Bioassays to investigate the effects of insect oviposition on a plant’s resistance to herbivores. BioProtocol. 2016;6:e1823. [Google Scholar]
- 59.Nguyen D, et al. Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara. Plant, Cell & Environ. 2016;39:1485–1499. doi: 10.1111/pce.12708. [DOI] [PubMed] [Google Scholar]
- 60.Gulati J, Kim S-G, Baldwin IT, Gaquerel E. Deciphering herbivory-induced gene-to-metabolite dynamics in Nicotiana attenuata tissues using a multifactorial approach. Plant Physiol. 2013;162:1042–1059. doi: 10.1104/pp.113.217588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruijter, J. M. et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Research37, 10.1093/nar/gkp045 (2009). [DOI] [PMC free article] [PubMed]
- 62.Ritchie ME, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritchie ME, et al. A comparison of background correction methods for two-colour microarrays. Bioinformatics. 2007;23:2700–2707. doi: 10.1093/bioinformatics/btm412. [DOI] [PubMed] [Google Scholar]
- 64.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 65.Wickham, H. ggplot2 Elegant graphics for data analysis (Springer, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.