Abstract
Signaling by members of the transforming growth factor-β (TGF-β) superfamily, such as TGF-β3 and BMP7, and oxygen tension play a pivotal role in chondrocyte biology. The objective of this research was to investigate the endogenous BMP7 expression in human osteoarthritis (OA) cartilage and the effect of oxygen tension on the single or combined treatment with TGF-β3 and BMP7 on OA chondrocyte redifferentiation in three dimensional (3D) pellet cultures. The results showed the expression of BMP7 and its intracellular signaling target SMAD1/5/8 was decreased in early OA, while it was increased in later stages of OA. The combined treatment with TGF-β3 and BMP7, both in normoxia and hypoxia, was more effective than TGF-β3 or BMP7 alone in redifferentiating chondrocytes. This was reflected by Alcian blue/Safranin O staining and collagen type II protein expression, as well as by gene expression. Hypoxia elevated TGF-β3 and BMP7-induced matrix formation of OA chondrocytes and alleviated the catabolic gene expression. Interestingly, cells cultured under normoxia displayed mild signs of an inflammatory stress response, which was effectively counteracted by culturing the cells under low oxygen tension. Our data underscores the important modulatory role of oxygen tension on the chondrocyte’s responsiveness to TGF-β3 and/or BMP7.
Introduction
Articular cartilage exhibits a poor self-regeneration capacity, and even minor cartilage defects may predispose to early onset osteoarthritis (OA). To break this vicious cycle, surgical intervention is often considered. Several surgical strategies have been developed to repair damaged cartilage. One of the most promising approaches is autologous chondrocyte implantation (ACI)1. This approach is based on isolation of chondrocytes, followed by expansion in monolayer culture to obtain sufficient cells for implantation into the lesion. However, monolayer expansion leads to a rapid loss of typical chondrocyte features, a process known as chondrocyte dedifferentiation, which eventually results in fibrous cartilage formation rather than hyaline cartilage after implantation2,3. Consequently, it is important to minimize the negative aspects of chondrocyte dedifferentiation or, alternatively develop effective strategies for chondrocyte redifferentiation.
Transforming Growth Factor-β (TGF-β) superfamily, including TGF-β and bone morphogenetic proteins (BMPs), is a family of secreted signaling factors with a remarkable ability to induce cartilage and bone formation. Generally, TGF-β induced signaling depends on Sma and Mad related proteins (SMAD) 2 and SMAD34,5. This results in stabilization of the SOX9 transcription complex while inhibiting RUNX2 expression6,7. BMP signaling depends on the SMAD1/5/8 pathway to stimulate the expression of typical cartilage hypertrophic markers, such as COL10A1, MMP13 and ALPL during chondrogenesis of mesenchymal stem cells (MSCs) and termimal differentiation of primary chondrocytes8. TGF-β is an effective inducer of chondrogenesis9,10 by stimulating chondrocyte proliferation while inhibiting chondrocyte hypertrophy and maturation in vitro11,12. BMPs, such as BMP2 and BMP4, are potent regulators of chondrocyte hypertrophy and matrix degradation13,14. In contrast, BMP7 distinctly stimulates chondrocyte proliferation and inhibits chondrocyte hypertrophy15–17.
There are a few studies that have shown the combinationatorial effect of TGF-β and BMPs on chondrogenesis18–20. However, all these studies have been performed in normoxia (21% O2) which is a supra-physiological oxygen concentration compared to the much lower oxygen tension in articular cartilage in vivo. Articular cartilage is a typical avascular tissue, and chondrocytes normally reside in a low oxygen environment21. Hypoxia has a positive influence on the chondrocyte phenotype and has been shown to stimulate cartilage matrix formation22,23. Furthermore, most studies have focused on chondrogenic differentiation of MSCs from different sources with treatment of BMPs or TGF-βs. Few studies have focused on the redifferentiation of chondrocytes under hypoxic conditions in the presence of these factors. The aim of this study was to identify the effects of exogenous BMP7 and TGF-β3 on the redifferentiation of osteoarthritic chondrocytes after expansion in monolayer, using an aggregate pellet culture under low oxygen, and to explore the possibility of using the combination of hypoxia with TGFβ and BMP7 to stimulate chondrocyte redifferentiation.
Materials and Methods
Cartilage samples collection
The collection and use of human cartilage was approved by the local hospital ethical committee (De Medisch Ethische Toetsingscommissie (METC) TWENTE) and for all samples informed written consent was obtained. Cartilage specimens were isolated from 12 patients (mean age ± SD: 68 ± 6 years) with osteoarthritis undergoing total knee replacement surgery. In order to get comparable cartilage samples, several cartilage pieces were removed from the same joint and for each of the specimens a histological cartilage score was determined as previously described24.
Cell culture and expansion
Human primary chondrocytes (hChs) were obtained from macroscopically healthy looking full thickness cartilage, dissected from knee biopsies of four OA patients (mean ± SD age 60 ± 3 years) undergoing total knee replacement, as described in25,26. hChs were used at passage 3 in this study.
Pellet cultures and chondrogenic differentiation
Pellets consisting of 2.5 × 105 cells were cultured for 3 weeks at 37 °C, 5% CO2, 21% O2 (for nomoxia) or 2.5% O2 (for hypoxia), in chondrogenic differentiation medium as described27. The medium was supplemented with 10 ng/mL TGF-β3 or 100 ng/ml BMP7 (R&D). The medium was refreshed twice per week.
Total RNA extraction and quantitative polymerase chain reaction (qPCR)
Cell pellets were crushed and RNA was isolated using Trizol reagent (Thermo Fisher Scientific). The concentration and purity of RNA samples were determined using the Nanodrop 2000 (Thermo scientific). Total mRNA was reverse-transcribed into cDNA using the iScript cDNA Synthesis kit (Bio-Rad). qPCR was performed as described28.
Alcian blue and Safranin O staining
Cartilage samples were fixed in 10% phosphate buffered formalin (pH = 7) overnight at room temperature (RT), dehydrated with a graded ethanol series and embedded in paraffin using routine procedures. Sections were cut at 5 μm thickness using a microtome (Shandon). Cell pellets samples were collected after 3 weeks of incubation and fixed with 10% buffered formalin. Sections of 7 μm thickness were directly cut using a cryotome (Shandon) after embedding in cryomatrix. Slides were stained as described24,29.
Immunofluorescent (IF) staining for collagen type II and collagen type X
Immunofluorescent staining of collagen type II and collagen type X was performed using 7 μm sections from pellets. Slides were stained as described26 using Rabbit anti-human collagen type II antibody (ab34712, Abcam) or mouse anti-human collagen type X antibody (BIOCYC GmbH & Co. KG, Cat. No. 2031501005). Slides were viewed by BD pathway confocal microscopy.
Immunohistochemistry (IHC) of BMP7 and SMAD 1/5/8 in cartilage or pellets
Immunohistochemistry staining of BMP7 and SMAD 1/5/8 for cartilage samples was performed using 5 μm sections, and the staining of SMAD 1/5/8 for pellet samples using 7 μm sections. The procedure was performed as described24. Mouse anti-human BMP7 (PeproTech) and mouse anti-human SMAD 1/5/8 antibody (Santa Cruz biotechnology) were used in this study. Images were taken using a Nanozoomer (Iwata City, Japan).
EdU and TUNEL staining
For labeling of newly synthesized DNA in proliferating cells, EdU (5-ethynyl-2′-deoxyuridine) was added to the culture media at a concentration of 10 μM, 24 hours before harvesting the samples. Cell pellets were embedded in cryomatrix after fixing, and were cut into 7 μM sections by cryotome (Shandon). Sections were permeabilized and stained for EdU with Click-iT® EdU Imaging Kit (ThermoFisher scientific). Cryo-sections were also stained for DNA fragments with DeadEnd Fluorometric TUNEL System (Promega). Nuclei were counterstained with Hoechst 33342.
GAG and DNA assays
After redifferentiation, pellets were digested and GAG was measured as previously described30. Relative cell number was determined by quantification of total DNA using a QuantiFluor® dsDNA System kit (Promega), according to the manufacturer’s instructions.
Enzyme- linked immunosorbent assay (ELISA)
The supernatant of pellet cultures was collected every week. The content of MMP1 dissolved in medium was measured by ELISA using a mouse anti-human MMP1 antibody (MAB901-SP, R&D systems) as described28.
Statistical analysis
Statistical differences between two groups were analyzed by two-tailed student’s t-tests or one-way ANOVA. P < 0.05 was considered statistically significant and indicated with an asterisk. Data are expressed as the mean ± SD.
Results
The expression of BMP7 and SMAD1/05/08 decreases in early OA while it increases in late stages of OA
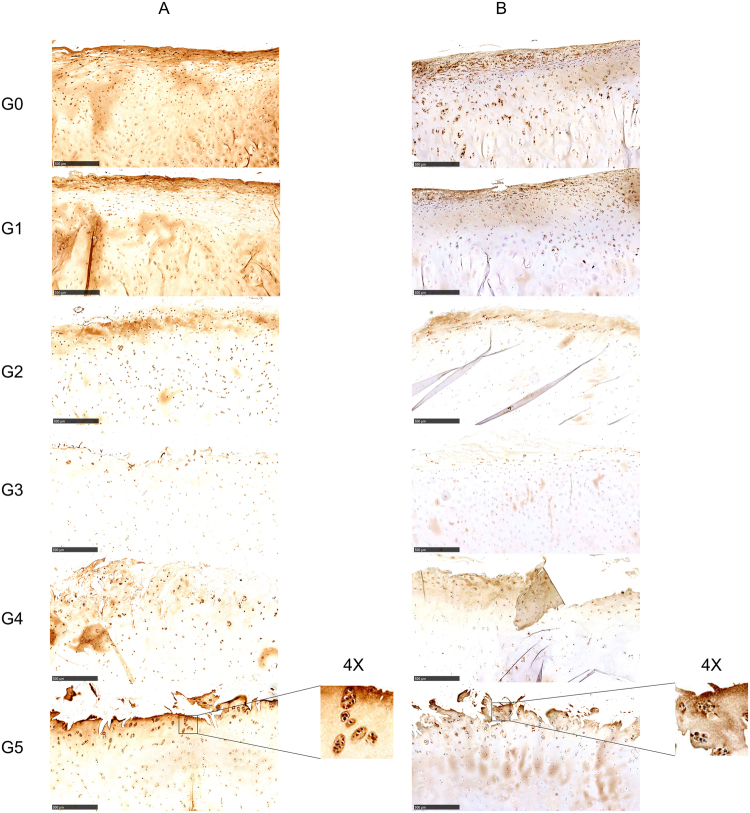
To better characterize the BMP7/SMAD signaling pathway and its involvement in cartilage degeneration, we performed IHC to detect protein expression of BMP7 and its downstream target SMAD1/5/8 in OA cartilage. The expression of BMP7 (Fig. 1A) distinctly decreased with increasing severity from 0 to 3 in the early OA development, while it was increased from grade 3 to 5. BMP7 was mainly expressed in the matrix of the superficial layer and highly expressed inside chondrocytes. BMP7 expression was decreased in the middle layer and deep layers, however, it was increased again in hypertrophic chondrocytes. In the early OA stages from grade 0 to 2, positive staining of BMP7 was observed in the whole cartilage section, high to low from the superficial layer to the deep layer. At grade 3, intensity of BMP7 staining was decreased. BMP7 was only detected in chondrocytes in the superficial layer and in hypertrophic chondrocytes in the deep layer. Interestingly, in cartilage specimens with OA stage 4 and 5, and especially in grade 5 specimens, BMP7 was highly expressed in cell clusters, which is a typical characteristic of late OA. The expression of BMP7 was measured by IHC in each patient (Supplemental Fig. 1).
Figure 1.
The protein expression of BMP7 and SMAD1/5/8 in human cartilage was visualized by IHC in each donor. The expression of BMP7 (A) and SMAD 1/5/8 (B) was visualized in differentially graded cartilage specimens of OA patients. Representative pictures are shown. Images were taken using the Nanozoomer (scale bar = 500 μm). G0, G1, G2, G3, G4, G5 = OARSI Grade 0, Grade 1, Grade 2, Grade 3, Grade 4, Grade 5. Magnified pictures were indicated in inserts.
SMAD1/5/8 expression showed a similar trend as BMP7 (Fig. 1B). Its expression gradually declined from grade 0 to 3, and increased from grade 3 to 5. SMAD1/5/8 was mainly present in the chondrocytes of the superficial layer, even in the relatively healthy cartilage (grade 0 and 1). In stage 2 to 4 OA specimens, positive staining of SMAD1/5/8 was hard to observe in the chondrocytes of the middle layer and in hypertrophic chondrocytes of the deep layer. However, at stage 5, SMAD1/5/8 was strongly expressed in the top layer of the cartilage and especially in the chondrocyte clusters. The expression of SMAD1/5/8 was measured by IHC in each patient (Supplemental Fig. 2).
Hypoxia enhanced matrix formation of OA chondrocytes with TGF-β3 or BMP7 treatment
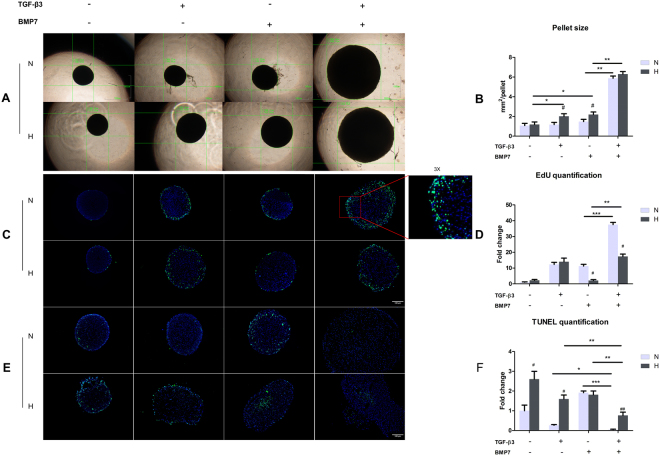
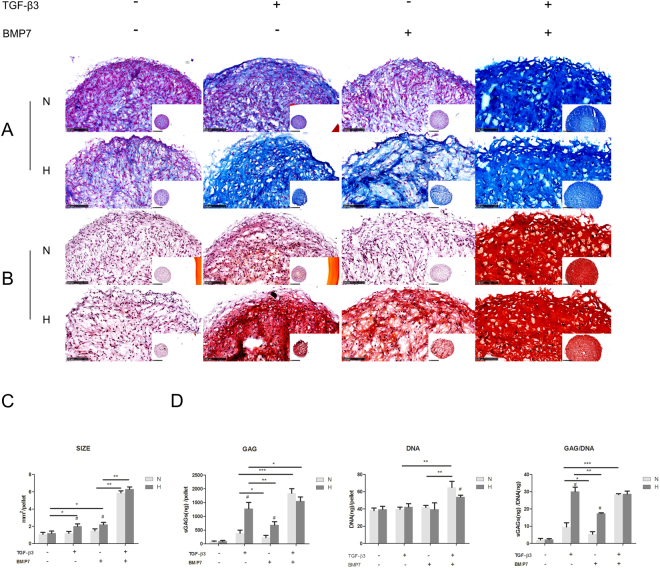
In 3D pellet cultures, the sizes of the pellets treated with either TGF-β3 or BMP7 under hypoxia were obviously larger than the pellets cultured under normoxia (Fig. 2A,B). There was no significant difference between normoxia and hypoxia in the TGF-β3 and BMP7 treated groups. To clarify whether the enhanced pellet size was due to cell proliferation or to matrix production, EdU proliferation assays, Alcian blue and Safranin O staining, and GAG assays were performed. As shown in Fig. 2C,D, the proliferation ratio after treatment with TGF-β3 did not change between normoxia and hypoxia, while BMP7 treated pellets under hypoxia presented less positive Edu staining than pellets in normoxia. Moreover, treatment of TGF-β3 or BMP7 pellets of hypoxia showed lower cell density than pellets cultured in normoxia, as can be derived from the DAPI staining. TUNEL assay showed that the amount of apoptotic cells was increased in hypoxia as compared to normoxia for TGF-β3 treatment and that no difference was observed for the BMP7 treated group (Fig. 2E,F). However, GAG deposition was increased in hypoxia after treatment with TGF-β3 or BMP7 (Fig. 3A,B). This was confirmed by GAG assays (Fig. 3C). Although we did not observe a difference in the amount of DNA between hypoxia and normoxia, GAG production was up to approximately 3 fold higher in hypoxia than in normoxia after treatment with TGF-β3 or BMP7. These observations indicated that the enlarged pellet size in hypoxia after treatment with TGF-β3 or BMP7 was mainly due to increased matrix production rather than increased cell proliferation.
Figure 2.
Cell proliferation and apoptosis in OA pellets. (A) Pellets imaged by light microscopy. Scale bar = 1000 μm. (B) Measurement of pellet size. Data represents the mean of three pellets in each group. #Indicates a significant difference between normoxia and hypoxia; *represents P < 0.05; **represents P < 0.01; ***represents P < 0.001. Error bar represents Standard Deviation (S. D.). (C) EdU staining of pellets. EdU incorporation into newly synthesized DNA was visualized by Alexa 488 (green). Nuclei were counterstained with Hoechst 33342 (blue). Scale bar = 100 μm. (D) Quantification of EdU positive chondrocytes. (E) TUNEL staining of pellets. TUNEL positive cells were visualized with fluorescent labeling (green). Nuclei were counterstained with Hoechst 33342 (blue). Scale bar = 100 μm. (F) Quantification of TUNEL positive chondrocytes. N, normoxia; H, hypoxia; #Indicates a significant difference between normoxia and hypoxia; *represents P < 0.05; **represents P < 0.01; ***represents P < 0.001. Error bar represents Standard Deviation (S. D.).
Figure 3.
GAG production in pellets. (A) Alcian blue staining, nuclei were counterstained by nuclear fast red; (B) Safranin O staining, nuclei were counterstained by haematoxylin; (C) GAG and DNA assay. N, normoxia; H, hypoxia; *p < 0.05; **p < 0.01; #represents a significant difference between normoxia and hypoxia (p < 0.05).
Synergistic effect of TGF-β3 and BMP7 on chondrocytes redifferentiation
As Fig. 2A,B shows, treatment with TGF-β3 or BMP7 alone did not significantly increase the pellet size in normoxia, which is in contrast to pellets treated in hypoxia. Interestingly, the combined treatment significantly increased the pellet size compared to the single treatments. The effect was more pronounced in normoxia than in hypoxia. EdU staining indicated that the treatment of TGF-β3 plus BMP7 greatly promoted cell proliferation in pellets in normoxia, while only a modest increase was found in hypoxia when compared to the TGF-β3 or BMP7 treated groups. In normoxia, BMP7 treated pellets showed more apoptotic cells. TGF-β3 increased the number of apoptotic cells in hypoxia as compared to nomoxia. Remarkably, co-treatment effectively decreased the number of apoptotic cells in both normoxia and hypoxia (Fig. 2E,F). Alcian blue (Fig. 3A) and safranin O staining (Fig. 3B) showed that TGF-β3 or BMP7 treatment dramatically enhanced GAG production in hypoxia, while this effect was not observed in normoxia. However, co-stimulation of TGF-β3 and BMP7 significantly increased GAG deposition both in normoxia and hypoxia. This similar trend was observed in the GAG assays, with the exception of the GAG/DNA content in the co-treatment with TGF-β3 plus BMP7 in hypoxia, which was not higher when compared to the treatment with TGF-ß3 alone in hypoxia. (Fig. 3C).
Anabolic and catabolic gene expression profiles of OA chondrocytes treated with TGF-β3 and/or BMP7
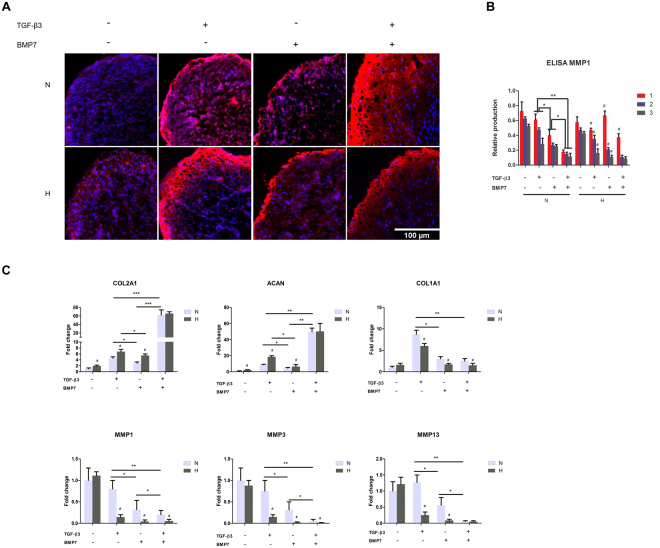
Next, we measured the expression and the distribution of type II collagen by immunofluorescence. Chondrocyte pellets treated with BMP7 or TGF-β3 alone increased the expression of type II collagen as compared to control (Fig. 4A) in normoxia. The most pronounced effect was found in the co-treatment group. In hypoxia, TGF-β3 was more effective in increasing the expression of type II collagen than BMP7. Co-treatment did not have an additive effect. The expression of collagen type X was also measured by immunofluorescence. As can be seen in Supplemental Fig. 3, TGF-β3 slightly induced the type-X collagen expression in normoxia only, BMP-7 alone did not induce collagen X expression. Both hypoxia as well as co-treatment with BMP-7 in normoxia effectively counteracted the induction of collagen X by TGF-β3.
Figure 4.
The anabolic and catabolic protein and gene expression profiles of OA chondrocytes after treatment with TGF-β3 and/or BMP7 in normoxia and hypoxia. (A) Measurement of type II collagen expression by immunofluorescence. Type II collagen was detected by rabbit anti-type II collagen antibody, followed by anti-rabbit secondary antibody coupled to Alex 564. Fluorescent images were taken by BD pathway confocal microscopy. Cell nuclei were counterstained with DAPI. Scale bar = 100 μm. (B) Secreted MMP1 was measured by ELISA. The numbers 1, 2, and 3 represent 1 week, 2 week, 3 week, respectively. (C) Gene expression of ACAN, COL2A1, COL1A1, MMP1, 3, 13 was measured by Q-PCR. N, normoxia; H, hypoxia; *p < 0.05; **p < 0.01; #indicates a significant difference with corresponding ones between normoxia and hypoxia (p < 0.05).
To measure MMP1 secretion, medium was collected every week and MMP1 was measured by ELISA. The expression of MMP1 gradually decreased in the order of control, TGF-β3, BMP7, TGF-β3 + BMP7 in normoxia (Fig. 4). In hypoxia, the same trend was observed with the exception that MMP1 expression was relatively high in the first week as compared to in the pellets of this time-point in normoxia.
The anabolic and catabolic gene expression profiles were measured by RT-Q-PCR. The cartilage markers ACAN and COL2A1 showed similar expression trends. Pellets with TGF-β3 treatment showed higher ACAN and COL2A1 expression than BMP7 treatment. Co-treatment of BMP7 and TGF-β3 showed the highest expression of ACAN and COL2A1 both in normoxia and hypoxia. The expression of ACAN and COL2A1 is higher in hypoxia than in normoxia irrespective of the treatment with either TGF-β3 or BMP7. Catabolic genes, such as MMP1, 3, 13 showed opposite trends compared to the anabolic genes. Their expression gradually decreased in the order of TGF-β3, BMP7, TGF-β3 + BMP7 both in normoxia and hypoxia. The combined group of BMP7 and TGF-β3 showed the lowest catabolic gene expression compared to single treatments. Catabolic gene expression in normoxia was higher than in the counter treatments cultured in hypoxia. This effect was less pronounced in the TGF-β3 and BMP7 co-treatment groups. The expression trend of the dedifferentiation marker COL1A1 was similar to that of the catabolic genes, except for the negative control group (no TGF-β3 and no BMP7), which showed extremely low expression of COL1A1.
SMAD 1/5/8 signaling in OA chondrocytes with treatment of TGF-β3 and BMP7
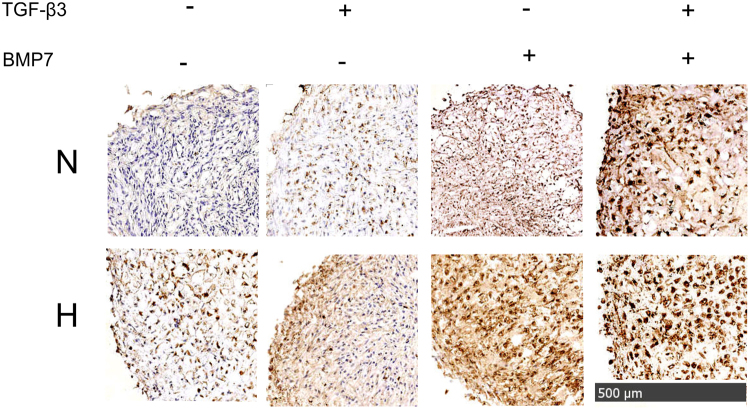
We next examined the translocation of SMAD 1/5/8 to the nucleus upon treatment with TGF-β3, BMP7 or in co-treatment in normoxia and hypoxia. The strongest positive staining was observed in the co-treatment group compared to the single treatments both in normoxia and hypoxia (Fig. 5). Stronger positive nuclear staining of SMAD 1/5/8 was observed in hypoxia than in normoxia.
Figure 5.
The expression of SMAD 1/5/8 in pellets in normoxia and hypoxia after treatment with TGF-β3 and BMP7. Protein expression of SMAD 1/5/8 was detected by mouse anti-SMAD 1/5/8 primary antibody, followed by a biotinylated anti-mouse secondary antibody followed by signal development using Horse Radish Peroxidase-Streptavidin. For visualization, DAB substrate kit was used. Images were taken by Nanozoomer. Nuclei were counterstained with haematoxylin. Scale bar = 500 μm. N, normoxia; H, hypoxia.
Discussion
Although intra-articular injection of BMP7 has been evaluated in clinical trials for the treatment of OA, only few studies have focused on the expression and role of endogenous BMP7 in human cartilage. To the best of our knowledge, this study has evaluated for the first time the protein expression of endogenous BMP7 and its intracellular signaling target SMAD 1/5/8 in different grades of OA in human cartilage. We show that BMP7 and SMAD1/5/8 are predominantly present inside cells and in the surrounding pericellular matrix of chondrocytes in the superficial and upper middle layers, and in chondrocytes of the transitional layer. Their expression initially decreased in early OA from grade 0 to 3 and then started to increase again from grade 3 to 5. At grade 5, BMP7 was especially highly expressed in chondrocyte clusters. Bobinac and his colleagues also found that BMP7 is strongly expressed in chondrocyte clusters31. Given the anabolic role of BMP7 in cartilage homeostasis, increased expression in chondrocyte clusters may be considered as a reparative response of the tissue. The expression of SMAD 1/5/8 followed the pattern of BMP7 expression albeit at a lower level.
Chondrocytes are the only cell type responsible for maintaining the surrounding matrix by synthesizing cartilage specific matrix proteins such as type II collagen and aggrecan32. Autologous Chondrocyte Implantation (ACI) has proven to be a pivotal approach as restorative cartilage surgery. However, even the most recent ACI techniques have some limitations33. It has been shown that the transplanted chondrocytes are able to form new cartilage, which appears to be of hyaline nature and expresses typical cartilage markers such as type II collagen, aggrecan and COMPs. However type I collagen and fibrous cartilage matrix are also detected in the newly formed tissue after two years34,35. There are plenty of cytokines and growth factors in the cartilage or synovial fluid to modulate the balance between the synthesis and degradation of ECM, or the balance between anabolism and catabolism. BMP/TGF-β signaling is extremely important in chondrocyte development. TGF-β induces collagen II and SOX9 deposition through SMAD2/3 phosphorylation pathway, while BMP7 promotes chondrocyte matrix production and inhibits chondrocyte hypertrophy via SMAD1/5/8 phosphorylation. The balance between SMAD 2/3 and SMAD 1/5/8 signaling, which are activated by TGF-β and BMP7 play a key role in cartilage homeostasis36–38. During ACI surgery, chondrocytes are isolated from a macroscopically healthy looking area in the joint, which may have limited matrix production capacity and the expansion process further reduces this matrix production potential. Therefore, to enable effective cartilage repair, efficient redifferentiation before or after implantation into the damaged area is a prerequisite.
BMP7 and TGF-β are important for the maintenance of cartilage homeostasis, and the loss of their expression could contribute to the development of OA. In this research, we mainly focus on the function of BMP7 and TGF-β in chondrocyte redifferentiation in 3D pellet cultures after expanding in monolayer. To do this, we cultured the pellets in serum free chondrogenic differentiation medium supplemented with TGF-β3 or BMP7 or both. In vivo, cartilage is exposed to a low oxygen tension. Despite this, chondrocytes are still cultured in normoxia in most labs. To study the effect of oxygen tension on the chondrocyte’s response to these growth factors, we compared the effects of growth factors on chondrocyte redifferentiation in both hypoxia (2.5% O2) and normoxia (21% O2). Our results indicated that TGF-β3 or BMP7 alone were not as efficient in redifferentiating chondrocytes as compared to the combined treatment with TGF-β3 and BMP7 both in normoxia and hypoxia. Alcian blue and Safranin O staining, GAG assay, type II collagen staining and gene expression analysis showed the same trend that the combined treatment with TGF-β3 and BMP7 positively influenced redifferentiation of OA chondrocytes. Previously, it was reported that BMP7 inhibited hMSC growth in expansion cultures39. We observed that the combination of BMP7 and TGF-β3 significantly promoted cell proliferation. However, the observed enlarged pellet size was mainly due to increased extracellular matrix production, with less tightly packed cells in the pellets.
In hypoxia, in the first week, MMP1 expression was relatively higher in the pellets treated with BMP7 alone or combined with TGF-β3, and lower in the TGF-β3 only treatment as compared to normoxia. This indicates that chondrocytes are more sensitive to the BMP7 stimulation in hypoxia in the early stages of redifferentiation. In this study, higher SMAD 1/5/8 expression was observed in hypoxia than in normoxia in all treatment conditions. While the expression of SMAD 1/5/8 was higher when BMP7 was present, the highest expression was observed in the combined treatment of BMP7 and TGF-β3 in hypoxia. Lafont and his colleagues reported that hypoxia inhibited BMP2 induced SMAD signaling in monolayer cultures of human articular chondrocytes40. These observations indicate that hypoxia modulates the sensitivity of the chondrocytes for BMP signaling.
The mechanism of the additive effects of BMP7 and TGF-β3 on chondrocyte redifferentiation and the influence of oxygen tension on this mechanism is still not clear. It might be that TGF-β3 upregulates the expression of BMP receptors in chondrocytes, which in turn promotes the cellular response to BMP7. It has been reported that TGF-β3 mediates the upregulation of the type I BMP receptor, ALK6, through which BMP7 and other BMPs are recruited to stimulate signal transduction in human MSCs41. Furthermore, it has been reported that the TGF-β3 receptor can also function as a BMP receptor by binding multiple members of the BMP subfamily including BMP742. The mutual regulation or receptor activation may amplify and enhance the effects of TGF-β3 and BMP7.
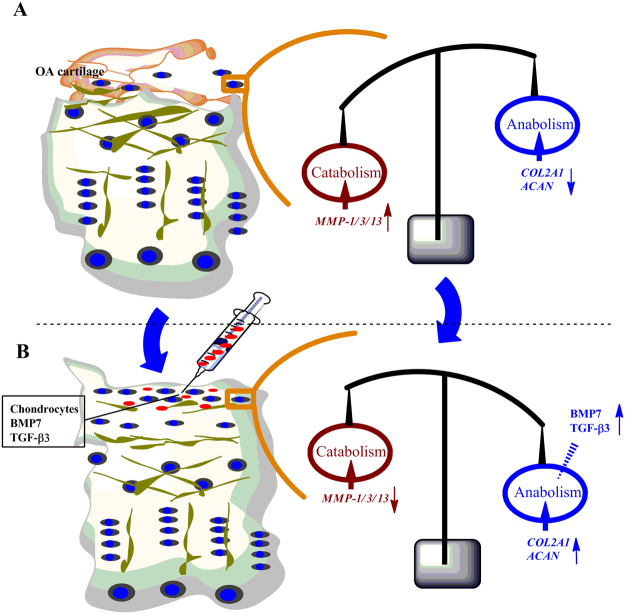
To the best of our knowledge, this is the first study using human OA chondrocyte to study the potential application of BMP7 and TGF-β in cartilage tissue regeneration in 3D-cultured pellets under hypoxia. As Fig. 6 shows, the combination of TGF-β3 and BMP7 might readjust the balance between catabolism and anabolism by upregulating anabolic activities and downregulating catabolic activities in the treatment of osteoarthritic cartilage. Taken together, the combined treatment of BMP7 and TGF-β3 was superior in chondrocyte redifferentiation compared to single treatments both in normoxia and hypoxia. This study also indicate that a balanced combination of cytokines or growth factors in combination with an appropriate oxygen tension is required for efficient chondrocyte redifferentiation in pellet cultures.
Figure 6.
The schematic diagram of OA treatment by TGF-β3 and BMP7. (A) Osteoarthritic cartilage with a degenerative superficial layer and loss of chondrocytes shows higher catabolism, reflected by elevated MMP-1/3/13 expression; (B) After treatment with the combination of TGF-β3 and BMP7, the superficial layer with chondrocytes with newly gained energy may exhibit higher anabolism, reflected by increased expression of COL2A1 and ACAN.
Electronic supplementary material
Author Contributions
Xiaobin Huang and Leilei Zhong performed the experiments. All authors analyzed the data and contributed to writing the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27602-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brittberg M, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Cournil-Henrionnet C, et al. Phenotypic analysis of cell surface markers and gene expression of human mesenchymal stem cells and chondrocytes during monolayer expansion. Biorheology. 2008;45:513–526. [PubMed] [Google Scholar]
- 3.Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue engineering. Part B, Reviews. 2014;20:641–654. doi: 10.1089/ten.teb.2014.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, et al. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. The Journal of cell biology. 2001;153:35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li TF, et al. Smad3-deficient chondrocytes have enhanced BMP signaling and accelerated differentiation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006;21:4–16. doi: 10.1359/JBMR.050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. The Journal of biological chemistry. 2005;280:8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 7.Kang JS, Alliston T, Delston R, Derynck R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. The EMBO journal. 2005;24:2543–2555. doi: 10.1038/sj.emboj.7600729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellingman CA, et al. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue engineering. Part A. 2011;17:1157–1167. doi: 10.1089/ten.tea.2010.0043. [DOI] [PubMed] [Google Scholar]
- 9.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental cell research. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 10.Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. Journal of cellular physiology. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 11.Song JJ, et al. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. Journal of cellular physiology. 2007;210:398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- 12.Tuli R, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. The Journal of biological chemistry. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, et al. BMP-2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. Journal of cellular biochemistry. 2009;108:896–905. doi: 10.1002/jcb.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retting KN, Song B, Yoon BS, Lyons KM. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development (Cambridge, England) 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caron MM, et al. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis Cartilage. 2013;21:604–613. doi: 10.1016/j.joca.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthritis and cartilage. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Haaijman A, et al. Inhibition of terminal chondrocyte differentiation by bone morphogenetic protein 7 (OP-1) in vitro depends on the periarticular region but is independent of parathyroid hormone-related peptide. Bone. 1999;25:397–404. doi: 10.1016/S8756-3282(99)00189-1. [DOI] [PubMed] [Google Scholar]
- 18.Shintani N, Siebenrock KA, Hunziker EB. TGF-ss1 enhances the BMP-2-induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PloS one. 2013;8:e53086. doi: 10.1371/journal.pone.0053086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen B, Wei A, Tao H, Diwan AD, Ma DD. BMP-2 enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue engineering. Part A. 2009;15:1311–1320. doi: 10.1089/ten.tea.2008.0132. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto C, Matsumoto T, Sakimura K, Shindo H. Osteogenic protein-1 with transforming growth factor-beta1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J Orthop Sci. 2007;12:555–561. doi: 10.1007/s00776-007-1176-4. [DOI] [PubMed] [Google Scholar]
- 21.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1975;271:261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 22.Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012;417:305–310. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 23.Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J. Biol. Chem. 2006;281:31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- 24.Zhong, L., Huang, X., Karperien, M. & Post, J. N. Correlation between Gene Expression and Osteoarthritis Progression in Human. Int J Mol Sci17, 10.3390/ijms17071126 (2016). [DOI] [PMC free article] [PubMed]
- 25.Nazem K, et al. Treatment of full thickness cartilage defects in human knees with Autologous Chondrocyte Transplantation. J Res Med Sci. 2011;16:855–861. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong L, et al. Endogenous DKK1 and FRZB Regulate Chondrogenesis and Hypertrophy in Three-Dimensional Cultures of Human Chondrocytes and Human Mesenchymal Stem Cells. Stem Cells Dev. 2016;25:1808–1817. doi: 10.1089/scd.2016.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Leijten J, Georgi N, van Blitterswijk CA, Karperien M. Trophic Effects of Mesenchymal Stem Cells Increase Chondrocyte Proliferation and Matrix Formation. Osteoarthritis and cartilage. 2011;19:S105–S105. doi: 10.1016/S1063-4584(11)60240-9. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, et al. Promoted Chondrogenesis of Cocultured Chondrocytes and Mesenchymal Stem Cells under Hypoxia Using In-situ Forming Degradable Hydrogel Scaffolds. Biomacromolecules. 2018;19:94–102. doi: 10.1021/acs.biomac.7b01271. [DOI] [PubMed] [Google Scholar]
- 29.Leijten J, et al. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci USA. 2014;111:13954–13959. doi: 10.1073/pnas.1410977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendriks JAA, et al. Primary chondrocytes enhance cartilage tissue formation upon co-culture with a range of cell types. Soft Matter. 2010;6:5080–5088. doi: 10.1039/c0sm00266f. [DOI] [Google Scholar]
- 31.Bobinac D, et al. Expression of bone morphogenetic proteins, cartilage-derived morphogenetic proteins and related receptors in normal and osteoarthritic human articular cartilage. Coll Antropol. 2008;32(2):83–87. [PubMed] [Google Scholar]
- 32.Spreafico A, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J. Cell. Biochem. 2009;108:1153–1165. doi: 10.1002/jcb.22344. [DOI] [PubMed] [Google Scholar]
- 33.Bernardini G, Chellini F, Frediani B, Spreafico A, Santucci A. Human platelet releasates combined with polyglycolic acid scaffold promote chondrocyte differentiation and phenotypic maintenance. J Biosci. 2015;40:61–69. doi: 10.1007/s12038-014-9492-2. [DOI] [PubMed] [Google Scholar]
- 34.Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. The American journal of sports medicine. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 35.Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. European journal of radiology. 2006;57:24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Blaney Davidson EN, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J. Immunol. 2009;182:7937–7945. doi: 10.4049/jimmunol.0803991. [DOI] [PubMed] [Google Scholar]
- 37.van der Kraan PM, Blaney Davidson EN, van den Berg WB. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12:201. doi: 10.1186/ar2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Kroon LM, et al. Activin Receptor-Like Kinase Receptors ALK5 and ALK1 Are Both Required for TGFbeta-Induced Chondrogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. PLoS One. 2015;10:e0146124. doi: 10.1371/journal.pone.0146124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen B, et al. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2010;109:406–416. doi: 10.1002/jcb.22412. [DOI] [PubMed] [Google Scholar]
- 40.Lafont JE, Poujade FA, Pasdeloup M, Neyret P, Mallein-Gerin F. Hypoxia potentiates the BMP-2 driven COL2A1 stimulation in human articular chondrocytes via p38 MAPK. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2016;24:856–867. doi: 10.1016/j.joca.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Xu D, et al. Potential involvement of BMP receptor type IB activation in a synergistic effect of chondrogenic promotion between rhTGFbeta3 and rhGDF5 or rhBMP7 in human mesenchymal stem cells. Growth Factors. 2006;24:268–278. doi: 10.1080/08977190601075865. [DOI] [PubMed] [Google Scholar]
- 42.Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. Bone morphogenetic proteins signal through the transforming growth factor-beta type III receptor. J. Biol. Chem. 2008;283:7628–7637. doi: 10.1074/jbc.M704883200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.