Abstract
T cell exclusion from cancers is a major problem for a proportion of patients suffering poor outcomes. Two papers recently published in Nature highlight the negative role of cancer associated fibroblast TGFβ. These studies indicate that TGFβ enables T cell exclusion in a proportion of colorectal and urothelial cancers, and in the latter disease may reduce their response to anti-PD-L1 immunotherapy.
Subject terms: Cancer microenvironment, Tumour immunology
Main
Transforming growth factor beta (TGFβ) is believed to have a pro-tumour role in advanced cancers by promoting fibroblast activation, desmoplasia, angiogenesis, immunosuppression, epithelial-to-mesenchymal transition, and metastasis,1–3 and is associated with poor prognosis in many cancer types.1, 4, 5 In a recent issue of Nature, two papers6, 7 have described a link between stromal TGFβ-signalling and T-cell exclusion from mouse and human tumours and showed that this can be inhibited to improve therapeutic outcomes. These studies, and other earlier studies, firmly indicate that inhibition of TGFβ at the tumour-stromal interface is likely to improve the efficacy of existing cancer immunotherapies in a broad range of patients.
Most human solid tumours exhibit distinct immunological phenotypes: one classification system denotes three types (immune inflamed, immune excluded, and immune desert);8 another system describes four (PD-L1 active TIL+, PD-L1 inactive TIL+, PD-L1 active TIL− and PD-L1 inactive TIL−).9 Prior evidence, mainly from melanomas, has suggested that inflamed tumours (PD-L1 active TIL+) are the most responsive to immune checkpoint blockade.8–10 Therapeutic antibodies that block the PD-1-PD-L1 pathway in inflamed tumours may induce durable responses in patients with various cancers; however, only a proportion of these patients see a response. Determining the mechanisms of resistance and response is key to improving outcomes and developing new therapeutic approaches. New immunotherapies are largely predicated on the anti-tumour effector functions of T cells. However, a large fraction of tumour types actively exclude T cells, and an even greater proportion of cancers have no T cells, when “T cell ignorant, cold, or T cell deserted” tumours are included. Therapeutically rectifying this represents one of the greatest challenges for T cell-based cancer immunotherapies today.
In the study by Tauriello et al, the authors analysed the relationship between gene alterations and the tumour microenvironment in colorectal cancer.6 Microsatellite stable (MSS) colorectal carcinomas (CRCs) were previously hypothesised to be immunologically ‘cold’, and therefore unlikely to benefit from immunotherapies.8 Lack of T-cell infiltration, low type 1 T-helper cell (TH1) activity, reduced immune cytotoxicity and increased TGFβ levels in the tumour microenvironment (TME) have been associated with adverse outcomes in CRC patients. Furthermore, progression of CRC most often correlates with changes in four signalling pathways: WNT, EGFR, p53 and TGFβ. Therefore, the authors of this study generated mice lacking the following conditional alleles: Apcfl/fl, KrasLSL-G12D, Tgfbr2fl/fl and Trp53fl/fl (abbreviated A, K, T and P, respectively) in intestinal stem cells using the Lgr5eGFP-creERT2 driver (abbreviated L). Most LAKTP mice developed cancers, with more than half the tumours breaching all intestinal layers, spreading to the lung and liver and resembling the features of human CRCs, including low mutation burden, T-cell exclusion, and a stroma with high levels of TGFβ-activity. Interestingly, T-cells significantly infiltrated the stroma of adenomas and normal mucosa but were mostly excluded from neighbouring invasive cancers. These new mouse models of genetic alterations in CRC will prove useful in studying the tumour microenvironment.
The authors went on to collect samples from these mouse tumours or metastases to create a biobank of mouse tumour organoids (MTO). They utilised these organoids in vitro, and by intra-caecum inoculation in vivo, to show four effects: 1) in vitro MTOs (LAKTP) expanded independently of factors that activate TGFβ, WNT and EGF pathways; 2) MTOs engrafted in 30% of mice and progressed to fully invasive tumours, with 40% spreading to the liver; 3) triple- and quadruple-mutant MTOs recapitulated features of human MSS CRCs, for example, exhibiting similar mutational burden and predicted high-affinity MHC class I-binding neoantigens; 4) obvious T-cell exclusion and increased TGFβ activity was observed in the tumour microenvironment. These findings also suggested that stromal gene expression was able to identify molecular subtypes with poor prognosis. The authors observed an enhanced metastatic burden in immune-deficient (nu/nu) mice compared with immune-competent mice, indicating that the cancers were sensitive to T-cell-mediated immune control. Furthermore, the micrometastases were characterised by many T-cells amongst the tumour cells, which were progressively excluded over time. Unfortunately, Tauriello et al did not perform in-depth immunological analysis on the transgenic tumour-prone mice; the immune history of the developing malignancy would be important. However, the MTOs represent a useful resource.
Tauriello and colleagues have shown in their study that a TGFβ-activated TME excludes T lymphocytes from tumours and metastases, a finding associated with a poor outcome across several cancer types.11, 12 They also corroborated their mouse data by analysing transcriptomic datasets of human MSS CRC samples (n = 981). Strikingly, the ratio of TH1 to naive T cells was inversely correlated with the mean expression of TGFB genes or a gene signature specific to cancer-associated fibroblasts (CAF), and predicted disease relapse in MSS CRC patients. Profiling of cell populations in mouse or human CRC samples uncovered that CAFs were the major source of TGFβ. Therefore, inhibited T-cell differentiation, upregulated TGFβ and CAF gene signatures characterised a significant subset of MSS CRC patients with a worse prognosis.
In a complementary article, Mariathasan et al. examined the transcriptome of 298 metastatic urothelial cancer samples from patients treated with an anti-PD-L1 antibody (atezolizumab), to identify novel indicators of clinical outcome.7 Metastatic urothelial cancer is characterised by a high somatic mutation rate, and tumour mutation burden is known to correlate with response to immunotherapy13. Mariathasan et al. confirmed that clinical outcome was associated with a CD8+ effector T-cell phenotype, tumour mutation burden, and neoantigen frequency. Furthermore, gene set enrichment analysis (GSEA) identified that TGFβ signalling in fibroblasts was associated with poor response. This observation was particularly relevant in patients that exhibited CD8+ T cell trapping in the fibroblast- and collagen-rich peritumoural stroma and exclusion from the tumour microenvironment; a common phenotype (47%) among metastatic urothelial cancer patients. Mariathasan et al. generated a pan-fibroblast TGFβ-response signature (F-TBRS) that was significantly associated with non-response, but only in T cell-excluded tumours. Taken together, these results suggested that metastatic urothelial cancer patient outcome of anti-PD-L1 therapy is dictated by three factors: pre-existing T-cell immunity, tumour mutation burden and TGFβ signalling in stromal cells.
The therapeutic opportunities from these two studies are obvious. The results build on previous studies that have led to the clinical development of agents such as M7824, a novel bifunctional anti-PD-L1/TGFβ-trap fusion protein (NCT02517398). Both studies, either using the MTO models or EMT6 mouse mammary carcinoma cells, demonstrated the benefit of co-targeting TGFβ and PD1-PD-L1 interactions. In the MTO CRC model, inhibition of the PD-1-PD-L1 immune checkpoint initiated a limited response, but inhibition of TGFβ (using Galunisertib) unleashed a potent anti-tumour response, even against the liver metastases. Using immunohistochemistry, it was shown that Galunisertib lowered the number of pSMAD3+ (TGFβ target) cells, and expression profiling illustrated reduced levels of TGFβ-response gene signatures in fibroblasts and T lymphocytes. In addition, Galunisertib treatment increased the recruitment, activation, proliferation and enhanced effector functions of cytotoxic T cells and TH1 cells. T cells that infiltrated liver metastases showed increased PD-1 expression associated with PD-L1+ stromal cells, which were mostly tumour-associated macrophages. Galunisertib and anti-PD-L1 antibody combination treatments induced an enhanced immune response, including increased Th1 transcription factor (T-bet) and IFNγ levels in CD4+ TH cells, and increased granzyme B production in cytotoxic T lymphocytes. This eliminated most metastases and prolonged the recurrence-free survival. Similar observations were made in the EMT6 mouse mammary carcinoma model, although antibody-mediated blockade of TGFβ alone had no effect. Mice treated with a combination of antibodies against PD-L1 and TGFβ exhibited reduced TGFβ-signalling in stromal cells, enabled T-cell penetration into the central core of tumours, strong anti-tumour immunity, and enhanced tumour regression (Fig. 1).
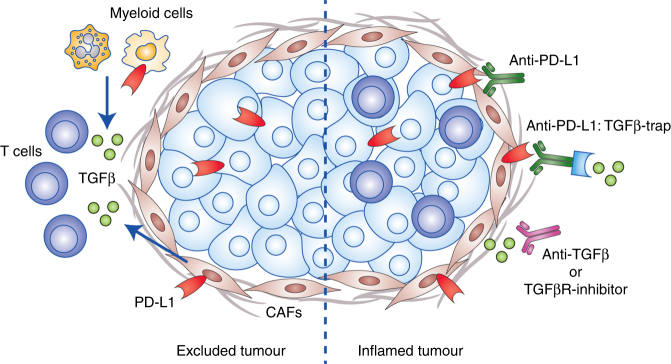
Fig. 1.
TGFβ shuts the door on T cells. Cancer associated fibroblasts (CAF) release TGFβ to exclude T cells from colorectal and metastatic urothelial cancer tumours. Co-targeting PD-L1 and TGFβ (via anti-TGFβ, TGFβR-inhibitor or TGFβ-traps) may overcome T cell exclusion and immune checkpoint function to convert excluded tumours to inflamed tumours and thus broaden the effectiveness of cancer immunotherapy
It was also interesting that deletion of Tgfbr2 in endothelial cells, leucocytes and fibroblasts inhibited metastasis by CRC MTOs. The study by Mariathasan et al. attempted to use gene signatures to suggest that fibroblasts producing TGFβ were the major host cells contributing to T cell exclusion; however, comparative specific genetic deletion of Tgfbr2 in fibroblasts would be necessary to ignore the important contributions from myeloid cells, granulocytes, and T cells. A major unanswered question now focuses on the mechanism by which TGFβ signalling blocks T cell exclusion. Some of the other T cell exclusion mechanisms involving β-catenin, Axl, STAT3, PTEN or p53 may interfere with intratumour dendritic cell function;14 however, it is not clear whether any commonality exists between TGFβ and these other players with respect to T cell exclusion from cancers.
These two papers illustrate the power of immunogenomics in combination with experimental mouse models and functional readouts. Closely linked pathways, and their relationships to one another, may in part explain why predicting outcomes from a single biomarker, such as PD-L1, is challenging. The observed multifactorial basis of immunotherapy response may be applied to different tumour types. Enabling T cell infiltration using TGFβ inhibitors may be enough to improve the success of PD-1–PD-L1 immune checkpoint-based therapies and may be a general approach for the treatment of cancers that grow in a TGFβ-rich environment.
Acknowledgements
M.J.S. was supported by a Senior Principal Research Fellowship (1078671). T.B. was supported by a NH&MRC Early Career Fellowship (1124690) and EMBO Long Term Fellowship (ALTF 945-2015).
Competing interests
M.J. Smyth has research agreements with Bristol Myers Squibb, Tizona Therapeutics, and Aduro Biotech. The other author declares no competing interests.
References
- 1.Massague J. TGFbeta in. Cancer Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 3.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-beta in cancer. Cancer Biol. Med. 2015;12:385–393. doi: 10.7497/j.issn.2095-3941.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calon A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 6.Tauriello DVF, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 7.Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 2016;22:1865–1874. doi: 10.1158/1078-0432.CCR-15-1507. [DOI] [PubMed] [Google Scholar]
- 9.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg JE, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilera TA, Giaccia AJ. Molecular pathways: oncologic pathways and their role in T-cell exclusion and immune evasion-a new role for the AXL receptor tyrosine kinase. Clin. Cancer Res. 2017;23:2928–2933. doi: 10.1158/1078-0432.CCR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]