Abstract
This study investigated whether common comorbidities or biochemical factors, such as allergic disease, anemia, inflammation, and neurotransmitters, are singly or additively associated with an increased risk of attention deficit–hyperactivity disorder (ADHD). We recruited 216 children diagnosed with ADHD and 216 age-, sex-, height-, weight-, and class-matched controls from 31 elementary schools in Taipei, Taiwan. The International Study of Asthma and Allergies in Childhood questionnaire was used to measure allergic symptoms. Fasting venous blood was collected and analyzed for complete blood count, white blood cell differential count, immunoglobulin (Ig) E level, and serotonin (5-HT) level. The results showed that symptoms of both rhinitis (OR = 2.08, 95% CI = 1.42–3.05) and eczema (OR = 1.72, 95% CI = 1.02–2.88) were significantly associated with increased risk of ADHD. Children with ADHD showed considerably lower levels of hemoglobin (p = 0.001) and 5-HT (p < 0.001) and higher IgE level (p < 0.001) and eosinophil count (p = 0.001) than did control children. ADHD risk increased with the number of aforementioned biochemical risk factors present (one factor: OR = 1.87, 95% CI = 0.87–4.18; two factors: OR = 2.90, 95% CI = 1.29–6.48; three factors: OR = 4.47, 95% CI = 1.97–10.13; four factors: OR = 6.53, 95% CI = 2.43–17.57). Findings suggest that either ADHD’s etiology is multidimensional or the aforementioned conditions have shared etiology with ADHD.
Introduction
Attention deficit–hyperactivity disorder (ADHD), one of the most frequent neuropsychiatric disorders found in children, is characterized by a lack of impulse control, inattention, and hyperactivity1. ADHD may have a substantial influence on children’s school performance, familial relationships, and social interactions2,3. Thorough investigation of ADHD’s complex etiology is vital for improving its diagnosis, prevention, and management4. ADHD is associated with various comorbidities, including allergic disease, immune dysregulation, neurotransmitter underproduction, anemia, and oxidative and endocrinological imbalances5,6.
Regarding the neurophysiological mechanisms, various allergic diseases, including allergic rhinitis, atopic dermatitis, and asthma, have already been associated with ADHD7–11. Immune responses resulting from these allergic diseases may affect the central nervous system (CNS) and predispose children with neurodevelopmental disorders, including ADHD12. Therefore, evaluating ADHD symptoms in children and adolescents with allergic diseases may facilitate ADHD identification9. Furthermore, identifying allergic diseases in children exhibiting ADHD symptoms provides an opportunity to control allergies. A recent randomized small-scale, placebo-controlled trial showed that rhinitis control combined with Ritalin administration can effectively alleviate ADHD endpoints13. Allergies result from immune responses that often involve chronic inflammation14. Immunoglobulin (Ig) E is an antibody found only in mammals, and an elevated IgE level is usually related to atopic diseases15. IgE-mediated allergic and inflammatory pathways are targets for intervention in the pathological processes of allergic diseases16. Furthermore, eosinophils, one of the five major leukocyte types, are responsible for tissue damage and inflammation in allergic diseases. However, little is known about whether the markers of allergy (i.e., peripheral IgE level or eosinophil count) may be linked to ADHD.
Atopic diseases, such as eczema, asthma, hay fever, and food allergies, are associated with anemia17. The most prevalent form of anemia is iron deficiency anemia. Low levels of serum ferritin, a biomarker of iron store, are correlated with risks of cognitive deficits and behavioral and psychiatric problems in pediatric populations18. A large-scale field study demonstrated that a lower ferritin level may be associated with parent-reported hyperactivity after controlling for confounding factors19. Furthermore, research, including a Taiwanese study, has indicated that iron deficiency or iron deficiency anemia may be a potential risk factor for neurodevelopmental disorders including ADHD20,21. Iron deficiency may also reduce the effectiveness of psychostimulant treatment in children with ADHD22. A routine complete blood count (CBC) can detect anemia, a late-stage marker of iron deficiency. This low-cost method may indicate poor iron nutrition and unsatisfactory response to psychostimulant treatment to prompt clinicians to treat these children with ADHD.
Serotonin (5-HT) is a monoamine neurotransmitter that induces metabolic effects in a variety of cell types both in the CNS and peripheral nervous system. A polymorphism on the 5-HT transporter gene (5-HTTLPR) can influence impulsivity in humans23,24. In addition, 5-HT can modulate several immunological events, such as activation, proliferation, cytokine secretion, and chemotaxis of leukocytes. The influence of 5-HT on immune cells may be associated with the pathologies of inflammatory diseases25 or atopic diseases (e.g., eczema or atopic dermatitis)26,27. Therefore, 5-HT deficits have been indicated as participating in the etiology of ADHD28. However, findings related to 5-HT circulation levels in ADHD patients have been inconsistent among previous case–control studies, with some studies indicating that children with ADHD tend to have lower blood levels of 5-HT29–31. However, other studies have not found this difference32,33. Therefore, additional studies with larger sample sizes (or greater statistical power) are required to confirm the association between 5-HT and ADHD.
Because ADHD etiology is multifaceted; each factor may make a partial contribution to ADHD susceptibility. Few studies have examined the joint effects of these factors. Understanding the complex relationships between ADHD and these conditions may elucidate ADHD pathogenesis and a comprehensive management plan. On the basis of the literature, we hypothesize that patients with ADHD have more allergic symptoms and higher levels of allergic and inflammation markers, such as IgE, histamine, CBC parameters, and 5-HT, compared with healthy controls. We aimed to investigate the dose–response relationship (cumulative effect) between the number of risk factors and susceptibility to ADHD. If links between the aforementioned risk factors and ADHD are established, routinely screening these underlying pathophysiological indicators may be helpful for devising a comprehensive treatment strategy for ADHD. Therefore, we conducted a case–control study by strictly matching age, sex, height, weight, and class to investigate whether ADHD is correlated with the aforementioned factors.
Method
Study participants
This study received approval from the Medical Research Ethical Committee of the Institute of Biomedical Sciences at Academia Sinica (AS-IBMS-MREC-92-01). All procedures involving participants were in accordance with the ethical standards of the institutional and national research committees and the Helsinki Declaration.
We mailed invitation letters to elementary schools in the Taipei metropolitan area and visited the schools agreeing to participate. Teachers distributed informed consent forms (ICFs). After completed ICFs were received from parents, we screened the students aged 8–10 years for potential ADHD on two validated scales: the Chinese version of the Conners Teacher Rating Scale (for teachers) and the Chinese version of the Werry–Weiss–Peters Activity Scale (for parents)34. Of all screened students, 287 students scored higher than the 85th-percentile values on both scales and were referred for further clinical evaluation. After being interviewed by a child psychiatrist on our research team, 252 students were diagnosed with ADHD according to the criteria of the Diagnostic and Statistical Manual, Fourth Edition, Text Revision (DSM-IV-TR). Control students were randomly selected from the same classes as the ADHD students were and matched by age, sex, height, and body weight (within a 5% range). After students with incomplete data were excluded, this analysis comprised 216 children with ADHD and 216 controls.
Questionnaire, allergic symptoms, and biomarker assessment
We obtained information on family education levels, family income, lifestyle, and disease history of students and their parents through a questionnaire. Body weights and heights of the participating children were measured by trained technicians. We had parents fill out the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire35, and symptoms of asthma, rhinitis, and eczema were identified.
Approximately 10 mL of fasting venous blood was drawn from each child and stored in darkness throughout the biospecimen handling process. CBC analysis was performed at each school using EDTA-blood with a blood autoanalyzer (ADVIA 120; Bayer, Tarrytown, NY, USA; coefficients of variation [CVs]: WBC, 1.07%; RBC, 0.40%; HGB, 0.36%; HCT, 0.43%; MCV, 0.16%; MCH, 0.50%; MCHC, 0.52%; PLT, 1.72%). All serum and plasma samples were kept at −70 °C until analysis. We measured routine blood clinical chemistry and plasma C-reactive protein (CRP) concentrations by using the Olympus AU 600 Autoanalyzer (Olympus Corp., Tokyo, Japan; CV: 3.6–3.7%). IgE levels in the serum were analyzed with the radioimmunosorbent test and capsulated hydrophilic carrier (CAP) method (Pharmacia & Upjohn, Uppsala, Sweden; CV: 2.5–2.8%). Finally, we obtained histamine levels (CV: 2.2–9.2%) and 5-HT levels (CV: 3.6–17.9%) using ELISA kits (RE59121 for histamine and RE59221 for HT-5, IBL-America, Inc., USA).
Statistical analysis
All statistical analyses were conducted using SAS (version 9.1; 2002, 2003, SAS Institute, Cary, NC, USA). We performed group comparisons between cases and controls using the chi-square test for categorical variables and t test for continuous variables. Logistic regression models were used to determine the relationships between each allergic disease (yes or no) and ADHD (present or absent). Eosinophil count and histamine, CRP, IgE, and 5-HT levels demonstrated a significant level of positive skewness. We applied arithmetic logarithmic transformations to approximate normal distributions for the aforementioned variables before preforming further statistical analyses. A p of < 0.05 was considered statistically significant. Bonferroni correction was applied to adjust for multiple testing in the group comparison, with regard to multiple biochemical indices (p = 0.05/16 = 0.003125).
Four indicators were identified: hemoglobin (Hb), eosinophil, IgE, and serotonin (5-HT). Risk conditions were defined by low levels of Hb and 5-HT, but high eosinophil count and IgE levels. Because no sex differences in these risk factors were noted among the children, we classified students into quartiles for each of the risk factors to compare effect sizes. We used logistic regression to determine the odds ratios of biochemical risk conditions as well as allergic conditions for their influences on ADHD. Potential confounders—namely jaundice after birth, mother with smoking habit, expenditure balanced with revenue, facing stressful situation (see Table 1)—were adjusted in each logistic regression model. Furthermore, we estimated the odds ratios for children with varying numbers of risk factors.
Table 1.
Demographics of children in the ADHD group and in the control group.
| Variables | Control (N = 216) | ADHD (N = 216) | p-valuea |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 9.2 (1.8) | 9.2 (1.7) | 0.95 |
| Height (cm) | 135.2 (10.7) | 135.2 (10.7) | 0.93 |
| Weight (kg) | 33.6 (9.8) | 33.6 (9.8) | 0.95 |
| Body mass index (kg/m2) | 18.0 (3.1) | 18.1 (3.1) | 0.83 |
| N (%) | N (%) | ||
| Male gender | 186 (86) | 186 (86) | 1.00 |
| Grades | |||
| 1–3 grades | 121 (56) | 121 (56) | 1.00 |
| 4–6 grades | 95 (44) | 95 (44) | 1.00 |
| Birth related conditions | |||
| Abortion symptoms in the 1st trimester | 21 (10) | 36 (17) | 0.03 |
| Maternal smoking habit in pregnancy | 2 (1) | 11 (5) | 0.01 |
| Jaundice after birth | 14 (6) | 35 (16) | 0.001* |
| Presence of hypoxia at birth | 6 (3) | 16 (7) | 0.03 |
| Breast feeding | 108 (50) | 100 (46) | 0.44 |
| Parental occupation | |||
| Non-blue collar | 156 (72) | 127 (59) | 0.006 |
| Blue collar | 60 (28) | 89 (41) | |
| Having smoking habit currently | |||
| Parental | 100 (46) | 106 (49) | 0.56 |
| Maternal | 11 (5) | 30 (14) | 0.002* |
| Familial situation | |||
| Expenditure balanced with revenue | 188 (87) | 162 (75) | 0.001* |
| Facing stressful situation | 128 (59) | 159 (74) | 0.002* |
aChi-square test for groups comparisons of binary variables and t-test for groups comparisons of continuous variables; *Statistical significant after Bonferroni correction (p < 0.003125).
Results
Characteristics of study participants
Because children with ADHD and control children were matched by age, height, weight, body mass index, grade level, and sex, the distributions and means of these variables were comparable (Table 1). Children with ADHD were more likely to have parents with lower education levels, blue-collar jobs, unstable incomes, higher levels of familial stress, conditions such as hypoxia or jaundice during or after delivery, and siblings with ADHD. Furthermore, children with ADHD tended to have mothers who were current smokers, smoked during pregnancy, and had experienced miscarriage symptoms in their first trimester of pregnancy.
Relationship between ADHD and allergic diseases
According to the ISAAC questionnaire, 24.5%, 61.6%, and 20.4% of our students with ADHD had symptoms of asthma, rhinitis, and eczema, respectively (Table 2). Rhinitis symptoms (OR = 2.08, 95% CI = 1.42–3.05) and eczema (OR = 1.72, 95% CI = 1.02–2.88) demonstrated a significant association with an increased risk of ADHD. Figure 1a shows that patients with at least one symptom of allergic diseases were more likely to receive an ADHD diagnosis (any one allergic disease: OR = 1.66, 95% CI = 1.06–2.60; two or three allergic diseases: OR = 2.34, 95% CI = 1.45–3.79).
Table 2.
Associations between allergic diseases, biochemical markers and ADHD.
| Allergic diseases | Control (N = 216) | ADHD (N = 216) | Logistic regression model | |
|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | p-value | |
| Asthma | 61 (28.2) | 53 (24.5) | 1.21 (0.79–1.86) | 0.383 |
| Rhinitis | 94 (43.5) | 133 (61.6) | 2.08 (1.42–3.05) | <0.001 |
| Eczema | 28 (13.0) | 44 (20.4) | 1.72 (1.02–2.88) | 0.040 |
Abbreviations: ADHD, attention-deficit hyperactivity disorder; OR, odds ratios; 95% CI, 95% confidence interval.
Figure 1.
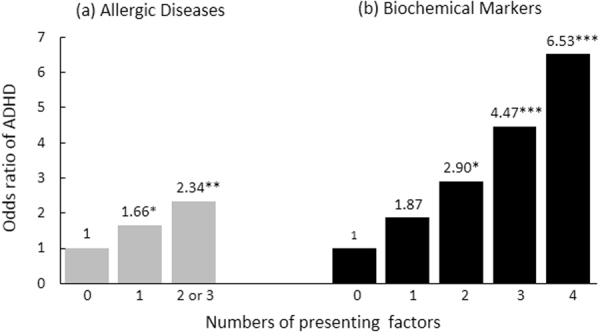
Effects of the number of allergic diseases (a) and clinical biochemical risk factors (b) on ADHD. (Numbers denoted above the bar are the exact odds ratios of corresponding groups; *p < 0.05, **p < 0.01, ***p < 0.001).
Relationship between ADHD and biochemical indices
The biochemical profiles of children with ADHD and controls are listed in Table 3. Significantly lower Hb and 5-HT levels were observed in students with ADHD. By contrast, these children had significantly higher IgE levels and eosinophil counts than the control children. We did not observe significant differences in histamine or CRP levels or CBC parameters, except Hb levels.
Table 3.
Comparisons of biochemistry profile between ADHD patients and healthy control children.
| Biochemical indexes | Control (N = 216) | ADHD (N = 216) | Statistic b | p-value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| RBC (1012/L) | 4.70 (0.27) | 4.64 (0.32) | 2.259 | 0.024 |
| Hb (g/dL) | 13.30 (0.85) | 13.00 (0.80) | 3.466 | 0.001* |
| MCV (fL) | 84.10 (3.6) | 83.00 (4.7) | 2.730 | 0.007 |
| MCH (pg/cell) | 28.20 (1.5) | 28.10 (1.9) | 0.392 | 0.70 |
| MCHC (g/dL) | 33.60 (1.4) | 33.90 (1.4) | 1.619 | 0.11 |
| WBC (109/L) | 7.02 (1.71) | 7.38 (2.11) | 1.919 | 0.06 |
| Neutrophil (%) | 49.20 (10.5) | 49.80 (9.8) | 0.682 | 0.50 |
| Lymphocyte (%) | 39.10 (9.5) | 38.30 (8.9) | 0.902 | 0.37 |
| Monocyte (%) | 6.83 (1.9) | 6.77 (1.83) | 0.346 | 0.73 |
| Eosinophil (%)a | 3.78 (2.67) | 4.93 (3.62) | 3.364 | 0.001* |
| Basophil (%) | 0.61 (0.85) | 0.57 (0.40) | 0.553 | 0.58 |
| Platelet (109/L) | 310.00 (66) | 314.00 (66) | 0.585 | 0.56 |
| Histamine (nmol/L)a | 9.54 (9.18) | 9.99 (9.27) | 0.461 | 0.65 |
| CRP (mg/L)a | 0.96 (1.66) | 1.35 (2.71) | 1.470 | 0.14 |
| IgE (KU/L)a | 262.38 (420.27) | 367.44 (483.36) | 3.559 | <0.001* |
| Serotonin (ng/mL)a | 191.61 (88.26) | 164.25 (88.39) | 4.025 | <0.001* |
aLog transformation has been done for statistical testing; bChi-square test for groups comparisons of binary variables or t-test for continuous variables, *Statistical significant after Bonferroni correction (p < 0.003125).
We defined the cutoff points for biochemical risk factors using quartile cutoff points. Although these points were situated in the subclinical (normal) range, logistic regression models indicated that Hb < 12.6 g/dL, 5-HT < 113.57 ng/mL, IgE > 367 KU/L, and eosinophil >5.85% were all significantly associated with an increased ADHD risk (Table 4). Furthermore, children with one or more of the aforementioned biochemical risk factors were more likely to be diagnosed with ADHD (any one factor: OR = 1.87, 95% CI = 0.87–4.18; any two factors: OR = 2.90, 95% CI = 1.29–6.48; any three factors: OR = 4.47, 95% CI = 1.97–10.13; four factors: OR = 6.53, 95% CI = 2.43–17.57; Fig. 1b).
Table 4.
Associations between biochemical markers and ADHD.
| Biochemical marker at risk | Control (N = 216) | ADHD (N = 216) | Logistic regression model | |
|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | p-value | |
| Hb (<12.6 g/dL) | 49 (22.7) | 69 (31.9) | 1.60 (1.04–2.45) | 0.031 |
| Eosinophil (>5.85%) | 41 (19) | 67 (31) | 1.92 (1.23–3.00) | 0.004 |
| IgE (>367 KU/L) | 44 (20.4) | 62 (28.7) | 1.57 (1.01–2.45) | 0.045 |
| Serotonin (<113.57 ng/mL) | 45 (20.8) | 63 (29.2) | 1.57 (1.01–2.43) | 0.046 |
Abbreviations: ADHD, attention-deficit hyperactivity disorder; OR, odds ratios; 95% CI, 95% confidence interval.
Discussion
To our knowledge, this study is the first to examine the relationship between ADHD and allergies along with multiple biochemical indices related to immune-mediated inflammation. Compared with control participants, children with ADHD had higher prevalence of allergic diseases, higher eosinophil counts, higher IgE levels, lower blood 5-HT concentration, and lower Hb levels. The higher the number more risk factors was, the higher the odds of being diagnosed with ADHD were. This finding suggests the coexistence of ADHD and several clinical conditions, including allergic diseases; biomarkers of immunological responses and the associated conditions, such as anemia; and lower 5-HT levels.
The results of our study indicate a significantly higher prevalence of rhinitis and eczema in the ADHD group than in the control group. This finding is consistent with that of a previous meta-analysis on allergic diseases and ADHD, although only eczema was independently related to ADHD in that study36. Furthermore, our study showed the number of allergic disease symptoms had a dose–response effect on ADHD, suggesting that patients with ADHD had more allergic conditions than their counterparts without ADHD. Various reasonable biological mechanisms linking ADHD and allergies were proposed37. Allergic reactions result in an increase in inflammatory cytokines from TH2 or TH17 cells38, which may stimulate the neuroimmune mechanism in the brain circuits, such as the prefrontal cortex and the anterior cingulate cortex, associated with emotional and behavior control39,40. ADHD symptoms resemble cognitive disturbances caused by functional and structural changes in the aforementioned two brain circuits. Although neither the existing literature nor this study could provide a chronological sequence from the development of atopic disease to that of ADHD, we found total IgE levels and percentage of eosinophils to be elevated in children suffering from ADHD. Because it is unlikely that ADHD causes allergy, these findings support the hypothesis that allergies precede or coexist with ADHD because of some shared etiology. Although it is not confirmed at the present time whether or how allergy is involved in the etiology of ADHD, allergic symptom assessment may be considered in the comprehensive appraisal and management of ADHD in children.
Iron deficiency may also result in an insufficient supply of neurotransmitters because iron is a cofactor of tyrosine hydroxylase and tryptophan hydroxylase41. Previous studies have also found iron deficiency to alter dopamine transporter function in rat striatum42. Not only is iron involved in neurotransmitter production but iron deficiency may also contribute to the development of ADHD by disrupting the oxygen supply to the brain43 and disturbing growth and cognitive ability44. Studies related to the iron status of patients with ADHD and controls have been controversial22,45,46. We did not directly assess ferritin levels, but rather performed a routine CBC assessment. We observed significantly lower Hb levels in children with ADHD. Although the threshold points for Hb (12.6 g/dL at 25th-percentile) did not reach the criterion for anemia, this finding indicates that subclinical anemia may be associated with certain aspects of ADHD. Iron deficiency is the only possible cause of microcytic anemia. The lower Hb levels in children with ADHD may have also resulted from other nutrient deficiencies, such as folate, vitamin B6 or B12, or other inflammatory or parasitic conditions. Moreover, asthma and infectious diseases have also been associated with anemia17,47,48, implying that allergies (immune responses) and anemia may share a biological process and environmental factors. Determining causes of anemia is not difficult clinically and may help to improve the overall well-being of patients with ADHD.
The CNS inhibitory neurotransmitter 5-HT correlates with impulse control and emotions23,49. We found that children with ADHD had lower 5-HT levels compared with healthy controls. This finding agrees with those of previous studies29–31 but disagrees with those of others32,33. Relative to previous studies that investigated a similar topic, the current study featured a larger sample and matched controls more strictly. Therefore, we believe that our study possessed greater statistical power compared with previous small-scale studies. Furthermore, a recent study indicated that the peripheral function of the 5-HT metabolic pathway can serve as a significant predictor of aggressive behavior50. It has also been found that 5-HT may also exert effects on immune cells and link to the pathologies of inflammatory diseases25 or atopic diseases (eczema or atopic dermatitis)26,27. Therefore, certain characteristics of children with ADHD, such as impulsivity, temper tantrums, or aggression, may be partially related to lower peripheral 5-HT levels.
Here, although each single factor showed significant differences between the ADHD group and control group (p < 0.05), the discriminative validity of each was not robust. We found that children with more of the aforementioned biochemical risk factors were more likely to be diagnosed with ADHD. The dose–response relationship between the number of risk factors and susceptibility to ADHD supports either the idea that the etiology of ADHD may be multifaceted or that ADHD coexists with allergy, immunological stress, anemia, and low 5-HT levels because of a shared etiology41,51. Notably, several of these biomarkers may also interact with each other. Studies25,52 have demonstrated that several nutritional factors and allergic reactions are associated with serotonin levels. Additional studies are required to clarify the underlying mechanisms of the relationships between these biochemical abnormalities and ADHD and assess their predictive values and applicability in clinical settings. Because allergic diseases and low levels of Hb and 5-HT may be modifiable through dietary intervention, nutritional supplements, or medical treatment, clinicians should monitor these factors in children exhibiting ADHD symptoms. Adequate correction for these risk factors may be beneficial to some children diagnosed with ADHD or who respond poorly to ADHD drug therapy (maybe phenocopies of ADHD).
This study has several limitations. First, this was a case–control study, and the causal relationships among ADHD, allergic symptoms, and 5-HT and Hb levels warrant further investigation. Second, although ADHD diagnoses were confirmed using the DSM-IV-TR, the ADHD severity and neuropsychological functions of the participants were not assessed. The complex relationships among clinical characteristics (e.g., severity of ADHD symptoms, comorbidity, or neurocognitive deficits) and severity of allergic diseases and biomarkers consequently remain uncertain. Third, allergy symptoms were only defined using the ISAAC. Nonetheless, findings on IgE and eosinophil were consistent with the questionnaire results. In addition, this study only measured Hb concentration in the blood. We cannot confirm whether it is anemia or iron deficiency that is involved in ADHD etiology. Finally, not only 5-HT but also other neurotransmitters (e.g., dopamine or norepinephrine) are involved in the pathophysiology of ADHD38; however, we did not determine the levels of neurotransmitters other than 5-HT.
In conclusion, ADHD is associated with several comorbidities, including allergic symptoms, subclinical anemia, and low 5-HT levels; furthermore, the number of biochemical risk factors is associated with the presence of ADHD in a dose–response manner. Thus, either ADHD etiology is potentially multidimensional or ADHD has shared etiology with the other included clinical conditions. Future longitudinal studies should investigate the causal relationships between these subclinical indicators and ADHD and determine whether ADHD risk can be modified by appropriately managing allergic symptoms and Hb and neurotransmitter levels.
Acknowledgements
The research was supported by grant from National Science Council (NSC 92-2320-B-001-027 and NSC93-2320-B-001-011). The authors thank the Institute of Biomedical Sciences, Academia Sinica, for administrative assistance.
Author Contributions
L.J.W., Y.H.Y., and W.T.Y. analyzed the data, participated in interpreting data, reviewing literatures, and drafting the manuscript. M.L.F. carried out patient recruitment and data collection, coordinated the lab data analysis and data entry, and executed preliminary data analysis. J.L.H. designed the ADHD screening protocol and confirmed the cases clinically. Y.H.Y. and B.L.C. designed the allergy protocol and confirm the allergy cases. W.J.C. interpreted the findings and provided statistical consultation. W.H.P. designed the study, interpreted the findings, suggested writing directions, and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Canu, W. H. & Eddy, L. D. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment (4th ed.). Cogn Behav Ther 1 (2015).
- 2.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. Ambul Pediatr. 2007;7:82–90. doi: 10.1016/j.ambp.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Classi P, Milton D, Ward S, Sarsour K, Johnston J. Social and emotional difficulties in children with ADHD and the impact on school attendance and healthcare utilization. Child Adolesc Psychiatry Ment Health. 2012;6:33. doi: 10.1186/1753-2000-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman HM, Reiff MI. Clinical practice. Attention deficit-hyperactivity disorder in children and adolescents. N Engl J Med. 2014;370:838–846. doi: 10.1056/NEJMcp1307215. [DOI] [PubMed] [Google Scholar]
- 5.Selek S, Savas HA, Gergerlioglu HS, Bulut M, Yilmaz HR. Oxidative imbalance in adult attention deficit/hyperactivity disorder. Biol Psychol. 2008;79:256–259. doi: 10.1016/j.biopsycho.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387:1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- 7.Strom MA, Fishbein AB, Paller AS, Silverberg JI. Association between atopic dermatitis and attention deficit hyperactivity disorder in U.S. children and adults. Br J Dermatol. 2016;175:920–929. doi: 10.1111/bjd.14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki C, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17:120. doi: 10.1186/s12888-017-1281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, et al. Association of pediatric allergic rhinitis with the ratings of attention-deficit/hyperactivity disorder. Am J Rhinol Allergy. 2017;31:161–167. doi: 10.2500/ajra.2017.31.4439. [DOI] [PubMed] [Google Scholar]
- 10.Schans JV, Cicek R, de Vries TW, Hak E, Hoekstra PJ. Association of atopic diseases and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. Neurosci Biobehav Rev. 2017;74:139–148. doi: 10.1016/j.neubiorev.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Yang, C. F., Yang, C. C. & Wang, I. J. Association between allergic diseases, allergic sensitization and attention-deficit/hyperactivity disorder in children: A large-scale, population-based study. J Chin Med Assoc (2017). [DOI] [PubMed]
- 12.Chang HY, et al. Allergic diseases in preschoolers are associated with psychological and behavioural problems. Allergy Asthma Immunol Res. 2013;5:315–321. doi: 10.4168/aair.2013.5.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melamed I, Heffron M. Attention Deficit Disorder and Allergic Rhinitis: Are They Related? J Immunol Res. 2016;2016:1596828. doi: 10.1155/2016/1596828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin RD, Robinson M, Sly PD, Holt PG. Childhood atopy and mental health: a prospective, longitudinal investigation. Psychol Med. 2017;47:317–325. doi: 10.1017/S0033291716001896. [DOI] [PubMed] [Google Scholar]
- 15.Salo PM, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1226–1235 e1227. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TW, Wu PC, Hsu CL, Hung AF. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007;93:63–119. doi: 10.1016/S0065-2776(06)93002-8. [DOI] [PubMed] [Google Scholar]
- 17.Drury KE, Schaeffer M, Silverberg JI. Association Between Atopic Disease and Anemia in US Children. JAMA Pediatr. 2016;170:29–34. doi: 10.1001/jamapediatrics.2015.3065. [DOI] [PubMed] [Google Scholar]
- 18.Gottfried RJ, Gerring JP, Machell K, Yenokyan G, Riddle MA. The iron status of children and youth in a community mental health clinic is lower than that of a national sample. J Child Adolesc Psychopharmacol. 2013;23:91–100. doi: 10.1089/cap.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oner P, Oner O, Azik FM, Cop E, Munir KM. Ferritin and hyperactivity ratings in attention deficit hyperactivity disorder. Pediatr Int. 2012;54:688–692. doi: 10.1111/j.1442-200X.2012.03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MH, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161. doi: 10.1186/1471-244X-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bener A, Kamal M, Bener H, Bhugra D. Higher prevalence of iron deficiency as strong predictor of attention deficit hyperactivity disorder in children. Ann Med Health Sci Res. 2014;4:S291–297. doi: 10.4103/2141-9248.141974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortese S, Angriman M, Lecendreux M, Konofal E. Iron and attention deficit/hyperactivity disorder: What is the empirical evidence so far? A systematic review of the literature. Expert Rev Neurother. 2012;12:1227–1240. doi: 10.1586/ern.12.116. [DOI] [PubMed] [Google Scholar]
- 23.Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- 24.Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Arreola R, et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015;2015:354957. doi: 10.1155/2015/354957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Li G, Xiang J, Yin D, Chi R. Immunohistochemical study of serotonin in lesions of chronic eczema. Int J Dermatol. 2004;43:723–726. doi: 10.1111/j.1365-4632.2004.02196.x. [DOI] [PubMed] [Google Scholar]
- 27.Vavrova K. Emerging small-molecule compounds for treatment of atopic dermatitis: a review. Expert Opin Ther Pat. 2016;26:21–34. doi: 10.1517/13543776.2016.1101451. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee E, Nandagopal K. Does serotonin deficit mediate susceptibility to ADHD? Neurochem Int. 2015;82:52–68. doi: 10.1016/j.neuint.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Coleman M. Serotonin concentrations in whole blood of hyperactive children. J Pediatr. 1971;78:985–990. doi: 10.1016/S0022-3476(71)80428-6. [DOI] [PubMed] [Google Scholar]
- 30.Saul RC, Ashby CD. Measurement of whole blood serotonin as a guide in prescribing psychostimulant medication for children with attentional deficits. Clin Neuropharmacol. 1986;9:189–195. doi: 10.1097/00002826-198604000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Spivak B, et al. Circulatory levels of catecholamines, serotonin and lipids in attention deficit hyperactivity disorder. Acta Psychiatr Scand. 1999;99:300–304. doi: 10.1111/j.1600-0447.1999.tb07229.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson HB, Pappas BA, Trites RL, Peters DA, Taub H. Plasma free and total tryptophan, blood serotonin, and the hyperactivity syndrome: no evidence for the serotonin deficiency hypothesis. Biol Psychiatry. 1981;16:231–238. [PubMed] [Google Scholar]
- 33.Bhagavan HN, Coleman M, Coursin DB. The effect of pyridoxine hydrochloride on blood serotonin and pyridoxal phosphate contents in hyperactive children. Pediatrics. 1975;55:437–441. [PubMed] [Google Scholar]
- 34.Chou W, Wang Y, Chen Y. The assessment of clinical application of activity rating scales in attention-deficit hyperactivity disorder. Chin Psychiatry. 1993;7:162–171. [Google Scholar]
- 35.Asher MI, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt J, Buske-Kirschbaum A, Roessner V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy. 2010;65:1506–1524. doi: 10.1111/j.1398-9995.2010.02449.x. [DOI] [PubMed] [Google Scholar]
- 37.Buske-Kirschbaum A, et al. Psychoendocrine and psychoneuroimmunological mechanisms in the comorbidity of atopic eczema and attention deficit/hyperactivity disorder. Psychoneuroendocrinology. 2013;38:12–23. doi: 10.1016/j.psyneuen.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 39.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009;6:18–22. [PMC free article] [PubMed] [Google Scholar]
- 40.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry. 2012;51:1003–1019 e1020. doi: 10.1016/j.jaac.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 43.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:1468s–1472s. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- 44.Lozoff, B. et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev64, S34-43; discussion S72-91 (2006). [DOI] [PMC free article] [PubMed]
- 45.Percinel I, Yazici KU, Ustundag B. Iron Deficiency Parameters in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Child Psychiatry Hum Dev. 2016;47:259–269. doi: 10.1007/s10578-015-0562-y. [DOI] [PubMed] [Google Scholar]
- 46.Cortese S, et al. Brain iron levels in attention-deficit/hyperactivity disorder: a pilot MRI study. World J Biol Psychiatry. 2012;13:223–231. doi: 10.3109/15622975.2011.570376. [DOI] [PubMed] [Google Scholar]
- 47.Liao SL, et al. Infant anemia is associated with reduced TLR-stimulated cytokine responses and increased nasopharyngeal colonization with Moxarella catarrhalis. Sci Rep. 2018;8:4897. doi: 10.1038/s41598-018-23264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasreen S, et al. Relationship of Hemoglobin Concentration in Adult Asthmatic Patients. Mymensingh Med J. 2016;25:601–606. [PubMed] [Google Scholar]
- 49.Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Comai S, et al. Tryptophan via serotonin/kynurenine pathways abnormalities in a large cohort of aggressive inmates: markers for aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:8–16. doi: 10.1016/j.pnpbp.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Faraone SV, Bonvicini C, Scassellati C. Biomarkers in the diagnosis of ADHD–promising directions. Curr Psychiatry Rep. 2014;16:497. doi: 10.1007/s11920-014-0497-1. [DOI] [PubMed] [Google Scholar]
- 52.Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29:2207–2222. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]


