Abstract
In prostate cancer (PCa), neuroendocrine cells (NE) have been associated with the progression of the disease due to the secretion of neuropeptides that are capable of diffusing and influence surrounding cells. The GABAergic system is enriched in NE-like cells, and contributes to PCa progression. Additionally, γ-aminobutyric acid (GABA) stimulates the secretion of gastrin-releasing peptide (GRP) in peripheral organs. For the first time, in this study we show the role of GABA and GABAB receptor 1 (GABBR1) expression in GRP secretion in NE-like prostate cancer cells. We demonstrated an increase in GRP levels in NE-like cell medium treated with GABAB receptor agonist. Moreover, the blocking of this receptor inhibited GABA-induced GRP secretion. The invasive potential of PC3 cells was enhanced by either GRP or conditioned medium of NE-like cells treated with GABA. Additionally, we confirmed a positive correlation between GABA and GRP levels in the serum of PCa patients with NE markers. Finally, using public available data sets, we found a negative correlation between GABBR1 and androgen receptor (AR) expression, as well as a strong positive correlation between GABBR1 and enolase 2. These results suggest that GABA via GABBR1 induces GRP secretion in NE like cells involved in PCa progression.
Introduction
Despite great efforts to improve treatment, prostate cancer (PCa) is the most frequently diagnosed cancer among men in developed countries1. Androgen ablation has been the main therapeutic intervention in managing hormone-sensitive prostate cancer2. However, in most cases, tumors tend to progress, despite treatment, to the castration-resistant prostate cancer (CRPC) stage. Once this occurs, the median survival rate is 18 to 24 months3. CRPC is a lethal stage, when prostate cancer progresses and metastasizes4.
Hormone-treated and hormone-refractory tumors can undergo neuroendocrine differentiation (NED)5,6. NED is characterized by an increase in a malignant subpopulation of cells with neuroendocrine (NE) features. Among CRPC tumors, it is estimated that 40–100% acquires NED. These tumors are referred to as neuroendocrine prostate cancer (NEPC)7,8. Experiments conducted in vitro and in vivo (animal models) have shown that prostate adenocarcinoma cells can transdifferentiate and acquire a NE phenotype through a process termed NE transdifferentiation9–12. These cells are typically referred to as “NE-like” cells, because their origin and biochemical characteristics are different from those of normal NE cells9,10. Wright et al. demonstrated that a knockdown of the androgen receptor (AR) in LNCaP cells induced a NE-like morphology and expression of NE markers. Those findings suggested that AR activity repressed the transdifferentiation process11.
NE transdifferentiation in PCa revealed an epithelial plasticity that gave rise to tumor adaptation in response to AR-targeted therapies. A recent study showed that NEPC occurred in 30/81 patients with CRPC. Moreover, AR expression was low in these tumors12. Although NE cells and NE-like cells do not express AR13, they can secrete many types of neuropeptides, like gastrin-releasing peptide (GRP), serotonin, and neurotensin, and they express NE markers, such as enolase 2 (ENO2), chromogranin A, and chromogranin B6,14. Hence, understanding the molecular etiology of NEPC and identifying novel therapeutic targets are of utmost importance, particularly because, at present, no effective therapy is available.
Neuropeptides, such as GRP, have been positively associated with PCa progression15. GRP, a 27-amino acid neuropeptide, is the mammalian homologue of the peptide known as bombesin, which was isolated from frog skin16. Neuroendocrine cells in tumors are considered the main source of GRP. In PCa, GRP stimulates mitogen-stimulated proliferation, migration, and invasion, through autocrine and paracrine signaling17,18. Studies have shown that serum GRP concentrations were elevated in patients with advanced PCa. Specifically, GRP concentrations were significantly elevated in advanced metastatic and CRPC tumors, but not in the early cancer stages19.
The Gordon group created transgenic mice (CR2-TAg) that developed a pattern of NEPC initiation and progression. Prostate samples from CR2-Tag mice were compared to prostate samples from normal mice with GeneChip arrays to identify candidate mediators of NE cell differentiation. One candidate gene was glutamic acid decarboxylase (Gad1 in mouse, GAD1 in human), which showed 40-fold higher expression in NEPC than in normal NE cells20,21. The GAD1 enzyme produces the most abundant inhibitory neurotransmitter in the mammalian brain: γ-aminobutyric acid (GABA). GABA plays a tissue-specific function22–28, and it is widespread throughout periphery organs, including the prostate.
The above-mentioned study also showed that GABA levels were enriched in NE-like cells, compared to normal NE cells. Likewise, NE-like cells expressed functional GABAB receptors (GABBR1), which regulated the invasion of PCa cells and promoted matrix metalloproteinase (MMP) expression29–31. GABBR1 is a metabotropic G-protein-coupled receptor that mediates the inhibitory effects of GABA; these effects play crucial roles in hepatocellular and pancreatic carcinomas32. However, how GABA participates in the invasion of PCa cells remains unknown.
GABA has been reported to regulate the release of neuropeptides and hormones in different peripheral organs. For instance, it governs somatostatin and glucagon secretion in pancreatic beta cells33; and in the stomach, it regulates the secretion of gastrin, somatostatin, and GRP by endocrine cells34. In this study, we investigated the role of GABA in GRP secretion in NE/NE-like cells derived from PCa samples, and its impact in PCa progression. We demonstrated that GABA, through GABBR1, induced GRP secretion. The results suggested that GABA might be a central player in regulating PCa progression when tumors lack the AR.
Results
Establishment of a NE-like cell line with siRNA-mediated AR silencing in LNCaP cells
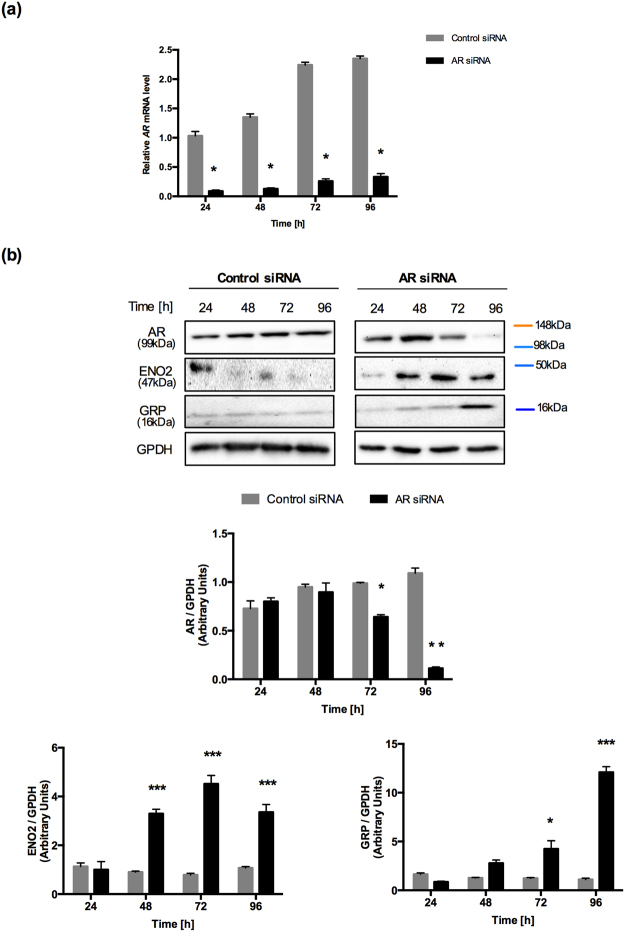
Wright et al. reported that an AR knockdown induced neuronal-specific expression of proteins, like ENO2, which led to a NE phenotype in LNCaP cells11. We decided to use AR-specific siRNA in LNCaP cells to establish a NE cell model. LNCaP cells transfected with an AR-specific siRNA exhibited successful AR mRNA knockdown at 24 h to 96 h (>70% knockdown) compared to cells transfected with control siRNA (*p < 0.0001; Fig. 1a). At the protein level, AR could not be detected at 96 h (**p < 0.01); in contrast, AR protein levels remained unchanged in control cells (Fig. 1b). Furthermore, we confirmed that the AR knockdown led to an elevation in the ENO2 content (***p < 0.001). GRP was also evaluated as a NE protein, as previously described35. We observed that GRP protein levels were also elevated in AR-silenced cells (*p < 0.05 and ***p < 0.001) (Fig. 1b). These results confirmed the hypothesis of a potential link between AR inactivation and the increased frequency of NE cells in androgen-independent tumors11.
Figure 1.
Establishment of NE-like cell line by siRNA targeted to the AR silence in LNCaP cell. (a) AR mRNA expression levels were analyzed by RT-qPCR in LNCaP cells transfected with AR-specific siRNA and control siRNA from 24 h to 96 h in RPMI supplemented with 10% FBS. Experiments were repeated four times. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.0001. (b) Upper panel, Representative Western blot analysis of AR, ENO2 and GRP expression in LNCaP cells transfected with AR-specific siRNA and Control siRNA from 24 h to 96 h. GPDH was used as a loading control. Lower panel, ImageJ software was used for densitometric quantification of proteins. Graphs represent the mean ± SEM (n = 3) *p < 0.05, **p < 0.01 and ***p < 0.001 compared to the control group. The samples derive from the same experiment and that gels/blots were processed in parallel. Full-length blots/gels are presented in Supplementary Fig. 1.
Presence of GABA in LNCaP cells after knocking down AR with siRNA
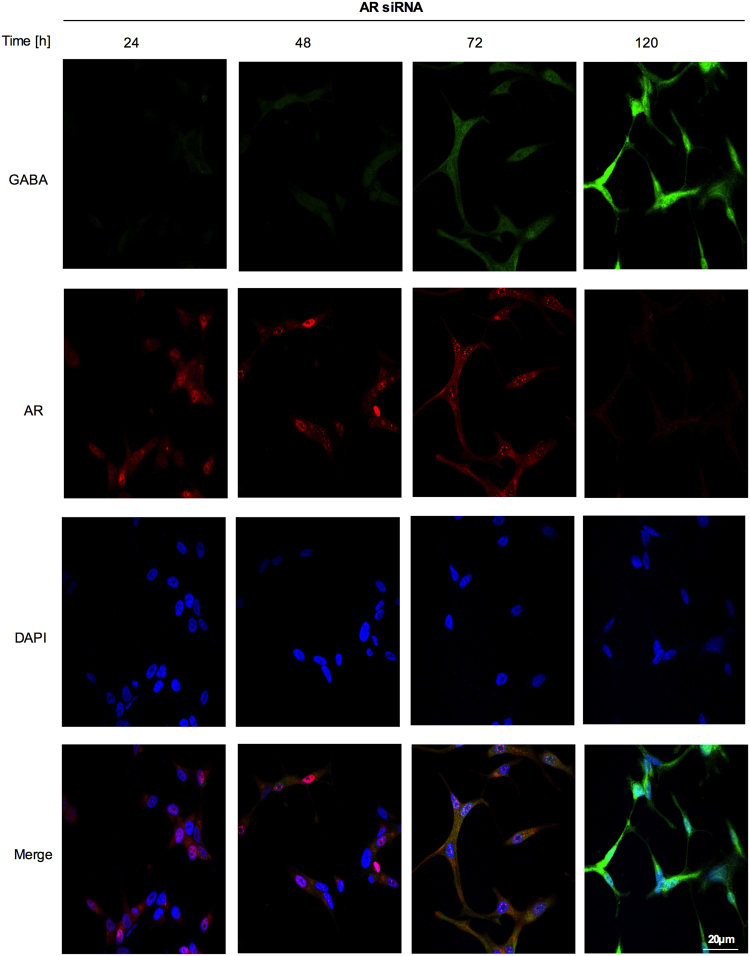
It has been reported that GABA and its receptor were present at higher levels in NEPC cells, compared to other prostate cell types30. This observation prompted to examine whether GABA was present in LNCaP cells after the AR knockdown. Thus, at 96 h, we found that AR levels decreased and GABA levels increased. We also observed that the AR knockdown induced morphological changes in LNCaP cells. At 96 h, cells showed increases in protrusions and extended processes (Fig. 2). Control siRNA did not lead to any morphology changes or changes in GABA levels (Supplementary Fig. 2). Taken together, our results suggested that the acquisition of the NE phenotype was linked to an increase in GABA levels and a decrease in AR expression in LNCaP cells.
Figure 2.
GABA expression is induced in NE-like cells. Fluorescence immunohistochemistry was performed to examine GABA and AR content in LNCaP cells transfected with AR-specific siRNA from 24 to 96 h. An anti-GABA antibody (green staining), anti-AR antibody (red staining) and 4,6-diamidino-2-phenylindole (DAPI) were used. GABA levels were increased after AR silencing. Morphological changes can be seen as well. The proportion of LNCaP cells with obvious protrusions showed an increase once GABA levels were increased and AR was silenced.
Secretion of GABA vesicles from NE-like cells mediated by GABBR1
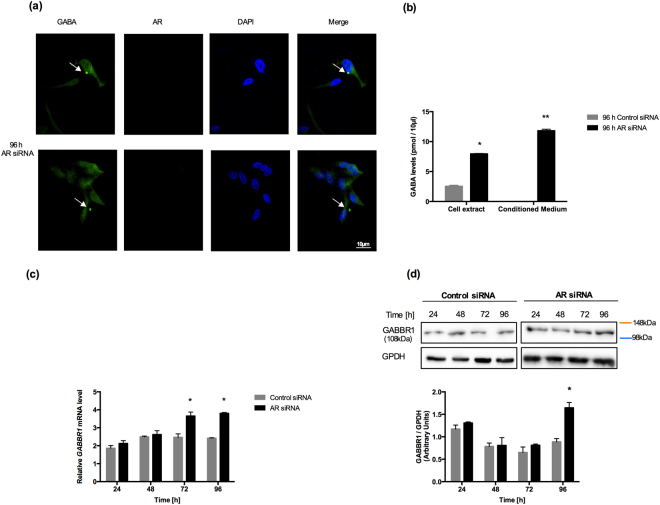
Interestingly, we found that LNCaP cells were able to secrete GABA vesicles 96 hours after the AR-knockdown (Fig. 3a, Supplementary Fig. 3). To evaluate further whether NE-like cells secreted GABA, we used HPLC to measure GABA levels in the cell extracts and conditioned medium of AR-knockdown and control cells at 96 h. We found increased GABA levels in cell extracts of AR-knockdown LNCaP cells (*p < 0.01; Fig. 3b), but not in the conditioned medium of cells transfected with control siRNA (**p < 0.001; Fig. 3b).
Figure 3.
Secretion of GABA vesicles in NE-like cells mediated by GABBR1. (a) Representative immunofluorescence detection of GABA vesicles (green staining) at 96 h in AR siRNA LNCaP cells. The presence of these vesicles was associated with the reduction in AR expression and the display of the neuroendocrine phenotype. Vesicles are indicated by arrows; anti-AR antibody (red staining) and 4,6-diamidino-2-phenylindole (DAPI) were used; (b) GABA levels were evaluated by HPLC in 96 h LNCaP cells transfected with AR-specific siRNA and control siRNA from an aliquot of total lysate proteins and conditioned medium. Experiments were repeated three times. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.01 and **p < 0.001; (c) GABBR1 mRNA expression levels were examined by RT-qPCR in LNCaP cells transfected with AR-specific siRNA and control siRNA. Experiments were repeated three times. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.0001. (d) Western blot analysis of GABBR1 after transfection with AR-specific siRNA and control siRNA from 24 h to 96 h. GPDH was used as a loading control; representative blots are shown of three replicated experiments (Upper panel). Intensity of the bands was quantified with ImageJ software and GABBR1/GPDH is shown in the bar graph (Lower panel) *p < 0.05. The samples derive from the same experiment and that gels/blots were processed in parallel. Full-length blots/gels are presented in Supplementary Fig. 1.
We then assessed the expression of GABAB receptors, based on evidence from previous reports, which suggested that these receptors were implicated in promoting metastasis, particularly in NEPC tumors12,36. GABBR1 expression was induced by ~1.2-fold at 72 h after AR-knockdown in LNCaP cells. This expression level was maintained until 96 h after silencing (*p < 0.0001; Fig. 3c). In contrast, GABBR2 mRNA levels did not change with the AR-knockdown in LNCaP cells (Supplementary Fig. 4). The GABBR1 protein content increased by ~0.8-fold at 96 h (*p < 0.05) (Fig. 3d), when AR was undetectable. Thus, we showed that both GABA levels and GABABR1 expression were elevated in NE-like cells.
Regulation of GRP by exogenous and endogenous GABA-GABBR1 in NE-like cells
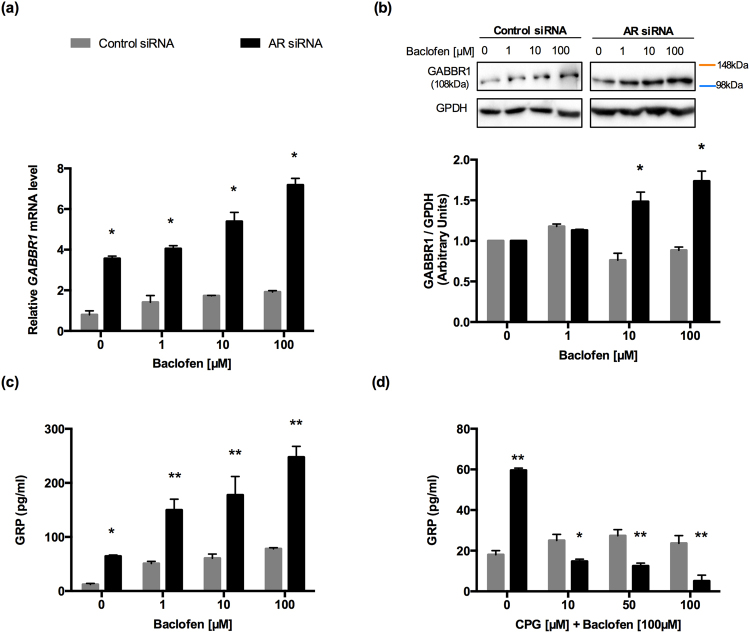
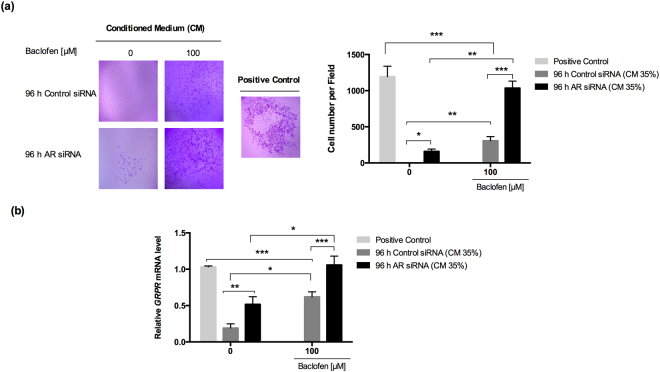
We explored whether GABBR1 participated in the secretion of GRP (involved in cell invasion), as described previously in studies on stomach endocrine cells34,36,37. We studied the expression of GABBR1 in NE-like and control cells treated with baclofen. The expression of GABBR1 in NE-like cells increased with increases in baclofen concentrations (*p < 0.0001; Fig. 4a). In a Western blot analysis, the GABBR1 content was elevated in NE-like cells treated with 10 and 100 µM of baclofen (*p < 0.001; Fig. 4b). Moreover, the GRP concentration was higher in the medium of NE-like cells treated with baclofen compared to that of control cells (Fig. 4c). To confirm the role of GABAB receptor in GRP secretion, we exposed NE-like cells and control cells to the GABAB receptor antagonist, CPG 35348 (10, 50, and 100 µM), for 12 h, combined with baclofen (100 µM). We observed that CPG 35348 blocked the baclofen effects on GRP secretion. In fact, at the highest concentration, CPG 35348 inhibited GRP secretion (Fig. 4d). These results suggested that GABBR1 activation stimulated GRP secretion in NE-like cells.
Figure 4.
GABA promotes GRP secretion mediated by GABBR1 in NE-like cells. (a) GABBR1 mRNA expression was evaluated by RT-qPCR of 96 h AR siRNA cells and 96 h control siRNA cells grown for 12 h in RPMI with indicated concentrations of baclofen. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.0001. (b) GABBR1 protein expression was evaluated by Western Blot in whole cell lysate. GAPDH was used as a loading control. Graphs represent the mean ± SEM (n = 4), *p < 0.001. The last result was normalized to 1 with respect to the vehicle. The concentrations of GRP in the medium of 96 h AR siRNA cells and 96 h control siRNA cells were examined by ELISA (n = 3/group). (c) GRP was present in the medium of both cells incubated with RPMI containing different concentrations of Baclofen (GABAB agonist) 1, 10 and 100 µM for 12 h. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.001 and **p < 0.0001. (d) GABAB antagonist (CGP 35348) was used, and cells were treated with 10, 50 and 100 µM in combination with baclofen (100 µm) for 12 h. Differences among groups were compared by two-tailed unpaired Student’s t test, *p < 0.001 and **p < 0.0001. The samples derive from the same experiment and that gels/blots were processed in parallel. Full-length blots/gels are presented in Supplementary Fig. 1.
Modification in the invasive capacity of PC3 cells by conditioned medium from NE-like cells
We collected conditioned media from NE-like and control cells treated with or without 100 µM of baclofen for 12 h. With a Boyden chamber assay, we evaluated whether the conditioned media could modify the invasive capacity of PC3 cells. We applied synthesized GRP as a positive control for inducing invasion. The upper chamber contained PC3 cells (6 × 104) suspended in 200 µl of culture medium. The lower chamber contained 700 µl of either conditioned medium or GRP (positive control). Samples were incubated for 6 h, as described previously38. We found that PC3 cell invasion was significantly augmented by the conditioned medium from NE-like cells (Fig. 5a). Interestingly, incubation in the conditioned medium from NE-like cells without baclofen treatment significantly increased PC3 cell invasion compared to conditioned medium from control cells (Fig. 5a).
Figure 5.
The conditioned medium of NE-like cells modifies the invasive capacity of PC3 cells through GRPR. (a) Invasion assay was performed by using a Boyden chamber. PC3 cells (6 × 104) suspended in 200 µl of culture medium without FBS were placed in the upper chamber. 700 µl of conditioned medium from 96 h Control siRNA and 96 h AR siRNA treated with or without baclofen (100 µM) was added in the lower chamber for 6 h. Results are represented as mean ± SEM of three independent experiments in triplicate wells, *p < 0.05, **p < 0.001 and ***p < 0.0001. (b) GRPR mRNA expression was analyzed by RT-qPCR. Results are presented as mean ± SEM of three independent experiments in triplicate, *p < 0.05 **p < 0.001 and ***p < 0.0001. GRP synthetized was used as positive control; the previous concentration represents 35% of conditioned medium from 96 h AR siRNA treated with baclofen (100 µM) that was used to perform the experiments.
In addition, we incubated PC3 cells with 35% of conditioned medium from either NE-like cells or control cells, treated with and without baclofen (100 µM) for 24 h. We performed RT-qPCR on cell extracts, and found a significant increase in GRPR expression in PC3 cells treated with conditioned medium from baclofen-treated NE-like cells (Fig. 5b). These results suggested that GRP secreted by NE-like cells increased PC3 invasion by activating GRPR in AR-deficient cells. Similar results were observed in AR-knockdown LNCaP cells at 96 h, when they were stimulated with the conditioned medium described above (Supplementary Fig. 5).
Positive correlations between GABA and GRP levels in patients with NE characteristics
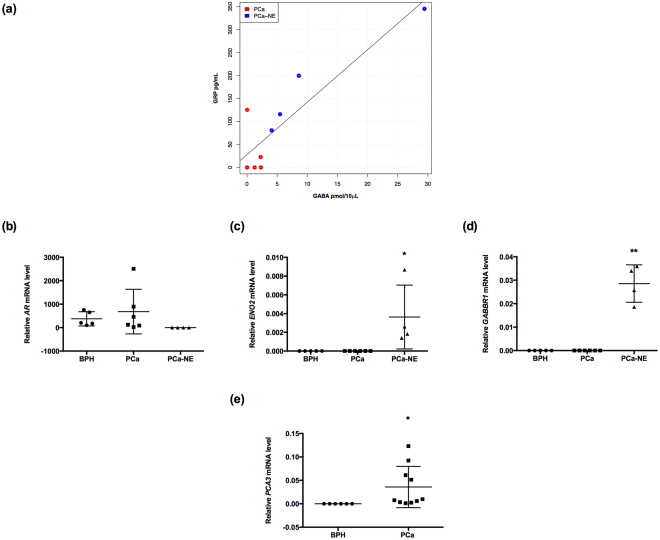
To investigate whether GABA and GRP levels were correlated, we evaluated serum samples from ten patients with PCa. We observed a positive correlation between GABA and GRP levels in all samples (correlation coefficient [r] = 0.894; p = 0.0004). The mean ± SEM serum concentrations were: GRP, 88.92 ± 35.91 pg/ml; and GABA, 5.33 ± 2.8 pmol/10 µl. When the samples were separated into two groups according to their characteristics, the group with NE characteristics showed the strongest positive correlation (r = 0.961; p = 0.03; Fig. 6a).
Figure 6.
GABA levels are positively correlated with GRP levels in patients with NE markers. (a) A positive correlation between GABA and GRP levels was found in PCa patients (r = 0.894; p = 0004; n = 10), and when patients were separated, PCa-NE patients had a strong positive correlation (r = 0.961; p = 0.03; n = 4). (b–d) Relative AR, ENO2 (*p < 0.05), GABBR1 (**p < 0.001) gene expression in BPH vs. PCa and BPH vs. PCa-NE, respectively. AR mRNA levels were not significantly different between groups. (e) PCA3 mRNA levels were significantly higher in PCa patients compared to BPH patients (*p < 0.05).
We also measured levels of AR, ENO2, and GABBR1 in urine samples from patients with PCa (n = 10) and patients with Benign Prostatic Hyperplasia (BPH) (n = 5). The NEPC group displayed the lowest AR levels, compared to the other two groups (PCa and BPH) (Fig. 6b). The NEPC group also displayed elevated ENO2 expression (*p < 0.05; Fig. 6c) and elevated GABBR1 mRNA levels (**p < 0.001; Fig. 6d). As a control for the diagnosis of PCA, PCA3 expression was assessed with RT-qPCR in urine samples from patients with BPH and PCa (*p < 0.05; Fig. 6e)39. Keeping in mind the limited number of samples, these results pointed to a strong correlation between GABA and GRP in patients with NE characteristics.
Correlations between GABBR1 and AR and GABBR1 and ENO2 expression in NEPCs
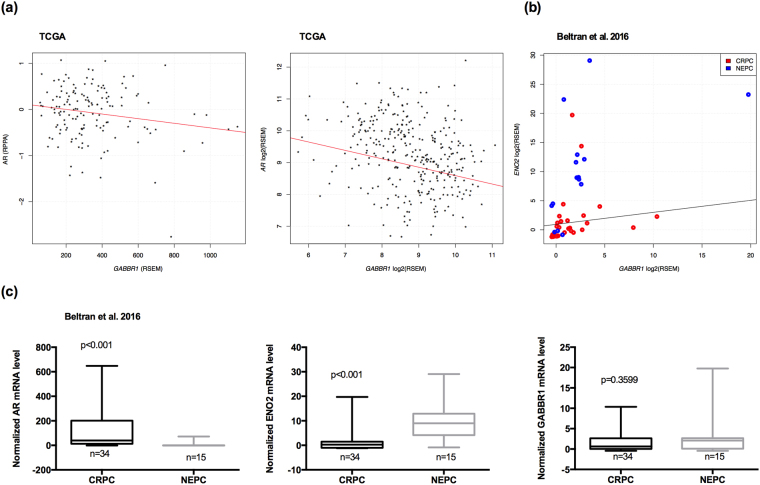
To evaluate further the role of GABBR1 in PCa progression to NEPC, we analyzed AR mRNA levels, AR protein levels, and GABBR1 gene expression in The Cancer Genome Atlas Research Network data set40. Interestingly, we observed a significant negative correlation between the expression of GABBR1 and AR, at the protein level (r = 0.179; p = 0.03; n = 146; Fig. 7a, left) and at the mRNA level (r = −0.274; p = 0.003677−4; n = 333; Fig. 7a, right) in primary PCa tissues. In addition, we examined a data set12 that included CRPC (n = 34) and NEPC (n = 15) samples to assess the regulation of GABBR1 in NED. Importantly, we found a highly significant positive correlation between the expression of GABBR1 and a NE marker (ENO2) in NEPC samples (r = 0.539; p = 0.03; n = 15; Fig. 7b) compared to CRPC samples (r = 0.223; p = 0.22; n = 32; Fig. 7b). Moreover, we observed that AR mRNA levels were indeed down-regulated in NEPC compared to CRPC (Fig. 7c), ENO2 expression was upregulated in NEPC compared to CRPC (Fig. 7c), and GABBR1 expression was similar in NEPC and CRPC (Fig. 7c). These results suggested a negative relationship between GABBR1 and AR expression in patients with PCa, but a positive relationship between GABBR1 and ENO2 expression (a neuronal marker) in patients with NEPC.
Figure 7.
GABBR1 is negatively correlated with AR expression and positively correlated with ENO2 expression in NEPC. (a) GABBR1 and AR expression are negatively correlated at protein level, left panel (r = −0.179; p = 0.03; n = 146) and mRNA level, right panel (r = −0.274; p = 0.003677−4; n = 333). Previously published prostate cancer gene and protein expression data set40 was acquired from cBioPortal51,52. GABBR1 and AR expression values were retrieved and plotted. Previously published RNA-seq of 49 CRPC12 with or without neuroendocrine differentiation was obtained from cBioPortal51,52. (b) GABBR1 and ENO2 mRNA expression are positively correlated in NEPC group (r = 0.539; p = 0.03; n = 15) compared to CRPC group (r = 0.223; p = 0.22; n = 32). (c) The AR, ENO2 and GABBR1 expression values were plotted comparing NEPC to CRPC.
Discussion
This study aimed to unveil the role of GABA in the secretion of GRP, mediated by GABBR1, in NE/NE-like cells from PCa samples. We showed that both endogenous and exogenous GABA could stimulate GRP secretion in NE/NE-like cells. This stimulation promoted invasion in AR-deficient cells.
There is a strong relationship between alterations in AR (both mRNA and protein) levels and the progression of PCa adenocarcinoma to NEPC. Certainly, a large number of studies have shown that, under selective pressure of potent AR inhibition in PCa adenocarcinoma cells, NE-like cells transdifferentiated to form a more aggressive tumor41–43. Recently, Beltran et al., demonstrated that divergent NEPC evolution occurred in association with reduced AR expression, in one or more CRPC cells, through either linear or independent clonal evolution12. Wright et al. showed that AR was required to repress the NE transdifferentiation process in PCa cells in vitro, and they suggested that AR might actively repress an analogous NE transdifferentiation process in PCa cells in vivo. Following this methodology, we also confirmed that, in most cases, transdifferentiation was associated with reduced AR expression11. Moreover, we demonstrated that NE-like cells expressed NE markers, such as ENO2 and GRP. We also found elevated levels of GABA after knocking down AR in cells. Moreover, NE-like cells could secrete GABA vesicles, which enriched the conditioned medium, compared to the conditioned medium of control cells.
GABA and its receptors, GABAA and GABAB, have been identified in NE-like cells derived from PCa samples21. Likewise, enriched GABA levels were found in NE cells and in primary tumors, compared to normal prostate tissues, in transgenic mice with NEPC. Also, elevated GABA levels were found in the serum of transgenic mice compared to that of normal mice30. Furthermore, we detected higher GABBR1 mRNA and protein levels in NE-like cells, which implied that GABBR1 might play a role in the GABAergic pathway of NE-like cells. In addition, the GABAB receptor was implicated in PCa metastasis, because it promoted MMP production36, metastatic spread, and treatment resistance, which was strongly associated with NEPC44.
Another important characteristic of tumors that display NED is the production of NE peptides, such as GRP, which play an important role in the development of castration resistance18,37. Studies have revealed that exogenous and endogenous GABA can stimulate the secretion of peptides such as gastrin, somatostatin and GRP in rat stomach34. Our results gave rise to the hypothesis that GABA and GRP secretion might be regulated by GABBR1 activity in NE-like PCa cells. Indeed, a GABAB antagonist inhibited the elevation in GRP levels induced by baclofen in NE-like cells. We showed that this regulation was mediated by GABBR1, also we found that baclofen increases the mRNA and protein expression of GABBR1 in a dose-dependent manner, in NE-like cells. In future studies, it would be interesting to test whether the GABAA receptor might be implicated in neuropeptide secretion, because it has been shown that GABAA regulates proliferation in PCa45.
GRP has been identified as a potent paracrine and autocrine growth factor in PCa. Moreover, GRP promoted PCa cell migration and protease expression46,47. We noticed higher invasiveness rates mediated by GRPR. This effect was induced by GRP obtained from NE-like cells’ conditioned medium. This finding supported a correlation between NE differentiation and the increased metastatic potential of androgen-insensitive prostate cancers. Keep in mind that cancer invasion is a critical step that leads to the lethality associated with metastasis.
Another important finding from our study was the strong correlation we found between GABA and GRP in the serum of patients with PCa, particularly in serum that contained NE markers. Four out of ten patients displayed lower AR and higher ENO2 gene expression levels than the levels observed in the PCa and the BPH groups, consistent with patients with NEPC in previous studies12,48. Moreover, the same four patients showed elevated GABBR1 mRNA levels.
Patients with NEPC exhibited a higher Gleason score.Azuma et al. observed that patients with PCa metastasis had higher Gleason scores and higher prostate GABA levels compared to patients with PCa without metastasis and patients with BPH36. Yashi et al. found that patients that displayed resistance to androgen blockage showed elevated ProGRP levels in the serum. They also demonstrated that ProGRP levels in a subset of patients tended to increase when the cancer became androgen independent, but remained unchanged or decreased during the androgen-dependent stage19. It would be interesting to follow our patients with NEPC, who displayed elevated GABBR1 and ENO2 gene expression levels, to evaluate the cancer progression. This study was the first to find a direct correlation between GABA and GRP levels, particularly in the NEPC group. However, a larger number of samples would be necessary to provide a more robust statistical analysis.
We found a novel negative association between GABBR1 and AR expression in PCa. Functionally, we showed that AR was downregulated and GABBR1 was upregulated in NE-like cells of PCa samples derived from patients that expressed serum NE markers. Furthermore, we confirmed that these genes were negatively correlated in a PCa data set from primary tumors. As mentioned above, the presence of NED implicated reduced AR activity and/or expression. Multiple studies have suggested that genes involved in NEPC progression were accompanied, or controlled, by the loss of AR expression and/or activity49. Consistent with our finding of this negative correlation, we observed a high positive correlation between GABBR1 and ENO2 expression in NEPC samples. GABBR1 was expressed in both CRPC and NEPC, although the expression was greater in NEPC. This result might be explained by the fact that, among the samples included in the NEPC group, some had low AR-signaling and a low NEPC classification score12.
Overall, our results confirmed a negative correlation between GABBR1 expression and AR (protein and mRNA) expression. It is well known that, once CRPC progresses to NEPC, PCa cells lose the AR, which makes them resistant to any drug that targets the AR35. GABBR1 may represent a new therapeutic strategy for treating late-stage castrate-resistant tumors that have lost the AR and have acquired a NE phenotype. Based on our results, we propose that GABBR1 is expressed when cells have a well-defined NE phenotype, which includes the presence of NE markers and low AR expression.
In conclusion, our results suggested that GABA, through GABBR1 activation in NE-like cells, induced GRP secretion, which in turn, increased cell invasion. The exact molecular mechanism through which the GABA/GABBR1 axis is involved in GRP secretion remains an important field of study and requires further investigation. However, we believe the results of this study will contribute to a better understanding of NEPC tumors.
Methods
Cells culture and treatment
The human prostate adenocarcinoma cell lines LNCaP and PC3 were obtained from the American Type Culture Collection (ATCC) used with passage number between 10 and 12. LNCaP was cultured in RPMI-1640 medium (Sigma-Aldrich), and PC3 was grown in DMEM medium (Corning). Both media contained 10% Fetal Bovine Serum (FBS) (Corning). Cells were maintained at 37 °C under 5% CO2. Baclofen at 1, 10 and 100 µM for 12 h (Sigma-Aldrich) was used to activate GABAB receptor, and CGP 35348 (Sigma-Aldrich) for antagonizing GABAB receptor. Cancer cells were pretreated with receptor antagonist for 2 h at concentrations of 10, 50 and 100 µM, to inhibit GRP secretion, and then incubated with GABA (100 µM) for 12 h.
Small interfering RNA (siRNA) transfection
LNCaP cells (2 × 105) were grown in phenol-red free RPMI medium (Sigma-Aldrich). Then, cells were transfected with Silencer select (Life Technologies) siRNA targeting AR (siRNA ID: s1538) and Negative control (siRNA ID: AM4615) using DharmaFECT 2 transfection reagent (Dharmacon) as described by manufacturer’s instructions. siRNAs final concentration was 12.5 nM per experiment. Cells were harvested every 24 h for 96 h.
Patient cohort
A total of 10 male Mexican patients, being treated at the Hospital General Dr. Manuel Gea Gonzalez (Mexico City, Mexico) between July 2012 and February 2013, were enrolled in the present study. Written informed consent was obtained from all patients. The protocol of the present study was approved by the ethics committee of Hospital General Dr. Manuel Gea Gonzales (No.CIEI/144/12), project registration number (28-53-2012). All experiments and methods were performed according to these relevant guidelines and regulations. All patients presented palpable nodules or induration of the prostate [suspicious digital rectal examination (DRE)] and blood levels of PSA > 2.5 ng/ml. Subsequently, an experienced pathologist assessed the cores to determine whether the biopsy specimens were malignant. For those samples pathologically diagnosed as PCa, Gleason scoring was performed by careful examination of the microscopic pattern of the cancer foci. The control group comprised 6 patients with Benign Prostatic Hyperplasia (BPH), whose biopsy specimens contained benign glands and normal prostate cells. Blood and urine samples were obtained. Patient’s clinical and pathological data were collected (Table 1).
Table 1.
Clinical features.
| Pt. | Age | Gleason score | PSA (ng/ml) | Surgery | Hormone therapy |
|---|---|---|---|---|---|
| Benign prostate hyperplasia | |||||
| 1 | 62 | − | 3.3 | Prostatectomy | − |
| 2 | 75 | − | 5.65 | Prostatectomy | − |
| 3 | 68 | − | 6.5 | Prostatectomy | − |
| 4 | 72 | − | 5.53 | Prostatectomy | − |
| 5 | 65 | − | 16.2 | Prostatectomy | − |
| Prostate cancer | |||||
| A | 65 | 3 + 3 | 4.87 | Prostatectomy | − |
| B | 65 | 4 + 4 | 12 | TURP | − |
| C | 64 | 4 + 4 | 4.5 | TURP | − |
| D | 83 | 5 + 4 | 31 | TURP | + |
| E | 80 | 3 + 3 | 8.4 | TURP | + |
| F | 74 | 3 + 3 | 16.0 | TURP | − |
| G*◆ | 64 | 4 + 4 | 147 | − | + |
| H* | 76 | 4 + 3 | 160 | − | + |
| I* | 74 | 3 + 3 | 32 | TURP | + |
| J* | 77 | 3 + 3 | 30 | TURP | + |
*Prostate cancer patients with NE characteristics; ◆Castration-resistant prostate cancer patient; PSA, prostate-specific antigen.
Urine sample processing and RNA extraction
Urine sample processing was performed as described39. The collected urine with prostate fluid was mixed with 5 ml RNAlater buffer (Qiagen) as an RNA stabilizing agent. RNA extraction was performed using the RNeasy Mini kit (Qiagen) according to manufacturer’s guidelines.
Real-time RT-PCR assay
Total RNA from cell lines was isolated using an RNeasy kit (Qiagen) according to manufacturer’s instructions. A total of 800 ng from cell lines and 250 ng from urine samples [as described previously39] of RNA were reverse transcribed in a thermal cycler using SuperScript II (Invitrogen) according to manufacturer’s instructions. All reagents for TaqMan PCR, including AR (Hs00171172_ml), GAPDH (Hs02758991_gl), KLK3 (Hs01105076_m1), PCA3 (Hs01371939_g1), GABBR1 (Hs00559488_m1), GRPR (Hs01055872_m1), ENO2 (Hs00157360_m1) were purchased from Life Technologies. Reactions were run in a 96-well plate format using a Viia 7 Real Time PCR-System (Life Technologies). The quantitative real-time PCR results were analyzed using the 2−∆∆Ct method, using GAPDH as housekeeping gene.
Protein extraction and Western blot analysis
Cells were lysed with RIPA buffer (Sigma-Aldrich) with added protease inhibitors (Sigma-Aldrich). Proteins were obtained by centrifugation at 12,500 rpm at 4 °C for 30 min and quantified using an EZQ Protein Quantitation Kit (Life Technologies) according to manufacturer’s instructions. Proteins (30 µg) were separated by electrophoresis on SDS-PAGE (Bio-Rad). SeeBlue Plus2 (Invirogen) was used for sizing and visualization of the gel running pattern and protein transfer. Resolved proteins were transferred on immobilon PVDF membranes (Millipore) at 20 V for 30 min. Membranes were air-dried, wet with methanol, washed with TBS-T and blocked at 4 °C overnight in 1% (w/v) non-fat skimmed milk/TBS-T. Membranes were incubated with antibodies against AR (1:500 ab108341), GABBR1 (1:500 ab75239) from ABCAM, GAPDH (1:20000 5174 s), ENO2 (1:200 9536 s) obtained from Cell Signaling and GRP (1:500 sc-271045) from Santa Cruz Biotechnology at 4 °C overnight.
Blots were then incubated with anti-rabbit secondary antibody (1:5000 ab99697) obtained from ABCAM or anti-mouse secondary antibody (1:300 7076 s) from Cell Signaling conjugated to horseradish peroxidase (HRP) at room temperature (RT) for 2 h. Chemiluminescence signals were detected using ChemiDoc XRS system (BioRad). Western immunoblots were quantified using ImageJ software.
Immunofluorescence
LNCaP cells were transfected as mentioned above, cells were collected every 24 h for 96 h. Next, cells were fixed with 4% (v/v) paraformaldehyde, permeabilized with1% (v/v) Triton X-100. Then, cells were blocked with 1% bovine serum albumin in PBS for 2 hat RT and labeled with rabbit polyclonal anti-GABA (1:100 A2052; Sigma-Aldrich) for 1 hat RT and mouse monoclonal anti-AR (1:50 ab9474; ABCAM) overnight at 4 °C in PBS. The anti-GABA antibody was detected with an Alexa Fluor 488 donkey anti-rabbit antibody (Invitrogen), and the anti-AR antibody was detected with an Alexa Fluor 568 anti-mouse antibody (Invitrogen), incubated for 1 h. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Immunohistochemistry was observed using a Confocal Microscope (Zeiss LSM 510 Axiovert 200 M Laser Scanning).
Preparation of conditioned medium (CM)
In order to collect conditioned medium (CM), LNCaP-siAR and LNCaP-Control were incubated in phenol-red free RPMI culture medium with different concentrations of Baclofen (1, 10, 100 and 500 µM), or vehicle (sterile water) in a humidified incubator for 12 h, after 96 h of transfection. CM was collected and centrifuged at 2000 g for 10 min at 4 °C; medium was filtered through a 0.2 µm nylon filter; then, protease inhibitors (Sigma-Aldrich) were added and immediately used or frozen at −80 °C until needed.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to quantify GRP concentrations in conditioned medium and serum patients. Serum samples were separated using ultra centrifuge filters 3 K (Amicon). In brief, analyses were performed in 96-well polyvinyl chloride (PVC) microtiter plates (Maxisorp, Nunc). Plates were coated at 4 °C overnight with conditioned medium. Plates were washed three times (PBS-Tween solution) and then non-specific sites were blocked with bovine serum albumin at 37 °C for 2 h. After one washing step, a monoclonal antibody specific for GRP human (0.5 µg/ml; GenScript) was added to the plates at 4 °C overnight. Plates were washed and the Anti-rabbit secondary antibody (1:8000 ab99697, ABCAM) conjugated to horseradish peroxidase (HRP) was incubated at RT for 2 h. After another wash, final reaction was determined by adding 100 µl of freshly prepared peroxidase substrate solution (TMB-ELISA; Thermo Scientific). Enzyme was blocked with 100 µl H2SO4·2 N. The optical density was measured at 450 nm using an Epoch Microplate spectrophotometer. GRP concentrations were determined according to a standard curve from synthesized peptide (GRP; GeneScript), and results were given in pg/ml.
Invasion assay
Cell invasion assay was performed in a transwell chamber (24-well, 8μm pore size; Corning), with the bottom coated with 1 mg/ml BD Matrigel Matrix (BD Biosciences). PC3 and LNCaP cells were re-suspended in serum free DMEM medium, and 200 µl of the cell suspension (6 × 104) was supplemented to the upper chamber membranes. 700 µl of indicated conditioned medium was put into the lower chamber as a chemoattractant. After 6 h or 24 h of PC3 and LNCaP cells incubation at 37 °C in a 5% CO2 humidified atmosphere, respectively, non-invaded cells on the surface of the membrane were removed with a cotton swab. Cells that had invaded through the filter to the lower surface were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Each experiment was performed 3 times in triplicate. The number of invading cells was counted in 4 fields within each trans-well. Determination of the number of invaded cells was performed using ImageJ software.
Amino acid analysis
The levels of GABA in total lysate proteins and conditioned medium were measured by HPLC as described previously by Salazar et al.50. An aliquot was used for protein determination and perchloric acid (0.6% final concentration) was added to the homogenates. Protein was sedimented in a microfuge. The supernatant was neutralized with KOH and centrifuged again to sediment the precipitated potassium perchlorate. Amino acids were derivatized with O-phthalaldehyde and after 3 min 20 µl were injected into a Beckman liquid chromatograph, using a reverse phase ODS column (25 cm + 4 mm) and a linear gradient (from 25% to 75% methanol) of a methanol mixture: 0.1 mM potassium acetate buffer, pH 5.5. The Amino acids were fluorometrically detected, and the identification and quantitation of peaks were compared according to their retention times taking into account standards for amino acid mixtures and additional internal standards.
Statistical analysis
Gene and protein expression levels from RT-qPCR, Western blot and data from invasion assay experiments were analyzed in Graph Pad 6.0 using the two-tailed unpaired Student t-test when comparing two groups and a one-way ANOVA for several groups at once. To assess the correlation between GABBR1 expression and AR (with gene and protein expression from TCGA and from our independent patient cohort) a test for association between paired samples, using Pearson’s product moment correlation coefficient was performed. In this context, correlation of CRPC and NEPC was assessed jointly as well as independently. The R language for statistical computing (http://www.R-project.org) was used for this analysis and statistical significance was assigned at p < 0.05.
Electronic supplementary material
Acknowledgements
We would like to thank the “Programa de Doctorado en Ciencias Biomédicas-UNAM” for providing academic support to Dr. Susana del Rocio Solórzano Rosales. The present study was supported by CONACYT (grants 177687 and 390027). We are grateful to Ana Rosshandler Fernandez for grammar review of the manuscripts as well as M.Sc. José Luis Cruz Colin and Dr. Valeria Quintanar Jurado for the technical assistance provided.
Author Contributions
S.R.S. carried out the experiments, analyzed data and manuscript writing. I.I.R. performed bioinformatics analysis and provided scientific discussion. P.G.T., P.S., and M.L.A.O. performed experiments and technical support. G.M.M. provided clinical samples and clinical support. I.C.A. provided scientific discussion and reviewed of manuscript. S.R.S., and M.R.D. conceived and designed the study. M.R.D. provided financial support, data analysis and interpretation. All authors approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28538-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 4.Dutt SS, Gao AC. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009;5:1403–1413. doi: 10.2217/fon.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 6.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clin Genitourin Cancer. 2011;9:73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Beltran H, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Frontiers in oncology. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.van Bokhoven A, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 11.Wright ME, Tsai MJ, Aebersold R. Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol. 2003;17:1726–1737. doi: 10.1210/me.2003-0031. [DOI] [PubMed] [Google Scholar]
- 12.Beltran H, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, et al. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. 2006;66:1399–1406. doi: 10.1002/pros.20434. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 15.Hoosein NM, Logothetis CJ, Chung LW. Differential effects of peptide hormones bombesin, vasoactive intestinal polypeptide and somatostatin analog RC-160 on the invasive capacity of human prostatic carcinoma cells. J Urol. 1993;149:1209–1213. doi: 10.1016/S0022-5347(17)36349-8. [DOI] [PubMed] [Google Scholar]
- 16.Ischia J, Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide: different forms, different functions. Biofactors. 2009;35:69–75. doi: 10.1002/biof.10. [DOI] [PubMed] [Google Scholar]
- 17.Bologna M, Festuccia C, Muzi P, Biordi L, Ciomei M. Bombesin stimulates growth of human prostatic cancer cells in vitro. Cancer. 1989;63:1714–1720. doi: 10.1002/1097-0142(19900501)63:9<1714::AID-CNCR2820630912>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21:8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yashi M, Muraishi O, Kobayashi Y, Tokue A, Nanjo H. Elevated serum progastrin-releasing peptide (31–98) in metastatic and androgen-independent prostate cancer patients. Prostate. 2002;51:84–97. doi: 10.1002/pros.10063. [DOI] [PubMed] [Google Scholar]
- 20.Garabedian EM, Humphrey PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc Natl Acad Sci USA. 1998;95:15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI. Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice. J Biol Chem. 2002;277:44462–44474. doi: 10.1074/jbc.M205784200. [DOI] [PubMed] [Google Scholar]
- 22.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8(721-737):715. doi: 10.1038/sj.mp.4001395. [DOI] [PubMed] [Google Scholar]
- 23.Tillakaratne NJ, Erlander MG, Collard MW, Greif KF, Tobin AJ. Glutamate decarboxylases in nonneural cells of rat testis and oviduct: differential expression of GAD65 and GAD67. J Neurochem. 1992;58:618–627. doi: 10.1111/j.1471-4159.1992.tb09763.x. [DOI] [PubMed] [Google Scholar]
- 24.Levy LJ, Leek J, Losowsky MS. Evidence for gamma-aminobutyric acid as the inhibitor of gamma-aminobutyric acid binding in the plasma of humans with liver disease and hepatic encephalopathy. Clin Sci (Lond) 1987;73:531–534. doi: 10.1042/cs0730531. [DOI] [PubMed] [Google Scholar]
- 25.Glassmeier G, et al. Expression of functional GABAA receptors in isolated human insulinoma cells. Ann N Y Acad Sci. 1998;859:241–248. doi: 10.1111/j.1749-6632.1998.tb11138.x. [DOI] [PubMed] [Google Scholar]
- 26.Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC. The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology. 1991;129:2521–2529. doi: 10.1210/endo-129-5-2521. [DOI] [PubMed] [Google Scholar]
- 27.Geigerseder, C. et al. Evidence for a GABAergic system in rodent and human testis: local GABA production and GABA receptors. Neuroendocrinology77, 314–323, doi:70897 (2003). [DOI] [PubMed]
- 28.Erdo SL, Nemet L, Szporny L. The occurrence of GABA in vas deferens, prostate, epididymis, seminal vesicle and testicle of the rat. Acta Biol Hung. 1983;34:435–437. [PubMed] [Google Scholar]
- 29.Abdul M, McCray SD, Hoosein NM. Expression of gamma-aminobutyric acid receptor (subtype A) in prostate cancer. Acta Oncol. 2008;47:1546–1550. doi: 10.1080/02841860801961265. [DOI] [PubMed] [Google Scholar]
- 30.Ippolito JE, et al. An integrated functional genomics and metabolomics approach for defining poor prognosis in human neuroendocrine cancers. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9901–9906. doi: 10.1073/pnas.0500756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ippolito JE, et al. Linkage between cellular communications, energy utilization, and proliferation in metastatic neuroendocrine cancers. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12505–12510. doi: 10.1073/pnas.0605207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longqiu Y, Pengcheng L, Xuejie F, Peng Z. A miRNAs panel promotes the proliferation and invasion of colorectal cancer cells by targeting GABBR1. Cancer Med. 2016;5:2022–2031. doi: 10.1002/cam4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun M, et al. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes. 2010;59:1694–1701. doi: 10.2337/db09-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weigert N, Schepp W, Haller A, Schusdziarra V. Regulation of gastrin, somatostatin and bombesin release from the isolated rat stomach by exogenous and endogenous gamma-aminobutyric acid. Digestion. 1998;59:16–25. doi: 10.1159/000007462. [DOI] [PubMed] [Google Scholar]
- 35.Qiao, J. et al. Activation of GRP/GRP-R signaling contributes to castration-resistant prostate cancer progression. Oncotarget, 10.18632/oncotarget.11326 (2016). [DOI] [PMC free article] [PubMed]
- 36.Azuma H, et al. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer research. 2003;63:8090–8096. [PubMed] [Google Scholar]
- 37.Levine L, et al. Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer research. 2003;63:3495–3502. [PubMed] [Google Scholar]
- 38.Nagakawa O, et al. Effect of prostatic neuropeptides on invasion and migration of PC-3 prostate cancer cells. Cancer Lett. 1998;133:27–33. doi: 10.1016/S0304-3835(98)00186-4. [DOI] [PubMed] [Google Scholar]
- 39.Salido-Guadarrama AI, et al. Urinary microRNA-based signature improves accuracy of detection of clinically relevant prostate cancer within the prostate-specific antigen grey zone. Mol Med Rep. 2016;13:4549–4560. doi: 10.3892/mmr.2016.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer Genome Atlas Research, N. The Molecular Taxonomy of Primary Prostate Cancer. Cell163, 1011–1025, 10.1016/j.cell.2015.10.025 (2015). [DOI] [PMC free article] [PubMed]
- 41.Lin D, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res. 2014;74:1272–1283. doi: 10.1158/0008-5472.CAN-13-2921-T. [DOI] [PubMed] [Google Scholar]
- 42.Frigo DE, McDonnell DP. Differential effects of prostate cancer therapeutics on neuroendocrine transdifferentiation. Mol Cancer Ther. 2008;7:659–669. doi: 10.1158/1535-7163.MCT-07-0480. [DOI] [PubMed] [Google Scholar]
- 43.Terry S, et al. Cross Modulation between the Androgen Receptor Axis and Protocadherin-PC in Mediating Neuroendocrine Transdifferentiation and Therapeutic Resistance of Prostate Cancer. Neoplasia. 2013;15:761–IN722. doi: 10.1593/neo.122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nouri M, et al. Androgen-targeted therapy-induced epithelial mesenchymal plasticity and neuroendocrine transdifferentiation in prostate cancer: an opportunity for intervention. Front Oncol. 2014;4:370. doi: 10.3389/fonc.2014.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young SZ, Bordey A. GABA’s control of stem and cancer cell proliferation in adult neural and peripheral niches. Physiology (Bethesda) 2009;24:171–185. doi: 10.1152/physiol.00002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Festuccia C, et al. In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer. 1998;75:418–431. doi: 10.1002/(SICI)1097-0215(19980130)75:3<418::AID-IJC16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 48.Kim J, et al. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene. 2017;36:4072–4080. doi: 10.1038/onc.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzelepi V, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clin Cancer Res. 2012;18:666–677. doi: 10.1158/1078-0432.CCR-11-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar P, Montiel T, Brailowsky S, Tapia R. Decrease of glutamate decarboxylase activity after in vivo cortical infusion of gamma-aminobutyric acid. Neurochem Int. 1994;24:363–368. doi: 10.1016/0197-0186(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 51.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.