Abstract
Background
Management advice for women with lobular carcinoma in situ (LCIS) is hampered by the lack of accurate personalised risk estimates for subsequent invasive breast cancer (BC). Prospective validation of the only tool that estimates individual BC risk for a woman with LCIS, the International Breast Cancer Intervention Study Risk Evaluation Tool (IBIS-RET), is lacking.
Methods
Using population-based cancer registry data for 732 women with LCIS, the calibration and discrimination accuracy of IBIS-RET Version 7.2 were assessed.
Results
The mean observed 10-year risk of invasive BC was 14.1% (95% CI:11.3%-17.5%). IBIS-RET overestimated invasive BC risk (p = 0.0003) and demonstrated poor discriminatory accuracy (AUC 0.54, 95% CI: 0.48 – 0.62).
Conclusions
Clinicians should understand that IBIS-RET Version 7.2 may overestimate 10-year invasive BC risk for Australian women with LCIS. The newer IBIS-RET Version 8.0, released September 2017, includes mammographic density and may perform better, but validation is needed.
Subject terms: Breast cancer, Cancer prevention
Introduction
Women diagnosed with lobular carcinoma in-situ (LCIS) have an elevated risk of subsequent invasive breast cancer (BC)1 that increases by about 1% every year after diagnosis to 13% after 10 years, 11%-26% at 15 years1–3 and 21%-26% risk after 20 years.4, 5
Most are managed with observation alone,6 but American Society of Clinical Oncology and Cancer Australia guidelines recommend that risk-reducing medications, specifically selective oestrogen receptor modulators (SERM) or aromatase inhibitors (AI) (the latter only in postmenopausal women), be discussed with LCIS patients.7–10 Risk-reducing bilateral mastectomy is pursued by only a minority of LCIS patients.11 Informed decision-making would be facilitated by accurate personalised risk estimates for future invasive BC.
The International Breast Cancer Intervention Study Risk Evaluation Tool (IBIS-RET) is the only tool available to estimate risk for an individual woman with LCIS. 12–14 Although validated in other populations,15–17 IBIS-RET, to our knowledge, has not been validated in LCIS patients. Using population-based data, we prospectively examined the performance of IBIS-RET Version 7.2 for estimating invasive BC risk for women with a history of LCIS.
Methods
The Victorian Cancer Registry (VCR) has collected data on all cancer diagnoses in Victoria, Australia since 1982. It determines vital status of all registrants by record linkage to the state and national death registries.18 De-identified data including dates of birth, death, LCIS and invasive BC diagnoses were obtained from the VCR for all women diagnosed with pure LCIS between 1982 and 2015, when aged 20–70 years. ‘Pure’ LCIS was defined as LCIS without previous or synchronous ductal carcinoma in-situ (DCIS) or invasive BC in either breast (including within 6 months after LCIS diagnosis). Women with other invasive cancer diagnoses (except non-melanotic skin cancer) prior to their pure LCIS diagnosis were excluded. The study was approved by the Peter MacCallum Cancer Centre ethics committee.
The calibration and discriminatory accuracy of the 10-year IBIS-RET Version 7.2 estimates were assessed by comparing IBISRET-assigned risks with observed invasive BC incidence. To assess calibration, the mean IBIS-RET-assigned risk was compared with the mean 10-year observed invasive BC incidence in each IBIS-RET-assigned risk group, using a chi-squared goodness-of-fit statistic,19 for the whole cohort (by tertiles) and also for two subgroups stratified by the diagnosis of LCIS before and at or after age 50 years. To evaluate discriminatory accuracy, the overall area under the receiver operating characteristic (ROC) curve for the development of invasive BC within 10 years of LCIS diagnosis was computed. RMAP (https://gailg.github.io/rmap/) and SAS software 9.4 (SAS Institute, Cary, NC) were used. Data were censored at date of invasive BC diagnosis, death and date that the most recently linked death data were considered complete (31st December 2015). Two exploratory analyses were also conducted; the first censored the data at date of any ductal carcinoma in situ (DCIS) diagnosis and the second included diagnosis of either invasive breast cancer and/or DCIS as the primary endpoint.
Results
There were 732 eligible women (median age at LCIS 50 years, range 25–70 years, mean follow-up 9.8 years, range 0.04–33.9 years, total 4855 person-years), of whom 73 were diagnosed with invasive BC within 10 years after their LCIS. 10 women died within 10 years without an invasive BC diagnosis, 293 women were invasive BC-free at 10 years and 356 women were last observed without invasive BC with less than 10 years follow-up. The mean observed risk of invasive BC at 10 years was 14.1% (95% confidence interval (CI) 11.3%-17.5%), whilst the mean assigned IBIS-RET 10-year risk was 20.9%.
Figure 1 shows the cumulative invasive BC incidence by 10-year IBIS-RET-assigned risk tertile (i.e. <18.8%, ≥18.8%–<23.5%, ≥23.5%).
Fig. 1.
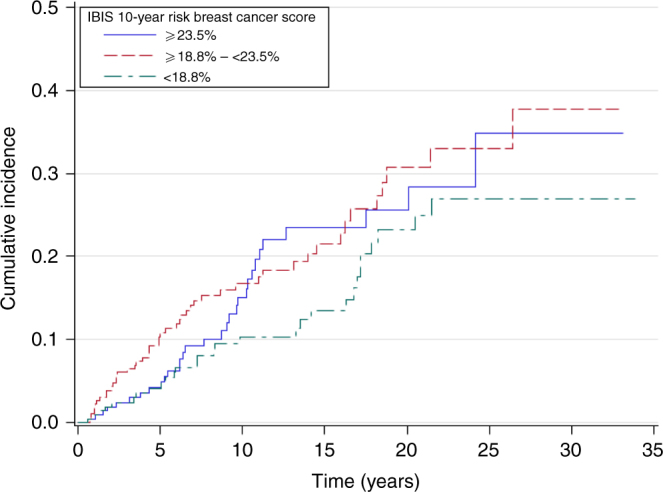
Cumulative incidence of invasive breast cancer in patients with LCIS, by tertile of IBIS-RET estimated risk
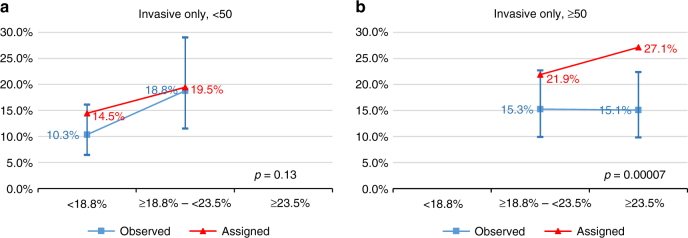
Figure 2 shows that the mean IBIS-RET-assigned invasive BC risks by tertile were significantly different to the observed BC incidence at 10 years (p = 0.0003). Overall the IBIS-RET Version 7.2 tended to overestimate invasive BC risk. When we compared the calibration for women below and above age 50 using internal cutpoints by age, we found it was well-calibrated for women diagnosed before age 50 years (p = 0.13), but not for those diagnosed at or after age 50 years mainly owing to the lack of fit and overestimation by IBIS-RET for older women in the highest quantile, (p = 0.00007) (Fig. 3a,b).
Fig. 2.
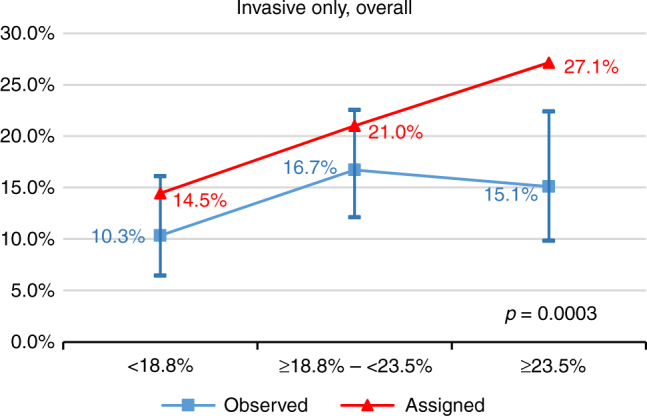
Calibration of IBIS-RET for estimates of invasive BC in women with LCIS. The assigned line (triangle symbol) is the mean 10-year predicted risks of the IBIS-RET for that tertile (<18.8%, ≥18.8%–<23.5%, ≥23.5%). The observed line (square symbol) is the estimates of 10-year breast cancer probabilities based on the womens' observed breast cancer status, and the bars denote 95% confidence intervals for the observed risk
Fig. 3.
a Calibration of IBIS-RET for estimates of invasive BC in women with LCIS diagnosed at <50 years (cut-off: <18.8%, ≥18.8%–<23.5%). b Calibration of IBIS-RET for estimates of invasive BC in women with LCIS diagnosed at >=50 years (cut-off: ≥18.8%–<23.5%, ≥23.5%). The assigned line (triangle symbol) is the mean 10-year predicted risks of the IBIS-RET for that tertile (<18.8%, ≥18.8%-<23.5%, ≥23.5%). The observed line (square symbol) is the estimates of 10-year breast cancer probabilities based on the womens’ observed breast cancer status, and the bars denote 95% confidence intervals for the observed risk
The area under the ROC curve (AUC) for the IBIS-RET 10-year invasive BC risk estimates was 0.54 (95% CI: 0.48 – 0.62) overall, and 0.55 (95% CI: 0.44 – 0. 0.63) and 0.48 (95% CI: 0.38 – 0.57) for women diagnosed with LCIS before age 50 years and at or after age 50 years, respectively.
Nine women were diagnosed with DCIS during the first 10 years after their LCIS diagnosis. The results were similar when the analyses were repeated either censoring at DCIS diagnosis or including the 9 cases of DCIS along with the invasive BC cases in the primary endpoint (Supplementary Figure 1).
Discussion
In this population-based study of women with LCIS, the IBIS-RET Version 7.2 tended to overestimate 10-year invasive BC risk and had poor discriminatory accuracy. However this study had several limitations that may have contributed to this finding.
The lack of information in our dataset regarding uptake of bilateral mastectomy or risk-reducing medication after LCIS diagnosis could have resulted in our study erroneously finding that IBIS-RET overestimates BC risk. However, uptake of these interventions is historically very low in Australia, even in very high-risk women,20 so this is unlikely to have been a major factor.
Histopathological diagnostic thresholds for atypical hyperplasia (AH) and LCIS have changed over time.21 If some of the cases included in this study were in fact AH misclassified as LCIS, this could have contributed to our finding that IBIS-RET V7.2 overestimates BC risk, because AH confers a lower BC risk than LCIS. No pathology review of cases was undertaken by the authors or the VCR.
The IBIS-RET model is calibrated to UK BC incidence rates for 2008–2010 (Supplementary Table 1).22 Although our study covers Victorian women over a period from 1982–2015, the average age-specific BC incidence figures for these Victorian women closely resembled those used in the IBIS-RET model, except for the lower incidence in those aged 50 years and over (Supplementary Table 1).23 This could have contributed to the overestimation and poorer calibration in our dataset for women diagnosed with LCIS at and over age 50 years, but it is unlikely to completely explain our findings.
Overdiagnoses from mammographic screening could have influenced our findings. However, Victorian women aged 50 years and over are screened 2-yearly as opposed to 3-yearly in the UK and this higher screening frequency should, if anything have resulted in a higher invasive BC incidence when compared to the UK population, but this was not observed in those aged older than 50 years (Supplementary Table 1).
Patient migration out of Victoria after LCIS diagnosis would mean that some subsequent invasive breast cancers were not captured in the VCR data. Using aggregate data from the Australian Institute of Health and Welfare, we estimated that this interstate migration would have resulted in approximately 3 cases of invasive cancer being missed in our dataset, which could in part have contributed to our finding that IBIS-RET overestimates BC risk.
The study dataset also did not include information on other BC risk factors, but because IBIS-RET Version 7.2 relies only on age to estimate subsequent BC risk in women with LCIS, this would not have impacted our findings. In fact there are conflicting reports on whether age at LCIS diagnosis affects subsequent BC risk. One study reported that the relative risk of BC tended to decrease with increasing age at LCIS diagnosis.4 Conversely, according to King et al,3 risk factors like family history, age and breast density were not associated with BC risk in women with LCIS. Instead, the authors found that chemoprevention was the major factor associated with lower BC risk (Hazard ratio, 0.27; 95% CI, 0.15 to 0.50). They also performed a subgroup nested case-control analysis, which showed that the volume of LCIS, which was defined as the ratio of slides with LCIS to total number of slides reviewed, was associated with BC development (p = 0.008). Therefore, volume of LCIS might provide further risk stratification in women with LCIS.
Mammographic density is an important risk factor for breast cancer and has been shown to refine the IBIS-RET model in predicting BC risk for women at increased risk, although not specifically in women with LCIS.24 A subsequent study using a UK prospective BC screening cohort showed that using mammographic density with the IBIS-RET improved the accuracy of BC risk prediction.25
Since starting this study, IBIS-RET, Version 8 22 has been released (September 2017). For women with LCIS, Version 8 differs from Version 7.2 in that it now uses cancer family history and mammographic density (if available) to predict BC risk, as well as age at diagnosis of LCIS. The addition of cancer family history means that, in the absence of mammographic density information, IBIS-RET Version 8 will always provide the same or higher 10-year BC risk estimates as Version 7.2 (which we have shown here already tends to overestimate risk). However, if mammographic density is known to be low, the risk estimate provided by Version 8 may be lower than that provided by Version 7.2.25 A validation study of IBIS-RET Version 8, using a dataset of women with LCIS and known mammographic density and family history, is highly desirable.
Electronic supplementary material
Supplementary Figure 1. Calibration of IBIS-RET for estimates of BC (invasive or DCIS) in women with LCIS
Supplementary Table 1. Average age-specific breast cancer incidence (per 100,000 women) for the Victorian population from 1982 to 2015 compared to those used in IBISRET model v7/8
Acknowledgements
We thank Helen Farrugia for her assistance in acquiring study data and Adam Brentnall for his valuable insights during the data interpretation phase of this study.
Author contributions
Conception and design – LLL, KAP. Acquisition of data – RLM. Data analysis – YL. Interpretation of data—LLL, RLM, YL, JC, MBT, KAP. Drafting and revising manuscript – LLL, RLM, YL, MBT, KAP. Final approval of manuscript—LLL, RLM, YL, JC, MBT, KAP.
Competing interests
The IBIS model is offered for commercial use by Cancer Research UK and Dr Cuzick receives a portion of the derived royalties. All other authors of this paper have declared no conflict of interest.
Funding
KAP is an Australian National Breast Cancer Foundation Practitioner Fellow.
Availability of data and material
The data used for this study are available from the Victorian Cancer Registry but restrictions apply to the availability of these data, which were used for the current study with appropriate approvals, and so are not generally publicly available. Data are however available from the authors upon reasonable request and with permission of the Victorian Cancer Registry.
Footnotes
Ethical approval and consent to participate This study was approved by the Peter MacCallum Cancer Centre Human Research Ethics Committee (LNR/16/PMCC/93) and was conducted in accordance with the Declaration of Helsinki 2008. As the study used Cancer Registry data the requirement for individual patient consent was waived.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information is available for this paper at 10.1038/s41416-018-0120-z.
References
- 1.Page DL, Kidd TE, Jr, Dupont WD, Simpson JF, Rogers LW. Lobular neoplasia of the breast: higher risk for subsequent invasive cancer predicted by more extensive disease. Hum. Pathol. 1991;22(12):1232–1239. doi: 10.1016/0046-8177(91)90105-X. [DOI] [PubMed] [Google Scholar]
- 2.Chuba PJ, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J. Clin. Oncol. 2005;23(24):5534–5541. doi: 10.1200/JCO.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 3.King TA, et al. Lobular carcinoma in situ: A 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J. Clin. Oncol. 2015;33(33):3945–3952. doi: 10.1200/JCO.2015.61.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodian CA, Perzin KH, Lattes R. Lobular neoplasia. Long term risk of breast cancer and relation to other factors. Cancer. 1996;78(5):1024–1034. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1024::AID-CNCR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.To T, Wall C, Baines CJ, Miller AB. Is carcinoma in situ a precursor lesion of invasive breast cancer. Int. J. Cancer. 2014;135(7):1646–1652. doi: 10.1002/ijc.28803. [DOI] [PubMed] [Google Scholar]
- 6.Degnim AC, King TA. Surgical management of high-risk breast lesions. Surg. Clin. North. Am. 2013;93(2):329–340. doi: 10.1016/j.suc.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer. Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 8.Vogel VG, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 9.Visvanathan K, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guidelines. J. Clin. Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 10.Cancer Australia . Clinical guidance for the management of lobular carcinoma in situ [Internet] NSW (AU): Cancer Australia; 2016. [Google Scholar]
- 11.Oppong BA, King TA. Oncology (Williston Park) 2011. Recommendations for women with lobular carcinoma in situ (LCIS) pp. 1051–1056. [PubMed] [Google Scholar]
- 12.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat. Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 13.Amir E, Freedman OC, Seruga B, Evans DG. Assessing women at high risk of breast cancer: A review of risk assessment models. J. Natl. Cancer. Inst. 2010;102(10):680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Preventive Services Task Force . Final Recommendation Statement: Breast Cancer: Medications for Risk Reduction [Internet] Rockville (US): U.S. Preventive Services Task Force; 2016. [Google Scholar]
- 15.Amir E, et al. Evaluation of breast cancer risk assessment packages in the family history evaluation and screening programme. J. Med. Genet. 2003;40(11):807–814. doi: 10.1136/jmg.40.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobi CE, de Bock GH, Siegerink B, van Asperen CJ. Differences and similarities in breast cancer risk assessment models in clinical practice: which model to choose? Breast. Cancer. Res. Treat. 2009;115(2):381–390. doi: 10.1007/s10549-008-0070-x. [DOI] [PubMed] [Google Scholar]
- 17.Quante AS, Whittemore AS, Shriver T, Strauch K, Terry MB. Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast. Cancer. Res. 2012;14(6):R144. doi: 10.1186/bcr3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Council Victoria . Victorian Cancer Registry [Internet] Melbourne (AU): Cancer Council Victoria; 2017. [Google Scholar]
- 19.Whittemore AS, Halpern J. Two-stage sampling designs for external validation of personal risk models. Stat. Methods. Med. Res. 2016;25(4):1313–1329. doi: 10.1177/0962280213480420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins IM, et al. Preventing breast and ovarian cancers in high-risk BRCA1 and BRCA2 mutation carriers. Med. J. Aust. 2013;199(10):680–683. doi: 10.5694/mja13.10848. [DOI] [PubMed] [Google Scholar]
- 21.Ginter PS, D’Alfonso TM. Current concepts in diagnosis, molecular features, and management of lobular carcinoma in situ of the breast with a discussion of morphologic variants. Arch. Pathol. Lab. Med. 2017;141(12):1668–1678. doi: 10.5858/arpa.2016-0421-RA. [DOI] [PubMed] [Google Scholar]
- 22.Cuzick J. IBIS Breast Cancer Risk Evaluation Tool [Internet] London (UK): Cuzick, J; 2017. [Google Scholar]
- 23.Cancer Council Victoria . Victorian Cancer Statistics. Age-specific cancer rates, Victoria (Incidence)[Internet] Melbourne (AU): Cancer Council Victoria; 2017. [Google Scholar]
- 24.Warwick J, et al. Mammographic breast density refines Tyrer-Cuzick estimates of breast cancer risk in high-risk women: findings from the placebo arm of the International Breast Cancer Intervention Study I. Breast. Cancer. Res. 2014;16(5):451. doi: 10.1186/s13058-014-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brentnall AR, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast. Cancer. Res. 2015;17(1):147. doi: 10.1186/s13058-015-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Calibration of IBIS-RET for estimates of BC (invasive or DCIS) in women with LCIS
Supplementary Table 1. Average age-specific breast cancer incidence (per 100,000 women) for the Victorian population from 1982 to 2015 compared to those used in IBISRET model v7/8