Abstract
Cellular condensates—phase-separated concentrates of proteins and nucleic acids—provide organizational structure for biochemistry that is distinct from membrane-bound compartments. It has been suggested that one major function of cellular condensates is to accelerate biochemical processes that are normally slow or thermodynamically unfavorable. Yet, the mechanisms leading to increased reaction rates within cellular condensates remain poorly understood. In this article, we highlight recent advances in microdroplet chemistry that accelerate reaction rates by many orders of magnitude as compared to bulk and suggest that similar mechanisms may also affect reaction kinetics in cellular condensates.
Introduction
Cellular compartmentalization into membrane-bound and non-membrane-bound organelles is a key aspect of intracellular organization and dictates spatial and temporal control of cellular biochemistry. Historically, membrane-bound organelles have been the archetypal examples of subcellular organization, in part because the stability imparted by the encompassing membrane facilitates investigation. Recently, however, more transient organizational structures of the cytoplasm, which lack lipid membranes, have been shown to be ubiquitous and of great physiological significance (1). They are found in the cytoplasm (e.g., P-bodies (2), stress granules (3), and centrosomes (4, 5)) and in the nucleus (e.g., nucleoli (6, 7), P-granules (8), Cajal bodies (9), paraspeckles (10), and nuclear speckles (11)) as well as near or on membranes (12, 13). Accumulating evidence indicates that these structures are formed through liquid-liquid phase separation (4, 5, 8, 13, 14, 15, 16, 17).
In response to perturbations, a well-mixed solution of molecules can phase-separate into condensed droplets, greatly increasing local solute concentration. For systems in thermodynamic states that lie near a phase boundary, small changes in conditions such as temperature or salt concentration can drive the system across the phase boundary, inducing large changes in local concentration within the phase-separated droplets. In most cases droplet formation is also readily reversible by returning the physicochemical properties of the system back to the initial state. However, some pathological condensates, for example FUS protein condensates implicated in amyotrophic lateral sclerosis (18), may become kinetically trapped in a condensed phase, preventing resolvation upon reversion of the cellular environment. It has been reported that the cytoplasm can also undergo phase separation in response to changes in pH and ion concentration because of its high macromolecular content (19, 20, 21). The discrete nature of liquid-liquid phase transitions as opposed to the continuous changes in thermodynamic variables away from phase boundaries makes condensation of cellular condensates exceptionally sensitive to changes in the cellular environment (22, 23).
Although classical theory for polymer phase transitions provides a useful conceptual framework to explain organelle formation, many details of organelle assembly and how they maintain distinct functional and physical identities remain to be understood. Protein multivalency (24, 25) and intrinsic disorder (26, 27) as well as ATP flux (14, 28) have been shown to modulate the phase diagrams and concentration profiles of cellular condensates made from ribonucleoproteins (RNPs). A full understanding of droplet formation requires extensions to the classical theory of liquid-liquid phase separations to incorporate these additional complexities. Advances on this front have been recently reviewed (22, 29, 30). In this Biophysical Perspective, we will instead focus on the function of these cellular condensates, specifically with regard to accelerating biochemical reactions.
The rates of biochemical reactions are greatly altered in crowded environments as compared to well-mixed assays (31, 32, 33). Macromolecular crowding limits diffusion, which slows the rates of diffusion-limited reactions and can lead to fractal-like kinetics (31, 34, 35). Crowded environments differ from concentrated environments in that crowded environments may have high molecular diversity. This means that although the volume fraction of solute molecules is high, the concentration of a specific species can be quite low. By concentrating specific interacting species, cellular condensates can overcome the diffusional barriers to reactions. Conversely, by sequestering specific species within condensates and excluding their partner reactants, typically favorable reaction can be retarded. Acceleration of reactions has been proposed as one of the main functions of phase-separated cellular compartments in the nucleus (36, 37, 38, 39, 40, 41), and in vitro model systems have demonstrated accelerated kinetics experimentally (13, 24, 42). Mounting evidence indicates that at least one function of cellular condensates is to enhance reaction kinetics. Although several plausible mechanisms for this acceleration have been proposed, many details of how and under what circumstances cells harness these mechanisms remain unclear. Insight may come from recent discoveries in the field of microdroplet chemistry.
Acceleration of reactions in microdroplets
Numerous recent studies have shown that confining reactants to micron-scale droplets using electrospray ionization (ESI) and microfluidic devices can accelerate the reaction rates by many orders of magnitude. For example, the reaction rate for the Pomeranz-Fritsch reaction, which synthesizes isoquinoline and is typically quite slow in bulk, increased by a factor of 106 when confined to aerosolized microdroplets (43). Many other reactions have been accelerated in microdroplets (see (44, 45, 46) for recent reviews), including several noncovalent-complex-forming reactions (44, 47) and the spontaneous phosphorylation of sugars and production of ribonucleosides (48). Despite numerous examples of microdroplet confinement accelerating reactions, the underlying cause of this acceleration remains debatable. Several factors have been proposed that would lead to greater reaction rates. Broadly speaking, these can be classified into two types of accelerating mechanisms: those that increase the local concentration of the reagents and those that alter the environment in which the reagents interact.
In microdroplet chemistry, concentration of the reagents can be accomplished in a number of ways. For one, the evaporation of the solvent has a concentrating effect on the reagents, speeding the reaction rate. The effect of evaporation rate has been demonstrated in thin-film mixtures (49) and with ESI droplets. In the latter case, altering the distance that microdroplets travel before reaching a mass spectrometer or altering the ambient temperature significantly alters the distributions of products, presumably because of solvent evaporation during transport (50).
In addition to the concentrating effect of evaporation on solutes within microdroplets, the nature of the droplet interface appears to play a significant role. Because of their small size, the surface-area-to-volume ratio of microdroplets is significantly larger than that of bulk mixtures. If the reagents are surface active, the reactions become localized to the surface of the droplets. Interfaces are well known to accelerate chemical reactions by increasing the likelihood of interparticle collisions and by orienting molecules so that reactions become more favorable when collisions do occur. For example, evidence suggests that the air-water interface lowers the entropic cost of uridine ribonucleoside formation, allowing the reaction to proceed in microdroplets without enzyme catalysis (48). The affinity of a molecule to the interface depends on the interfacial charge (51), which is dictated by the chosen solvent and reagents and hence can be experimentally manipulated. Thin-film experiments in which the surface affinity of rate-limiting negative ions is modulated by applying an external voltage show that when the ions are forced into the interior of the film, as opposed to localizing at the interface, the acceleration of the reaction is markedly decreased (though it is still faster than the bulk) (52). Hence, surface charges on microdroplet interfaces may serve to attract and orient polar reagents, making the affinity of the reagents for the interface a key factor in microdroplet-based reaction acceleration.
In addition to concentrating reacting species, microdroplets provide a means for controlling the pH of the reaction environment. During the production of microdroplets through ESI, the pH can be significantly altered through both the electric fields required for ionization and preferential evaporation of solvent during flight. This creates a vastly different reaction environment within microdroplets than in the bulk solution from which they were formed. For instance, lowered pH has been used to control and measure the unfolding of proteins within microdroplets (44, 53). Additionally, the Pomeranz-Fritsch synthesis of isoquinoline, which requires an acidic catalyst in bulk solution, can proceed at an accelerated rate without additional acid when confined to microdroplets, presumably because of the altered pH (43). Altering the pH affects the rate of a reaction through two main mechanisms. First, different pH can lead macromolecules such as proteins or RNA to reorganize into different conformations that may be more reactive. Second, as the Pomeranz-Fritsch reaction shows, altered pH within microdroplets can catalyze reactions that would otherwise be either slow or thermodynamically unfavorable.
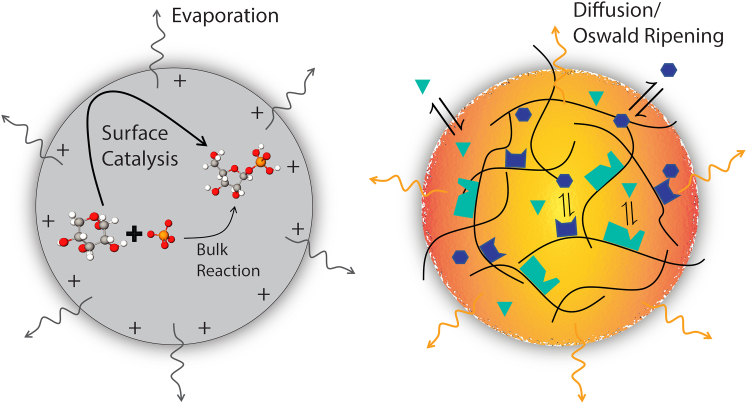
Although both the chemical environment and the concentrating effects of microdroplets suggest mechanisms by which acceleration can occur, it is important to note that the dominant effect is system specific. For example, although pH clearly affects the rate of acid catalyzed formation of isoquinoline, the acceleration of acid-induced chlorophyll demetallation was recently shown to be insensitive to droplet pH and solvent composition (54). Instead, the acceleration likely resulted from localization of the reaction to the air-water interface. In general, it is likely that several mechanisms may be interacting or reinforcing one another to achieve the large accelerations observed in microdroplets (see Fig. 1).
Figure 1.
Comparison of reactions within micro- and cellular condensates. The left schematic shows a microdroplet accelerating the production of ribose-1-phosphate from ribose and phosphate (48). The reaction is thought to be accelerated by the affinity of the polar reagents for the charged droplet interface, which localizes and orients the molecules. The right schematic depicts a cellular condensate composed of a scaffolding of multivalent RNPs. The scaffolding has a high density of enzymatic sites that interact with diffusing client molecules. The client molecules are able to readily cross the interface of the condensed phase and are more mobile in the droplet interior than the RNP scaffolding. Whereas microdroplets lose solvent through evaporation, thereby concentrating the reactants, cellular condensates exchange mass through Ostwald ripening, which could similarly have a concentrating effect. Lastly, whereas the air-water interface of the microdroplet is well defined, the interface of the cellular condensate is more diffuse and has thus far been less well-characterized. To see this figure in color, go online.
How do cellular condensates utilize confinement to accelerate reactions?
The mechanisms leading to acceleration of reactions in microdroplets can provide useful insight into the function of cellular condensates. Liquid-liquid phase transitions within the cytoplasm concentrate certain species (often RNPs) into liquid droplets with markedly different properties from those of the well-mixed cytoplasm. A description of the structure of cellular condensates has emerged lately in which multivalent scaffolding proteins and RNA separate into a condensed phase characterized by a high density of noncovalent cross-links through a process called complex coacervation (22, 29, 55). In simple coacervation, a solute phase-separates once its concentration surpasses the solubility limit. The volume of the separated phase increases as solute is added in a manner that maintains the relative concentration between the phases. In complex coacervation, however, oppositely charged soluble macromolecules phase-separate as complexes to reduce net charge, making phase separation highly sensitive to charge distribution of the constituent macroions (56). After separation of scaffolding molecules, client molecules such as enzymes and their substrates are then recruited to these condensates, effectively concentrating specific species in a scaffold-mediated manner (see Fig. 1) (25).
Whereas reactants within microdroplets are concentrated by solvent evaporation, cellular condensates do not possess an air-water interface at which evaporation can occur. However, a similar concentrating effect could be achieved through Ostwald ripening, in which solvent molecules from smaller droplets diffuse toward larger droplets because of concentration gradients resulting from interfacial curvature. If certain species diffuse more rapidly, the remaining species within the shrinking small droplets would be concentrated in an analogous manner to solvent evaporation in microdroplets.
Cellular condensates also allow for confined regions of the cytoplasm to possess markedly different environments than the rest of the cell. The charged, proteinaceous solvents within cellular condensates act more similarly to organic solvents than water and have been shown to alter the configuration of nucleic acids and proteins within the condensates (57, 58). The high charge density and competition for cation-π bonds within the condensate melts DNA double helices and has a diverse range of effect on proteins within the condensates (57). Whereas the conformational changes induced by the internal condensate solution can enhance the reactivity of certain macromolecules, high viscosity within the condensed phase should retard reactions (58, 59).
Evidence suggests, however, that the net effect of crowding because of complex coacervation of multivalent macromolecules is to increase reaction rates. For example, controlled partitioning of RNA in a model system mimicking a crowded, phase-separated environment increased ribozyme activity 70-fold (42). In another case, the rate of transcription in cellular condensates formed from cell lysate was shown to increase as a function of crowding (60). More recently, it was shown that the histone locus body, a small nuclear droplet, regulated histone messenger RNA biosynthesis by concentrating the protein FLASH and U7 small nuclear RNP (41). Modulating the concentration of a modified FLASH mutant provided direct evidence for the acceleration of processing kinetics due to localization in a cellular condensate. These results open an interesting question as to how differentiated diffusion and conformational selection might be controlled by scaffolding molecules to facilitate specific reactions. Both theoretical and experimental results show that clustering of enzymes enhances the rate and efficiency of sequential biochemical pathways (61, 62) as well as the rate of small nuclear RNPs in Cajal bodies (63). Potentially, the meshwork of scaffolding could operate in much the same way as air-water interfaces within microdroplets in that they induce particular conformations of reactants as well as spatially localizing them. Whereas the microdroplet interface provides these advantages through a charged two-dimensional interface, the cellular condensate scaffolding provides the same advantages through a charged three-dimensional lattice. In this regard, the structure of the cross-linked network of multivalent cellular condensate scaffolding may directly influence reaction rates by altering the mobility of diffusing client molecules. Additionally, the geometry and flexibility of the network, which has been shown to be strongly influenced by the solvation volume of network cross-links (64), may introduce similar crowding effects those as discussed by Kopelman (34) and Schnell and Turner (31), leading to anomalous reaction kinetics. Interestingly, recent evidence indicates that in some cases the density of macromolecules within cellular condensates is not necessarily that much greater than the cytoplasm (65). In the case of LAF-1 droplets, intrinsically disordered regions of proteins allow for significant conformational flexibility within condensates, suggesting many cellular condensates may be dilute or semidilute mixtures. One can speculate that these semidilute mixtures balance concentration or localization of reactants with conformational sampling to optimize the frequency of productive interactions between reactants. Additionally, relatively rapid diffusion within semidilute droplets may magnify the importance of the droplet interface.
In microdroplets, the surface affinity of the reactants plays a significant role in the observed acceleration. However, characterization of the surface properties of cellular condensates is sparse. Several studies have measured surface tension (8, 14), including direct microfluidic measurements that show droplet surface tension can vary over several orders of magnitude depending on the specific constituent macromolecules (59). Additionally, client molecules are observed to diffuse much more freely than scaffolding molecules and exchange rapidly across condensate interfaces (66, 67). However, beyond surface tension and interfacial flux, there exists little characterization of cellular condensate surface properties such as charge or molecule composition. Although the concentrating effect of scaffolds with condensed phases appears to be an important biochemical modulator, it is unclear how surfaces of cellular condensates may also concentrate specific molecules and act as molecular sieves for the interior of orient macromolecules. Based on the evidence from microdroplet chemistry, it seems likely that cellular biochemistry would take advantage of the special physicochemical environment afforded by the surfaces of cellular condensates.
In fact, subcellular compartmentalization frequently takes a hierarchical form, with compartments themselves being subdivided into distinct phases (7, 68). Spatial organization within cellular droplets is known to exist in the nucleolus and nuclear speckles as well as in stress granules in the cytoplasm. This additional layer of organization may play a crucial functional role (69). Presumably, interfacial effects are further amplified as greater organization requires increased boundaries between phases. The role that these nestled phases and the interfaces between them play in controlling biochemistry remains an open question.
Conclusions
Microdroplet chemistry may be an ideal testing ground for theories regarding reactions in cellular condensates and non-membrane-bound organelles. Microdroplets offer a more-controlled and less-complex analogy for the myriad interactions occurring in phase-separated cellular compartments. Understanding the physicochemical features of microdroplets that allow for enormous increases in reaction rates could clarify the evolutionary advantages dictating the size, chemical makeup, and structure of cellular condensates and may also provide insights into the self-organization principles leading to the origin of life (48, 70).
Acknowledgments
This work was partially supported by the University of Michigan Protein Folding Diseases Initiative. Dr. Stroberg is a fellow of the Michigan Institutional Research and Academic Career Development Awards program (National Institutes of Health grant K12 GM111725).
Editor: Brian Salzberg.
References
- 1.Stroberg W., Schnell S. On the origin of non-membrane-bound organelles, and their physiological function. J. Theor. Biol. 2017;434:42–49. doi: 10.1016/j.jtbi.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo Y., Na Z., Slavoff S.A. P-Bodies: composition, properties, and functions. Biochemistry. 2018;57:2424–2431. doi: 10.1021/acs.biochem.7b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Protter D.S., Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26:668–679. doi: 10.1016/j.tcb.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zwicker D., Decker M., Jülicher F. Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles. Proc. Natl. Acad. Sci. USA. 2014;111:E2636–E2645. doi: 10.1073/pnas.1404855111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodruff J.B., Wueseke O., Hyman A.A. Centrosomes. Regulated assembly of a supramolecular centrosome scaffold in vitro. Science. 2015;348:808–812. doi: 10.1126/science.aaa3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber S.C., Brangwynne C.P. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr. Biol. 2015;25:641–646. doi: 10.1016/j.cub.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feric M., Vaidya N., Brangwynne C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 9.Gall J.G. Cajal bodies: the first 100 years. Annu. Rev. Cell Dev. Biol. 2000;16:273–300. doi: 10.1146/annurev.cellbio.16.1.273. [DOI] [PubMed] [Google Scholar]
- 10.Fox A.H., Lamond A.I. Paraspeckles. Cold Spring Harb. Perspect. Biol. 2010;2:a000687. doi: 10.1101/cshperspect.a000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spector D.L., Lamond A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3:a000646. doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y.R., King O.D., Gitler A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banjade S., Rosen M.K. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife. 2014;3:e04123. doi: 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brangwynne C.P., Mitchison T.J., Hyman A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wippich F., Bodenmiller B., Pelkmans L. Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell. 2013;152:791–805. doi: 10.1016/j.cell.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Patel A., Lee H.O., Alberti S. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Banjade S., Wu Q., Rosen M.K. Conserved interdomain linker promotes phase separation of the multivalent adaptor protein Nck. Proc. Natl. Acad. Sci. USA. 2015;112:E6426–E6435. doi: 10.1073/pnas.1508778112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami T., Qamar S., St George-Hyslop P. ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron. 2015;88:678–690. doi: 10.1016/j.neuron.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks D.E. Can cytoplasm exist without undergoing phase separation? Int. Rev. Cytol. 2000;192:321–330. doi: 10.1016/s0074-7696(08)60532-x. [DOI] [PubMed] [Google Scholar]
- 20.Johansson H.O., Brooks D.E., Haynes C.A. Macromolecular crowding and its consequences. Int. Rev. Cytol. 2000;192:155–170. doi: 10.1016/s0074-7696(08)60525-2. [DOI] [PubMed] [Google Scholar]
- 21.Walter H. Consequences of phase separation in cytoplasm. Int. Rev. Cytol. 2000;192:331–343. doi: 10.1016/s0074-7696(08)60533-1. [DOI] [PubMed] [Google Scholar]
- 22.Hyman A.A., Weber C.A., Jülicher F. Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- 23.Alberti S., Hyman A.A. Are aberrant phase transitions a driver of cellular aging? BioEssays. 2016;38:959–968. doi: 10.1002/bies.201600042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P., Banjade S., Rosen M.K. Phase transitions in the assembly of multivalent signalling proteins. Nature. 2012;483:336–340. doi: 10.1038/nature10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banani S.F., Rice A.M., Rosen M.K. Compositional control of phase-separated cellular bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nott T.J., Petsalaki E., Baldwin A.J. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uversky V.N., Kuznetsova I.M., Zaslavsky B. Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Lett. 2015;589:15–22. doi: 10.1016/j.febslet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Elbaum-Garfinkle S., Kim Y., Brangwynne C.P. The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. USA. 2015;112:7189–7194. doi: 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banani S.F., Lee H.O., Rosen M.K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017;18:285–298. doi: 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry J., Brangwynne C.P., Haataja M. Physical principles of intracellular organization via active and passive phase transitions. Rep. Prog. Phys. 2018;81:046601. doi: 10.1088/1361-6633/aaa61e. [DOI] [PubMed] [Google Scholar]
- 31.Schnell S., Turner T.E. Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws. Prog. Biophys. Mol. Biol. 2004;85:235–260. doi: 10.1016/j.pbiomolbio.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H.X., Rivas G., Minton A.P. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourão M.A., Hakim J.B., Schnell S. Connecting the dots: the effects of macromolecular crowding on cell physiology. Biophys. J. 2014;107:2761–2766. doi: 10.1016/j.bpj.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kopelman R. Fractal reaction kinetics. Science. 1988;241:1620–1626. doi: 10.1126/science.241.4873.1620. [DOI] [PubMed] [Google Scholar]
- 35.Pitulice L., Vilaseca E., Mas F. Monte Carlo simulations of enzymatic reactions in crowded media. Effect of the enzyme-obstacle relative size. Math. Biosci. 2014;251:72–82. doi: 10.1016/j.mbs.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Hancock R. A role for macromolecular crowding effects in the assembly and function of compartments in the nucleus. J. Struct. Biol. 2004;146:281–290. doi: 10.1016/j.jsb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Staněk D., Pridalová-Hnilicová J., Neugebauer K.M. Spliceosomal small nuclear ribonucleoprotein particles repeatedly cycle through Cajal bodies. Mol. Biol. Cell. 2008;19:2534–2543. doi: 10.1091/mbc.E07-12-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matera A.G., Izaguire-Sierra M., Rajendra T.K. Nuclear bodies: random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev. Cell. 2009;17:639–647. doi: 10.1016/j.devcel.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mao Y.S., Zhang B., Spector D.L. Biogenesis and function of nuclear bodies. Trends Genet. 2011;27:295–306. doi: 10.1016/j.tig.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawyer I.A., Dundr M. Nuclear bodies: built to boost. J. Cell Biol. 2016;213:509–511. doi: 10.1083/jcb.201605049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatomer D.C., Terzo E., Duronio R.J. Concentrating pre-mRNA processing factors in the histone locus body facilitates efficient histone mRNA biogenesis. J. Cell Biol. 2016;213:557–570. doi: 10.1083/jcb.201504043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strulson C.A., Molden R.C., Bevilacqua P.C. RNA catalysis through compartmentalization. Nat. Chem. 2012;4:941–946. doi: 10.1038/nchem.1466. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee S., Zare R.N. Syntheses of isoquinoline and substituted quinolines in charged microdroplets. Angew. Chem. Int. Ed. Engl. 2015;54:14795–14799. doi: 10.1002/anie.201507805. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.K., Banerjee S., Zare R.N. Acceleration of reaction in charged microdroplets. Q. Rev. Biophys. 2015;48:437–444. doi: 10.1017/S0033583515000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan X., Bain R.M., Cooks R.G. Organic reactions in microdroplets: reaction acceleration revealed by mass spectrometry. Angew. Chem. Int. Ed. Engl. 2016;55:12960–12972. doi: 10.1002/anie.201602270. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee S., Gnanamani E., Zare R.N. Can all bulk-phase reactions be accelerated in microdroplets? Analyst (Lond.) 2017;142:1399–1402. doi: 10.1039/c6an02225a. [DOI] [PubMed] [Google Scholar]
- 47.Lee J.K., Kim S., Zare R.N. Microdroplet fusion mass spectrometry for fast reaction kinetics. Proc. Natl. Acad. Sci. USA. 2015;112:3898–3903. doi: 10.1073/pnas.1503689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam I., Lee J.K., Zare R.N. Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc. Natl. Acad. Sci. USA. 2017;114:12396–12400. doi: 10.1073/pnas.1714896114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badu-Tawiah A.K., Campbell D.I., Cooks R.G. Accelerated C-N bond formation in dropcast thin films on ambient surfaces. J. Am. Soc. Mass Spectrom. 2012;23:1461–1468. doi: 10.1007/s13361-012-0394-y. [DOI] [PubMed] [Google Scholar]
- 50.Bain R.M., Pulliam C.J., Cooks R.G. Accelerated Hantzsch electrospray synthesis with temporal control of reaction intermediates. Chem. Sci. (Camb.) 2015;6:397–401. doi: 10.1039/c4sc02436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenn J.B. Ion formation from charged droplets: roles of geometry, energy, and time. J. Am. Soc. Mass Spectrom. 1993;4:524–535. doi: 10.1016/1044-0305(93)85014-O. [DOI] [PubMed] [Google Scholar]
- 52.Li Y., Yan X., Cooks R.G. The role of the interface in thin film and droplet accelerated reactions studied by competitive substituent effects. Angew. Chem. Int. Ed. Engl. 2016;55:3433–3437. doi: 10.1002/anie.201511352. [DOI] [PubMed] [Google Scholar]
- 53.Banerjee S. Induction of protein conformational change inside the charged electrospray droplet. J. Mass Spectrom. 2013;48:193–204. doi: 10.1002/jms.3148. [DOI] [PubMed] [Google Scholar]
- 54.Lee J.K., Nam H.G., Zare R.N. Microdroplet fusion mass spectrometry: accelerated kinetics of acid-induced chlorophyll demetallation. Q. Rev. Biophys. 2017;50:e2. doi: 10.1017/S0033583517000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brangwynne C.P., Tompa P., Pappu R.V. Polymer physics of intracellular phase transitions. Nat. Phys. 2015;11:899–904. [Google Scholar]
- 56.Pak C.W., Kosno M., Rosen M.K. Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell. 2016;63:72–85. doi: 10.1016/j.molcel.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nott T.J., Craggs T.D., Baldwin A.J. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat. Chem. 2016;8:569–575. doi: 10.1038/nchem.2519. [DOI] [PubMed] [Google Scholar]
- 58.Brady J.P., Farber P.J., Kay L.E. Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc. Natl. Acad. Sci. USA. 2017;114:E8194–E8203. doi: 10.1073/pnas.1706197114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor N., Elbaum-Garfinkle S., Brangwynne C.P. Biophysical characterization of organelle-based RNA/protein liquid phases using microfluidics. Soft Matter. 2016;12:9142–9150. doi: 10.1039/c6sm01087c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sokolova E., Spruijt E., Huck W.T. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl. Acad. Sci. USA. 2013;110:11692–11697. doi: 10.1073/pnas.1222321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellana M., Wilson M.Z., Wingreen N.S. Enzyme clustering accelerates processing of intermediates through metabolic channeling. Nat. Biotechnol. 2014;32:1011–1018. doi: 10.1038/nbt.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis B.W., Aumiller W.M., Jr., Keating C.D. Colocalization and sequential enzyme activity in aqueous biphasic systems: experiments and modeling. Biophys. J. 2015;109:2182–2194. doi: 10.1016/j.bpj.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klingauf M., Staněk D., Neugebauer K.M. Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol. Biol. Cell. 2006;17:4972–4981. doi: 10.1091/mbc.E06-06-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmon T.S., Holehouse A.S., Pappu R.V. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife. 2017;6:e30294. doi: 10.7554/eLife.30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei M.T., Elbaum-Garfinkle S., Brangwynne C.P. Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 2017;9:1118–1125. doi: 10.1038/nchem.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dundr M., Hebert M.D., Misteli T. In vivo kinetics of Cajal body components. J. Cell Biol. 2004;164:831–842. doi: 10.1083/jcb.200311121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weidtkamp-Peters S., Lenser T., Hemmerich P. Dynamics of component exchange at PML nuclear bodies. J. Cell Sci. 2008;121:2731–2743. doi: 10.1242/jcs.031922. [DOI] [PubMed] [Google Scholar]
- 68.Fei J., Jadaliha M., Ha T. Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolution. J. Cell Sci. 2017;130:4180–4192. doi: 10.1242/jcs.206854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holehouse A.S., Pappu R.V. Functional implications of intracellular phase transitions. Biochemistry. 2018;57:2415–2423. doi: 10.1021/acs.biochem.7b01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brangwynne C.P., Hyman A.A. In retrospect: the origin of life. Nature. 2012;491:524–525. [Google Scholar]