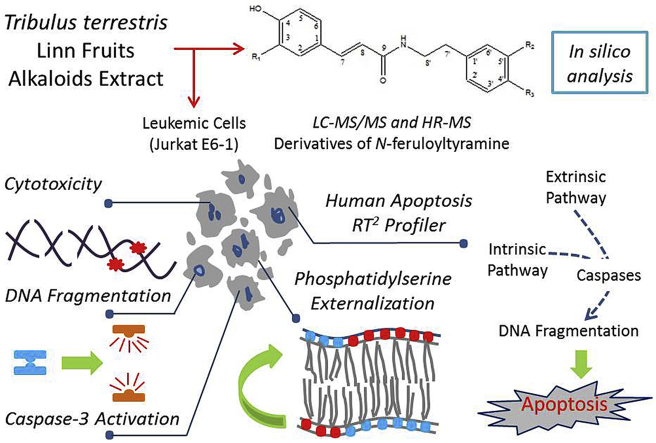
Abstract
The present study demonstrates apoptosis-inducing potential and mechanism of action of Tribulus terristris alkaloid extract in Jurkat E6-1 cancer cell line. Liquid Chromatography-Mass Spectrometry and High Resolution-Mass Spectrometry analysis identified the presence of four N-feruloyltyramine derivatives, namely trans-N-feruloyl-3-hydroxytyramine (1), trans-N-coumaroyltyramine (2), trans-N-feruloyltyramine (3) and trans-N-feruloyl-3-ethoxytyramine (4) in the alkaloid extract. Compounds 2 and 3 have not been yet reported in the alkaloid extract of T. terristris. In silico analysis revealed therapeutic potential of N-feruloyltyramine derivatives and strong binding efficiency to both chains of Tumor Necrosis Factor Receptor 1. Treatment of alkaloids extract to Jurkat E6-1 clone induced dose-dependent cytotoxicity (LC50 140.4 μg mL−1). Jurkat cells treated with alkaloids extract at sub-lethal concentration showed DNA fragmentation, enhancement in caspase-3 activity and phosphatidylserine translocation (apoptosis indicator) compared to control cells. Gene expression analysis using Human Apoptosis RT2 Profiler PCR Array analysis upon alkaloid treatment was found to significantly alter expression of critical genes such as TNFR1, FADD, AIFM, CASP8, TP53, DFFA and NFKB1. These genes are predicted to mediate apoptotic cell death via both intrinsic and extrinsic apoptosis pathway. In summary, we report the identification of new N-feruloyltyramine derivatives from alkaloid extract of T. terristris fruit with probable anti-leukemic and pharmacological potential.
Keywords: Tribulus terristris L., Cytotoxicity, Apoptosis, N-feruloyltyramine, In silico
Graphical abstract
Abbreviations
- AIFM
Apoptosis Inducing Factor, Mitochondria Associated
- CIDEB
Cell Death-Inducing DFFA-Like Effector B
- DFFA
DNA Fragmentation Factor Subunit Alpha
- KFKB1
Nuclear Factor Kappa B Subunit 1
- NOD1
Nucleotide Binding Oligomerization Domain Containing 1
- TRAF2
TNF Receptor Associated Factor 2
- TNFR1
Tumor Necrosis Factor Receptor 1
- TP53
Tumor Protein 53
- BAX
BCL2 Associated X
- TP53BP
Tumor Protein P53 Binding Protein
1. Introduction
Leukemia has been reported as the prime cause of cancer deaths in United States in 2010 for men aged below 40 years and in women aged between 20 to 59 years.1 Unlike solid tumours constraints to particular organs, leukemia cells are mobile through blood stream and can infiltrate in other tissues. Therefore, management of leukemia becomes complex. Leukemic cancer can be managed through the employment of stem cell transplantation, chemotherapy and drug therapy. However, these therapies are known to associated with side effects like low blood cell counts, infection, graft versus host diseases and formation of kidney stones. Long term effects include increased risk to heart muscle injury, infertility, thyroid dysfunction, chronic fatigue and secondary cancer.2 Therefore, worldwide researchers are trying to develop effective anticancer drug analogues with minimal side effects.
Natural compounds are promising candidates for small molecule drugs due to their minimum side effects as compared to synthetic analogues. Natural compounds contributed 64% among all the approved drugs during 1981-2010.3 The natural bioactive compounds include alkaloids, terpenoids, flavonoids, saponins, tannins, phenolics, etc. Alkaloids have comparatively higher bioactive potential due to their structural peculiarity.4 Anticancer properties of alkaloids have been reported in various scientific literatures.5 The alkaloids of Camptotheca acuminata alkaloids are marketed as irinotecan (CPT-11, Campto®) and Topotecan (TPT, Hycamtin®) as antitumor agents.6 Isoquinoline alkaloids, like Berberine, Jatrorrhizine and Sanguinarine interact with nucleic acid have importance in antineoplastic research.7 In bladder cancer, for second line treatment a bifluorinated semisynthetic vinca alkaloid, Vinflunine was approved in September 2009.8 The fruit extracts of Tribulus terristris L. (Sanskrit name: Gokshura) has been preliminary researched for aphrodisiac, spermatogenesis, antioxidant, antimicrobial, antidiabetic, anti-inflammatory, analgesic, antihypertensive, and for ameliorative properties.9 However, the alkaloids of T. terristris fruits have not been investigated for apoptosis inducing properties against leukemic cell.
In the present study, we demonstrate isolation and characterization of alkaloids from T. terristris. We report trans-N-feruloyl-3-hydroxytyramine and trans-N-feruloyl-3-ethoxytyramine as newly identified derivatives of N-feruloyltyramine in T. terristris fruit alkaloids extract. Further we examine their apoptosis induction potential in leukemic cancer cell line. In addition, we catalogue the expression profile of genes associated with apoptosis. Molecular docking studies were performed to investigate interaction and binding efficiency of identified compound in alkaloid extract with critical regulators of apoptotic pathway. N-feruloyltyramine derivatives in T. terristris fruit alkaloids may presume to be potential anti-leukemic drug molecule. However the results need to be further investigated and validated in order to strengthen the present findings.
2. Material and methods
2.1. Plant sample
T. terristris L. (family Zygopyllaceae) plant fruits were purchased from local traditional medicinal shop in Nagpur, Maharashtra (India). The plant sample was authenticated by Department of Botany, University Campus, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur, Maharashtra. The plant sample was deposited in the Herbarium with voucher specimen no. 9978 dated on 25thMay 2016.
2.2. Preparation of T. terristris fruits alkaloids extract
The fruit alkaloids extract of Tribulus terrestris was prepared according to Maldoni10 with slight modifications in solvent usage. Fruits were dried in an incubator below 50 °C, finely pulverized and defatted with n-hexane in Soxhlet apparatus to get rid of lipophilic contents. The defatted powder was again extracted in soxhlet using methanol until colourless extract was obtained. The extract was concentrated at 100 mbar pressure and 45 °C in a rotary evaporator. Presence of alkaloids was confirmed by Bouchardt, Dragendorff and Mayer's tests.10 After confirmatory test, 0.5 N HCl (2:1 v/v) was added to the flask containing extract. The flask was kept in an ice bath with continuous stirring for 3 h and then kept overnight at 4 °C to settle down gummy material. Extract was filtered; the gummy material was washed with 0.5 N HCl and the washed solutions were added to the filtrate. The extract was further basified drop wise with 15% NaOH to pH 10 and the liberated alkaloids were extracted in chloroform by liquid-liquid extraction. Finally, chloroformic extracts were combined, concentrated and dried by vacuum and turbo evaporator, successively. Resulting dried extract represented tertiary alkaloids extract form the fruits of T. terrestris.
2.3. Ultra-performance liquid chromatography (UPLC)-Electrospray ionization Mass Spectrometry
Liquid Chromatography-Mass Spectrometry (LC-MS) and LC-tandem MS/MS analysis of alkaloids fraction from fruits of T. terrestris plant was performed using Waters TQD triple quadrupole mass spectrometer (USA) equipped with H-Class Acquity UPLC system and electrospray ionization (ESI) source. Thermo Betasil C-18 (50 × 2.1 mm, 3 μm) column was used to achieve separation of analytes. One microliter of sample was injected through auto sampler into UPLC. Acetonitrile (A) and 5 mM ammonium acetate in 95% water with 5% acetonitrile (B) were used as solvents of mobile phase. Elution was performed at the flow rate of 0.25 mL min−1 with linear gradients of 90–70% B in 0–3 min, 70-60% B in 3–6 min, 60% B in 6–8 min, 60-30% B in 8–10 min, 30–90% B in 10–13 min, and 90% B up to 16 min. Nitrogen was used as the nebulizing and drying gas at flow rates of 50 and 750 L h−1, respectively. The ESI source parameters were capillary voltage of 3.5 kV and cone potential at 30 V. Source and desolvation temperatures were at 120 and 350 °C, respectively. The mass analyser was scanned between 150 to 1000 Th in 0.6 s. Tandem mass spectra of compounds were measured by precursor ion selection in MS1 followed by Collision Induced Dissociation (CID) and analysis of the product ions by MS2. Argon was used as the collision gas and collision energy was ramped between 25 to 15 eV to achieve significant fragmentation. Data acquisition and processing were carried out using MassLynx V4.1 SCN 714 software. The spectra were accumulated from the top of TIC (Total Ion Chromatogram) peak.
2.4. High Resolution Mass Spectrometry (HR-MS)
The accurate mass measurement was recorded on Thermo Scientific Orbitrap Velos Pro hybrid mass spectrometer equipped with Accela UPLC system and electrospray ionization source. The sample was injected into LC and chromatographic conditions were maintained same as above. The capillary voltage and temperature were set to 4 kV and 320 °C, respectively. Full scan mass spectra were recorded from m/z 150–1000 Th and processed with Xcalibur software.
2.5. Cell culture
Acute T cell leukemic (Jurkat E6-1, passage number 29) cell line was procured from National Centre of Cell Science (NCCS), Pune, India. Cells were cultured in Roswell Park Memorial Institute-1640 (RPMI) medium with 10% FBS supplemented with 100 U mL−1 penicillin and 50 μg mL−1 streptomycin. Cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
2.6. Cytotoxicity assays
Cytotoxicity of alkaloids extract in Jurkat E6-1 cells was determined using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and later with Trypan blue exclusion assay. Cells were seeded at a density of 2.5 × 104 per 200 μL RPMI medium in a flat transparent 96-well plate and incubated at 37 °C and 5% CO2. Cells were treated with different concentrations of alkaloids extract (50, 100, 150, 200 and 250 μg mL−1), 0.1 % dimethyl sulfoxide (DMSO; vehicle control) and Staurosporine (5 μM) as positive control for 24 h in triplicates. MTT dye (20 μL) prepared at 5 μg mL−1 in phosphate buffer was added in each well and the plate was incubated at 37 °C for 4 h. After incubation, plate was centrifuged at 650 g and formazan crystals were dissolved in 150 μL DMSO. Finally, absorbance was read at 565 nm and the percent viability was calculated using the dose response curve analysis. Lethal Concentration-50 (LC50) and sub-LC50 doses were used to test cell death in Trypan blue exclusion.11
2.7. DNA laddering assay
DNA fragmentation was studied in Jurkat E6-1 (1.5 × 105 cells mL−1) after treatment with different concentrations of alkaloids extract (70.2 and 140.4 μg mL−1) for 24 h. DMSO (0.1 %) and Staurosporine (5 μM) were taken as vehicle and positive controls respectively. DNA was isolated as per the method used by Sain et al12 and electrophoresed in 2% agarose gel containing ethidium bromide (0.5 μg mL−1). The gel was visualized under ultra violet light and image was captured using ChemiDoc (Bio-Rad)
2.8. Caspase-3 activity assay
The caspase-3 enzyme activity was estimated by fluorimetric assay kit (Sigma-Aldrich, USA). Jurkat E6-1 cells (4 × 105) were treated with T. terrestris alkaloids extract at 70.20 μg and 140.40 μg concentrations per mL for 12 h and 24 h respectively. Vehicle and positive controls were taken as DMSO (0.1%) and Staurosporine (5 μM), respectively. After the incubation period, cells were lysed and the enzyme activity was estimated according to manufacturer's instructions. Caspase-3 activity was represented in pmol min−1 mL−1.
2.9. Annexin V-FITC assay
Phosphatidylserine (PS) translocation from inner to the outer leaflet of plasma membrane of apoptotic cells was measured by FITC Annexin V Apoptosis Kit II (BD Pharmingen). Jurkat E6-1 cells were seeded in 24-well plate at a concentration of 5 × 105 perwell. Cells were exposed to 70.2 μg mL−1 of alkaloids extracts for 12 h and 24 h. Staurosporine (5 μM) exposed cells were considered as positive control. Control cells were exposed to 0.1 % DMSO. After incubation, 10,000 cells were analysed using flow cytometer (BD Accuri) as per manufacturer's instructions.
2.10. Gene expression profiling for genes associated with programmed cell death
Jurkat E6-1 cells were exposed to T. terrestris fruit alkaloids extract at 70.2 μg mL−1 concentration for 24 h and RNA was isolated using TRIzol Reagent (Invitrogen, USA) according to manufacturer's instructions. Complementary DNA (cDNA) was synthesized using RT2 First Strand Kit (QIAGEN). Human Apoptosis RT2 Profiler PCR Array (QIAGEN) profiles the expression of 84 genes associated with programmed cell death pathway. Each RT2 Profiler PCR array contains 12 controls that were used for the normalization of RT-PCR results. Real-Time qPCR was performed on ABI 7300 thermal cycler. The experiment was performed in biological and technical duplicates. The Ct values obtained for target genes were normalized and processed using Qiagen Data Analysis Center. Ct values of target genes were normalized with housekeeping gene and the relative fold change was calculated using ddCt method (2ˆ-ddCt). Statistical significance of genes was calculated using p value test. Scatter plot and clustergram were generated with the fold change of regulated genes.
2.11. In silico molecular docking analysis
In silico analysis was carried out in order to determine the probable interaction of compounds identified in the alkaloid extract (ligands) with putative target protein. This activity was carried out in four steps: a) the retrieval of protein structures from Protein Data Bank (PDB) database; b) extraction of the ligand conformers from PubChem database; c) docking data preparation; and d) docking by Patchdock web service. All database and web server information updated on 15th June 2016 were used. The chemical compounds with 3D conformation were downloaded from PubChemin SDF format. The octanol-water partition coefficient (log P) of unknown compound was estimated by Chem Axon Marvin Sketch. The smile code of unknown chemical compound, obtained from MarvinSketch, was submitted to Frog v2.14 in order to obtain the optimal 3D conformer. UCSF Chimera suite was applied to prepare the protein structure for docking. The interaction between selected proteins and chemicals was carried out using web service of Patchdock. The PatchDock/FireDock data were used to evaluate the degree of protein-ligand interactions, taking into account the number of atomic contacts. Predicted Global Contact Energy (GCE) was estimated according to the Kuntz approach.13 Ligand Efficiency (LE) was calculated as below:
Where, numerator is the contact energy and denominator represents the number of non-hydrogen atoms in the ligands.
3. Results
3.1. Identification and characterization of alkaloids in T. terristris fruit extract
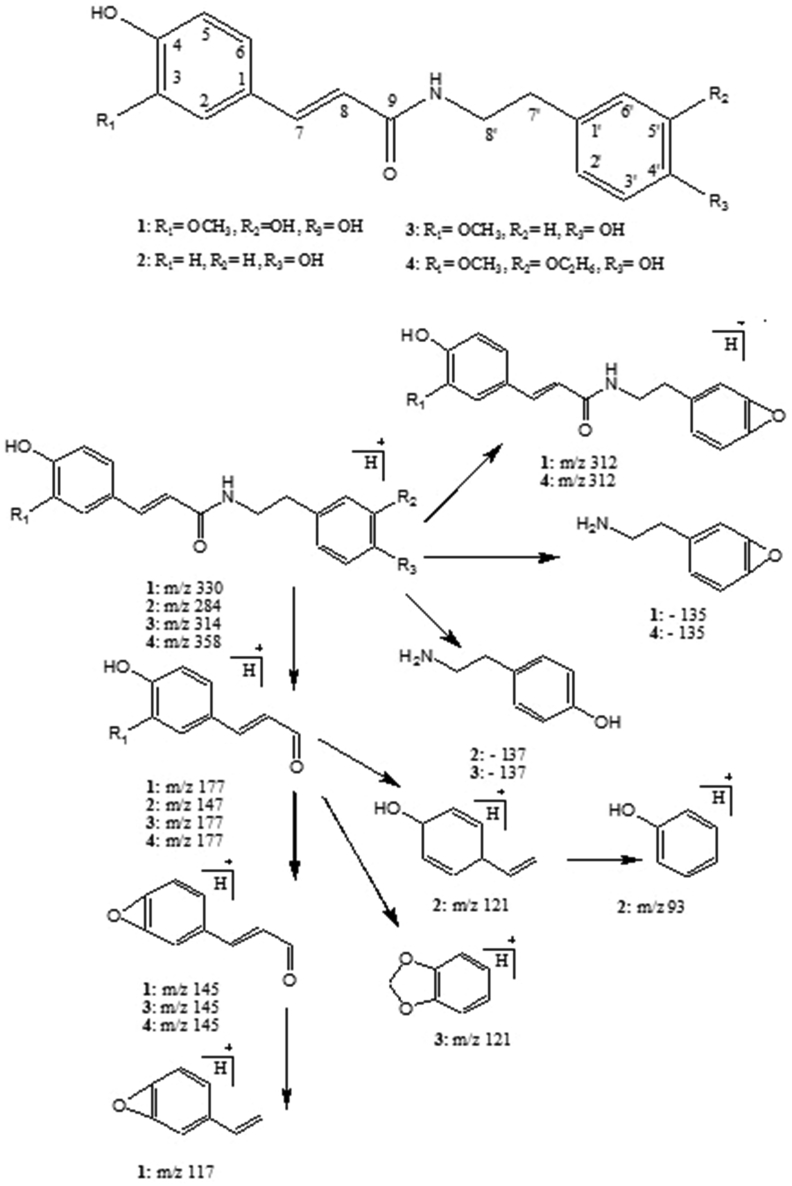
The presence of alkaloids in the T. terrestris extract was confirmed by Bouchardt, Dragendorff and Mayer's tests which gives brown, orange and white precipitate respectively (not shown in figure). The percent yield of alkaloids extract from T. terristris fruit was found to be 1.2 % (w/w). LC-MS/MS and HR-MS analysis revealed the presence of N-feruloyltyramine derivatives namely, trans-N-feruloyl-3-hydroxytyramine (1), trans-N-coumaroyltyramine (2) and trans-N-feruloyltyramine (3) trans-N-feruloyl-3-ethoxytyramine (4), in the alkaloid extract. These molecules consist of a tyramine unit linked by amide bond to a coumaraldehyde unit, and differ from each other in the nature of substituents (Fig. 1). Out of these four compounds, structures of 2 and 3 were previously reported by 2D-NMR spectroscopy from of the fruits of T. terrestris14. The retention time, protonated molecule, calculated and observed accurate mass, molecular formula with MS/MS data of identified compounds are shown in Table 1. The compounds eluted at retention times (Rt) of 5.88 (2) and 6.04 (3), with protonated m/z of 284 and 314, were further transmitted to a CID (Collision Induced Dissociation) cell. This resulted in prominent product molecules at m/z of 147 and 177 for the above parent ions, respectively. This indicated loss of tyramine unit (137 amu) from parent ions by dissociation at amide group (Fig. 1). These results are in agreement with the earlier findings.15 Peak with m/z of 147 indicated the presence of coumaraldehyde in 2, while m/z of 177 corresponded to coniferaldehyde with additional O-methyl group in coumaraldehyde unit of 3. Therefore, the compound eluting at Rt of 5.88 and 6.04 were characterised as trans-N-coumaroyltyramine and trans-N-feruloyltyramine respectively. Besides, compounds eluting at Rt of 4.74 and 6.96 min showed prominent loss of H2O (18 amu) and C2H5OH (46 amu) with parent ion peak of m/z 312 [M + H-H2O]+ and [M + H-C2H5OH]+, which was fragmented to coniferaldehyde at m/z 177 in MS/MS spectrum. It was predicted that hydroxyl and ethanolic functional group were present in the tyramine unit of the parent compound. On the basis of fragmentation pattern, accurate mass measurement, chemical formula and previously known derivative of trans-N-feruloyltyramine, the compounds eluting at Rt of 4.74 and 6.96 were characterised as trans-N-feruloyl-3-hydroxytyramine (1) and trans-N-feruloyl-3-ethoxytyramine (4) respectively.
Fig. 1.
Compounds identified in T. terrestris fruits alkaloids extracts by LC-MS/MS and HR-MS analysis.1 = Trans-N-feruloyl-3-hydroxytyramine; 2 = Trans-N-coumaroyltyramine; 3 = Trans-N-feruloyltyramine; 4 = Trans-N-feruloyl-3-ethoxytyramine. Proposed MS/MS fragmentations of N-feruloyltyramine derivatives are illustrated.
Table 1.
Chromatographic retention time and mass spectrometry detection of N-feruloyltyramine and their derivative in the alkaloids extract of the fruits of T. terristris.
| Sr. No. | Retention Time (Rt) | Name | Molecular Weight | ESI (+) [M+H]+ [2 M + H]+ | Calculate Mass | Observed Mass | Chemical Formula | |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.74 | Trans-N-feruloyl-3-hydroxytyramine | 329 | 330 | 659 | 330.1336 | 330.1333 | C18H20NO5+ |
| 2 | 5.88 | Trans-N-coumaroyltyramine | 283 | 284 | 567 | 284.1281 | 284.1281 | C17H18NO3+ |
| 3 | 6.04 | Trans-N-feruloyltyramine | 313 | 314 | 627 | 313.1387 | 314.1384 | C18H20NO4+ |
| 4 | 6.96 | Trans-N-feruloyl-3-ethoxytyramine | 357 | 358 | 715 | 358.1649 | 358.1655 | C20H24NO5+ |
| Sr. No. | Name | Molecular Weight | ESI (+) Parent Ion | MS/MS Daughters ion (Intensity) | Neutral Losses in amu |
|---|---|---|---|---|---|
| 1 | Trans-N-feruloyl-3-hydroxytyramine | 329 | m/z 312; [M + H-H2O]+ | 177 (100), 145 (40), 117 (12) | 135, 167, 195 |
| 2 | Trans-N-coumaroyltyramine | 283 | m/z 284; [M+H]+ | 147(100), 121(70), 93(8) | 137, 163, 193 |
| 3 | Trans-N-feruloyltyramine | 313 | m/z 314; [M+H]+ | 177(100), 145(30), 121(60) | 137, 169, 193 |
| 4 | Trans-N-feruloyl-3-ethoxytyramine | 357 | m/z 312; [M + H-46]+ | 177(100), 145(30) | 135, 167 |
3.2. In silico analysis of N-feruloyltyramine derivatives
In silico approach was used to evaluate therapeutic potential of compounds identified in alkaloid extract of T. terrestris plant fruits. The drug likeliness of compounds was evaluated by the Lipinsky's rule of five.16 Octanol-water values expressed as XlogP3 (Table 2) were predicted by computational approach.17 For unknown compounds, the physico-chemical parameters were estimated by ChemAxon Marvin suite. The canonical Lipinsky's rule of five corroborated potential pharmacological activities of N-feruloyltyramine derivatives for drug development in further clinical assays. Molecular docking was used to evaluate binding affinities of the identified N-feruloyltyramine derivatives with the core death domain receptor, TNFR1 (Table 3). It is ubiquitous membrane receptor that activates key members of apoptotic signalling pathway (FADD, Caspase-8, caspase 3 etc.). The compounds were predicted to bind to the part of TNFR1 receptor located at cell periphery, indicating that these compounds may initiate conformational changes and trigger apoptotic pathway.
Table 2.
Chemical-physics properties of N-feruloyltyramine and its derivative selected for docking analysis.
| Compound | IUPAC Name | CAS Number | Molecular Mass | Number of Heavy Atoms | Number of Rotable Bonds | Number of Hydrogen Bonds Donors Acceptors |
XlogP3 | |
|---|---|---|---|---|---|---|---|---|
| Donors | Acceptors | |||||||
| 1 | Trans-N-feruloyl-3-hydroxytyramine | 66648-44-0 | 329 | 24 | 6 | 4 | 5 | 1.8 |
| 2 | Trans-N-coumaroyltyramine | 36417-86-4 | 283 | 21 | 5 | 3 | 3 | 2.7 |
| 3 | Trans-N-feruloyltyramine | 66648-43-9 | 313 | 21 | 5 | 3 | 4 | 2.7 |
| 4 | Trans-N-feruloyl-3-ethoxytyramine | Unknown | 357 | 25 | 7a | 3a | 5a | 2.5a |
Predicted value for an unknown compound.
Table 3.
Interactions of N-feruloyltyramine derivatives with 1NCF chains of Tumor Necrosis Factor Receptor-1 (TNFR1).
| Gene ID | Compounds | GCE | aVdW | rVdW | ACE | Number of Contacts Clashes−1 | Ligand Efficiency (kcal mol−1 per non-H) | Amino Acid Residues with Contacts |
|---|---|---|---|---|---|---|---|---|
| 1NCF Chain A |
1 | -40.43 | -17.38 | 3.06 | -12.76 | 90/8 | -1.68 | G87, C88, K90, N91, Q92, Y93, R94, C107, C119, Q120, E121 |
| 2 | -38.02 | -14.94 | 1.83 | -12.37 | 82/6 | -1.81 | I75, C88, R89, K90, N91, Q92, C107, C119, Q120, E121 | |
| 3 | -37.07 | -18.22 | 2.44 | -8.43 | 85/5 | -1.76 | Y93, F105, N106, C107, S108, L109, L111, N124, N138, E139 | |
|
4 |
-40.42 |
-19.04 |
5.03 |
-12.07 |
100/9 |
-1.79 |
I75, G87, C88, K90, N91, Q92, R94, C107, S118, C119 |
|
| 1NCF Chain B | 1 | -41.82 | -18.14 | 4.74 | -14.11 | 89/6 | -1.74 | N228, N243, C244, S245, L246, C247, V252, S255, C256, T262 |
| 2 | -41.89 | -18.05 | 4.02 | -13.63 | 86/3 | -1.99 | R226, K227, N228, Q229, C244, S245, L246, C247, V252, S255, C256, T262 | |
| 3 | -39.45 | -18.65 | 5.94 | -12.54 | 93/3 | -1.87 | R226, K227, N228, Q229, C244, S245, L246, V252, S255, C256, T262 | |
| 4 | -32.66 | -17.49 | 3.57 | -8.02 | 85/11 | -1.3 | R204, Q209, T221, V222, C223, R231, Y233, E236, F239 |
Note: GCE, Global Contact Energy; aVdW, Van der Waals attractive; rVdW, Van der Waals repulsive; ACE, Atomic Contact Energy.
3.3. Induction of apoptosis by alkaloid extracts from T. terristris fruit
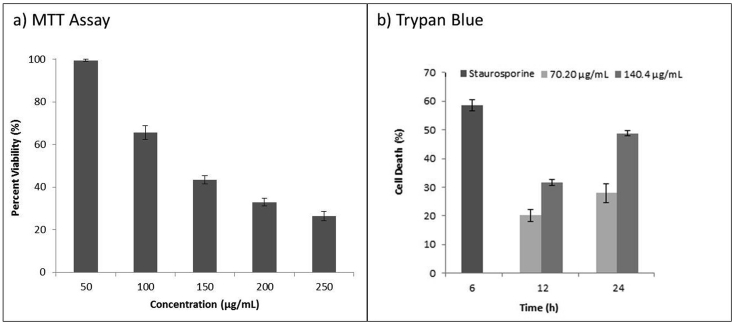
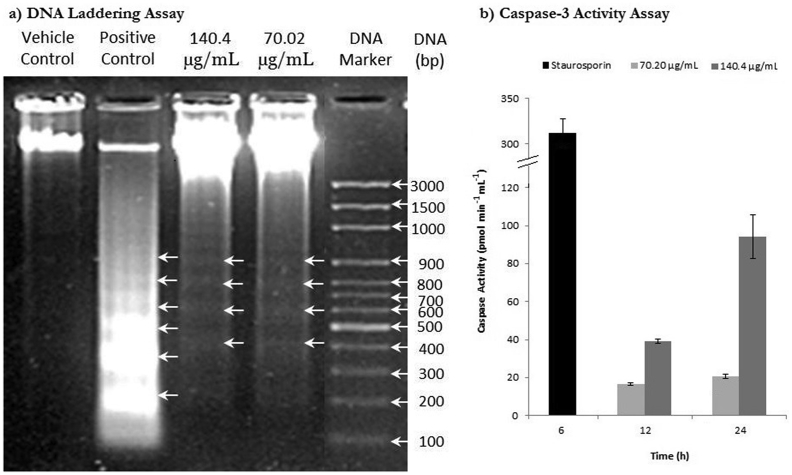
The apoptosis inducing potential of alkaloids extract was demonstrated experimentally. Crude alkaloid extract of T. terrestris shows dose dependent cytotoxicity in Jurkat cells (Fig. 2). Lethal concentration-50 (LC50; 24 h) value was found to be 140.4 μg mL−1. In further studies, we have selected LC50 and sub-lethal LC50 concentration. Cytotoxicity estimation by Trypan blue exclusion assay demonstrated concentration and time-dependant increase in cell death at lethal and sub-lethal dose of T. terrestris alkaloids extract. DNA ladder formation is the hallmark of cell death via apoptosis and it was observed in Jurkat cells exposed to 70.2 and 140.4 μg mL−1 concentrations of alkaloids extract for 24 h (Fig. 3a). Caspases are a family of cysteine-aspartic proteases essential for initial and final events of apoptosis. Executioner caspase (caspase-3) triggers activation of Caspase-Activated DNase (CAD). CADs are involved in the DNA fragmentation in apoptotic cells.18 The effect of alkaloids extract on caspase-3 activity in Jurkat E6-1 cells were measured at different concentrations and time intervals (Fig. 3b). In treated samples the caspase-3 activity gradually increased with time and concentration of alkaloids extract. Cells treated with 70.2 and 140.4 μg mL−1 of alkaloid extract for 12 h showed 16.66 ± 0.63 and 38.98 ± 1.18 pmol min−1 mL−1 caspase activities respectively. The highest caspase-3 activity was recorded in cells treated with 140.4 μg mL−1 of alkaloids extract for 24 h.
Fig. 2.
Cytotoxicity of T. terrestris alkaloids extract on Jurkat E6-1 cell line. a) MTT Assay: Exposure (24 h) of alkaloids extract resulted in dose-dependent cytotoxicity in Jurkat E6-1 cells. b) Trypan Blue Test: Time and concentration dependant increase in Jurkat cell's death exposed to T. terrestris alkaloids extract along with staurosporine treated positive control cells. Error bars represent variations in three independent measurements.
Fig. 3.
Agarose gel electrophoresis (2 %) of total DNA (a) and caspase-3 activity in pmolmin−1mL−1(b) in Jurkat cells exposed to different concentrations of T. terrestris alkaloids extract at respective time intervals. Vehicle control, positive control and DNA ladder represents DMSO, 5 μM Staurosporine and 1 kb DNA ladder, respectively. The arrowheads represent DNA fragments of 180–200 base pairs and multiples thereof. In caspase-3 assay, the values were normalized from vehicle control and the error bars represents standard error (n = 3) with level of significance (*p < 0.05).
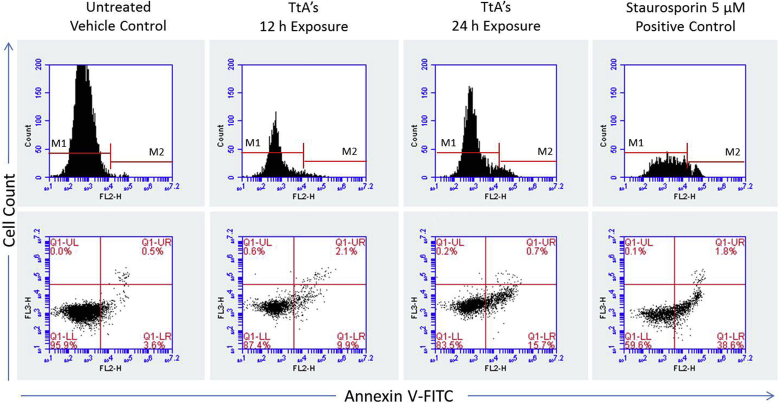
Flipping of phosphatidylserine from inner leaflet to outer cell membrane is another hallmark of apoptosis. Apoptotic cells undergo peculiar morphological changes which aid in engulfment of these cells by phagocytes. Flow cytometric analysis using annexin V antibody revealed that alkaloids extract (70.2 μg mL−1) induced time dependant apoptosis in Jurkat E6-1 cells (Fig. 4). Cells exposed for 12 h showed 9.9% apoptotic cells (lower right quadrant), whereas those exposed for 24 h showed 15.7% apoptotic cells.
Fig. 4.
Flow cytometric analysis. Scatter plot represents FITC Annexin V staining in untreated (left), Staurosporin treated (left) and cells treated with T. terristris alkaloids extract for 12 h and 24 h. Upper panel indicate viable or non-apoptotic cells (M1) and the cells undergoing for early or late apoptosis (M2). In the lower panel, Q1-LL represents live, Q1-LR represents apoptotic cells; Q1-UR represents dead cells and Q1-UL represent slate apoptotic cells.
3.4. Mechanism of intrinsic and extrinsic apoptosis pathway induction
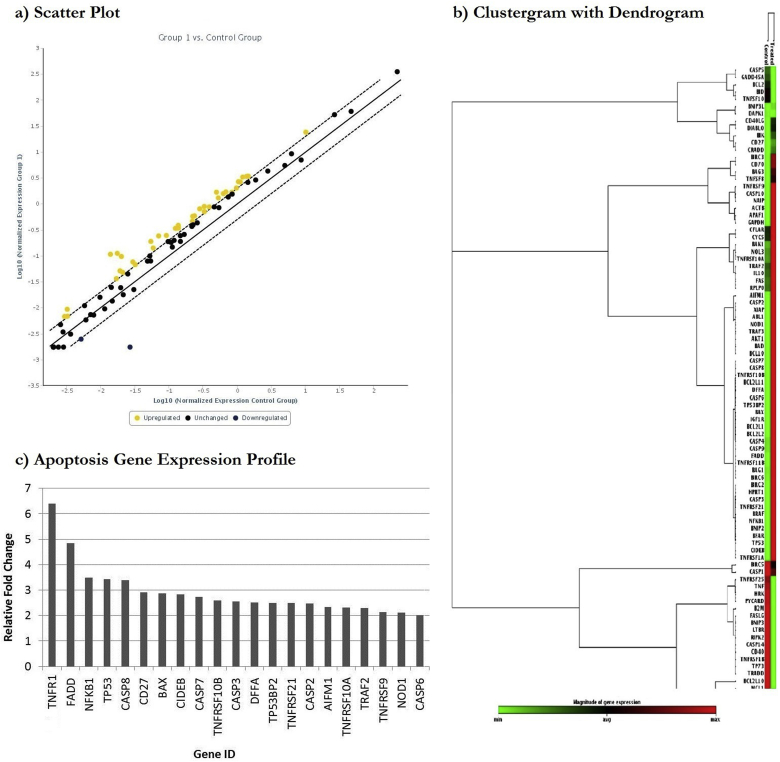
The mechanism of induction of apoptosis by T. terrestris alkaloids extract in Jurkat cells was studied by analysing the expression of genes involved in apoptotic signalling pathways. The expression profile of 84 genes involved in apoptotic signalling pathway is shown in scatter plot (Fig. 5a). The fold regulation threshold was set to 2.0, and genes above the cut-off value (yellow dots) were considered to be differentially expressed. Clustergram indicates non-supervised hierarchical clustering in co-regulated genes across control groups and treated samples, whereas dendrogram represents correlations between the genes (Fig. 5b). The green and red colours indicate minimum and maximum change in magnitude of gene expressions between the two groups. In clustergram, TNFR1, TP53 and NFKB1 were found to be closely co-regulated, whose magnitudes of gene expression in the treated sample were high. Change in expression for FADD and CASP8 was also observed. Major up-regulated genes involved in apoptosis induction are further represented in Fig. 5c. Results indicated that exposure to sub-lethal concentration of alkaloid extract elevated expression of key genes of extrinsic apoptotic pathway such as tumor necrosis factor receptor superfamily genes (TNFR1, TNFRSF10B, TNFRSF21, TNFRSF10A and TNFRSF9), pro-apoptotic genes (FADD, CD27, BAX, TP53BP2, AIFM, TRAF2 and NOD1) and apoptosis executioners/initiator like,caspase-8, CIDEB, caspase-7, caspase-3, caspase-6 and DFFA and caspase-2). The genes TNFR1, FADD, NFKB1, TP53 and caspase-8 showed more than 3 fold change compared to control. These genes drive the major pathways for apoptosis induction. The proposed mechanism of action of alkaloid extract induced apoptosis is represented in a schematic form in Fig. 6.
Fig. 5.
Scatter plot compares normalized expression of each gene on array between the two selected groups by plotting them against one another to visualize large gene expression changes (a). The central line indicates unchanged gene expression. The dotted lines indicate selected fold regulation threshold. Data points beyond the dotted lines in upper left and lower right sections represents differentially expressed genes. The clustergram performs non-supervised hierarchical clustering of the entire dataset to display a heat map with dendrogram indicating co-regulated genes across groups or individual samples (TNFRSF1A = TNFR1) (b). Expression of key genes involved in apoptotic signalling pathway analysed through RT2 PCR array profiler (c).
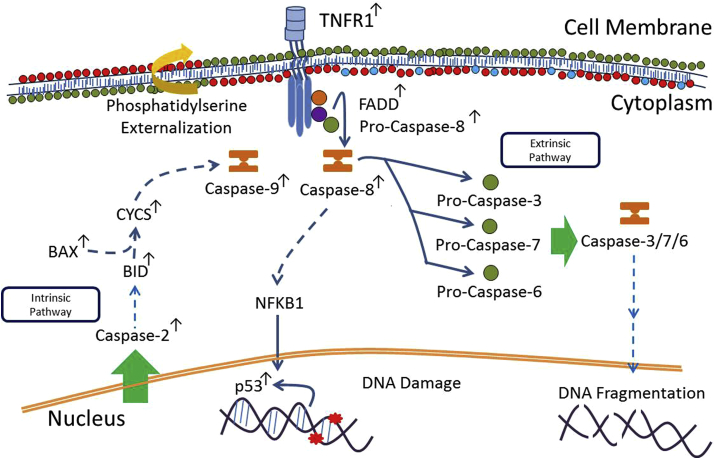
Fig. 6.
Proposed apoptosis pathway in Jurkat E6-1 cells exposed to T. terristris alkaloids extract. The upward arrowheads (↑) indicate up regulation of the genes involved in apoptosis pathways against exposure of alkaloids extract. Biomolecule interactions of all compounds identified in T. terristris alkaloids fraction were analysed by in silico studies. The extrinsic pathway of apoptosis was initiated by the upregulation of TNFR1 gene followed by FADD and Caspase-8 activation while TP53 trigger the intrinsic pathway. The fold-change expressions of some genes (BID, CYCS and caspase-9) of intrinsic pathways were between 1.5–1.9. The caspase activated DNase catalyses the fragmentation of genomic DNA. The schematic pathway was proposed based on the results obtained by RT2 Profiler array, DNA fragmentation assay, caspase-3 activation assay and apoptosis assay.
4. Discussion
Cancer therapy involves the application of natural compounds, synthetic analogues or other physical and biological methods to selectively target cancer cells. In recent years, attention has been directed towards identification and application of natural compounds to treat leukemia and lymphoma due to their promising role in clinical trials.19 In the current study, an attempt was made to isolate and evaluate apoptosis inducing potential of alkaloids extract of T. terristris fruit against leukemic cells.
LC-MS/MS and HR-MS analysis identified four N-feruloyltyramine derivatives in the alkaloid extracts. The compound 4 was found to be novel on the basis of database search in PubChem, ZINC or other common chemical repositories. Compounds 2 and 3 were previously isolated and characterised form various plants like Annona montana, Physalis minima and Polygonum sachalinensis.20, 21, 22 In addition to compounds 2 and 3, the alkaloid trans-N-Feruloyloctopamine (compound 1) was found to be present in roots of bell pepper (Capsicum annuum var. grossum).23 In silico analysis revealed that the compounds identified in alkaloid extract can bind to extracellular domain of TNFR1. The activation of TNFR1 results in the recruitment of FADD with dimerization of its death effector domain and association with pro-caspase-8, leading to apoptosis via extrinsic pathway.24 Caspase-8 is an initiator caspase, whose activation catalyses degradation of inactive proenzyme caspase-3 into large and small subunits, and renders active enzyme after their dimerization.25 Ma et al. reported apoptosis induction by furanodiene, a compound extracted from Curcuma wenyujin, against HL60 cells26. Furanodiene was found to induce TNFR1 mediated apoptosis. Furanodiene exposure was found to up-regulate caspase-3, caspase-8, caspase-9 and poly (ADP-ribose) polymerase (PARP) cleavage in HL60 cells. In this study, the caspase-3 activity was found to increase upon treatment with alkaloid extract by both caspase-3 assay kit and gene expression profile. The expression of TNFR1 was found to be elevated (6.4 fold) in present study. Caspase-3 further cleaves and activates effectors caspase-6 and caspase-7, which are reported to cleave the target molecules and confer the morphological changes of programmed cell death.24 In present study, caspase-6 and caspase-7 were found to be up regulated by 2.01 and 2.73-fold respectively. Also, DFFA was up regulated, which is the caspase-3 substrate and initiates DNA fragmentation and chromatin condensation during apoptosis.27 The altered expression of pro-apoptotic, executioner and TNF family genes upon alkaloid treatment depicts induction of extrinsic apoptotic pathway.
The initiation of intrinsic apoptosis pathway was also speculated by the up-regulation of TP53 gene in Jurkat cells (Fig. 6). TP53 is a crucial tumour suppressor protein regulator in cell cycle against diverse cellular stress signals including DNA damage and oxidative stress.28 Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 is another modulator transcription factor that protects or contributes to apoptosis. The expression of NFKB1 was found to be elevated with 3.49- fold in the present study. NFKB1 induced apoptotic cell death involves the TNFR1 mediated signalling pathway and interaction with caspase-8.29, 30 Present study demonstrates up regulation of NFKB1 along with TP53 which indicates possibility of activating intrinsic pathway of apoptosis via alkaloid extracts in Jurkat cells.
Metastatic cells often acquire resistance to apoptosis. Evasion of apoptosis results in tumor proliferation and treatment resistance. Cancer cells escapes apoptosis by different mechanisms such as loss/down regulation of TP53 tumor suppressor, differential expression of pro-apoptotic/anti-apoptotic genes and cell cycle check points. Thus, researchers are trying to develop drug analogues with multiple mechanisms of action to impede evasion of apoptosis by cancer cells. Chen et al. also reported that baicalein (plant flavone) induced programmed cell death in Human Hepatoma Hep3B cells via both intrinsic and extrinsic apoptotic pathways.31
Results of present study suggested that N-feruloyltyramine derivatives identified in alkaloid extract of T. terrestris possibly trigger both intrinsic and extrinsic apoptotic pathways in leukemic cells. The dual mechanism of action may effectively target cancer cells, minimizing the development of tumour resistance. However, the result may need to be further validated and replicated in other in vitro cancer cell models.
5. Conclusion
The present study demonstrated apoptosis inducing activity of alkaloids extract of T. terrestris against Jurkat E6-1 cells. In addition, new N-feruloyltyramine derivative (trans-N-feruloyl-3-ethoxytyramine) was identified in the alkaloid extract of T. terrestris. In vitro and in silico studies revealed cytotoxic properties and potential pharmacological activity of alkaloid extract of T. terrestris. The alkaloids extract may probably induce extrinsic (TNFR1 mediated) as well as intrinsic (TP53 induced) apoptosis pathways in leukemic cell line. Future research may be focussed on the pure forms of identified chemical constituents of the extract with validation of apoptosis markers involved. Pure forms of the identified N-feruloyltyramine derivatives may have enhanced cytotoxic activities than the whole alkaloid extract analysed in this study.
Conflict of interest
None.
Acknowledgement
The authors are grateful to Council of Scientific and Industrial Research (CSIR) for providing necessary facilities (KRC No.: KRC∖2016∖AUG∖EHD∖1). Mr. Shriniwas S. Basaiyye is thankful to the Department of Science and Technology (DST) for providing fellowships (IF120278). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A.D.V.M. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.LLS . 2011. “Acute Myeloid Leukemia.” Wikipedia, the Free Encyclopedia. Retrieved (Accessed 10 August 2016) [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordell G.A., Beattie M.L.Q., Farnsworth N.R. The potential of alkaloids in drug discovery. Photother Res. 2001;15:183–205. doi: 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- 5.Mohan K., Jeyachandran R., Deepa Alkaloids as anticancer agents. Ann Phytomed. 2012;1(1):46–53. [Google Scholar]
- 6.Bailly B. Topoisomerase I poisons and suppressors as anticancer drugs. Curr Med Chem. 2000;7:39–58. doi: 10.2174/0929867003375489. [DOI] [PubMed] [Google Scholar]
- 7.Bhadra K., Kumar G.S. Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: binding aspects and implications for drug design. Med Res Rev. 2011;31(6):821–862. doi: 10.1002/med.20202. [DOI] [PubMed] [Google Scholar]
- 8.Gerullis H. Vinflunine: a fluorinated vinca alkaloid for bladder cancer therapy. Drugs Today. 2011;47(1):17–25. doi: 10.1358/dot.2011.47.1.1576693. [DOI] [PubMed] [Google Scholar]
- 9.Sivapalan S.R. Biological and pharmacological studies of Tribulus terrestris Linn- A review. Int J Multidiscip Res Dev. 2016;3(1):257–265. [Google Scholar]
- 10.Maldoni B. Alkaloids: isolation and purification. J Chem Educ. 1991;68(8):700–703. [Google Scholar]
- 11.Crowley L.C., Marfell B.J., Christensen M.E., Waterhouse N.J. Measuring cell death by trypan blue uptake and light microscopy. Cold Spring Harb Protoc. 2016:643–646. doi: 10.1101/pdb.prot087155. [DOI] [PubMed] [Google Scholar]
- 12.Sain S., Naoghare P.K., Devi S.S. Beta caryophyllene and caryophyllene oxide, isolated from Aegle marmelos, as the potent anti-inflammatory agents against lymphoma and neuroblastoma cells. Antiinflamm Antiallergy Agents Med Chem. 2014;13:45–55. doi: 10.2174/18715230113129990016. [DOI] [PubMed] [Google Scholar]
- 13.Kuntz I.D., Chen K., Sharp K.A., Kollman P.A. The maximal affinity of ligands. Proc Natl Acad Sci. 1999;96:9997–10002. doi: 10.1073/pnas.96.18.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J.X., Sh Q., Xiong Q.B. Tribulusamide A and B, new hepatoprotective lignanamides from the fruits of Tribulus terrestris: indications of cytoprotective activity in murine hepatocyte culture. Planta Med. 1998;64:628–631. doi: 10.1055/s-2006-957535. [DOI] [PubMed] [Google Scholar]
- 15.Sun J., Song Y., Zhang J. Characterization and quantitative analysis of phenylpropanoid amides in eggplant (Solanum melongena L.) by high performance liquid chromatography coupled with diode array detection and hybrid ion trap-time of flight mass spectrometry. J Agric Food Chem. 2015;63(13):3426–3436. doi: 10.1021/acs.jafc.5b00023. [DOI] [PubMed] [Google Scholar]
- 16.Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Cheng T., Zhao Y., Li X. Computation of octanol-water partition coefficients by guiding an additive model with knowledge. J Chem Inf Model. 2007;47:2140–2148. doi: 10.1021/ci700257y. [DOI] [PubMed] [Google Scholar]
- 18.Vaculova A., Zhivotovsky B. Caspases: determination of their activities in apoptotic cells. Methods Enzymol. 2008;442:157–181. doi: 10.1016/S0076-6879(08)01408-0. [DOI] [PubMed] [Google Scholar]
- 19.Lucas D., Still P., Perez L., Grever M., Kinghorn A. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr Drug Targets. 2010;11(7):812–822. doi: 10.2174/138945010791320809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y.C., Chang G.Y., Ko F.N., Teng C.M. Bioactive constituents from the stems of Annona Montana. Planta Med. 1995;61:146–149. doi: 10.1055/s-2006-958035. [DOI] [PubMed] [Google Scholar]
- 21.Neelam S., Gokara M., Sudhamalla B., Amooru D.G., Subramanyam R. Interaction studies of coumaroyltyramine with human serum albumin and its biological importance. J Phys Chem B. 2010;114:3005–3012. doi: 10.1021/jp910156k. [DOI] [PubMed] [Google Scholar]
- 22.Fan P., Terrier L., Hay A.E., Marston A., Hostettmann K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae) Fitoterapia. 2010;81:124–131. doi: 10.1016/j.fitote.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Yoshihara T., Yamaguchi K., Takamatsu S., Sakamura S. A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossurri) Agric Biol Chem. 1981;45(11):2593–2598. [Google Scholar]
- 24.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gnesutta N., Minden A. Receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol Cell Biol. 2003;23(21):7838–7848. doi: 10.1128/MCB.23.21.7838-7848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma E., Wang X., Li Y. Induction of apoptosis by furanodiene in HL60 leukemia cells through activation of TNFR1 signalling pathway. Cancer Lets. 2008;271:158–166. doi: 10.1016/j.canlet.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Zou H., Slaughter C., Wang X.D.F.F. A heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 28.Bieging K.T., Mello S.S., Attardi L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–370. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micheau O., Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhary P.M., Eby M.T., Jasmin A., Kumar A., Liu L., Hood L. Activation of the NF-kB pathway by caspase 8 and its homologs. Oncogene. 2000;19:4451–4460. doi: 10.1038/sj.onc.1203812. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y.N., Cheng C.C., Yu C.T.R., Chou S.J., Chen H.C., Hsu S.L. Involvement of intrinsic and extrinsic apoptotic pathways in baicalein-induced apoptosis in human hepatoma Hep3B cells. J Cancer Mol. 2005;1(1):37–45. [Google Scholar]