Abstract
Phenolic compounds and other antioxidants have been implicated in protection against non-communicable diseases (NCDs) in which oxidative stress is a main contributor. The extracts of Bael (Aegle marmelos) flower were examined for their phenolic content, free radical scavenging efficacy and inhibition of low density lipoprotein (LDL) cholesterol oxidation and DNA scission activities. The extracts of Bael flowers were prepared using different solvent systems and their total phenolic content (TPC) and total flavonoid content (TFC) determined. Selected extracts which showed high TPC were subsequently used to determine their efficacy in scavenging hydroxyl, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, using electron paramagnetic resonance (EPR) spectroscopy. The corresponding peroxyl radical scavenging activity was measured using oxygen radical absorbance capacity (ORAC) assay. The potency of the extracts in inhibiting hydroxyl and peroxyl radical-induced supercoiled DNA scission and inhibition of LDL cholesterol oxidation was also evaluated. The chemical identity of phenolic compounds present in the extracts was tentatively unraveled using HPLC-MS. Phenolic extracts of Bael flowers effectively inhibited hydroxyl, and peroxyl radicals. Phenolic extracts demonstrated notable inhibitory activity against hydroxyl and peroxyl radical-induced DNA scission and LDL oxidation. Vanillic, p-coumaric, chlorogenic, caffeic, and gentisic acids were identified as major phenolic acids, along with flavonoids, mainly catechin, and quercetin. The knowledge gained here may help better use of Bael flower extracts as functional herbal beverage ingredients in the prevention of NCDs.
Keywords: Hydroxyl radical, Peroxyl radical, TPC, Liposome oxidation, HPLC
Graphical abstract
1. Introduction
Herbal beverages play a significant role in the traditional dietary habits in many countries, especially among health-conscious consumers. They also penetrate into an emerging niche market along with other beverages. A majority of herbal beverages are prepared from natural ingredients using different morphological parts, namely leaves, stems, roots, fruits, buds and flowers of plants. The type of herbal beverages popular in different regions of the world could be different and may be affected by local habits and availability of certain plants. Herbal teas/beverages are rich sources of natural bioactive compounds such as carotenoids, phenolic acids, flavonoids, coumarins, alkaloids, polyacetylenes, saponins and terpenoids, among others. Health Canada categorizes herbal beverages under natural health products (NHPs).
Excessive generation of reactive oxygen species (ROS) in the body causes oxidative stress, a detrimental cascade of events leading to the oxidation of biomolecules such as proteins, lipids, carbohydrates and DNA in organisms. Oxidative stress is now well known for its pivotal role in the etiology of several chronic diseases such as cardiovascular diseases, arthritis, and diabetes, different types of cancers, autoimmune diseases and neurodegenerative disorders.
Studies have shown that major cardiovascular risk factors are associated with a significant increase of vascular production of ROS and lipid oxidation1 In addition to the oxidative modification of low-density lipoprotein (LDL), there are a number of additional, likely important, oxidative events in cardiovascular disease (CVD). For instance increased ROS production has been identified as a major cause of endothelial dysfunction which is associated with a rapid loss of anti-atherogenic and anti-inflammatory properties of endothelium-derived nitric oxide (·NO) in part due to its inactivation by superoxide. In addition, ROS are involved in signaling cascades leading to vascular pro-inflammatory and pro-thrombotic gene expression.2 It is known that some dietary constituents may provide protection against cancer by eliminating/neutralizing certain ROS that damage DNA or initiate cancer. Therefore, external sources of antioxidants are required to prevent oxidative damage in the human body once internal antioxidant defense systems are challenged by over exposure to free radicals and other ROS. Phenolic compounds which are ubiquitous in plant foods and beverages as well as spices and herbs are known for their antioxidant and other bioactivities such as antibacterial, antiviral, anti-inflammatory, anti-allergic, anti-thrombotic and vasodilatory action, as well as anti-mutagenicity, anti-carcinogenicity and anti-aging effects.3, 4
Aegle marmelos is a tropical plant belonging to the family Rutaceae and is grown in Southeast Asian countries, including India, Sri Lanka, Pakistan, Bangladesh, Burma, and Thailand. It is also known as Bengal quince, Indian quince, holy fruit, golden apple in English, and Bael or Bilva in Hindi. Different parts of Bael tree are used for the treatment of a number of ailments in traditional Ayurvedic medicine. The fruit is a rich source of vitamin C, and riboflavin. The bark as well as fruit are used as Ayurvedic medicine for dysentery and various intestinal complaints. A number of bioactivities, namely microfilarial, radioprotective, analgesic, anti-hyperglycemic, anti-dyslipidemic, anticancer and antidiabetic activities have been reported.5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Coumarins (marmelosin, marmesin, imperatorin), alkaloids (aeglin, aegelenine), tannins (skimmianine), and other phenolic compounds are some of bioactive classes of compounds reported in different parts of Bael tree.15
The flowers of Bael tree are fragrant and are found in clusters along the young branches (plate 1). Herbal tea prepared using dried Bael flower in boiling water serves as a popular beverage in Sri Lanka since ancient times. In traditional medical systems distillation of flowers has been used to formulate a drug which is used as tonic for stomach and intestine. Further, anti-dysenteric, antidiabetic, and diaphorectic properties of Bael flower have also been reported.5, 12 However, information on antioxidant activities of Bael flower extracts is limited. Thus, the present study was aimed to determine the antioxidant activity as well as the biological activities, reflected in the inhibition of DNA scission and LDL cholesterol oxidation activities, of the extracts of dried Bael flower.
Plate 1.
Flower and buds of Aegle marmelose used for preparation of herbal teas.
2. Materials and methods
Dried flowers of A. marmelos purchased from a local market in North Western Province in Sri Lanka were used in this study. Folin Ciocalteu's reagent, vanillic, p-coumaric, syringic, chlorogenic, caffeic, and gentisic acids, as well as catechin, quercetin, aluminium chloride, sodium nitrite, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) (ABTS), 2,2′-azobis-(2-methylpropionamidine) dihydrochloride (AAPH), sodium chloride, trolox, potassium ferricyanide, trichloroacetic acid (TCA), ferric chloride, ferrous chloride, ascorbic acid, ethylenediaminetetraacetic acid trisodium salt (Na3EDTA), mono- and dibasic potassium phosphates, butylated hydroxyanisole (BHA), 3-(2-pyridyl)-5-6-diphenyl-1,2,4,-triazine-4,4-disulphonic acid sodium salt (Ferrozine), were purchased from Sigma-Aldrich Canada Ltd. (Oakville, ON, Canada). Diethyl ether, ethyl acetate, hexane, acetone, methanol, chloroform, acetonitrile, formic acid, hydrochloric acid, sodium hydroxide and sodium carbonate were purchased from Fisher Scientific Ltd. (Ottawa, ON, Canada).
2.1. Sample preparation
Dried flowers of Bael (A. marmelos), cleaned to remove soil and other particles, were ground using a coffee bean grinder (Model CBG5 series, Black & Decker, Canada Inc. Brockville, ON, Canada) to obtain a fine powder which passed through mesh 16 (sieve opening 1 mm, Tylor test sieve, Mentor, OH, USA).
2.2. Extraction of soluble phenolic compounds
Ground powder of Bael dried flower was used to extract soluble phenolic compounds under reflux conditions using different solvent systems. Briefly, for refluxing, 1 g of dried powder was placed in a round bottom flask, and extraction was carried out using 80% (v/v) methanol, 80% (v/v) ethanol, 50% (v/v) ethanol, 80% (v/v) acetone, or distilled water under reflux conditions in a thermostated water bath at 60 °C for 30 min. Meanwhile, another sample was extracted with distilled water in a thermostated water bath at 100 °C for 30 min under reflux conditions. After centrifugation of the resulting slurry for 10 min at 4000 × g (IEC Centra MP4, International Equipment Co. Needham Heights, MA, USA), the supernatant was collected and extraction was repeated two more times. Combined supernatants were evaporated in vaccuo at 40 °C (Buchi, Flawil, Switzerland) and lyophilized for 72 h at -44 °C and 46 × 10−3 mbar (Freezone, Model 77530, Labconco Co. Kansas City, MO, USA). During all stages, extracts were minimally exposed to light by covering the containers with aluminium foil. Lyophilized crude phenolic extracts were stored at -20 °C until used for further analysis.
2.3. Determination of total phenolic content (TPC)
The total phenolic content of each extract was determined according to Singleton and Rossi16 with slight modifications as explained previously.17 The content of total phenolics in each extract was determined using a standard curve prepared for gallic acid and expressed as micromoles of gallic acid equivalents (GAE) per gram of crude extract.
2.4. Determination of total flavonoid content (TFC)
Total flavonoid content was determined using a colourimetric method described by Kim etal.18 and detailed by Chandrasekara and Shahidi.17 A standard curve prepared with catechin was used to calculate total flavonoid content which was expressed as micromoles of catechin equivalents (CE) per gram of crude extract.
2.5. DPPH radical scavenging activity
The effect of extracts on the scavenging of DPPH radicals was determined according to the method adapted from Chandrasekara and Shahidi.17 The phenolic extracts (0.5 mL) of Bael were added to 2 mL of methanolic solution of DPPH (0.19 mM), vortexed and allowed to stand at room temperature in the dark. The mixture was passed to the sample cavity of a Bruker E-scan electron paramagnetic resonance (EPR) spectrometer (Bruker E-scan, Bruker Biospin Co. Billercia, MA, USA) and the spectrum was recorded after 10 min. Methanol was used as the control in place of the extract. The parameters of Bruker E-scan EPR spectrometer were set as follows; 5.02 × 102 receiver gain, 1.86 G modulation amplitude, 2.621 s sweep time, 8 scans, 100.00 G sweep width, 3495.53 G centre field, 5.12 ms time constant, 9.795 GHz microwave frequency, 86.00 kHz modulation frequency, and 1.86 G modulation amplitude. DPPH radical scavenging capacities of the extracts were calculated using the following equation. DPPH radical scavenging capacity (%) = {(EPR signal intensity for the control – EPR signal intensity for the sample)/EPR signal intensity for the control} x 100.
2.6. Hydroxyl radical scavenging activity
The hydroxyl radical scavenging capacity was determined according to Chandrasekara and Shahidi.17 Crude extracts of Bael flower were dissolved in deionized water and diluted appropriately. Extracts (100 μL) were mixed with 100 μL of H2O2, (10 Mm), and 200 μL of DMPO (17.6 mM) and 100 μL of FeSO4 (0.1 mM). After 1 min the mixtures were introduced into the sample cavity of the EPR spectrometer and the spectrum was recorded. Deionized water was used as the control in place of the extract. Hydroxyl radical scavenging capacities of the extracts were calculated using the following equation. Hydroxyl radical scavenging capacity (%) = {(EPR signal intensity for the control – EPR signal intensity for the sample)/EPR signal intensity for the control} x 100.
2.7. Oxygen radical absorbance capacity (ORAC)
Oxygen radical absorbance capacity measures scavenging activity of test compounds against peroxyl radical generated by AAPH. The ORAC assay was based on the method explained byChandrasekara and Shahidi.17 The samples, standards and other reactants were prepared in 75 mM phosphate buffer solution (PBS) (pH 7.0) and the final reaction mixture (295 μL) contained 200 μL (0.11 μM) of fluorescein as oxidizable substrate, 20 μL of extract or trolox and 75 μL of AAPH (63.4 mM). The reaction was carried out in a Costar® 3695 flat bottom 96 well black microplates (Corning Incoperated, Corning, NY,USA). Determination of ORAC was carried out using a plate reader equipped with a built-in incubator (FLUOstar OPTIMA, BMG LABTECH GmbH, Offenburg, Germany). Fluorescein (200 μL) was manually pipeted into the wells containing the extract or standards (20 μL) followed by incubation for 15 min at 37 °C. The injector pump was programmed to inject APPH at the end of incubation during the first cycle. The plate was automatically shaken for 4 s after each addition and the microplate reader was programmed to perform additional shaking of the contents in wells before each reading was taken. A gain adjustment was performed before the beginning of the measurements to optimize the maximum sensitivity, by manually pipetting 200 μL of fluorescein into the wells. Fluorescence recorded every minute for 25 cycles and each cycle was 210s. A control (phosphate buffer, fluorescein and AAPH) and different concentrations of trolox (6.25–50 μM) as the standard were performed in each assay. All reactions mixtures were prepared in duplicate and three independent runs were performed for each sample. ORAC values of extracts were expressed as micromoles trolox equivalents (TE) per gram of crude extract.
2.8. Ferrous ion chelating capacity
The ability of Bael extracts to chelate ferrous ions was measured according to the method described by Dinis et al.19 and as explained by Chandrasekara and Shahidi.17 The inhibition percentage of ferrozine-ferrous ion complex formation was calculated by the following equation. Metal chelating effect (%) = {1-(Absorbance of the sample/Absorbance of the control)} x 100. The results were expressed as micromoles EDTA equivalents per gram of crude extract.
2.9. Inhibition of liposome oxidation induced by UVA light
The liposome suspension was prepared according to a previously described method20 To evaluate the inhibition of liposome oxidation, 0.8 mL of α-phosphatidylcholine (PC) and 0.1 mL of extract (1 mg/mL) were mixed in an Eppendorf tube (1.5 mL) and total volume was made to 1 mL with PBS. The control was prepared with PC mixture and PBS. The mixtures were incubated for 5 min at 37 °C before exposing to UV light for 60 min21 using a Spectroline® UV lamp (364 nm, UVA) (Model ENF-240 C, Spectronics Co. Westbury, NY, USA) at room temperature. At the end of incubation conjugated diene hydroperoxides of a 100 μL of sample diluted to 1 mL with methanol formed were measured (Model HP 8452A diode array spectrophotometer, Agilent Technologies, Palo Alto, CA, USA) at 234 nm. The results were expressed as percentage inhibition of oxidation.
2.10. Inhibition of copper-mediated human LDL peroxidation
Inhibitory activities of Bael flower extracts against human low density lipoprotein (LDL) cholesterol oxidation was determined by measuring conjugated dienes (CD) produced in the system using the method described by Andreasen et al.22 Human LDL cholesterol (in PBS, pH 7.4 with 0.01% EDTA), was dialyzed against 10 mM PBS (pH 7.4, 0.15 M NaCl) for 12 h under nitrogen at 4 °C and EDTA-free LDL cholesterol was subsequently diluted to obtain a standard protein concentration of 0.1 mg/mL in PBS. The diluted LDL cholesterol solution (0.8 mL) was mixed with 100 μL of extract (0.5 mg/mL in PBS) in an Eppendorf tube. Oxidation of LDL cholesterol was initiated by adding 0.1 mL of 100 μM CuSO4 solution in distilled water. The mixture was incubated at 37 °C for 20 h. The initial absorbance (t=0) was read at 234 nm immediately after mixing and CD hydroperoxides formed were measured at 12, and 20 h. The concentration of CD formed was calculated using molar extinct coefficient of 29500 M−1 cm−1. The corrected absorbance at 12 and 20 h against 0 h was employed to calculate the percentage inhibition of CD formation using the following equation. Percentage inhibition of CD formation = (Abs oxidative – Abs sample)/Abs oxidative – Abs native)*100, where, Abs oxidative = absorbance of LDL mixture and distilled water with CuSO4 only; Abs sample = absorbance of LDL with extract and CuSO4; Abs native = absorbance of LDL with distilled water.
2.11. Supercoiled plasmid DNA strand scission inhibition
Inhibition activity of Bael extracts against supercoiled strand DNA scission induced by peroxyl and hydroxyl radicals was evaluated according to a previously described method20 Supercoiled plasmid DNA (pBR 322 from Escherichia coli RRI) was dissolved at a concentration of 50 μg/mL in 0.5 M, pH 7.4 phosphate buffer solution (PBS). Bael extracts (0.25 and 0.5 mg/mL) were prepared in PBS. In an Eppendorf tube (500 μL), 2 μL of a solution of supercoiled plasmid DNA, PBS, phenolic extract, H2O2 (1 mM) and FeSO4 (0.5 mM) were added in the order stated to determine the inhibitory activity of millet extracts against hydroxyl radical induced DNA strand scission. The mixture was incubated at 37 °C for 1 h in the dark.20 The loading dye (2 μL), consisting of 0.25% bromophenol blue, 0.25% xylene cyanol and 50% glycerol in distilled water, was added to the reaction mixture at the end of the incubation period.
In another experiment, inhibitory effect of extracts against peroxyl radical induced DNA scission was investigated. In this, AAPH was dissolved in PBS in order to attain a final concentration of 9 mM which was then mixed with DNA and the extracts to a final volume of 10 μL. A control with DNA alone and a blank devoid of phenolic extracts were prepared with each set of phenolic extracts tested.
The samples were electrophoresed using a 0.7% (w/v) agarose gel prepared in Tris-acetic acid-EDTA (TAE) buffer (40 mM Tris acetate, 1 mM EDTA, pH 8.5). SYBR safe was added at a concentration of 100 μL/L of TAE buffer as a gel stain. Submarine gel electrophoresis was run at 60 V for 5 h using a model B1A horizontal mini gel electrophoresis system (Owl Separation systems Inc. Portsmonth, NH, USA) and a model 300 V power supply (WMR International Inc. West Chester, PA, USA) at room temperature in TAE buffer. The bands were visualized under transillumination of UV light using AlphaImager™ gel documentation system (Cell Biosciences, Santa Clara, CA, USA). The images were analyzed using Chemilmager 4400 software (Cell Biosciences, Santa Clara, CA, USA) to quantify DNA scission. The protective effect of phenolic extracts was calculated using retention percentage of the normalized supercoiled DNA as given below. DNA retention % = (Intensity of supercoiled DNA with the oxidative radical and extract/Intensity of supercoiled DNA in control) X 100.
2.12. HPLC-DAD analysis
Phenolic composition of phenolic fractions of Bael flower extracts was determined by HPLC analysis. The RP-HPLC analysis were carried out using an Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a G1311A quaternary pump, a G1379A degasser and a G1329A ALS automatic sampler, a G1330B ALS Therm, a G1316A Colcom column compartment, a G1315B diode array detector (DAD) and a system controller linked to Chem Station Data handling system (Agilent Technologies, Palo Alto, CA, USA). Separations were conducted with a SUPERLCOSILTM LC-18 column (4.6 × 250 mm, 5 μm; Merck, Darmstad, Germany). The mobile phase consisted of 1% formic acid (eluent A) and 100 % methanol (eluent B). Gradient elution was used as follows; 0 min, 5% B; 5 min, 25% B; 20 min, 50% B; 24 min, 55% B; 28 min, 60% B; 30 min, 70% B and 40 min, 95% B. The flow rate was adjusted to 1 mL/min and the detection of compounds was performed at 254, 280, and 320 nm. All samples were filtered through a 0.45 μm PTFE membrane syringe filter (Whatman Inc. Florham Park, NJ, USA) before injection.
Phenolic acids, namely, vanillic, syringic, gentisic, chlorogenic, caffeic, and p-coumaric, were identified by comparing their relative retention times (RT), and UV spectra and ESI-MS spectra with authentic compounds. Flavonoids used as analytical standards included catechin, epigallocatechin, epicatechin, quercetin, and apigenin.
2.13. Statistical analysis
All experiments were carried out in triplicates and data were reported as mean ± standard deviation. The Student's t test at p ≤ 0.05 was used to determine significance of differences between different time intervals and concentrations. All statistical analyses were performed using SPSS version 13.0 (SPSS Inc. Chicago, IL, USA).
3. Results and discussion
To the best of our knowledge this study is the first report oninhibition of LDL oxidation and DNA scission byBael flower extracts. The results showed the potential of using herbal teas prepared from Bael flower as therapeutic dietary agents in attenuating NCDs in which oxidative stress plays a pivotal role. Table 1 presents the percentage yield of the crude extracts obtained using different solvent systems and their phenolic contents. The average yield of crude extract ranged from 6.8 to 24.7 % and the extraction obtained with water at 100 °C for 30 min gave the highest crude yield.
Table 1.
The yield, total phenolic (TPC) and total flavonoid (TFC) contents of phenolic extracts of Bael flower.
| Average yield of crude extract (%) | TPC (micromoles gallic acid equiv/g crude extract) | TFC (micromoles catechin equiv/g crude extract) | |
|---|---|---|---|
| 80% ethanol | 6.8 ± 1.3 | 1061.5 ± 28.8d | 678.7 ± 26.5d |
| 50% ethanol | 22.3 ± 2.3 | 1615.2 ± 61.0a | 1095.7 ± 24.9a |
| 80% methanol | 15.2 ± 1.9 | 1279.9 ± 32.8c | 891.0 ± 19.6c |
| 80% acetone | 10.6 ± 1.5 | 1522.8 ± 29.4b | 948.8 ± 41.3b |
| Water at 60 °C | 18.4 ± 0.9 | 1027.7 ± 41.6d | 564.2 ± 16.2e |
| Water at 100 °C | 24.7 ± 1.7 | 1496.7 ± 42.8b | 938.7 ± 11.9b |
Values in each column having the same letter are not significantly different (p > 0.05).
3.1. Phenolic contents
The TPC of crude extracts were determined using Folin Ciocalteu's reagent and the values ranged from 1028 to 1615 μmol GAE/g extract (Table 1). The order of TPC of extracts with different extraction conditions were 50% ethanol >80% acetone > water at 100 °C > 80% methanol > 80 % ethanol > water at 60 °C. Crude extracts obtained with 50% ethanol, and water at 100 °C showed 1.5 times higher TPC than those obtained with 80% ethanol and water at 60 °C suggesting the compositional effect of extraction medium and temperature on TPC. The information generated in the present study lends further support on the preparation method of herbal teas from dried Bael flower to get the optimum utility as a functional beverage giving health benefits. Thus, boiling for 30 min produces Bael flower herbal beverages with higher TPC compared to those that can be prepared as infusions.
The TFC of crude extracts ranged from 564 to 1096 mg CE/g extract (Table 1). The crude extract obtained with 50% ethanol showed the highest TFC followed by those with 80% acetone and water at 100 °C. TPC (r2 = 0.837; p < 0.01) of extracts positively and significantly associated with TFC suggesting their contribution to the TPC of crude extracts. The crude extracts which showed the highest TPC and TFC were selected to determine their antioxidant activities using different analytical methods. In agreement with the present study, Rajan et al23 reported that alcoholic extracts of Bael fruit pulp had higher levels of TPC and TFC than those of aqueous extracts. Furthermore, it was revealed that TPC and TFC of different parts of the plants varied.24
3.2. Phenolic compounds identified
Table 2 presents individual phenolic compounds and their contents identified from Bael flower extracted in 50% aqueous ethanol. Hydroxycinnamic acids, hydroxybenzoic acids as well as some flavonoids were identified. Among hydroxycinnamic acids, cholorogenic, p-coumaric and caffeic acids were identified as major components. Gentisic, vanillic and syringic acids were among hydroxybenzoic acids identified. Catechin and quercetin were two major flavonoids identified in 50% ethanolic extracts of Bael flower and the contents were 5.80 and 1.35 mg/g crude extract, respectively. The results show that compounds belonging to flavonoids are predominant in the 50% ethanolic extracts of Bael flower compared to those of phenolic acids (Table 2).
Table 2.
Antioxidant activity of phenolic extracts of Bael flower.
| Antioxidant activity | Extraction with 50% ethanol | Extraction with Water at 100 °C |
|---|---|---|
| DPPH radical scavenging capacity (%) | 59.5 ± 3.3a | 50.5 ± 2.5a |
| Hydroxyl radical scavenging capacity (%) | 26.5 ± 3.9a | 41.5 ± 3.4b |
| Inhibition of UVA induced liposome peroxidation (%) | 77.3 ± 0.9a | 81.1 ± 0.8a |
| Ferrous ion chelation activity (%) | 16.9 ± 1.2a | 20.4 ± 1.2a |
| Oxygen radical absorbance capacity (Trolox equivalents per g of crude extract) | 930 ± 23a | 942 ± 29a |
Values in each raw having the same letter are not significantly different (p > 0.05).
3.3. Antioxidant activities
Antioxidant activities of phenolics extracted with 50% ethanol and boiling water at 100 °C are presented in Table 3. Except for hydroxyl radical scavenging activity, significant differences in the antioxidant activities were not observed between the aqueous-ethanol and aqueous Bael extracts used for the analysis in this study. Aqueous extracts showed 42% hydroxyl radical scavenging activity whereas aqueous-ethanol extract showed only 27%. Hydroxyl radical (˙OH) is extremely reactive and generates other radicals by reacting with biomolecules such as proteins, DNA and lipids. Further it plays a significant role as an initiator of lipid peroxidation.
Table 3.
Retention percentage of supercoiled pBR 322 plasmid DNA in peroxyl radical-mediated and hydroxyl radical-mediated in vitro systems with phenolic extracts of Bael flower.
| Peroxyl radical |
Hydroxyl radical |
|||
|---|---|---|---|---|
| 0.5 mg/mLa | 0.25 mg/mLa | 0.5 mg/mLa | 0.25 mg/mLa | |
| Control | 18±2 | 16±1 | ||
| Gallic acid | 98 ± 2a | 99 ± 1a | 90 ± 2a | 41 ± 2a |
| Extraction with 50% ethanol | 91 ± 1a | 81 ± 1a | 22 ± 3b | 30 ± 1 b |
| Extraction with water at 100° C | 92 ± 1a | 83 ± 2a | 23 ± 2 b | 21 ± 3b |
Values in each column having the same letter are not significantly different (p > 0.05).
Concentrations of extracts.
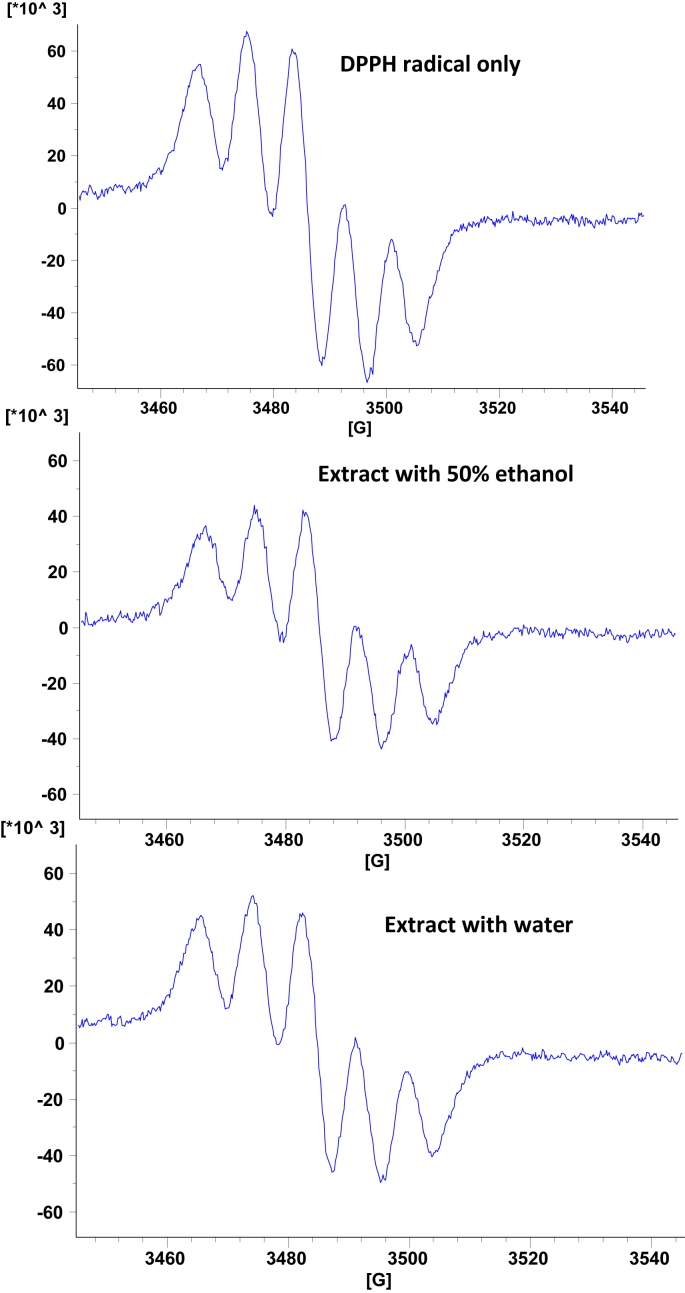
DPPH, as a stable synthetic radical compared to highly reactive and transient peroxyl and hydroxyl radicals, is widely used to evaluate free radical scavenging properties of antioxidant compounds. In the present study EPR spectroscopy was used to detect the signal intensity of DPPH radicals left upon reacting with phenolic compounds in the assay. Fig. 1 shows representative EPR spectra of DPPH radicals alone and in the presence of phenolic extracts of Bael flower. DPPH radical scavenging capacities were 51 and 60% for aqueous and aqueous-ethanol extracts of Bael flower extracts, respectively.
Fig. 1.
Electron Paramagenetic Resonance signals of DPPH radicals alone and in the presence of Bael flower phenolic extracts.
The oxygen radical absorbance capacity (ORAC) as trolox equivalents per gram of crude extract of aqueous and aqueous-ethanol extracts of dried Bael flower were 942 and 930, respectively. In the ORAC assay peroxyl radicals are generated by thermal decomposition of the azo compound, AAPH. Fluorescein (FL) is employed as the probe and the fluorescence decay indicates its reaction with peroxyl radical. In the presence of antioxidative compounds FL decay is inhibited and their intensity can be measured at 485 nm excitation and 525 nm emission. The protective effect of phenolic extracts against peroxyl radicals is calculated from net integrated area under the kinetic curve (AUC) and compared to that of the trolox, a water soluble analogue of α-tochopherol.
The results in this study demonstrated that phenolic extracts of Bael flower scavenge peroxyl radicals effectively (Table 3). This could be attributed to the high phenolic content in the crude extract of dried Bael flowers. Several in vivo studies have demonstrated the inhibition of lipid peroxidation by hydroalcoholic extracts of Bael. Jagetia et al25 reported that the intraperitoneal administration of the hydroalcoholic extract of the fruit pulp (20 mg/kg body weight) for five consecutive days before exposure to different doses of γ-radiation (6–9 Gy) could prevent radiation-induced lipid peroxidation in the liver, kidney, intestine and spleen of mice.
3.4. Liposome oxidation inhibition
Antioxidant activity of phenolics of Bael flower extracts were determined by their ability to inhibit conjugated diene hydroperoxide formation in phosphatidylcholine (PC) liposome membranes which were oxidized under UVA light. Ultraviolet radiation is known to damage lipid membranes directly or via a process mediated by ROS.26, 27
The results of the present study showed that Bael flower phenolics were effective against liposome peroxidation induced by UVA light at a concentration of 1 mg/mL of the extracts (Table 3). Phenolics of Bael flower extracts inhibited lipid oxidation ranging from 77 to 81%. The antioxidant activity of phenolic compounds may be related to both their structural characteristics as well as ability to interact with the liposome. It was reported that hydrophilicity of the phenolic compounds, degree of flavonol oligomerization and the number of hydroxyl groups in the respective molecule were associated with the protection against membrane oxidation.28 Several authors have shown that flavonol and quercetin may protect against UV radiation through absorbance of UV radiation, antioxidant activity and interaction with signal transduction pathways.29, 30 Furthermore, Fahlaman and Krol21 showed that quercetin inhibited UVA and UVB radiation induced lipid oxidation mainly by scavenging radical species. Thus, peroxidation inhibition demonstrated by both aqueous and aqueous-ethanolic phenolic extracts in the present study could be due to their ability to scavenge free radicals generated during UV radiation.
3.5. Ferrous ion chelating activity
Ferrous ion contributes to lipid peroxidation as well as DNA oxidation by generating hydroxyl radical through Fenton reaction. Further, ferrous ion accelerates decomposition of lipid hydroperoxides into peroxyl and alkoxyl radicals.31 In this study, Bael flower extracts showed chelating activity of ferrous ions in the range of 17–21%. It is noteworthy that phenolic extracts of Bael flower which displayed a relatively high ORAC and liposome oxidation inhibition activities (Table 3) demonstrated a low ferrous ion chelating activity. This could be due to deficit of compounds which are responsible for low ferrous ion chelating in the extract. In a previous study, Chandrasekara and Shahidi20 showed that finger millet varieties which had high chelating activity were reported to contain flavan-3-ol monomers as well as dimmers such as procyanidin B1 and B2.
3.6. Supercoiled plasmid DNA strand scission inhibition
Irreversible modification of DNA occurring as a result of oxidative damage has been identified as the first step in pathological conditions such as cancer and aging. The free radical mediated damage to DNA may vary and includes base modification, production of base-free sites, strand breaks, DNA-protein cross links and abnormal chromosomal arrangements, among others.32 Hydroxyl radical is able to abstract a hydrogen atom from the deoxyribose sugar moiety as well as pyrimidine and purine bases of DNA, producing single strands.33 Double strand breaks occur near to each other on both strands, could be due to the multiple hydroxyl radical attacks and lead to lethal damage of the cells.34 In the present study, free radical induced DNA strand scission inhibition was tested for two concentrations of crude phenolic Bael flower extracts, 0.25 and 0.5 mg/mL, and determined the percentage of the retention of supercoiled DNA. The results showed that both peroxyl and hydroxyl radicals induced DNA strand scission.
Phenolic compounds can act as chemopreventive agents through antioxidative and a number of other mechanisms.35 It is noted that phenolic extracts of Bael flower demonstrated effective inhibitory activity against peroxyl radical induced DNA scission at both concentrations tested. Furthermore, both extractions with 50% ethanol and water at 100 °C showed more than 90% DNA scission inhibition and in this respect were close to that of gallic acid at concentrations of 0.25 and 0.5 mg/mL (Table 4). It is interesting to note that both extracts had high (81–83%) inhibition activity against peroxyl radical-mediated DNA scission even after the extracts were diluted by a factor of two. The inhibition of DNA scission induced by peroxyl radicals has previously been reported for a number of cereal phenolic extracts.20, 36 Inhibition of peroxyl radical induced supercoiled DNA scission of barley extracts ranged from 78 to 92% at a concentration of 4 mg/mL.36 In a similar concentration schedule as in the present study, Chandrasekara and Shahidi20 showed a 45–99% inhibition of peroxyl radical induced supercoiled DNA scission of millet extracts. The observed scission inhibition could be due to the scavenging of peroxyl radicals by phenolic compounds present in the extract. This was further confirmed by the results of the ORAC assay which showed superior peroxyl radical scavenging activity in the present study.
Table 4.
Individual phenolic compounds identified in Bael flower extracted with 50% ethanol.
| Phenolic compounds | Molecular weight | [M-H] ˉ (m/z) | ESI negative ions (m/z) | Content (mg/g crude extract) |
|---|---|---|---|---|
| Gentisic acid | 154 | 153 | 329 | 0.95 ± 0.04 |
| Vanilllic acid | 168 | 167 | 123 | 0.94 ± 0.01 |
| Syringic acid | 198 | 197 | 153 | 0.07 ± 0.01 |
| Chlorogenic acid | 354 | 353 | 191 | 0.19 ± 0.01 |
| Caffeic acid | 180 | 179 | 135, 167 | 0.03 ± 0.01 |
| p-Coumaric acid | 164 | 163 | 119, 139 | 0.95 ± 0.01 |
| Catechin | 290 | 289 | 245 | 5.80 ± 0.21 |
| Quercetin | 302 | 301 | 121, 179 | 1.35 ± 0.04 |
For hydroxyl radical induced DNA scission, Bael flower extracts showed low inhibitory activity although gallic acid showed 90% inhibition at a concentration of 0.5 mg/mL (Table 4). The supercoiled DNA retention ranged from 21 to 30% against hydroxyl radical induced oxidation in the presence of Bael flower extracts. At a similar concentration of the extract (0.5 mg/mL), Bael flower phenolics inhibited DNA scission induced by peroxyl radicals 3 to 4 times higher than that of hydroxyl radicals. The same pattern was observed at a lower concentration (0.25 mg/mL) of the extracts in this study. These results further suggest that phenolic compounds present in the crude extracts of Bael flower could be more effective against the scavenging of peroxyl radicals compared to those of hydroxyl radicals.
It is demonstrated that both the quantity and the concentration of different phenolic compounds in the extracts may be responsible for the observed effects. In a previous study, Noroozi et al37 reported that flavonoids were effective against hydrogen peroxide-initiated oxidative DNA damage to human lymphocytes. In the present study, both extracts showed high inhibition ability against peroxyl radical-mediated DNA oxidation, possibly due to their high content of flavonoids. HPLC analysis revealed that catechin and quercetin were the major flavonoids in Bael flower extract (Table 2).
3.7. Inhibition human LDL cholesterol oxidation
Fig. 2 shows the inhibitory activities of Bael flower extracts at a concentration of 0.5 mg/mL against human LDL cholesterol oxidation induced by cupric ion. Extracts of dried Bael flower showed more than 70% inhibition of oxidation of LDL cholesterol. Furthermore, both aqueous and aqueous-ethanol extracts showed effective inhibition of oxidation after 12 and 20 h and aqueous-ethanol extracts showed superior inhibition than that of aqueous extract.
Fig. 2.
Percentage inhibition of LDL cholesterol oxidation by phenolic extracts of dried Bael flowers.
Uptake of oxidized LDL by macrophages and smooth muscle cells leads to the formation of fatty streaks or vascular lesions which accumulate lipids further in the progression of atherosclerosis. Therefore, natural antioxidants from dietary sources that may inhibit LDL cholesterol oxidation are of great importance in the prevention of cardiovascular diseases.38, 39
Inhibitory effects of phenolic compounds against LDL cholesterol could be due to their free radical scavenging activity or metal ion chelating properties. Further, phenolic compounds may protect endogeneous antioxidative compounds such as β-carotene, tochopherols, and ubiquinol of LDL cholesterol molecule. In addition, phenolics can inhibit enzymes such as xanthine oxidase involved in the initiation of oxidation or cell mediated LDL cholesterol oxidation. The ability of phenolic compounds to inhibit copper ion-mediated LDL cholesterol oxidation may be attributed to their capacity to remove cupric ions from the LDL cholesterol.40 It has been shown that copper mediated oxidation of tryptophan residues in apolipoprotein B could be a leading cause for initiating cholesterol oxidation in the LDL cholesterol.41
4. Conclusion
The phenolic extracts of dried Bael flower extracts serve as a natural source of antioxidants and effectively inhibit the DNA strand scission induced by peroxyl radicals and human LDL cholesterol oxidation induced by cupric ions. Phenolic extracts from Bael flower showed high liposome peroxidation inhibition activity. However, they exhibited low hydroxyl radical scavenging and ferrous ion chelation activities, suggesting the differential effects of constituent phenolics of the extract in diverse assay systems. There is anincreasing demand for natural sources of antioxidants which can be used for managing several pathological conditions and maintenance of health. Dried Bael flower has a great potential to be utilized as a functional beverage in health promotion and disease risk reduction.
Conflict of interest
The authors state that there is no conflict of interest to declare.
Acknowledgements
This research was supported by the Natural Science and Engineering Research Council (NSERC) of Canada through a Discovery grant to FS.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Anoma Chandrasekara, Email: anomapriyan@yahoo.ca.
Fereidoon Shahidi, Email: fshahidi@mun.ca.
References
- 1.Darley-Usmar V., Halliwell B. Blood Radicals: reactive nitrogen species, reactive oxygen species, transition metal ions and the vascular system. Pharm Res. 1996;13:649–662. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 2.Valko M., Leibfritz D., Moncola J., Cronin M.D. Free radicals and antioxidants in normal physiological functions and human disease. Rev Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Shahidi F., Naczk M. CRC press; Boca Raton, FL: 2004. Phenolics in Food and Nutraceuticals; pp. 1–82. [Google Scholar]
- 4.Halliwell B., Rafter J., Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 5.Jagetia G.C., Venkatesh P. Inhibition of radiation-induced clastogenicity by Aegle marmelos (L.) correa in mice bone marrow exposed to different doses of gamma-radiation. Hum Exp Toxicol. 2007;26:111–124. doi: 10.1177/0960327107071867. [DOI] [PubMed] [Google Scholar]
- 6.Shankarananth V., Balakrishnan N., Suresh G., Sureshpandian E.E., Sheeja E. Analgesic activity of methanol extract of Aegle marmelos leaves. Fitoterapia. 2007;78:258–259. doi: 10.1016/j.fitote.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Narender T., Shweta S., Tiwari P. Antihyperglycemic and antidyslipidemic agent from Aegle marmelos. Bioorg Med Chem Lett. 2007;17:1808–1811. doi: 10.1016/j.bmcl.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Costa-Lotufo L.V., Khan M.T., Ather A. Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol. 2005;99:21–30. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam D., Giridharan P., Murmu N. Activation of apoptosis by 1-hydroxy-5, 7-dimethoxy-2-naphthalene-carboxaldehyde, a novel compound from Aegle marmelos. Cancer Res. 2008;68:8573–8581. doi: 10.1158/0008-5472.CAN-08-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabu M.C., Kuttan R. Antidiabetic activity of Aegle marmelos and its relationship with its antioxidant properties. Indian J Physiol Pharmacol. 2004;48:81–88. [PubMed] [Google Scholar]
- 11.Narendhirakannan R.T., Subramanian S., Kandaswamy M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clin Exp Pharmacol Physiol. 2006;33:1150–1157. doi: 10.1111/j.1440-1681.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 12.Dhankhar S., Ruhil S., Balhara M., Dhankhar S., Chhillar A.K. Aegle marmelos (linn.) correa: a potential source of phytomedicine. J Med Plants Res. 2011;5:1497–1507. [Google Scholar]
- 13.Gohil T., Pathak N., Jivani N., Devmurari V., Patel J. Treatment with extracts of Eugenia jambolana seed and Aegle marmelos leaf extracts prevents hyperglycemia and hyperlipidemia in alloxan induced diabetic rats. Afr J Pharm Pharmacol. 2010;4:270–275. [Google Scholar]
- 14.Baliga M.S., Bhat H.P., Joseph N., Fazal F. Phytochemistry and medicinal uses of the bael fruit (Aegle marmelos Correa):A concise review. Food Res Int. 2011;44:1768–1775. [Google Scholar]
- 15.Suvimol C., Pranee A. Bioactive compounds and volatile compounds of Thai bael fruit (Aegle marmelos (L.) Correa) as a valuable source for functional food ingredients. Int Food Res. 2008;15:45–63. [Google Scholar]
- 16.Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 17.Chandrasekara A., Shahidi F. The content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem. 2010;58:6706–6714. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 18.Kim D., Jeong S.W., Lee C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- 19.Dinis T.C.P., Madeira V.M.C., Almeida L.M. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekara A., Shahidi F. Bioactivities and antiradical properties of millet grains and hulls. J Agric Food Chem. 2011;59:9563–9571. doi: 10.1021/jf201849d. [DOI] [PubMed] [Google Scholar]
- 21.Fahlman B.M., Krol Ed.S. Inhibition of UVA and UVB radiation-induced lipid oxidation by quercetin. J Agric Food Chem. 2009;57:5301–5305. doi: 10.1021/jf900344d. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen M.F., Kroon P.A., Williamson G., Garcia-Conesa M.R. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic Biol Med. 2001;31:304–3011. doi: 10.1016/s0891-5849(01)00585-8. [DOI] [PubMed] [Google Scholar]
- 23.Rajan S., Gokila M., Jency P., Brindha P., Sujatha R.K. Antioxidant and phytochemical properties of Aegle marmelos fruit pulp. Int J Curr Pharm Res. 2011;3:65–70. [Google Scholar]
- 24.Siddique N.A., Mujeeb M., Najmi A.K., Akram M. Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. Afr J Plant Sci. 2010;4:014–018. [Google Scholar]
- 25.Jagetia G.C., Venkatesh P., Baliga M.S. Fruit extract of Aegle marmelos protects mice against radiation-induced lethality. Integr Cancer Ther. 2004;3:323–332. doi: 10.1177/1534735404270641. [DOI] [PubMed] [Google Scholar]
- 26.Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23:S22–S26. doi: 10.1016/s0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 27.Einspahr S., Stratton P., Bowden G.T., Alberts D.S. Chemoprevention of human skin cancer Janine G. Crit Rev Oncol/Hematol. 2002;41:269–285. doi: 10.1016/s1040-8428(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 28.Erlejman A.G., Verstraeten S.V., Fraga C.G., Oteiza P.I. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 2004;38:1311–1320. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- 29.Bachelor M.A., Bowden G.T. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Casagrande R., Georgetti S.R., Verri W.A., Dorta D.J., Jr., dos Santos A.C., Fonseca M.J. Protective effect of topical formulations containing quercetin against uvb-induced oxidative stress in hairless mice. J Photochem Photobiol B. 2006;84:21–27. doi: 10.1016/j.jphotobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Biochemistry of oxidative stress. Buiocem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 32.Valko M., Izakovic M., Mazur M., Rhodes C.J., Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;2676:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 33.Breen A.P., Murphy J.A. Reactions of hydroxyl radicals with DNA. Free Radic Biol Med. 1995;18:1033–1077. doi: 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- 34.Ward J.E. The complexity of DNA damages; relevance to biological consequences. Int J Radiat Biol. 1994;66:427–432. doi: 10.1080/09553009414551401. [DOI] [PubMed] [Google Scholar]
- 35.Fresco P., Borges F., Diniz C., Marques M.P.M. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 36.Madhujith T., Shahidi F. Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J Agric Food Chem. 2006;54:8048–8057. doi: 10.1021/jf061558e. [DOI] [PubMed] [Google Scholar]
- 37.Noroozi M., Angerson W.J., Lean M.E. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am J Clin Nutr. 1998;67:1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg D. Low density lipoprotein oxidation and its pathological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 39.Kume N., Cybulsky M., Gimbrone M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kume N., Gimbrone M.A., Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J Clin Invest. 1994;93:907–911. doi: 10.1172/JCI117047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirico T.L., Omaye S.T. Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem Toxicol. 2006;l 44:510–516. doi: 10.1016/j.fct.2005.08.025. [DOI] [PubMed] [Google Scholar]