Abstract
Mitochondrial dysfunction and oxidative stress are two factors that are thought to contribute to the pathogenesis of Parkinson's disease (PD), a debilitating progressive neurodegenerative disorder that results in the loss of catecholamine producing cells throughout specific regions of the brain. In this study we aimed to compare the effects of hydrogen peroxide (H2O2) and rotenone (a pesticide and mitochondrial complex 1 inhibitor) on cell viability and the expression of tyrosine hydroxylase (TH) in a cellular model of PD. We also sought to investigate the potential neuroprotective benefits of bioactive constituents from cinnamon, hemp seed and polygonum cuspidatum. To create a model, SH-SY5Y cells transfected with human TH isoform 1 were treated with varying concentrations of H2O2 and rotenone, in the presence or absence of bioactive constituents. The effect of these toxins and constituents on cell viability, apoptosis and protein expression was assessed using MTT viability assays and western blotting. Rotenone treatment caused a significant decrease in cell viability but a significant increase in TH in the remaining cells. H2O2 treatment caused a significant decrease in cell viability but had no significant effect on TH expression. Curcumin, cinnamaldehyde, caffeoyltyramide (hemp seed extract) and piceatannol glucoside (polygonum cuspidatum extract) were unable to attenuate rotenone induced cell death, however they were able to provide protection against H2O2 induced cell death. This is the first study to demonstrate the neuroprotective properties of cinnamaldehyde, caffeoyltyramide and piceatannol glucoside in a dopaminergic cell line in response to H2O2.
Keywords: Cinnamon, Hemp seed, Oxidative stress, Polygonum cuspidatum, Parkinson's disease, Rotenone
Abbreviations: DA, Dopamine; DMSO, Dimethyl sulfoxide; GSH, Glutathione; H2O2, Hydrogen peroxide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine; MTT, Methylthiazolyldiphenyl-tetrazolium bromide; PARP-1, Poly (ADP-ribose) polymerase-1; PBS, Phosphate buffered saline; PD, Parkinson's disease; ROS, Reactive oxygen species; SDS, Sodium dodecyl sulphate; TH, Tyrosine hydroxylase; tTH, Total TH
Graphical abstract
1. Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder worldwide, affecting 1–2% of the population over the age of 65.1 The condition is characterised by the progressive loss of dopaminergic neurons from the substantia nigra pars compacta.2
There are a number of studies that link the development of PD with the exposure of certain pesticides such as rotenone.3, 4 As a result, rotenone is commonly used to create in vivo and in vitro models to study the disease.5, 6 H2O2 is a compound commonly used to model oxidative stress in vitro and in vivo.7, 8 As mitochondrial dysfunction and oxidative stress are thought to contribute to cell death in PD, we aimed to assess the effects of both rotenone and H2O2 on SH-SY5Y neuroblastoma cells.
The SH-SY5Y neuroblastoma cell line has been previously used to create a cellular model of PD.9, 10 The cells share many biochemical and functional characteristics with mature dopaminergic neurons and have the ability to differentiate into a dopaminergic phenotype. As tyrosine hydroxylase (TH) and dopamine (DA) seem to be central to the pathogenesis of PD and dopaminergic neurons are specifically targeted in the condition, we opted to utilise a cell line that had been transfected with human TH isoform 1 (TH1).11
There were two main aims to be addressed in this study, firstly we aimed to compare the effects of rotenone and H2O2 treatment on cell viability and TH expression and once we had established these changes we would then assess the ability of a number of potentially neuroprotective compounds to protect against this toxicity.
Cinnamon is a spice commonly used in food throughout the world. The spice has been demonstrated to have anti-diabetic and anti-inflammatory effects12, 13 as well as some neuroprotective properties.14 For instance a previous study demonstrated that treatment with cinnamon prevented the development of PD like symptoms and pathology in 1-methyl-4-phenyl-1,2,3,6-tetra hydropyridine (MPTP) treated mice,15 however the effect of cinnamon on rotenone is yet to be investigated. Hemp seed and its oil have been used as both a food and medicine in China for at least 3000 years and hemp seed extracts have been found to demonstrate antioxidant and antiaging effects16, 17, 18 as well as improve cognitive impairment induced by chemicals in mice.19 In addition to all of this, epidemiological studies suggest societies that commonly use curcumin, cinnamon and hemp seed appear to demonstrate a lower incidence of PD and neurodegenerative disorders.14, 20 We included the use of curcumin within our study as a positive control as this substance has been shown previously to provide protection against rotenone and H2O2 toxicity.10, 21, 22 Polygonum cuspidatum is widely distributed in the world and has been shown to possess antiviral, antimicrobial, anti-inflammatory, neuroprotective, and cardioprotective properties,23 however these properties are yet to be investigated using a cellular model of PD.
In this study we assessed the effect of rotenone and H2O2 on SH-SY5Y cell survival and TH protein expression. We also evaluated the protective effects of curcumin, cinnamaldehyde, and constituents isolated from hemp seed (caffeoyltyramide) and polygonum cuspidatum (piceatannol glucoside).
2. Materials and methods
2.1. Cell culture
TH1 transfected SH-SY5Y cells (kindly provided by Associate Professor Phil Dickson, University of Newcastle, Australia) were cultured in Dulbecco's Modified Eagles Medium nutrient mixture F-12 Ham (Sigma Aldrich, USA) supplemented with 10% foetal calf serum (Assay Matrix, AUS) and 1X Antibiotic-Antimycotic (Gibco, USA (Penicillin, Streptomycin, Fungizone® antimycotic)) in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were seeded and allowed to adhere for 2 days. Media was replaced with 2% foetal calf serum media 1.5 h before treatments began and cells were left to adjust.
2.2. Treatments
Caffeoyltyramide and piceatannol glucoside were isolated and purified as described previously from hemp seed and Polygonum cuspidatum24, 25 respectively. Their purity (>98%) was confirmed by high performance liquid chromatography and their structures were well identified using spectroscopic techniques.
Concentrated stocks of rotenone, curcumin, cinnamaldehyde, caffeoyltyramide and piceatannol glucoside were prepared using Dimethyl Sulfoxide (DMSO) (Sigma Aldrich, USA) while stocks of H2O2 were prepared using PBS. The compounds were added to the media in the wells to obtain the desired treatment concentrations. Cells were pre-treated with curcumin, cinnamaldehyde, caffeoyltyramide or picceatannol glucoside 1 h before rotenone or H2O2 exposure. All controls received the same concentration of vehicle with the DMSO not exceeding 0.5% (v/v) in any experiment.
2.3. MTT cell viability assay
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) powder (Sigma-Aldrich, USA) was used as a means to assess cell viability as previously described.26 Briefly, at the conclusion of the 24 h treatment duration, MTT was added to culture medium at a final concentration of 0.5 mg/ml and the plate was mixed gently for 1 min before 2 h incubation in a 37 °C, 5% CO2 incubator. After the incubation, media was removed and 100 μL of DMSO was added to each well for 10–15 min while shaking. The intensity of the purple colour produced in each well was measured colourimetrically using a plate reader at 595 nm. The values of absorbance are expressed as a proportion of the controls.
2.4. Trypan blue assay
The Trypan Blue (Sigma Aldrich, USA) assay was used as another means of visually assessing cell viability to support the MTT assay findings. Cells were seeded into 12 well plates and treated as described above. When the treatment protocol had ended the media was removed from the wells and 30 μL of Trypan Blue was added and left for 30 s. After 30 s an image of the plate was taken using ‘Cell Pad’. While not quantitative this protocol provided a means of visually assessing the viability of cell cultures.
2.5. SDS PAGE and western blots
Whole cell lysates were used for western blotting experiments to analyse total TH (tTH) and Poly-ADP ribose-polymerase (PARP-1) protein levels. Cells were seeded in a 24 well plate and treated as described above. At the end of the treatment protocol media was removed and 110 μL of 2% SDS stop buffer with inhibitors (50 mM Tris HCL (pH 6.8), 2% SDS, 2 mM EDTA, 1 mM Na orthovanadate, 1 mM Na fluoride, 10 mM Na pyrophosphate) was added. The lysed cells were collected and heated for 10 min at 100 °C. Samples were then frozen and stored at -20 °C for later analysis.
Samples were prepared for electrophoresis by dilution with sample buffer (20% DTT, 40% glycerol, 50 mM Tris, bromophenol blue, pH 6.8). Samples were run on an 8 or 10% SDS-polyacylamide gel and transferred to nitrocellulose membrane (0.45 μm and 0.2 μm, GE Healthcare, UK). To minimise non-specific binding membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.075% tween 20 (TBST) for 1.5 h at room temperature. Membranes were then incubated with anti-PARP-1 (1:1000, Santa Cruz Biotechnology catalogue no. sc-7150) or anti-total TH (tTH; 1:7,000, Sigma-Aldrich catalogue no. T2928) antibodies for 1 h at room temperature. Blots were washed and exposed to appropriate secondary antibody for 1 h at room temperature. Blots were then exposed to enhanced chemiluminescence detection reagent for 1 h and developed using a LAS 4000 imaging system (GE Healthcare, UK). Later, membranes were washed and then immunoblotted with β-actin antibody (1:10,000, Sigma-Aldrich, catalogue no. A3854) as a marker of the total protein loaded per lane. The density of the protein bands was quantified using ImageQuant TL software (GE Healthcare, UK). Quantitation of tTH and PARP-1 were normalised relative to β-actin levels.
2.6. Statistical analysis
For data with only two variables a two-tailed unpaired t-test was used. For data with more than two variables a one-way ANOVA was used to estimate the overall significance of the data; this was followed by post hoc Tukey's tests for multiple comparisons. All analyses were performed using GraphPad Prism software (USA) and the data is presented as mean ± SEM. All results were confirmed from a minimum of three independent experiments. Each experimental condition was conducted at least in triplicate. A probability of less than 5% (p < 0.05) was considered statistically significant.
3. Results
3.1. The effect of rotenone and H2O2 on cell viability
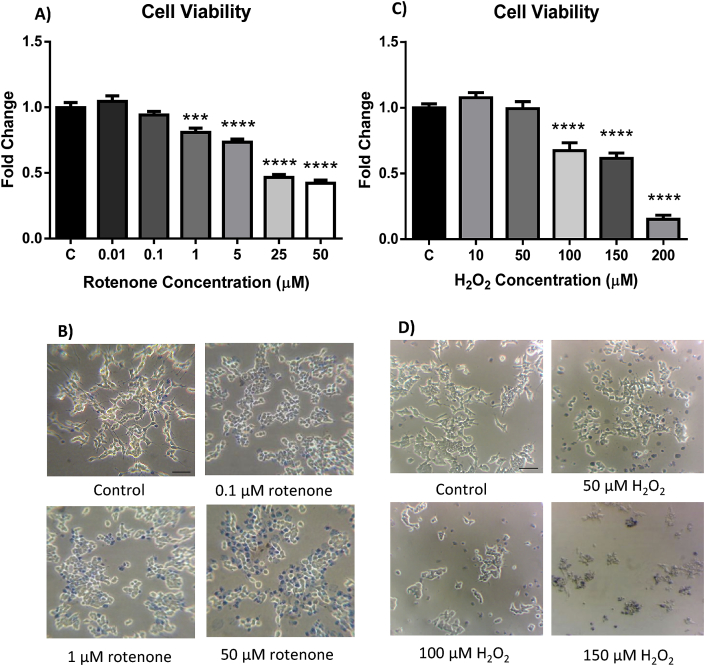
To determine the effect of rotenone and H2O2 on cell viability, cells were exposed to varying concentrations of the toxins for 24 h before viability was assessed by MTT assay. Fig. 1A depicts the effect of various concentrations of rotenone on the viability of SH-SY5Y cells. While exposure to 0.01 μM and 0.1 μM rotenone did not have a significant effect on cell viability, exposure to 1 μM and 5 μM rotenone decreased cell viability by approximately 20% (p = 0.0015) and 25% (p < 0.0001) respectively. The higher rotenone doses of 25 μM (p < 0.0001) and 50 μM (p < 0.0001) induced greater cell loss with an approximate 55% decrease of viability observed.
Fig. 1.
The effect of rotenone and H2O2on cell viability. Cells were exposed to varying concentrations of rotenone (A) or H2O2 (C) for 24 h before viability was assessed using the MTT assay. (B, D) Representative light microscopy images of trypan blue staining demonstrating the effect of the toxins on cell viability and morphology. Scale bar = 50 μm, C = control. Data presented as mean ± SEM. ***p < 0.005, ****p < 0.001 vs control.
Rotenone treatment also induced a change in cell morphology. Cells treated with rotenone lost their extensions and became small and rounded when compared to controls (Fig. 1B).
Fig. 1C depicts the effect of various concentrations of H2O2 on cell viability. No change in cell viability was detected after exposure to 10 μM or 50 μM H2O2 for 24 h. Treatment with 100 μM and 150 μM H2O2 resulted in the reduction of cell viability to approximately 67% (p < 0.0001) and 61% (p < 0.0001) of control cells respectively while treatment with 200 μM decreased cell viability to just 15% (p < 0.0001) of control cells.
Like rotenone, treatment with H2O2 also induced significant changes in cell morphology. Cells became smaller in size, lost their cellular extensions and became rounded when compared to controls (Fig. 1D).
3.2. The effect of rotenone and H2O2 on poly (ADP-ribose) polymerase-1 (PARP-1) cleavage
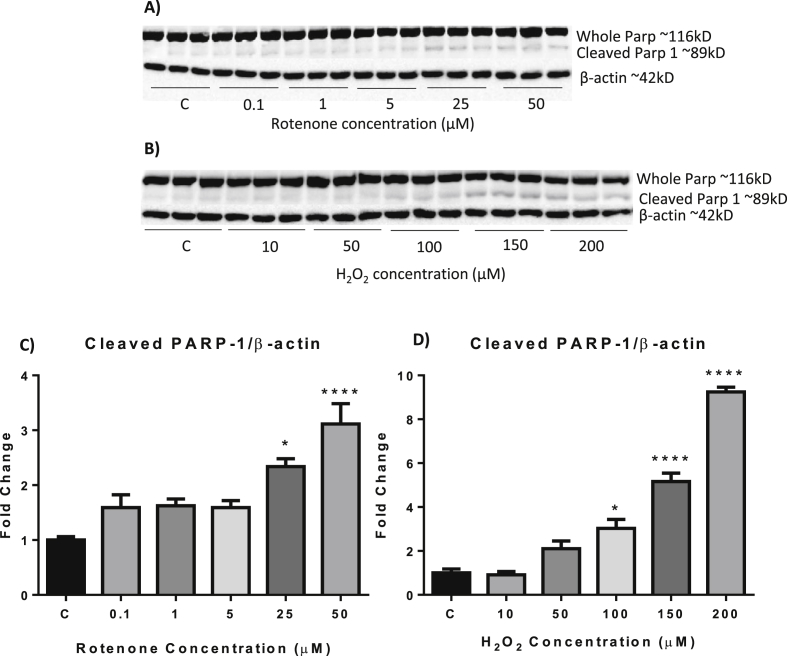
Poly (ADP-ribose) polymerase-1 (PARP-1) is a nuclear protein with a wide range of physiological functions. Cleavage of PARP-1 by caspases results in the production of an 89 kD protein fragment and is a major hallmark of apoptosis (Pacher and Szabo, 2008). Western blot analysis of our samples demonstrated 2 bands (Fig. 2A and B). The upper band was around 116 kD and corresponded to the expected size of uncleaved PARP-1. The other band was around 89 kD and corresponded to the expected size of the major fragment of cleaved PARP-1.
Fig. 2.
The effect of rotenone and H2O2treatment on PARP-1 cleavage as determined by western blot. (A, B) Representative western blots. (C, D) Cells were exposed to rotenone (C) or H2O2 (D) for 24 h before cells were harvested for western blot analysis. C = control. Data presented as mean ± SEM. *p < 0.05, ***p < 0.005, ****p < 0.001 vs control.
Treatment of SH-SY5Y cells with rotenone for 24 h caused a dose dependent increase in PARP-1 cleavage with 25 μM and 50 μM treatments resulting in approximately 2.5 (p = 0.0240) and 3-fold (p < 0.0001) increases in PARP-1 cleavage respectively compared to control cells (Fig. 2C).
Treatment of SH-SY5Y cells with varying concentrations of H2O2 for 24 h also resulted in a dose dependent increase in PARP-1 cleavage indicating the occurrence of apoptosis within these cells. While treatment with 10 μM and 50 μM H2O2 caused an increase in PARP-1 cleavage that failed to reach significance, treatment with 100 μM, 150 μM and 200 μM H2O2 resulted in 3 fold (p = 0.0174), 5 fold (p < 0.0001) and 9 fold (p < 0.0001) increases in PARP-1 cleavage respectively (Fig. 2D).
3.3. The effect of rotenone and H2O2 on tyrosine hydroxylase (TH) protein expression
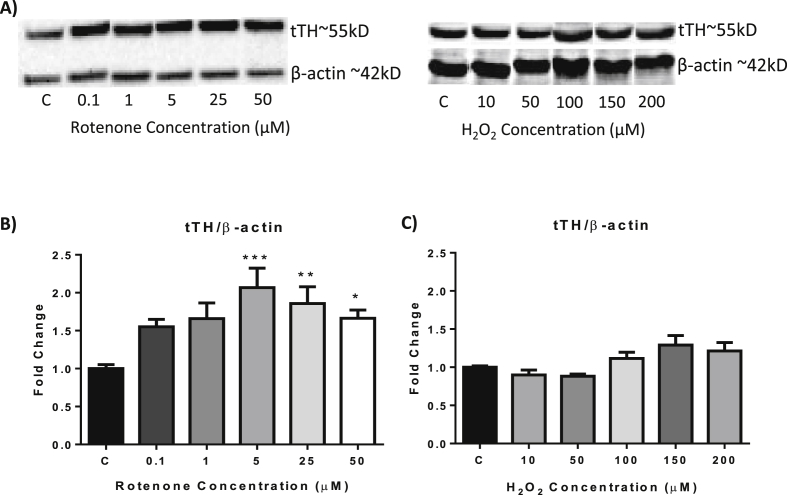
Tyrosine hydroxylase (TH) is the rate limiting enzyme in DA biosynthesis. The SH-SY5Y cells used in this project have been transfected with human TH-1 and hence have the ability to express the TH protein.11
Western blot analysis using a mouse anti-TH antibody revealed a prominent band at approximately 55 kD; the expected size of TH (Fig. 3A). 0.01 μM, 0.1 μM and 1 μM rotenone treatment did not induce any significant changes in total TH (tTH) expression (Fig. 3B). However, treatment with 5 μM, 25 μM and 50 μM rotenone resulted in a significant increase in TH expression (p = 0.0005, 0.0099 and 0.0367 respectively).
Fig. 3.
The effect of rotenone and H2O2on TH expression as determined by western blot. A) Representative western blots. (B, C) Cells were exposed to rotenone (B) or H2O2 (C) for 24 h before cells were harvested for western blot analysis. C = control. Data presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005, vs control.
In contrast to the rotenone treatment, H2O2 did not induce any significant changes in TH expression (Fig. 3C).
3.4. The effect of curcumin, cinnamaldehyde, caffeoyltyramide and piceatannol glucoside on rotenone toxicity
In this study we assessed the effect of curcumin, cinnamaldehyde (a component of cinnamon), caffeoyltyramide (a component of hemp seed) and piceatannol glucoside (a component of polygonum cuspidatum) on rotenone treatment to explore their ability to reduce rotenone induced cell death and therefore their potential as a preventative strategy and/or natural therapy to prevent DA degeneration in PD.
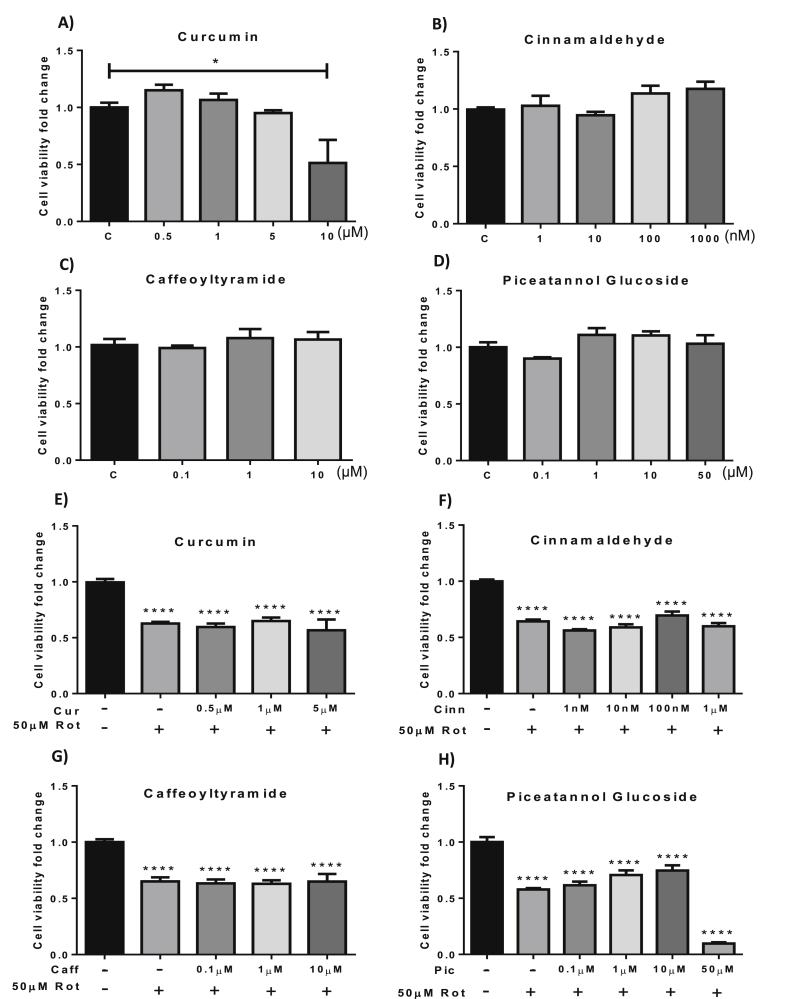
Exposing the cells to cinnamaldehyde (1 nM - 1 μM), caffeoyltyramide (0.1 μM–10 μM) or piceatannol glucoside (0.1 μM–50 μM) did not have any significant effect on cell viability (Fig. 4 B, C and D). Treatment with 10 μM curcumin demonstrated toxicity reducing cell viability to approximately 50% (p = 0.0149) (Fig. 4A) and hence this concentration was not used in further investigations.
Fig. 4.
The effect of natural compounds on cell viability and rotenone toxicity. Cells were exposed to curcumin (A), cinnamaldehyde (B), caffeoyltryramide (C), or piceatannol glucoside (D) for 24 h before viability was assessed using the MTT viability assay. Cells were exposed to curcumin (E), cinnamaldehyde (F), caffeoyltyramide (G) or piceatannol glucoside (H) before rotenone treatment to assess the effect of each compound on rotenone toxicity. C = control. Data presented as mean ± SE. *p < 0.05, ****p < 0.001 vs control. Cur-curcumin; Cinn-cinnamaldehyde; Caff-caffeoyltyramide; Pic-piceatannol glucoside; Rot-rotenone.
As demonstrated in Fig. 4 E, F, G and H, pre-treatment with curcumin, cinnamaldehyde, caffeoyltyramide or piceatannol glucoside did not have any significant effect on rotenone toxicity in SH-SY5Y cells.
3.5. The effect of curcumin, cinnamaldehyde, caffeoyltyramide and piceatannol glucoside on H2O2 toxicity
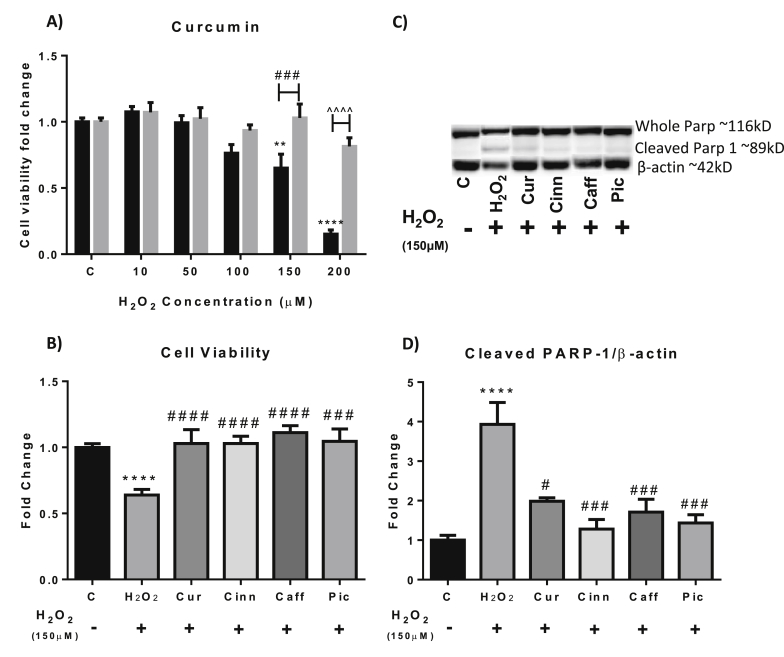
Pre-treatment with curcumin provided protection against every H2O2 concentration tested (10 μM - 200 μM), notably curcumin increased the viability of cells treated with 150 μM H2O2 from approximately 60%–100% (p = 0.0006) and the viability of 200 μM H2O2 treated cell from 15% to approximately 80% (p < 0.0001) (Fig. 5A). While curcumin did have the ability to protect against treatment with 200 μM H2O2, we found that the reduction in cell viability caused by this concentration of H2O2 was too robust and made further protein analysis difficult therefore we proceeded with 150 μM H2O2 treatments.
Fig. 5.
The effect of compounds on H2O2toxicity. A) The effect of 1 μM curcumin on the viability of H2O2 treated cells. Black columns = H2O2 alone, Grey columns = H2O2 + 1 μM Curcumin. B) The effect of various compounds on the viability of H2O2 treated cells. C) Representative western blots for the effect of various compounds on PARP-1 cleavage. D) Quantitative data on the effect of various compounds on PARP-1 cleavage. C = control. Data presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001 compared to control. #p < 0.05, ###p < 0.005 vs 150 μM H2O2 treatment group. ˆˆˆˆp < 0.0001 vs 200 μM H2O2 treatment group. Cur-curcumin; Cinn-cinnamaldehyde; Caff-caffeoyltyramide; Pic-piceatannol glucoside.
In contrast, pre-treatment with all of the compounds tested provided the cells with protection against cellular toxicity induced by treatment with 150 μM H2O2 (Fig. 5B).
In addition, we also evaluated the effect of these compounds on the increase in PARP-1 cleavage seen in H2O2 treated cells. We found that 1 μM curcumin, 100 nM cinnamaldehyde, 1 μM piceatannol glucoside and 10 μM caffeoyltyramide were able to reduce PARP-1 cleavage, indicating a reduction in apoptosis (Fig. 5C and D). These findings suggest that the tested compounds could provide protection against H2O2 induced apoptosis and that they potentially possess antioxidant properties.
4. Discussion
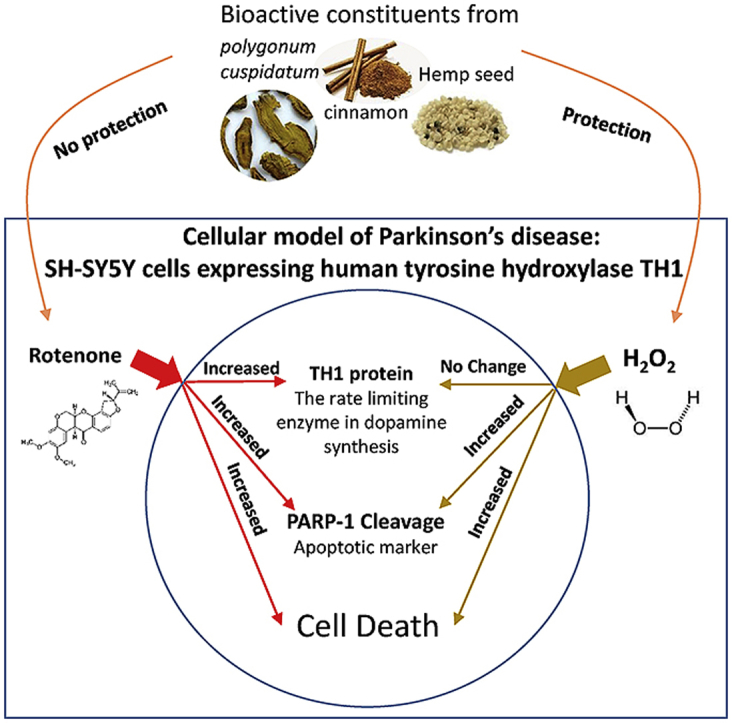
This study demonstrates that rotenone and H2O2 have markedly different effects on SH-SY5Y cells. H2O2 treatment does not appear to have any significant effect on TH protein expression and the cell death induced by H2O2 can be prevented by a number of compounds tested. In contrast, rotenone treatment was associated with an increase in TH protein levels and its toxicity could not be prevented with any of the compounds.
Mitochondrial dysfunction and oxidative stress are two factors that are thought to play a significant role in the development of PD. As seen in Fig. 1, rotenone and H2O2 both induced dose dependant decreases in cell viability in the TH1 transfected SH-SY5Y cells. The concentrations of rotenone and H2O2 needed to induce this change were higher than those noted in previous studies using the SH-SY5Y cell line22, 27; this could be due to the transfection of TH1 within these cells incurring increased resistance to rotenone. This hypothesis is supported by our previous studies using the TH1 transfected SH-SY5Y cell line reporting an increased resistance to oxidative stress and treatment with 6-OHDA and H2O2.28
Cleavage of PARP-1 is commonly used as an index of apoptosis. In this study, treatment with rotenone and H2O2 both induced a dose dependent increase in PARP-1 cleavage indicating the occurrence of apoptosis. Interestingly while oxidative stress is thought to play a major role in cell death induced by both rotenone and H2O2, the two toxins had significantly different effects on TH expression. Rotenone induced an increase in TH expression within our SH-SY5Y cells, this is in contrast to previous studies that suggest rotenone treatment results in a decrease in TH expression in both animal models and cells.29, 30 It should be emphasised, however, we measured TH protein only in the cells that were still attached to the bottom of the plate (which we assume were still viable) but not in the cells that had detached from the plates (due to death) as these were removed with the media at the completion of rotenone treatment. Therefore, our results suggest that TH protein was increased only per remaining cell (surviving cell) and not per total number of cells present at the beginning of the treatment. In contrast H2O2 had no effect on TH protein levels in the remaining cells, suggesting that while oxidative stress may play a role in rotenone induced cell death, the increase in TH seen was not induced by it. Rotenone is also thought to cause inhibition of the proteasome system,31 therefore it is possible that the levels of TH protein are increasing in these cells because breakdown and removal of the protein have been altered; however, this requires further investigation.
Natural compounds with antioxidant and anti-inflammatory properties have become of interest with regards to PD as the current treatments are associated with harmful side effects. Curcumin, cinnamon, hemp seed and Polygonum cuspidatum are all naturally occurring products that have been used in traditional Chinese medicine for many years.14, 16, 32 We found that pre-treatment of our SH-SY5Y cells with compounds isolated from these products did not have an effect on rotenone toxicity. This is in contrast to previous studies that have demonstrated the protective effect of curcumin against rotenone in SH-SY5Y cells.10, 22 It should be noted that while both studies have utilised SH-SY5Y cells, our cells contain human TH1. Therefore, it is possible that the transfected TH1 and the increase in TH protein expression seen with rotenone treatment could be potentiating rotenone induced cell death and playing a role in our inability to protect against rotenone toxicity. As TH is the rate limiting enzyme in DA synthesis, it is possible that the increased TH protein expression in response to rotenone could have induced an increase in DA production. The increased DA could possibly accumulate within the cytoplasm of the cells and lead to increased oxidative stress, proteasomal inhibition and mitochondrial dysfunction that could be contributing to rotenone induced cell death. This hypothesis is similar to a popular hypothesis that suggests an involvement of hyper-activation of TH and DA production in early PD pathogenesis.33 Interestingly, the same compounds did display the ability to protect against H2O2 induced toxicity. In addition, all tested compounds also prevented the increase in PARP-1 cleavage seen with H2O2 treatment indicating a reduction in apoptosis and supporting the findings of the viability assay as well as the findings of previous studies that suggest the novel compounds possess antioxidant activity.24, 25 Moreover a previous study has demonstrated the ability of cinnamaldehyde to modulate the release of catecholamines from a rat pheochromocytoma cell line,34 indicating this compound has the potential to not only be neuroprotective but may also further ease the symptoms of PD by promoting catecholamine release. While the in vitro antioxidant capabilities of these compounds have been demonstrated previously, this is the first study to demonstrate the neuroprotective properties of cinnamaldehyde, caffeoyltyramide and piceatannol glucoside in a dopaminergic cell line in response to H2O2.
In summary, we demonstrated that the effect of rotenone on these cells is more complicated than just the induction of oxidative stress and suggest that perhaps TH may be involved. Curcumin, cinnamaldehyde, caffeoyltyramide and piceatannol glucoside successfully prevented H2O2 induced cell death, making this the first study to demonstrate the neuroprotective potential of these natural compounds in a SH-SY5Y cellular model of oxidative stress.
Conflicts of interest
None.
Acknowledgements
This work was supported by the China-Australia Centre for Health Science Research (Grant No. 2015GJ04).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Pringsheim T., Jette N., Frolkis A., Steeves T.D.L. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29(13):1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 2.Maguire-Zeiss K.A., Federoff H.J. Convergent pathobiologic model of Parkinson's disease. Ann N. Y Acad Sci. 2003;991(1):152–166. doi: 10.1111/j.1749-6632.2003.tb07473.x. [DOI] [PubMed] [Google Scholar]
- 3.Tanner C.M., Kamel F., Ross G.W. Rotenone, paraquat, and Parkinson's disease. Environ health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezzoli G., Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology. 2013;80(22):2035–2041. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 5.Choi B.-S., Kim H., Lee H.J. Celastrol from ‘thunder god vine’ protects SH-SY5Y cells through the preservation of mitochondrial function and inhibition of p38 MAPK in a rotenone model of Parkinson's disease. Neurochem Res. 2014;39(1):84–96. doi: 10.1007/s11064-013-1193-y. [DOI] [PubMed] [Google Scholar]
- 6.Jang W., Kim H.J., Li H. 1,25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophysical Res Commun. 2014;451(1):142–147. doi: 10.1016/j.bbrc.2014.07.081. [DOI] [PubMed] [Google Scholar]
- 7.Koppenhöfer D., Kettenbaum F., Susloparova A. Neurodegeneration through oxidative stress: monitoring hydrogen peroxide induced apoptosis in primary cells from the subventricular zone of BALB/c mice using field-effect transistors. Biosens Bioelectron. 2015;67:490–496. doi: 10.1016/j.bios.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Hu X.-L., Gao L.-Y., Niu Y.-X. Neuroprotection by Kukoamine A against oxidative stress may involve N-methyl-d-aspartate receptors. Biochimica Biophysica Acta (BBA) - General Subj. 2015;1850(2):287–298. doi: 10.1016/j.bbagen.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Xie H.R., Hu L.S., Li G.Y. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson's disease. Chin Med J. 2010;123(8):1086–1092. [PubMed] [Google Scholar]
- 10.Liu Z., Li T., Yang D., Smith W.W. Curcumin protects against rotenone-induced neurotoxicity in cell and drosophila models of Parkinson's disease. Adv Parkinson's Dis. 2013;2(1):19–27. [Google Scholar]
- 11.Gordon S.L., Bobrovskaya L., Dunkley P.R., Dickson P.W. Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: isoform 2 is not phosphorylated at Ser35. Biochimica Biophysica Acta (BBA) Mol Cell Res. 2009;1793(12):1860–1867. doi: 10.1016/j.bbamcr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.H., Hyun S.H., Choung S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104(1–2):119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Tung Y.-T., Chua M.-T., Wang S.-Y., Chang S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol. 2008;99(9):3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 14.Kannappan R., Gupta S.C., Kim J.H., Reuter S., Aggarwal B.B. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Mol Neurobiol. 2011;44(2):142–159. doi: 10.1007/s12035-011-8168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khasnavis S., Pahan K. Cinnamon treatment upregulates neuroprotective proteins parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neuroimmune Pharmacol. 2014;9(4):569–581. doi: 10.1007/s11481-014-9552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T., He J., Zhang J. The isolation and identification of two compounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.) Food Chem. 2012;134(2):1030–1037. doi: 10.1016/j.foodchem.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Gowran A., Noonan J., Campbell V.A. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci Ther. 2011;17(6):637–644. doi: 10.1111/j.1755-5949.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldzio R., Pacher T., Krewenka C. Effects of cannabinoids Δ(9)-tetrahydrocannabinol, Δ(9)-tetrahydrocannabinolic acid and cannabidiol in MPP+ affected murine mesencephalic cultures. Phytomedicine. 2012;19(8–9):819–824. doi: 10.1016/j.phymed.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Luo J., Yin J.-H., Wu H.-Z., Wei Q. Extract from Fructus cannabis activating calcineurin improved learning and memory in mice with chemical drug-induced dysmnesia. Acta Pharmacol Sin. 2003;24(11):1137–1142. [PubMed] [Google Scholar]
- 20.Muangpaisan W., Hori H., Brayne C. Systematic review of the prevalence and incidence of Parkinson's disease in asia. J Epidemiol. 2009;19(6):281–293. doi: 10.2188/jea.JE20081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uğuz A.C., Öz A., Nazıroğlu M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J Recept Signal Transduct. 2016;36(4):395–401. doi: 10.3109/10799893.2015.1108337. [DOI] [PubMed] [Google Scholar]
- 22.Qualls Z., Brown D., Ramlochansingh C., Hurley L.L., Tizabi Y. Protective effects of curcumin against rotenone and salsolinol induced toxicity: implications for Parkinson's disease. Neurotox Res. 2014;25(1):81–89. doi: 10.1007/s12640-013-9433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H., Li C., Kwok S.-T., Zhang Q.-W., Chan S.-W. A review of the pharmacological effects of the dried root of polygonum cuspidatum (Hu Zhang) and its constituents. Evidence-Based Complementary Altern Med. 2013;2013:13. doi: 10.1155/2013/208349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X., Tang J., dos Santos Passos C. Characterization of lignanamides from hemp (cannabis sativa l.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J Agric Food Chem. 2015;63(49):10611–10619. doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- 25.Fan P., Marston A., Hay A.-E., Hostettmann K. Rapid separation of three glucosylated resveratrol analogues from the invasive plant Polygonum cuspidatum by high-speed countercurrent chromatography. J Sep Sci. 2009;32(17):2974–2984. doi: 10.1002/jssc.200900057. [DOI] [PubMed] [Google Scholar]
- 26.Wang X.J., Wang L.Y., Fu Y. Promising effects on ameliorating mitochondrial function and enhancing Akt signaling in SH-SY5Y cells by (M)-bicelaphanol A, a novel dimeric podocarpane type trinorditerpene isolated from Celastrus orbiculatus. Phytomedicine. 2013;20(12):1064–1070. doi: 10.1016/j.phymed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.-J., Park H.J., Park H.K., Chung J.-H. Tranexamic acid protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Toxicology. 2009;262(2):171–174. doi: 10.1016/j.tox.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Franco J.L., Posser T., Gordon S.L. Expression of tyrosine hydroxylase increases the resistance of human neuroblastoma cells to oxidative insults. Toxicol Sci. 2010;113(1):150–157. doi: 10.1093/toxsci/kfp245. [DOI] [PubMed] [Google Scholar]
- 29.Qin J., Wu M., Yu S. Pyrroloquinoline quinone-conferred neuroprotection in rotenone models of Parkinson's disease. Toxicol Lett. 2015;238(3):70–82. doi: 10.1016/j.toxlet.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Cannon J.R., Tapias V., Na H.M., Honick A.S., Drolet R.E., Greenamyre J.T. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34(2):279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamoto-Nagai M., Maruyama W., Kato Y. An inhibitor of mitochondrial complex I, rotenone, inactivates proteasome by oxidative modification and induces aggregation of oxidized proteins in SH-SY5Y cells. J Neurosci Res. 2003;74(4):589–597. doi: 10.1002/jnr.10777. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.-C., Chen Y.-T., Chiu C.-C., Liao W.-T., Liu Y.-C., David Wang H.-M. Polygonum cuspidatum extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. J Biosci Bioeng. 2015;119(4):464–469. doi: 10.1016/j.jbiosc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima A., Ota A., Kaneko Y.S., Mori K., Nagasaki H., Nagatsu T. A possible pathophysiological role of tyrosine hydroxylase in Parkinson's disease suggested by postmortem brain biochemistry: a contribution for the special 70th birthday symposium in honor of Prof. Peter Riederer. J Neural Transm. 2013;120(1):49–54. doi: 10.1007/s00702-012-0828-5. [DOI] [PubMed] [Google Scholar]
- 34.Tsai C.-C., Liu I.-M., Cheng J.-T. Stimulatory effect of trans- cinnamaldehyde on norepinephrine secretion in cultured pheochromocytoma (PC-12) cells. Acta Phamacol Sin. 2000;21(12):1174–1178. [PubMed] [Google Scholar]