Abstract
Sesamum indicum, one of the first recorded plants used for its seeds, is reported to have analgesic, antioxidant, anticancer, anti-obesity as well as hepato and nephro protective activities. The current study evaluated the effects of two doses (400 and 800 mg/kg) of ethanolic extract of S. indicum seeds in Freund's complete adjuvant induced arthritis in rats in comparison with diclofenac and methotrexate by the changes produced in body weight, body temperature, paw volume and spontaneous activity, hemoglobin, erythrocyte sedimentation rate, total white blood cells, red blood cells, Interleukin-6 and Tumor necrosis factor-α as well as joint changes in X-ray and histological changes in joint tissue. Unlike the untreated group, the groups treated with S. indicum showed significant decrease in paw volume, body weight, white blood cell count, erythrocyte sedimentation rate, Interleukin-6 and Tumor necrosis factor-α and an increase in body weight, spontaneous activity, hemoglobin level, and red blood cell count. Histopathological examination showed gross reduction in synovial inflammation and cartilage damage. X-ray revealed significant improvement in joint space. The effect of ethanolic extract of S. indicum was found to be equivalent to methotrexate and greater than diclofenac.
Keywords: Sesamum indicum, Rheumatoid arthritis, Freund's complete adjuvant, Interleukin-6, Tumor necrosis factor-α
Graphical abstract

1. Introduction
Rheumatoid arthritis (RA) is a chronic multi system disease characterized by persistent inflammatory synovitis involving peripheral joints leading to progressive functional impairment.1 About 1 % of the world's population and 0.7 % (88 lakhs) of Indian population are afflicted with RA. It is more common in women and generally occurs between 40 and 60 years of age.2, 3
A panel of drugs such as non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease modifying anti rheumatoid drugs (DMARDs), biologicals such as TNF-α and IL antagonists and JANUS kinase inhibitors (JKIs) have been used to relieve pain and reduce immunological reaction mediated inflammation and joint damage.4, 5 A step wise approach starting with NSAIDs followed by glucocorticoids and DMARDs, either alone or in combinations are used in the current management of RA.6 However these drugs neither offer a complete cure nor are free from adverse effects. Hence finding out new drugs is essential.
In this context, a well known plant from indigenous system, Sesamum indicum (SI) has been chosen for the current study.
SI, an ancient spice and one of the first recorded plants belongs to the family Pedaliaceae. It is used for its seeds for thousands of years and is still an oil seed of worldwide significance. Sesame oil is commonly used in margarine production and cooking.7 SI contains sesamin 31.3 %, Sesamol 34.2 % sesamolin 46.7 % per 100 g and also contain oleic acid, α-tocopherol, γ-tocophereol, palmitic acid, stearic acid and linoleic acid, α-linolenic acid.8, 9, 10 In addition SI contains vitamin B1: 0.28 mg, 1.48 mg of copper, 0.88 mg of manganese, 120 mg of tryptophan, 351.00 mg of calcium, 126.36 mg of magnesium, 5.24 mg of iron, 226.44 mg of phosphorus, 2.80 mg of zinc and dietary fiber.11 The pharmacological activities reported include analgesic, antioxidant, anticancer, anti-obesity as well as hepato and nephro protective activities.12, 13, 14, 15, 16
Considering its traditional use, the phytochemical constituents and the reported activities the present study was undertaken to evaluate the effects of ethanolic extract of SI seed in Freund's complete adjuvant-induced rheumatoid arthritis in rats.
2. Materials and methods
2.1. Extract preparation
The black seeds of SI were collected from Chennai (Tamil Nadu state, India) and authenticated by Dr. Narasimhan, Associate Professor of Botany, Madras Christian College, Tambaram, Chennai, Tamil Nadu.
The seeds were washed thoroughly, dried under shade and powdered. 1.5 kg of the air-dried seeds was subsequently pulverized to uniform powder using an electric blender (25–28 °C). Pulverized seed (1.5 kg) was then defatted by mixing with n-hexane (3000 ml) using a magnetic stirrer at room temperature for 6 h. The resultant slurry was filtered and the residue was air dried for 24 h. The dried defatted residue (1000×g obtained from 1.5 kg) was then subjected to continuous extraction with 5 L of 95% v/v ethanol using Soxhlet apparatus at a temperature of (60–70° C) for 15 cycles. This process was repeated for 3 times. The extract thus obtained was dried by using rotary evaporator. 1000 g of dried defatted residue yielded 113.4 g of extract and the percentage of extraction was 11.34 %. The extract was brown in colour and it was transferred to a clean bottle and stored at 4 °C in a refrigerator until further use.
2.2. Drugs and chemicals
Freund's Complete Adjuvant (FCA) was procured from Sigma chemicals Co. ELISA kits of TNF-α and IL-6 were purchased from Ray Biotech, diclofenac and methotrexate from M/S Alkem laboratories ltd. All other chemicals were of the highest purity and analytical grade.
2.3. Animals
Wistar albino rats were obtained from the animal house of Chettiand Hospital and Research Institute. The study was initiated after obtaining approval from the Institutional Animal Ethics Committee (IAEC2/Desp.No.49/Dt.29.07.2013).
Rats were used according to the guidelines given by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) in India. They were housed in clean poly propylene cages at 23–25 °C with relative humidity of 50–60 % in natural 12 h light-dark cycle with food and water.
2.4. Experimental design
A total of 36 male Wistar albino rats weighing 250–300 g were selected and allocated to 6 groups of 6 rats in each group. Group I was used as normal control. Group 2 to 6 were RA-induced and treated as given in Table 1.
Table 1.
Experimental design.
| Group | Treatment |
|---|---|
| Group 1 | Normal control (NC) |
| Group 2 | RA control (RAC) |
| Group 3 | FCA + Diclofenac (D) 25 mg/kg |
| Group 4 | FCA + Methotrexate (M) 50 μg/kg/week |
| Group 5 | FCA + EESI 400 mg/kg |
| Group 6 | FCA + EESI 800 mg/kg |
Drugs were administered daily orally from day 8–28.
2.4.1. Dose selection
Acute toxicity studies were not conducted as its safety up to 2000 mg/kg has been reported in an earlier study.13 The two doses 400 and 800 mg/kg were selected based on earlier studies which have reported better response for doses above 400 mg/kg for analgesic, hepato and nephro protective activities.12, 13, 14
2.5. Evaluation of anti-rheumatoid activity
2.5.1. Freund's complete adjuvant-induced arthritis
Freund's complete adjuvant contains heat-killed dead mycobacterium tuberculosis bacteria in liquid paraffin in the concentration of 10 mg/ml. All the rats, except those in the normal control group, were injected intradermally with 0.1 ml of FCA into the left hind paw on day ‘0’. An interval of 7 days was given for arthritis to develop. All the animals developed the signs of arthritis such as swelling, redness and restricted movement during this period.17 On day 8, 3 ml of blood samples were collected by retro-orbital puncture for baseline biochemical assays and treatment was started. The treatment was ended on day 28. Body weight, temperature, spontaneous activity and paw volume of rats were measured once in 7 days from day ‘0’ to ‘28’. On day 28, X-ray of the hind paw was taken and blood sample was again collected for biochemical assays. The rats were then sacrificed by administering high dose of halothane and the ankle joints were dissected for histological studies.
2.6. Determination of serum IL-6 and TNF-α levels
Serum was separated from blood samples by centrifugation (3000 rpm for 10 min) and stored at -20 °C. IL-6 and TNF-α were determined by ELISA kits according to the manufacturer's protocol (Ray Biotech, USA). Optical density (OD) was measured by Bio-Rad ELISA reader at 450 nm.
2.7. Radiological changes
The x-ray of lower limbs were taken with Siemens, Heliphos D X-ray machine and joint changes were assessed based on joint space and soft tissue swelling.
2.8. Histopathological study of joints
Rats were sacrificed by administering high dose of halothane. Ankle joints were removed and fixed in 10 % buffered formalin. The bones were decalcified in 5 % formic acid, processed for paraffin embedding, sectioned at 5 μm thickness and subsequently stained with haemotoxylin-eosin for examination under a light microscope for the presence of changes in synovium, cartilage and joint space.
2.9. In-vivo anti oxidant activity
The antioxidant activity was assessed using joint tissue homogenate by the following assays.
200 mg of joint tissue was cut into small pieces, crushed using mortar and pestle and homogenized at 4 °C in 1.5 ml of 0.1 M phosphate buffer (pH 7.2) to prepare joint homogenate. The homogenate was centrifuged at 8000 rpm for 15 min at 4 °C and the supernatant was stored at 80 °C.
-
A)
Lipid peroxidation
0.5 ml of 10% joint tissue homogenate was added to 100 μl of 0.2 mM FeCl3 which was then added to 2 ml reaction mixture (0.25N HCl containing 15% TCA, 0.30% TBA and 0.05% BHA). The suspension was heated at 80 0C for 1 h, cooled and then centrifuged at 1,500 rpm. The supernatant was collected and lipid peroxidation was estimated by measuring the concentration of thiobarbituric acid reaction substances (TBARS) in fluorescence at 530 nm.18
-
B)
Superoxide dismutase
50 μL of the above prepared joint tissue homogenate supernatant was added to 2.8 ml Tris-EDTA (50 mM Tris, 1.2 mM EDTA, pH = 8.5) and 100 μL of 2 mM pyrogallol at 25 °C. The optical density of the mixture was read at zero and at three minutes at 420 nm and compared with control containing Tris-EDTA and pyrogallol alone. The amount of SOD required to inhibit auto oxidation of pyrogallol by 50% is considered one unit. The activity of SOD is expressed as units/mg protein.19
-
C)
Catalase
0.1 ml of tissue homogenate was added to1.0 ml of 0.01 M phosphate buffer (PH 7.0) and 0.4 ml of 2 M H2O2 to make 1.5 ml of reaction mixture. Catalase in the tissue homogenate converts H2O2 to H2O and O. As the known amount of hydrogen peroxide was added the amount that is left unconverted will indicate the extent of antioxidant activity. 2 ml dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio) was added to stop the reaction. Then the absorbance was measured at 530 nm; CAT activity was expressed as μM of H2O2 consumed/min/mg protein.20
-
D)
Glutathione peroxidase (GPx)
0.5 ml of the tissue homogenate was added to 0.2 ml of phosphate buffer, 0.2 ml EDTA, 0.1 ml sodium azide to make 1 ml of reaction mixture. To this mixture 0.2 ml of glutathione and 0.1 ml of H2O2 were added and incubated at 37 °C for 10 min. The reaction was arrested by the addition of 0.5 ml of 10% TCA. GPx activity was calculated by the change of the absorbance at 340 nm for 3 min. GPx activity is expressed as nmol/min/mg protein.21
2.10. Statistical analysis
The results were expressed as mean ± SEM and difference among the means was analyzed using One-way ANOVA followed by post hoc (Tukey's) test. The difference in values at p < 0.05 was considered statistically significant.
3. Results
3.1. Physical parameters
Paw volume (PV) was increased in all the rats except the normal rats when assessed on day 7. Following treatment there was a reduction in PV in both standards and test groups. The reduction in PV on day 28 was found to be significant when compared to RA control and the reduction was equal in EESI (800 mg/kg) and D, M treated groups (p < 0.05) (Table 2).
Table 2.
Effect on paw volume.
| Groups | Treatment | Paw volume (ml) |
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| I | Normal control | 0.14 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 |
| II | RA control | 0.12 ± 0.02 | 1.03 ± 0.28 | 0.77 ± 0.03 | 0.85 ± 0.03 | 0.82 ± 0.02 |
| III | Diclofenac | 0.12 ± 0.02 | 1.03 ± 0.03 | 0.87 ± 0.09 | 0.40 ± 0.03* | 0.28 ± 0.02** |
| IV | Methotrexate | 0.17 ± 0.03 | 1.03 ± 0.09 | 0.87 ± 0.03 | 0.42 ± 0.03* | 0.23 ± 0.02** |
| V | SI 400 mg/kg | 0.15 ± 0.02 | 1.07 ± 0.05 | 0.83 ± 0.03 | 0.39 ± 0.02* | 0.38 ± 0.02* |
| VI | SI 800 mg/kg | 0.16 ± 0.02 | 0.97 ± 0.05 | 0.88 ± 0.03 | 0.38 ± 0.02* | 0.20 ± 0.02** |
Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
A significant decrease in body weight was observed in RAC group. The groups treated with EESI, D and M showed a decrease in BW on day 7 and day 14, but a gradual increase from day 21–28. This increase was not found to be statistically significant between standards and tests (Fig. 1).
Fig. 1.
Effect on body weight. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
The mean body temperature in NC was 34.67 ± 0.33 °C and there was no change in temperature during the experiment. After induction of arthritis temperature increased to 39.33 ± 0.88 °C. Treatment with EESI, D and M produced a reduction in temperature of 4–5 °C (Fig. 2). The reduction in temperature was equal among the groups.
Fig. 2.
Effect on body temperature. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
The baseline spontaneous activity (SA) on day 0 was 163.5 ± 5.95. On day 7, SA was reduced to 60 ± 9.54 in RAC group. Standard and test drugs improved the SA significantly. There was no significant difference between test and standard groups (Fig. 3).
Fig. 3.
Effect on spontaneous activity. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
3.2. Hematological parameters
Induction of arthritis resulted in significant decrease in RBC count (cells/mm3), Hb% and an increase in total WBC count and ESR. Treatment with EESI and D showed significant increase in Hb% and RBC count and decrease in total WBC count and ESR. The group treated with M had the lowest Hb% of 8.64 ± 0.68 and RBC count 6.62 ± 0.32; whereas with EESI 400 and 800 mg/kg Hb% was 10.71 ± 0.44, 10.90 ± 0.42 and RBC count 7.54 ± 0.38, 8.35 ± 0.23 respectively (Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 4.
Effect on RBC count. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
Fig. 5.
Effect on Hb %. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
Fig. 6.
Effect on total WBC count. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
Fig. 7.
Effect on ESR level. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
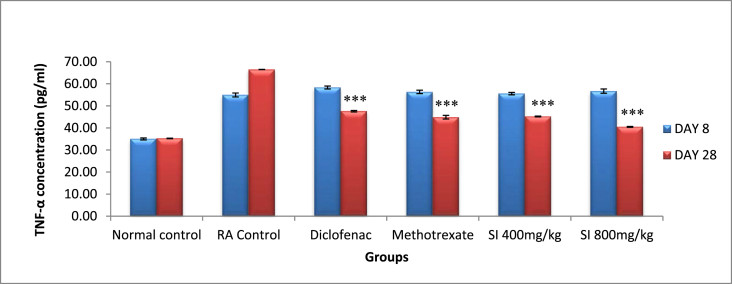
3.3. IL-6 and TNF-α levels
Serum IL-6 and TNF-α levels significantly increased when arthritis was fully developed as compared to normal rats. In arthritic rats, the levels of the two cytokines were 68.64 ± 0.84 pg/ml and 66.50 ± 0.04 pg/ml, respectively on day 28. The post treatment with EESI 800 mg/kg the levels of both IL-6 and TNF-α were the reduced to 39.87 ± 0.29 pg/ml and 40.46 ± 0.19 pg/ml, respectively and found to be greater than D and equal to M (Fig. 8, Fig. 9).
Fig. 8.
Effect on IL-6 levels. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA control, ***P < 0.001 with RA control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
Fig. 9.
Effect on TNF-α levels. Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA control, ***P < 0.001 with RA control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
3.4. Radiological changes
RA-induced joint showed soft tissue swelling and narrowing of joint space. The joint space was intact and soft tissue swelling was reduced in rats treated with D, M and EESI. But there was no difference in joint space between standards and tests (Fig. 10).
Fig. 10.
Radiological changes of joints. ↑ – Soft tissue swelling; ↓ – Joint space.
3.5. Histopathological changes
The joints of hind paw in NC rats had shown intact articular cartilage and normal joint space without inflammation. Inflammatory changes, distorted articular cartilage and narrowing of joint space were seen in RAC rats. There was varying degrees of reduction in inflammation and improvement in joint space in groups treated with SI at both the doses as well as with D and M. But the histological changes produced in EESI were better than D and M (Fig. 11).
Fig. 11.
Histopathological changes of joints. NC – Negative Control, D – Diclofenac, M – Methotrexate, SI 400 – Sesamum Indicum 400 mg/kg, SI 800 – Sesamum Indicum 800 mg/kg. B – Bone, C – Cartilage, SP – Synovial Space.  – Cartilage,
– Cartilage,  – Joint space,
– Joint space,  – cellular infiltration.
– cellular infiltration.
3.6. In-vivo antioxidant activity
Increased levels of SOD, CAT, GPx and decreased level of LPO confirmed the antioxidant property of the extract. The antioxidant activity was found to be significantly higher with SI 800 mg/kg (Table 4).
Table 4.
Effect on In-vivo antioxidant activity.
| Groups | Treatment | LPO (nmol/mg of protein) | SOD (U/mg of protein) | CAT (nmol/min/mg of protein) | GPx (nmol/min/mg protein) |
|---|---|---|---|---|---|
| I | Normal control | 8.23 ± 0.77 | 14.34 ± 1.08 | 301.49 ± 45.89 | 170.62 ± 10.89 |
| II | RA control | 13.34 ± 1.2 | 6.2 ± 0.51 | 134.96 ± 16.61 | 90.13 ± 1.37 |
| III | Diclofenac | 10.45 ± 1.32* | 10.24 ± 0.72* | 217.51 ± 40.5 | 119.22 ± 1.22* |
| IV | Methotrexate | 10.11 ± 1.7* | 10.11 ± 0.19* | 235.62 ± 35.48* | 127.88 ± 5.69** |
| V | SI 400 mg/kg | 10.98 ± 1.01 | 8.8 ± 0.72 | 201.87 ± 44.25 | 114.45 ± 2.98 |
| VI | SI 800 mg/kg | 8.93 ± 1.09* | 11.5 ± 0.89** | 262.24 ± 44.75* | 137.36 ± 3.82** |
Values expressed as Mean ± SEM, n = 6, *P < 0.05, **P < 0.01 with RA Control, #P < 0.05 with diclofenac, a P < 0.05 with methotrexate.
Thus the treatment with SI extract at both the doses resulted in reduction in PV, BT and increase in Hb level, RBC count and decrease in WBC count, ESR, IL-6 and TNF-α from baseline value. Radiological examination revealed significant improvement in joint space. Histologically there was a gross reduction in synovial inflammatory changes. The percentage changes observed with SI 800 mg/kg were comparatively greater than that of standard drugs D and M exhibiting its highly significant anti-rheumatoid activity (Table 3).
Table 3.
Percentage difference in physical and biochemical parameters.
| Sl no | Parameters | Control | RA control | Diclo | Metho | SI 400 | SI 800 |
|---|---|---|---|---|---|---|---|
| 1 | Paw volume | −18.75 | −20.39 | −72.82 | −77.67 | −64.49 | −79.38 |
| 2 | Body weight | 5.10 | −3.59 | 4.69 | 4.90 | 5.10 | 4.02 |
| 3 | Body temperature | 1.41 | −7.73 | −25.53 | −28.97 | −24.04 | −12.84 |
| 4 | Spontaneous activity | 3.99 | 66.12 | 133.49 | 148.68 | 164.13 | 165.48 |
| 5 | Total WBC | −2.70 | 16.78 | −14.81 | −16.56 | −15.60 | −15.91 |
| 6 | Total RBC | −0.64 | −6.47 | 8.72 | −4.61 | 16.36 | 30.47 |
| 7 | Hb | 0.86 | −26.69 | 33.62 | −7.59 | 19.93 | 16.33 |
| 8 | ESR | 0.98 | 12.11 | −37.57 | −33.06 | −34.01 | −43.98 |
| 9 | TNF-α | 0.71 | 21.04 | −18.43 | −20.35 | −18.65 | −28.59 |
| 10 | IL-6 | 3.15 | 30.72 | −26.10 | −29.36 | −23.17 | −35.28 |
Percentage change was calculated using the formula = [(Day 28 value − Day 8 value) ÷ Day 8 value] × 100.
Symbol (−) indicates reduction.
4. Discussion
Rheumatoid arthritis (RA) is a chronic multi system disease characterized by persistent inflammatory synovitis involving peripheral joints leading to progressive destruction of cartilages and bones resulting in functional impairment. The exact cause of RA is not known, but the mediators reported to be involved in pathogenesis include pro inflammatory cytokines, IL-1, IL-6, IL-17, IL-18, TNF-α22 and inflammatory mediators prostaglandins,23 acetyl choline24 and substance P.25 However histamine is not identified as a mediator in RA.26
NSAIDs inhibit the production of prostaglandins from arachidonic acid by inhibiting cyclooxygenase (COX) enzyme. When non selective COX inhibitors such as Indomethacin, Ibuprofen and Diclofenac are used, though they reduce joint swelling and pain, they cause adverse effects such as peptic ulcer, renal toxicity and bleeding diathesis due to inhibition of COX-1. Hence selective COX-2 inhibitors like celecoxib, valdecoxib which act only at the site of inflammation have been introduced but as these caused lethal cardiac toxicity most of them are withdrawn from use.27
Glucocorticoids act at a higher level in the inflammatory process by inhibiting the production of arachidonic acid from cell membrane phospho lipid. The immunosuppressive effects of glucocorticoids further help the treatment of RA. But long term use of glucocorticoids leads to several side effects such as peptic ulcer, hypertension, immune dysfunction and osteoporosis.28
DMARDs include methotrexate, azathioprine, sulfasalazine, hydroxychloroquine, gold salts, d-penicillamine and leflunomide. These agents decrease the inflammatory process and the consequent destructive changes in RA by their immuno-modulatory effects. But long term use of DMARDs is known to produce myelosuppression, stomatitis, alopecia, diarrhoea and thrombocytopenia.29
Biologic DMARDs like etanercept, adalimumab, infliximab, certolizumab and golimumab act by inhibiting tumor necrosis factor, an important inflammatory mediator of RA.29 The first direct and selective IL-1 receptor antagonist, anakinra is used alone or in combination with any of the DMARDs. In addition, abatacept (CTLA4-Ig fusion protein), rituximab, (anti CD20 antibody) tocilizumab (anti-IL-6 receptor antibody), and tofacitinib (Janus kinase inhibitor) including anakinra are often combined with methotrexate or other DMARDs to improve efficacy.30 All these biological DMARDs can also cause hypotension, severe infections, organ toxicity and others.
As each group of above mentioned drugs has its own limitations, an ideal drug for treatment of RA still awaits discovery. Hence this study was undertaken to find out the anti-rheumatoid activity of SI in FCA-induced rheumatoid arthritis in wistar albino rats. FCA-induced arthritic rat is the most widely used experimental model for RA as it closely mimics the features of human rheumatoid disease. It was first described by Newbold BB in 1963. Administration of FCA produced changes in rats similar to what would occur in humans with RA such as joint swelling (paw swelling), restricted joint movement, biochemical and histological changes.
4.1. Dose selection
Acute toxicity studies were not conducted with the extract in this study as its safety up to 2000 mg/kg has been reported in an earlier study.13 The two doses 400 and 800 mg/kg were selected based on earlier studies which have reported better response for doses above 400 mg/kg for analgesic, hepato and nephro protective activities.12, 13, 14
Paw swelling, ESR and inflammatory markers are indices of rheumatoid activity. TNF-α, IL-6 and IL-1 are well known inflammatory mediators of RA. These cytokines activate synoviocytes and chondrocytes and result in the secretion of matrix metalloproteinase into the synovial fluid leading to destruction of cartilage and synovial membrane.31, 32 Increase in the levels of all these parameters confirms the induction of arthritis.
Anemia is a common feature of RA. Increased level of IL-6 is associated with higher level of hepcidin, an iron regulatory hormone produced by hepatocytes. Hepcidin inhibits the release of iron from macrophages in spleen and iron uptake in duodenum resulting in anemia.33, 34 Treatment with EESI improved Hb level to 10.9% indicating that it had reversed the hematological changes. Hence EESI can be a safer alternative in this regard.
Oxidative stress contributes to the pathogenesis of RA. Excessive generation of ROS and RNS produced by activated neutrophils and macrophages inflicts damage to joints mainly through up regulation of matrix metalloproteinases and activation of osteoclast activity.35 Reduction of oxidative stress markers (SOD, LPO, CAT and GPx levels) in the joint tissue of arthritic rats shows that the antioxidant property of EESI has additionally contributed to its anti-arthritic efficacy.
Among phytochemicals, sesamol and sesamin have been evaluated for anti-inflammatory activity against FCA-induced arthritis and antioxidant activity and established by previous investigators.36, 37 Sesamin is also found to be present in other plants such as Ginkgo biloba and Vitex negundo and both these plants are reported for their anti-inflammatory activity.38, 39 Further both sesamin and sesamolin, are found to inhibit hepatic fatty acid oxidation. However sesamolin is found to be a better antioxidant than sesamin.40 Sesamol is a unique phyto-constituent present in S. indicum and it inhibits pro-inflammatory mediators, TNF-α, IL-1β, IL-6 and COX-2 resulting in anti-inflammatory activity.41 SI contains sesamin 31.3 %, Sesamol 34.2 % sesamolin 46.7% per 100 g. Sesamin and sesamolin are found to be present in higher quantity in black SI seeds compared to white and brown seeds.42
Another important phyto-constituent present in SI, α-linolenic acid (ALA) is reported to have anti-inflammatory and anti nociceptive effects mediated through down regulation of inflammatory mediators iNOS, COX-2 and TNF-α gene expression.43 γ-tocopherol, present in SI acts in the aracadonic acid pathway and inhibits the production of prostaglandins and other eicosanoids exhibiting anti-inflammatory activity.44 There are four different tocopherol homologues (α, β, γ, δ) and all have antioxidant activity. Among them γ-tocophereol is found to be a stronger inhibitor of cyclooxygenase and possibly lipoxygenase as well. It also scavenges the reactive nitrogen species more efficiently than α-tocophereol.45 A previous study has reported that Nimesulide and Diclofenac reduced the elevated TNF-alpha levels.46 In another study reduction in IL-6 was observed following administration of Diclofenac and Nimesulide.47 Inhibition of cytokine production especially TNF-alpha by methotrexate has also been reported.48
As the S. indicum seed extract contains all of these phytochemicals, which when given individually produce anti-inflammatory, antioxidant and anti-nociceptive effects, the whole extract will certainly have higher therapeutic benefit than the individual components. Hence SI can be a safer alternative in the treatment of rheumatoid arthritis.
5. Conclusion
Based on the results of the current study it can be concluded that, S. indicum seed extract has anti-rheumatoid activity comparable to diclofenac and methotrexate. EESI 800 mg/kg has better effect than 400 mg/kg. The anti-inflammatory, antioxidant, analgesic activities of SI have contributed to its anti-rheumatoid activity. The mechanism of action is attributed to inhibition of release of pro-inflammatory cytokines- IL-6 and TNF-α and the inflammatory mediators. Hence S. indicum seed extract can be further evaluated for its use as an effective future alternative in the treatment of rheumatoid arthritis.
Conflict of interest
Authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgment
We thank the faculty of Department of Radiology, Chettinad Hospital and Research Institute for helping us take the X-rays of the joints of rats as well as providing the radiological opinion.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
List of abbreviations
- FCA
Freund's complete adjuvant
- SI
Sesamum indicum
- HB
Haemoglobin
- ESR
Erythrocyte sedimentation rate
- WBC
White blood cell
- RBC
Red blood cell
- IL-6
Interleukin- 6
- TNF-α
Tumor necrosis factor- α
- COX
Cyclooxygenase
References
- 1.Aletaha D., Neogi T., Silman A.J. 2010 rheumatoid arthritis classification criteria: an American College of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 2.Alghuweri A., Marafi A., Alhiary M. Use of serological markers 2 for evaluation patients with rheumatoid arthritis. Int J Biol Med Res. 2012;3:1397–1398. [Google Scholar]
- 3.Chopra A. Disease burden of rheumatic diseases in India: COPCORD perspective. Indian J Rheumatology. 2015;10:70–77. [Google Scholar]
- 4.Singh J.A., Saag K.G., Bridges S.L. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 5.He Y., Wong A.Y., Chan E.W. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2013;14:298. doi: 10.1186/1471-2474-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka H., Tanaka Y., Takeuchi T. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34. doi: 10.1186/s13075-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross I.A. vol. 3. Humana press; New York: 2005. (Medicinal Plants of the World: Chemical Constituents, Traditional and Modern Medicinal Uses). [Google Scholar]
- 8.Moazzami Ali A., Kamal-Eldin Afaf . Sesame seed is a rich source of dietary lignans. J Am Oil Chem Soc. 2006;83:719–723. [Google Scholar]
- 9.Vijay, Sharmila K.P., Bekal Mahesh Prasad, Suchetha Kumari N., Pushpalatha K.C. Evaluation of phytochemical constituents and fatty acid content in Sesamum indicum L. J Pharm Chem Biol Sci. 2015;3:84–90. [Google Scholar]
- 10.Hajimahmoodi M., Oveisi M.R., Sadeghi N., Jannat B., Bahaeddin Z., Mansoori S. Gamma tocopherol content of Iranian sesame seeds. Iran J Pharm Res. 2010;20:135–139. [Google Scholar]
- 11.Chakraborthy G.S., Sharma G., Kaushik K.N. Sesamum indicum: a review. J Herb Med Toxicol. 2008;2:15–19. [Google Scholar]
- 12.Nahar L. Investigation of the analgesic and antioxidant activity from an ethanol extract of seeds of Sesamum indicum. Pak J Biol Sci. 2009;12:595–598. doi: 10.3923/pjbs.2009.595.598. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M. Hepatoprotective activity of Sesamum indicum Linn. against CCl4-induced hepatic damage in rats. Int J Pharm Biol Archive. 2011;2:710–715. [Google Scholar]
- 14.Bhuvaneswari P., Krishnakumari S. Nephroprotective effects of ethanolic extract of Sesamum indicum seeds (Linn.) in streptozotocin induced diabetic male albino rats. Int J Green Pharm. 2012;6:330–335. [Google Scholar]
- 15.Chinnala K.M., Elsani M.M., Mekala S.K. Evaluation of anti obesity activity of Sesamum indicum linn. in high fat diet induced obesity in rats. Int J Phytopharm. 2014;1:179–182. [Google Scholar]
- 16.Ghani Noor A., Umran Mahfoodha A., Shawkat Muayad S. Cytotoxic activity of ethanol extract of Sesamum indicum seeds to cancer cell lines in vitro. Iraqi J Cancer Med Genet. 2012;5:28–33. [Google Scholar]
- 17.Newbold B.B. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol. 1963;21:127–136. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 20.Sinha A. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 21.Rotruck J., Pope A., Ganther H., Swanson A., Hafeman D., Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 22.Nanjundaiah S.M., Astry B., Moudgil K.D. Mediators of inflammation-induced bone damage in arthritis and their control by herbal products. Evid Based Complement Alternat Med. 2013:1–20. doi: 10.1155/2013/518094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattahi M.J., Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012:1–7. doi: 10.1155/2012/239310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Maanen M.A., Stoof S.P., Larosa G.J., Vervoordeldonk M.J., Tak P.P. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010;69:1717–1723. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 25.Garrett N.E., Mapp P.I., Cruwys S.C., Kidd B.L., Blake D.R. Role of substance P in inflammatory arthritis. Ann Rheum Dis. 1992;51:1014–1018. doi: 10.1136/ard.51.8.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adlesic M., Verdrengh M., Bokarewa M., Dahlberg L., Foster S.J., Tarkowski A. Histamine in rheumatoid arthritis. Scand J Immunol. 2007;65:530–537. doi: 10.1111/j.1365-3083.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 27.Grosser Tilo, Smyth Emer, AF Garret. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill; , New York, NY: 2011. Anti-inflammatory, antipyretic and analgesic agents; Pharmacotherapy of gout; pp. 959–1004. [Google Scholar]
- 28.Bernard P.S., John W.P. Goodman and Gilman's the Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill; , New York, NY: 2011. ACTH, Adrenal steroids and pharmacology of adrenal cortex; pp. 1209–1235. [Google Scholar]
- 29.Singh J.A., Furst D.E., Bharat A. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Keyser F. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr Rheumatol Rev. 2011;7:77–87. doi: 10.2174/157339711794474620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 32.Smolen J.S., Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2:473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaisen C., Figenschau Y., Nossent J.C. Anemia in early rheumatoid arthritis is associated with interleukin 6-mediated bone marrow suppression, but has no effect on disease course or mortality. J Rheumatol. 2008;35:380–386. [PubMed] [Google Scholar]
- 35.Mirshafiey A., Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 36.Hemshekhar M., Thushara R.M., Jnaneshwari S., Devaraja S., Kemparaju K., Girish K.S. Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status. Eur J Nutr. 2013;52:1787–1799. doi: 10.1007/s00394-012-0482-6. [DOI] [PubMed] [Google Scholar]
- 37.Utsunomiya T., Chavali S.R., Zhong W.W., Forse R.A. Effects of sesamin-supplemented dietary fat emulsions on the ex vivo production of lipopolysaccharide-induced prostanoids and tumor necrosis factor α in rats. Am J Clin Nutr. 2000;72:804–808. doi: 10.1093/ajcn/72.3.804. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y.H., Yu J.P., Liu Y.F. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediat Inflamm. 2006:1–9. doi: 10.1155/MI/2006/92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng C.J., Zhao X.X., Ai H.W. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund's adjuvant induced arthritis in rats. Phytomedicine. 2014;21:838–846. doi: 10.1016/j.phymed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Lim J.S., Adachi Y., Takahashi Y., Ide T. Comparative analysis of sesame lignans (sesamin and sesamolin) in affecting hepatic fatty acid metabolism in rats. Br J Nutr. 2007;97:85–95. doi: 10.1017/S0007114507252699. [DOI] [PubMed] [Google Scholar]
- 41.Jnaneshwari S., Hemshekhar M., Thushara R.M. Sesamol ameliorates cyclophosphamide-induced hepatotoxicity by modulating oxidative stress and inflammatory mediators. Anticancer Agents Med Chem. 2014;14:975–983. doi: 10.2174/1871520613666131224123346. [DOI] [PubMed] [Google Scholar]
- 42.Dar A.A., Verma N.K., Arumugam N. An updated method for isolation, purification and characterization of clinically important antioxidant lignans–Sesamin and sesamolin, from sesame oil. Industrial Crops Prod. 2015;64:201–208. [Google Scholar]
- 43.Ren J., Chung S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55:5073–5080. doi: 10.1021/jf0702693. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q., Ames B.N. γ-Tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 45.Reiter E., Jiang Q., Christen S. Anti-inflammatory properties of α- and γ-tocopherol. Mol Asp Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dogan M.D., Ataoglu H., Akarsu E.S. Nimesulide and diclofenac inhibit lipopolysaccharide-induced hypothermia and tumour necrosis factor-alpha elevation in rats. Fundam Clin Pharmacol. 2002;16:303–309. doi: 10.1046/j.1472-8206.2002.00093.x. [DOI] [PubMed] [Google Scholar]
- 47.Henrotin Y.E., Labasse A.H., Simonis P.E. Effects of nimesulide and sodium diclofenac on interleukin-6, interleukin-8, proteoglycans and prostaglandin E2 production by human articular chondrocytes in vitro. Clin Exp Rheumatol. 1999;17:151–160. [PubMed] [Google Scholar]
- 48.Gerards A.H., de Lathouder S., de Groot E.R., Dijkmans B.A., Aarden L.A. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatol Oxf. 2003;42:1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]