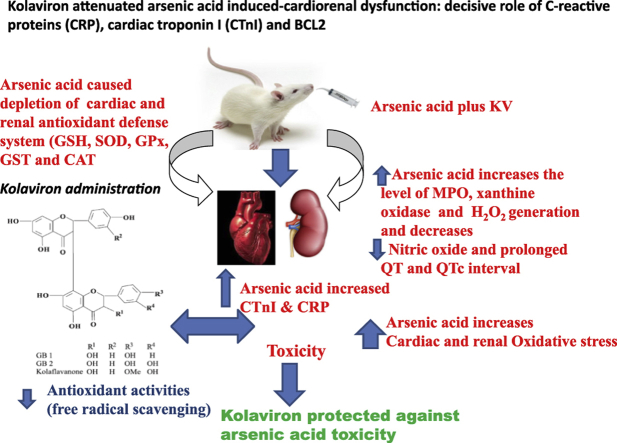
Abstract
Arsenic acid is one of the abundant environmental pollutants present in soil, water and the air. Undoubtedly, it has found its way to the food chain in which humans and animals are the final targets thereby causing arrays of disease conditions including cardiovascular and renal dysfunction. Hence, the use of phytochemicals present in medicinal plants has gained global acceptance as chemotherapeutic agents that can prevent, ameliorate, reverse or treat diseases. From our study, arsenic acid intoxication led to significant increase in heart rate (HR), QRS, together with prolonged QT and QTc interval. However, Kolaviron (KV) at the dosage of 100 and 200 mg/kg body weight reversed the aforementioned electrocardiographic (ECG) changes. KV pre-treatment also ameliorated cardiorenal dysfunction via significant reduction in cardiac and renal markers of oxidative stress such as malondialdehyde, hydrogen peroxide generation, myeloperoxidase activity and nitric oxide contents. Immunohistochemistry revealed expressions of renal C-reactive proteins (CRP) and expressions of anti-apoptotic protein BCL2 in KV treated rats. Furthermore, cardiac troponin I (CTnI) expressions were lower in KV treated rats. Taken together, KV mitigated arsenic-acid induced cardiovascular dysfunction via up-regulation of antioxidant defense system and down-regulation of inflammatory and apoptotic signaling pathways.
Keywords: Kolaviron, Arsenic acid, Oxidative stress, Cardiovascular dysfunction, Chemoprevention
Graphical abstract
1. Introduction
Arsenic has been recorded as one of the ubiquitous environmental pollutants that nearly affect all organs in the body.1 In a study elsewhere, exposures to inorganic arsenic (iAs) in drinking water was found to be associated with both carcinogenic and non-carcinogenic effects.2 Epidemiological studies revealed that population can be exposed to inorganic arsenic via water and also through consumption of food with higher arsenic concentrations.3 Several other epidemiological studies reported associations between arsenic exposure and skin, bladder, lung, liver and kidney cancer as well as cardiovascular diseases, diabetes reproductive and developmental effects.4
The trivalent methylated arsenicals are known to be responsible for the toxicity and carcinogenicity of environmental arsenic toxicity.5 Furthermore, both inorganic and organic trivalent arsenicals have been shown to be more potent toxicants than pentavalent forms of arsenic acid.6, 7 However, mechanism of arsenic-induced cardiac abnormalities has been linked to NF-kappaB activation via IKK, p38 and JNK MAPK signaling pathways respectively.8 Also, exposure to arsenic is influenced by the environmental factors together with individuals exhibiting different urinary arsenic metabolism patterns.9 In an in vivo experiment, animals exposed to NaAsO2 (10 mg/kg) orally for 10 days were found to have significantly inhibited superoxide dismutase, catalase, glutathione-S-transferase, glutathione peroxidase, glutathione reductase and reduced glutathione level in myocardial tissues of rats.10 In addition, NaAsO2 was found to significantly increase oxidized glutathione (GSS), malondialdehyde (MDA) and protein carbonyl (PCO) content in myocardial tissues.10, 11 Furthermore, hepatorenal damage and the influence of arsenic-induced DNA fragmentation of hepatic and renal tissues has also been documented.12, 13 Recently, arsenic exposure has been shown to be higher in meat and folate consumption, diet rich in green leafy and red-orange vegetables and eggs.14
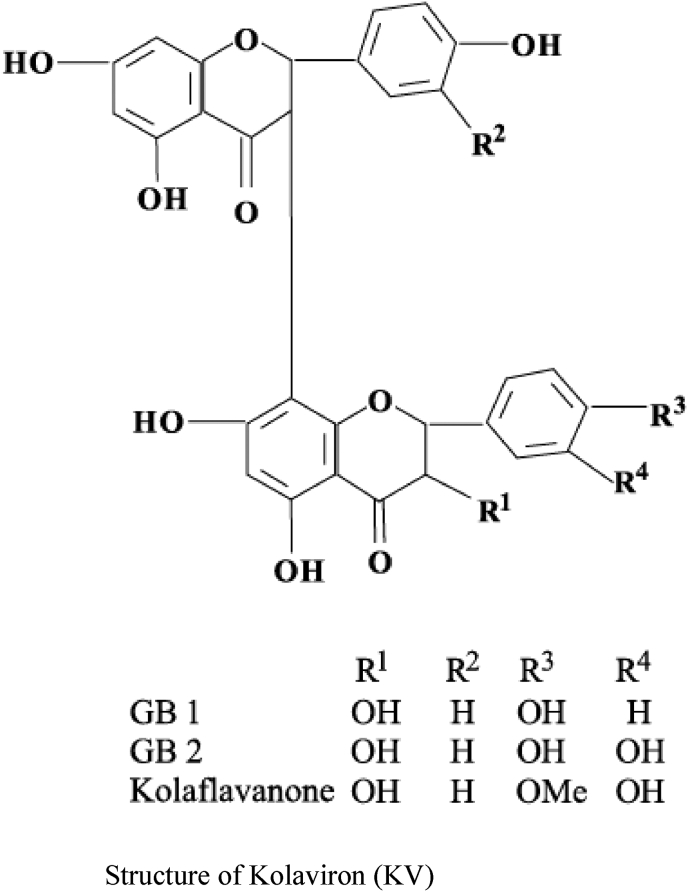
Kolaviron (KV) is a biflavonoid complex from the methanol extract from Garcinia kola seed.15 The antioxidant and immunomodulatory properties of KV against influenza virus have been reported.16 The protective effect of KV against benzo[a]pyrene-induced reproductive and neurotoxicity has been documented.17, 18, 19 From our laboratory, we demonstrated the gastro-protective effect against sodium arsenite toxicity.20 KV has been reported to demonstrate a novel chemotherapeutic potential together with different pharmacological properties.21, 22, 23, 24, 25 Abarikwu et al.26 reported the anti-inflammatory effect of KV via inhibition of transcription factors ERK1/2, p-JNK, NF-κB, and activate Akt expressions in the 93RS2 Sertoli cell lines. Oluwatosin et al.27 reported anti-malaria property of Kolaviron against Plasmodium berghei infection in Swiss albino mice. Adaramoye and Lawal28 reported cardio-protective effect against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats.
2. Objectives
The present study was designed to investigate the ameliorative effect of KV against sodium arsenite-induced cardiorenal dysfunction and the underlying mechanism of action.
3. Materials and methods
3.1. Chemicals
Trichloroacetic acid, Ellman's reagent (DTNB), NaAsO2, fetal calf serum, O-dianisidine, reduced glutathione (GSH), potassium dichromate, Hydrogen peroxide (H2O2), hydrochloric acid, sulphuric acid, xylenol orange, sodium hydroxide, thiobarbituric acid (TBA), ammonium ferrous sulphate, potassium iodide, sodium potassium tartrate, copper sulphate, glacial acetic acid, ethanol, sodium azide, 2-dichloro-4-nitrobenzene (CDNB) and sorbitol were purchased from Sigma (St Louis, MO, USA). Normal goat serum, Biotinylated antibody and Horse Radish Peroxidase (HRP) System was purchased from (KPL, Inc., Gaithersburg, Maryland, USA). Caspase 3 was purchased from (Bioss Inc. Woburn, Massachusetts, USA) while 3, 3′-Diaminobenzidine (DAB) tablets were purchased from (AMRESCO LLC. OHio, USA). All other chemicals used were of analytical grade and obtained from British Drug Houses (Poole, Dorset, UK).
3.2. Extraction of G. kola and isolation of kolaviron (KV)
KV was extracted from the seeds of Garcinia Kola according to the method of Iwu et al.29 The seeds were sliced, air-dried and ground to a fine powder using a pestle and mortar. The powdered seeds were defatted by extraction using n-hexane in a Soxhlet extractor apparatus for 24 h. The defatted dried marc was repacked and extracted with methanol. KV was fractionated from concentrated methanol extract using chloroform to give Garcinia biflavanones-GB1, GB2 and kolaflavanone.
3.3. Experimental design and animal treatment
Forty male Wistar rats were obtained from the Experimental Animal Unit of Faculty of Veterinary Medicine, University of Ibadan, Nigeria and were randomly divided into four groups of ten animals per group. The animals were kept in wire mesh cages under controlled light cycle (12 h light/12 h dark) and fed with commercial rat chow ad libitum and liberally supplied with water.
Group A received normal saline. Group B received sodium arsenite at 10 mg/kg single dose intraperitoneally on day 7. Rats in Groups C and D were pre-treated orally with Kolaviron (KV) 100 mg/kg and 200 mg/kg for 7 days. The electrocardiograph (ECG) recording was taken on day 8 and the rats were sacrificed after the ECG measurement.
3.4. Care of animals
All the experimental animals received humane care according to the criteria outlined in the Guide for the Care and the Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institute of Health. The ethics regulations were followed in accordance with national and institutional guidelines for the protection of the animals' welfare during experiments according to Public health service.30
3.5. Blood collection and serum preparation
Fresh whole blood (5 mls) was collected from each rat through the retro-orbital venous plexus into sterile plain tubes and left for about 30 min to clot. The clotted blood was thereafter centrifuged at 4,000 rpm for 10 min. Serum was harvested into sample bottles and stored at −20°C till the time of analysis.
3.6. Electrocardiogram (ECG) tracing
Standard lead II electrocardiogram was recorded in conscious rats using a 7-lead ECG machine (EDAN VE-1010, Shanghai, China). The machine was calibrated at 20 mm/mV paper speed and 50 mm/s paper speed. From the electrocardiogram, parameters such as heart rate, P-wave duration, PR-interval, QRS duration, R-amplitude, QT segment and Bazett's correction of the QT interval were determined.
3.7. Biochemical assays
Cardiac and renal post mitochondrial fractions (PMFs) preparation for biochemical assays.
The rats were sacrificed 24 h after the last administration. The heart and the kidneys were removed, weighed and portion of it rinsed in 1.15% KCl and homogenized in potassium phosphate buffer (0.1 M, pH 7.4) and centrifuged at 12,000 g for 15 min to obtain the post mitochondria fractions (PMFs). The PMFs of the heart and the kidneys were obtained and subsequently stored at −20°C until the time of use.
3.7.1. Determination of cardiac and renal catalase (CAT) activity
Catalase (CAT) activity was determined according to the method of Sinha.31 Briefly, 1 ml portion from the reaction mixture 2 ml of H2O2 solution, 2.5 ml of 0.01 M potassium phosphate buffer (pH 7.0) and 1 ml of properly diluted PMF was blown into 1 ml dichromate/acetic acid solution by a gentle swirl at room temperature at 60 s interval for 3 times. The mixture was incubated in the water bath at 100 °C for 10 min. The absorbance was read at 570 nm using distilled water as blank. One unit of CAT activity represents the amount of enzyme required to decompose 1 μmol of H2O2/minute.
3.7.2. Determination of cardiac and renal superoxide dismutase (SOD) activity
Superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich32 with slight modification from our laboratory.33, 34 Briefly, 100 mg of epinephrine was dissolved in 100 ml distilled water and acidified with 0.5 ml concentrated hydrochloric acid. This preparation prevents oxidation of epinephrine and is stable for 4 weeks. 0.01 ml of hepatic PMF was added to 2.5 ml 0.05 M carbonate buffer (pH 10.2) followed by the addition of 0.3 ml of 0.3 mM adrenaline. The increase in absorbance at 480 nm was monitored every 30 s for 150 s 1 unit of SOD activity was given as the amount of SOD necessary to cause 50% inhibition of the auto-oxidation of adrenaline to adrenochrome during 1 min.
3.7.3. Determination of cardiac and renal reduced glutathione (GSH)
The content of hepatic reduced glutathione (GSH) was estimated by the method of Jollow et al.35 Briefly, 0.5 ml of 4% sulfosalicylic acid (precipitating agent) was added to 0.5 ml of PMF and centrifuged at 4,000 rpm for 5 min. To 0.5 ml of the resulting supernatant 4.5 ml of Ellman's reagent (0.04 g of DTNB in 100 ml of 0.1M phosphate buffer, pH 7.4) was added. The absorbance was read at 412 nm against distilled water as blank.
3.7.4. Determination of cardiac and renal glutathione peroxidase (GPX) activity
The cardiac and renal glutathione peroxidase (GPX) activity was also measured according to Buetler et al.36 The reaction mixtures contain 0.5 ml of potassium phosphate buffer (pH, 7.4), 0.1 ml of Sodium azide, 0.2 ml of GSH solution, 0.1 ml of H2O2, 0.5 ml of PMF and 0.6 ml of distilled water. The mixture was incubated in the water bath at 37 °C for 5 min and 0.5 ml of Trichloroacetic acid (TCA) was added and centrifuged at 4,000 rpm for 5 min 1 ml of the supernatant was taken and added to 2 ml of K2PHO4 and 1 ml of Ellman's reagent. The absorbance was read at 412 nm using distilled water as blank.
3.7.5. Determination of glutathione-S-transferase (GST) activity
The activity of Glutathione-S-transferase (GST) was determined according the method of Habig et al.37 To 2.82 ml of 0.1M Potassium Phosphate buffer (pH 6.5), 150 μL of CDNB solution was added. Then, 30 μL of GSH solution was added to the reaction mixture together with 30 μL of PMFs of cardiac and renal tissues. The cuvette was quickly inverted; the increase in absorbance at 340 nm was monitored every 60 s for 150 s.
3.7.6. Determination of cardiac and renal thiobarbituric acid reactive substances (TBARS)
Thiobarbituric acid reactive substance was quantified as Malondialdehyde (MDA) in the cardiac and renal PMF. The MDA was determined according to the method of Varshney and Kale.38 To 1.6 ml of Tris–KCl, 0.5 ml of 30% TCA, 0.4 ml of samples and 0.5 ml of 0.75% Thiobarbituric acid (TBA) prepared in 0.2 M HCl were added. The reaction mixture was incubated in the water bath at 80 °C for 45 min, cooled on ice and centrifuged at 4,000 rpm for 15 min. The absorbance was measured against a blank of distilled water at 532 nm. Lipid peroxidation in units/mg protein was calculated with a molar extinction coefficient of 1.56 × 105 m−1cm−1.
3.7.7. Measurement of cardiac and renal hydrogen peroxide (H2O2) generation
Hydrogen peroxide generation was determined according to the method of Wolff's.39 To 2.5 ml of 0.1M potassium phosphate buffer (pH 7.4), 0.250 ml of Ammonium ferrous sulphate (AFS), 0.1 ml of sorbitol, 0.1 ml of Xylenol Orange (XO), 0.025 ml of H2SO4 and 0.050 ml of cardiac and renal PMF were added. The mixture was mixed thoroughly by vortexing until a light pink colour of the reaction mixture was observed. The reaction mixture was subsequently incubated at room temperature for 30 min. The absorbance was measured at 560 nm using distilled water as blank. The hydrogen peroxide (H2O2) generated was extrapolated from hydrogen peroxide standard curve.
3.7.8. Determination of serum nitric oxide (NO) contents
The serum nitric oxide (NO) was measured as described by Olaleye et al.40 by indirectly measuring the nitrite concentration. After incubation at room temperature for 20 min, the absorbance at 540 nm was measured by spectrophotometer. The concentration of nitrite in the sample was determined from a sodium nitrite (NaNO2) standard curve and was expressed as μmol nitrite/mg protein.
3.7.9. Determination of serum myeloperoxidase activities
Myeloperoxidase (MPO) as marker of inflammation was measured according to the method of Xia and Zweier.41 To 2 ml of O-dianisidine mixture (16.7 mg of O-dianisidine, 100 ml of 0.05 M potassium phosphate buffer and 50 μL of diluted H2O2) put into the cuvette, 70 μL of serum was added. The increase in absorbance was monitored every 30 s for 1 min. The absorbance was read at 450 nm. One unit of MPO activity can be defined as the quantity of enzyme able to convert/degrade 1 μmol of hydrogen peroxide to water in 1 min at room temperature.
3.7.10. Determination of cardiac xanthine oxidase activity
The cardiac xanthine oxidase was determined according to the method of Akaike et al.42 To 2, 950 μL of the substrate (xanthine-buffer) was added 50 μL of the post mitochondrial fraction (PMF) of the cardiac tissues making a total reaction mixture of 3,000 μL. The reaction mixture was prepared in duplicate and incubated for 40 min at room temperature. The reaction mixture was terminated with the addition of 100 μL of TCA at 0 min and 20 min respectively. The reaction mixture was centrifuged at 4,000 rpm for 20 min and the clear supernatants was read at 293 nm wavelengths.
3.7.11. Determination of serum enzymes and proteins
Determination of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP). The activities of Aspartate aminotransferase (AST) and total bilirubin were determined using the Randox Kit (Randox Laboratories Limited, UK). The manufacturer instruction was followed in the assay protocol.
3.7.12. Determination of cardiac and renal protein determination
Protein concentrations were determined by means of the described by Gornal et al.43 Briefly, 1 ml of diluted serum was added to 3 ml of the biuret reagent. The reaction mixture was incubated at room temperature for 30 min. The mixture was thereafter read with spectrophotometer at 540 nm using distilled water as blank. The final value for total protein was extrapolated from total protein standard curve.
3.8. Immunohistochemistry of cardiac troponin I (CTnI), BCL2 and C-reactive proteins (CRP) cardiac and renal tissues
The paraffin-embedded heart and kidney tissues were placed on charged slides and then dewaxed by immersion in xylene for 5 min (twice). This was followed by dehydration in ethanol of 100%, 90% and 80% concentrations for 5 min each. The slides were placed in distilled water tank for 5 min before incubating with endogenous peroxidase for 10 min. After incubation, they were rinsed with water and placed in wash buffer tank for 5 min and then rinsed with distilled water. The slides were placed in citrate buffer tank at pH 6.0 to retrieve antigen and afterwards were allowed to cool. They were then rinsed and placed in distilled water tank for 2 min. The sections were cleaned with tissue paper, goat serum (KPL, Inc., Gaithersburg, Maryland, USA) was added and the slides were incubated in a humidifying chamber for 15 min. After the incubation, the slides were shaken to remove excess goat serum, and probed with cardiac troponin I (CTnI), BCL2 and C-reactive proteins (CRP) (1: 200; Bioss Inc. Woburn, Massachusetts, USA) and incubated overnight in humidifying chamber. Following overnight incubation, the slides were rinsed with wash buffer and placed in wash buffer tank for 5 min. Biotinylated antibody (KPL, Inc., Gaithersburg, Maryland, USA) was added and incubated in humidifying chamber for 30 min. Then, they were rinsed and placed in wash buffer tank for 5 min. The slides were removed, shaken and incubated with streptavidin HRP system (KPL, Inc., Gaithersburg, Maryland, USA) for 30 min. They were later rinsed and placed in phosphate buffer saline tank for 5 min. Slides were shaken and DAB (AMRESCO LLC. OH, US) was added for 3 min. The reaction was stopped by rinsing with distilled water. The sections were counter-stained with HIGHDEF® IHC hematoxylin (Enzo Life Sciences, NY, USA) for 3 s. The slides were then transferred to 80%, 90% and 100% ethanol for 3 min each after which they were transferred to xylene (100 %) tank for 5 min (twice). The immunoreactive positive expression of cardiac troponin I (CTnI), BCL2 and C-reactive proteins (CRP) was observed on each slide under ×400 magnification with a digital microscope.
3.9. Histopathology
The heart and kidneys tissues were collected in 10% buffered formalin (pH 7.4) for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5–6 μm in thickness were made and stained with haematoxylin and eosin for histopathological examination.44
3.10. Statistical analysis
All values are expressed as mean ± S.D. “One-way ANOVA with Dunnett's post-test was also performed using GraphPad Prism version 4.00. The level of statistical significance was considered as p < 0.05.
4. Results
4.1. Serum nitric oxide and aminotransferases and phosphatase
Table 1 shows that NaAsO2 significantly (p < 0.05) increased serum ALT, AST and ALP when compared to the control or rats pre-treated with KV (100 and 200 mg/kg), respectively. However, pre-treatment with KV (100 and 200 mg/kg) significantly (p < 0.05) reduced serum ALT, AST and ALP activities. In another experiment, NaAsO2 intoxication led to a significant (p < 0.05) reduction in serum nitric oxide (NO) level relative the control and rats pre-treated with KV as indicated in Table 1. The improvement in NO level (bioavailability) by KV was dose-dependent (Table 1).
Table 1.
Effect of Sodium arsenite (NaAsO2) on serum aminotransferases, phosphatase and nitric oxide content.
| Parameters | Control | NaAsO2 | Kolaviron (100 mg/kg) | Kolaviron (200 mg/kg) |
|---|---|---|---|---|
| ALT (U/L) | 115.11.33 ± 1.33 | 133.04 ± 18.87 | 83.89 ± 4.99 | 70.77 ± 2.67 |
| AST (U/L) | 158.00 ± 0.37a | 168.53 ± 5.95a | 133.00 ± 7.44a | 108.44 ± 5.38a |
| ALP (U/L) | 358.80 ± 26.46a,b | 427.80 ± 19.51b | 386.40 ± 39.03b | 294.40 ± 31.87a,b |
| NO (μmol/mg protein) | 19.75 ± 1.31a,b | 13.31 ± 3.68b | 15.17 ± 4.21b | 15.17 ± 1.58a,b |
Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
4.2. Electrocardiogram (ECG)
From the present study, administration of NaAsO2 led to a significant (p < 0.05) increase in heart rate, QRS together with prolonged QT and QTc intervals in comparison with the control and rats administered with KV (100 and 200 mg/kg) (Table 2). On the other hand, pre-treatment with 100 and 200 mg/kg significantly lowered the heart rate, QRS and reversed the prolonged QT and QTc intervals (Table 2).
Table 2.
Effect of Sodium arsenite (NaAsO2) on electrocardiogram (ECG).
| Parameters | Control | NaAsO2 | Kolaviron (100 mg/kg) | Kolaviron (200 mg/kg) |
|---|---|---|---|---|
| Heart rate (Beats/mins) | 251.33 ± 13.61 | 495.00 ± 7.07a | 455.00 ± 26.46a,b | 490.00 ± 17.32a,b |
| QRS (ms) | 17.25 ± 4.57 | 21.33 ± 3.51a | 15.25 ± 3.30b | 14.67 ± 2.06b |
| QT (ms) | 62.20 ± 9.25b | 71.67 ± 8.02a | 62.20 ± 9.25 | 63.50 ± 5.97 |
| QTc (ms) | 136.02 ± 12.70 | 198.61 ± 16.31a | 164.08 ± 19.71a,b | 172.23 ± 4.43a,b |
Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
4.3. Cardiac and renal markers of oxidative stress and antioxidant status
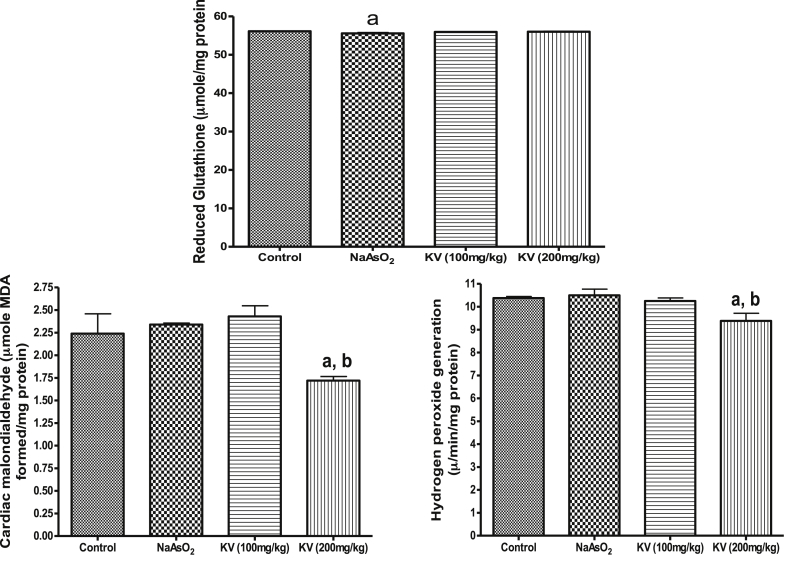
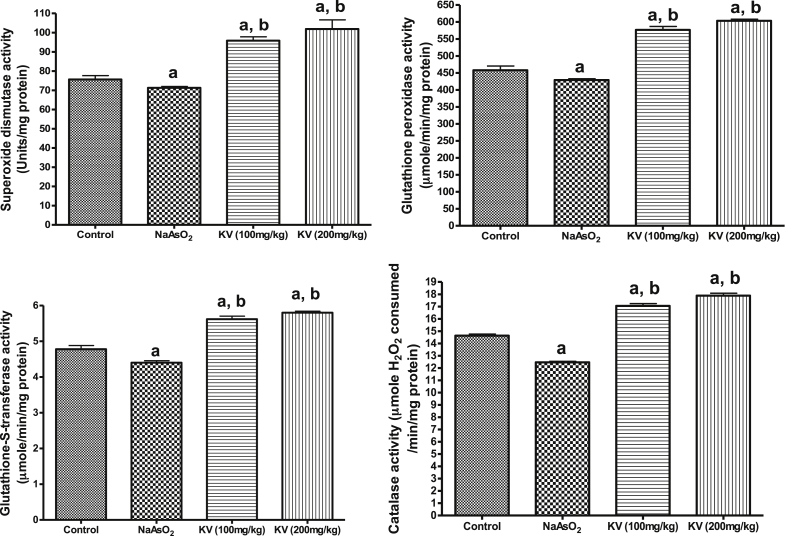
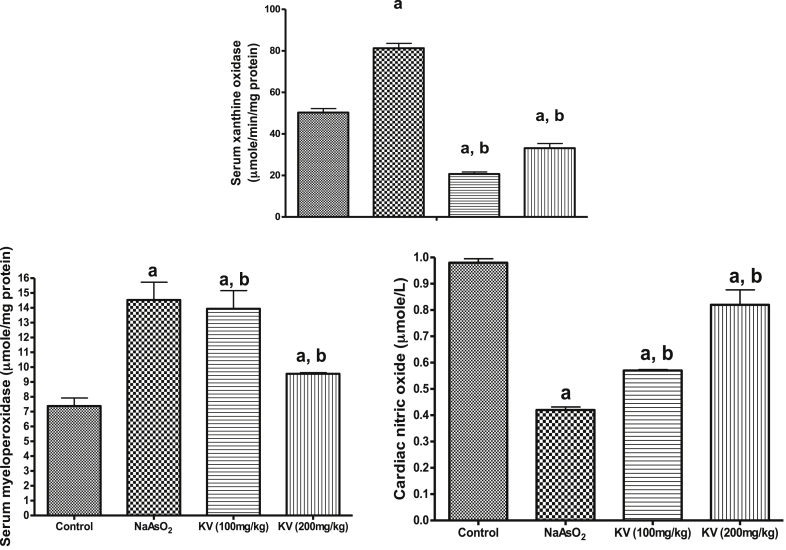
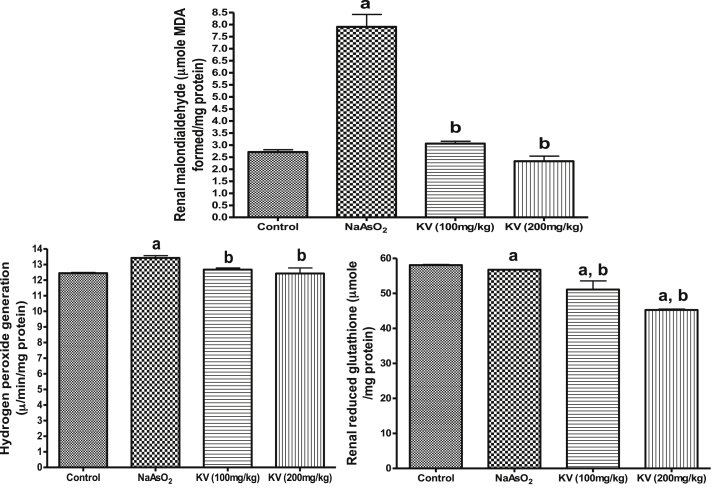
Fig. 1 shows that NaAsO2 significantly (p < 0.05) increased cardiac MDA contents and H2O2 generation whereas KV (100 and 200 mg/kg) significantly (p < 0.05) reduced these markers of oxidative stress. Similarly, the cardiac reduced glutathione (GSH) content declined in NaAsO2 only treated rats compared to the control group and KV treated rats (Fig. 2). The activities of cardiac superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and catalase (CAT) were significantly (p < 0.05) reduced in animals administered NaAsO2 alone relative to the control group and KV treated rats (Fig. 2). However, pre-treatment with KV (100 and 200 mg/kg) dose-dependently increased the activities of these antioxidants relative to the control group. In another experiment, a significant (p < 0.05) increase in serum xanthine oxidase (XO) activity was obtained in rats administered NaAsO2 alone (Fig. 3). However, the aforementioned increased serum XO activity was significantly inhibited by KV (100 and 200 mg/kg) pre-treatment.
Fig. 1.
Effect of Kolaviron on cardiac reduced glutathione (GSH), hydrogen peroxide generation and malondialdehyde (MDA) in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
Fig. 2.
Effect of Kolaviron on cardiac catalase (CAT), superoxide dismutase (SOD), glutathione-S-transferase (GST) and glutathione peroxidase (GPx) in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
Fig. 3.
Effect of Kolaviron on cardiac nitric oxide, serum myeloperoxidase (MPO) and xanthine oxidase (XO). Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
The result obtained from this study showed that NaAsO2 administration led to a significant (p < 0.05) reduction in cardiac NO levels compared to the control or rats pre-treated with KV (100 and 200 mg/kg) as shown in Fig. 3. Interestingly, KV pre-treatment dose-dependently improved the cardiac NO level. Our data also showed a significant (p < 0.05) increase in serum myeloperoxidase (MPO) activity in rats that received NaAsO2 alone (Fig. 3). On the contrary, rats treated with KV showed a significant (p < 0.05) reduction in MPO activity.
As indicated in Fig. 4, NaAsO2 administration led to a significant (p < 0.05) increase in renal MDA content and H2O2 generation along with a significant (p < 0.05) reduction in renal GSH (Fig. 4). In the same vein, KV treated rats showed significant (p < 0.05) reduction in renal MDA content and H2O2 generation together with significant improvement in renal GSH content (Fig. 4).
Fig. 4.
Effect of Kolaviron on renal reduced glutathione (GSH), hydrogen peroxide generation and malondialdehyde (MDA) in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
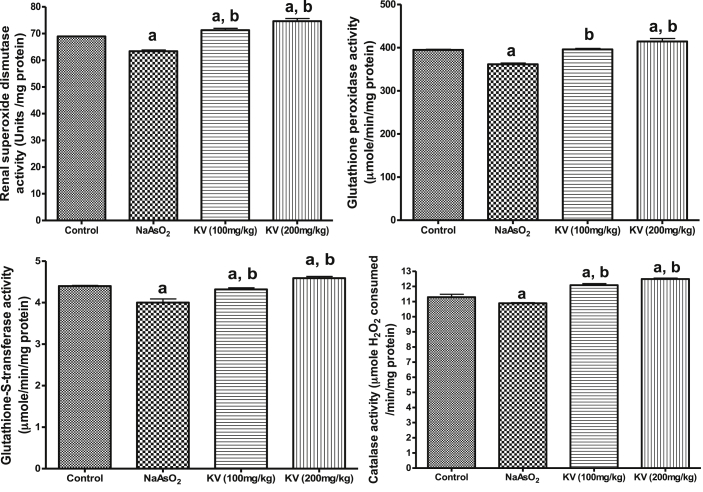
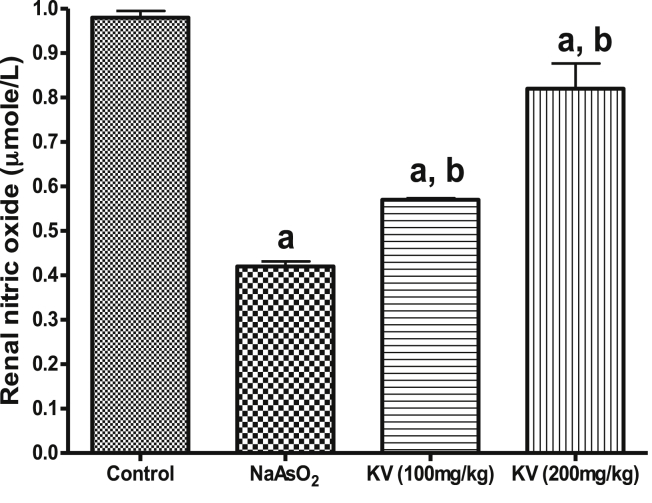
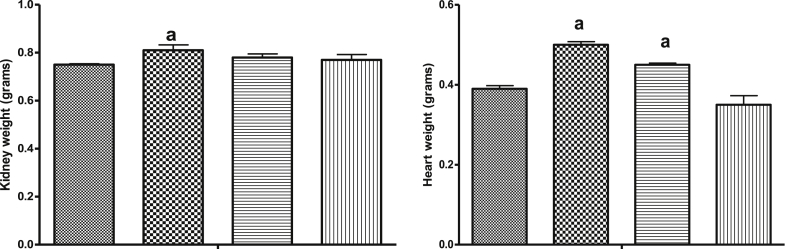
Furthermore, NaAsO2 intoxication caused a significant (p < 0.05) reduction in renal SOD, GPx, GST and CAT activities relative to the control and rats pre-treated with KV (100 and 200 mg/kg) as shown in Fig. 5. However, the activities of these antioxidant enzymes were reversed to near control values by KV pre-treatment in a dose-dependent manner. Also, NaAsO2 administration led to a significant (p < 0.05) reduction in renal NO levels compared to the control or rats pre-treated with KV (100 and 200 mg/kg) whereas KV pre-treatment caused a significant improvement in the renal NO level as shown in Fig. 6. Again, a significant (p < 0.05) increase in kidney and heart weights in NaAsO2 alone treated rats relative to the control and KV pre-treated rats (Fig. 7).
Fig. 5.
Effect of Kolaviron on renalcatalase (CAT), superoxide dismutase (SOD), glutathione-S-transferase (GST) and glutathione peroxidase (GPx) in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
Fig. 6.
Effect of Kolaviron on renal nitric oxide in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
Fig. 7.
Effect of Kolaviron on kidney weight and cardiac weight in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Values are mean ± SD, n = 7, aP < 0.05 compared with control, bP < 0.05 compared with NaAsO2 alone.
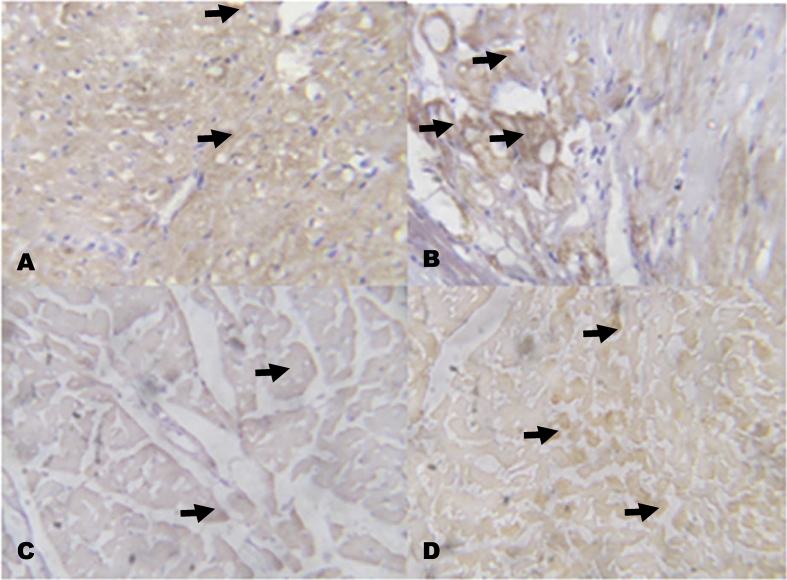
4.4. Immunohistochemical analysis of C-reactive protein (CRP), cardiac troponin I (CTnI) and BCL2
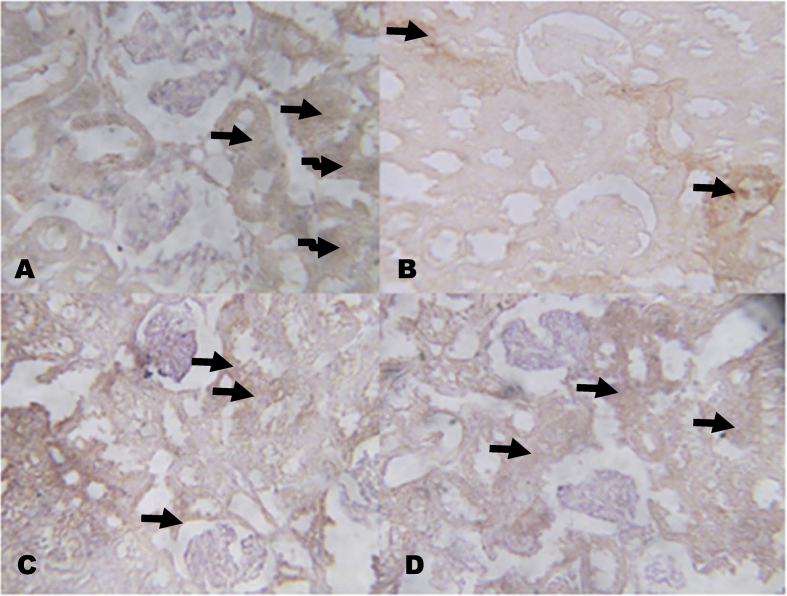
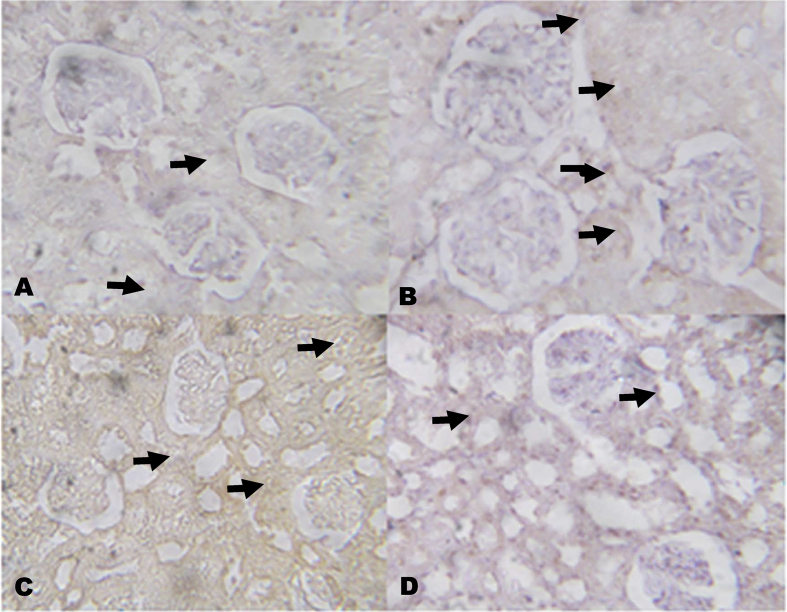
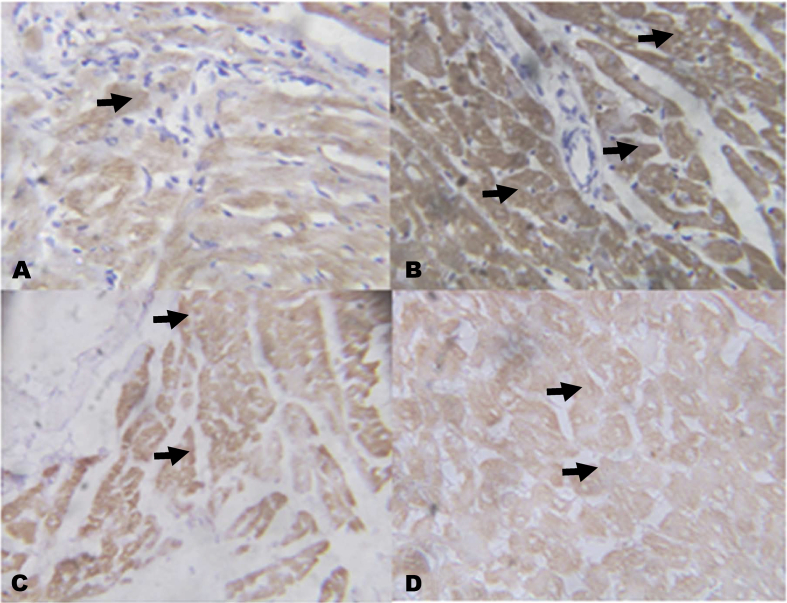
The immunohistochemistry revealed lower expressions of renal anti-apoptotic protein (BCL2) and higher expressions of C-reactive protein (CRP), respectively in NaAsO2 alone treated rats (Fig. 8, Fig. 9). However, KV (100 and 200 mg/kg) gave higher expressions of renal anti-apoptotic protein (BCL2) and lower expressions of CRP. In a similar experiment, higher expressions of immune-positive reactions of cardiac troponin I (CTnI) and cardiac CRP were observed in NaAsO2 alone treated rats when compared with control and KV treated rats (Fig. 10, Fig. 11).
Fig. 8.
The effect of Kolaviron on BCL2 protein expressions in the Kidney in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). The slides were counterstained with high definition hematoxylin and viewed ×100 objectives.
Fig. 9.
The effect of Kolaviron on C-reactive protein (CRP) expressions in the Kidney in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Plates are stained with H and E stains and viewed with 100 objectives. The slides were counterstained with high definition hematoxylin and viewed ×100 objectives.
Fig. 10.
The effect of Kolaviron on Cardiac Troponin I (CTnI) expressions in the heart in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). The slides were counterstained with high definition hematoxylin and viewed ×100 objectives.
Fig. 11.
The effect of Kolaviron on C-reactive protein (CRP) expressions in the heart in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Plates are stained with H and E stains and viewed with 100 objectives.
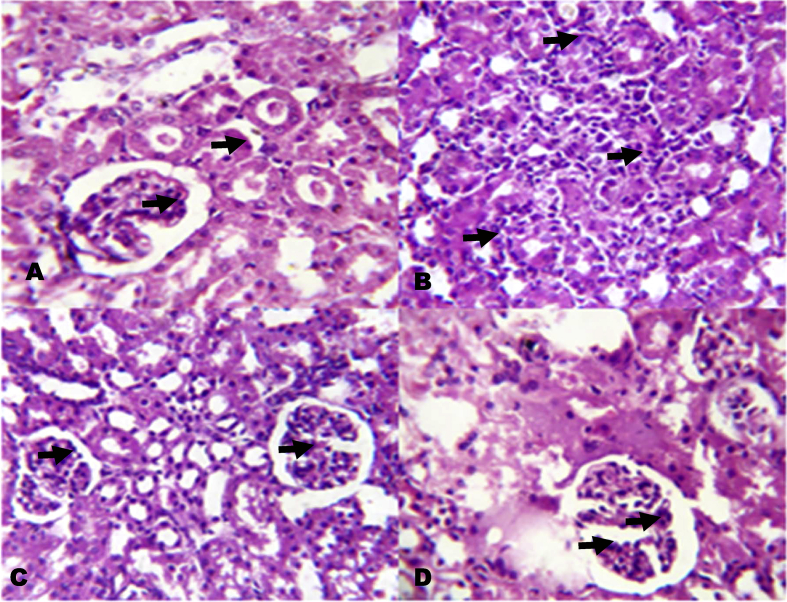
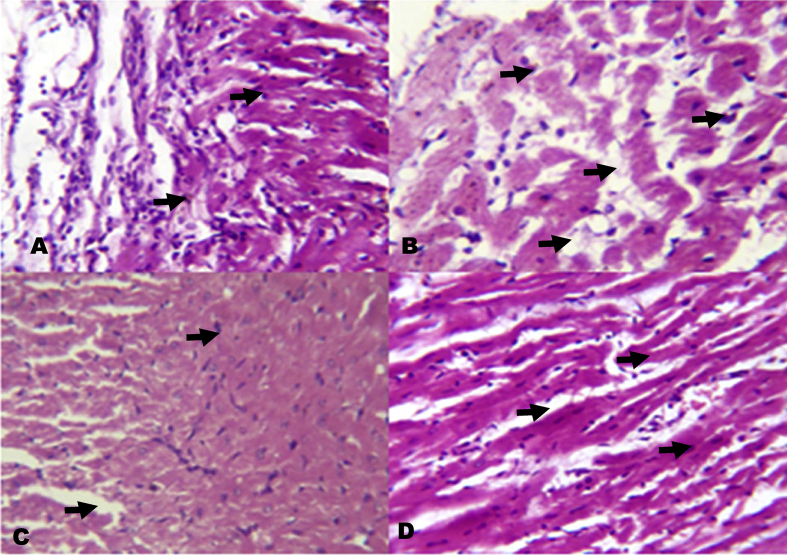
4.5. Histology
The photomicrographs of renal tissues show severe inflammatory cells infiltration into the renal interstitium and periglomerular inflammation (Fig. 12). The photomicrographs of KV pre-treated rats however show mild inflammatory cells infiltration into the renal interstitium. Also, the photomicrographs of heart show inflammation of the pericardium and myocardium of the NaAsO2 alone treated rats (Fig. 13). On the other hand, rats pre-treated with KV showed mild inflammation of the pericardium and myocardium with little cellular infiltration.
Fig. 12.
The histology of the kidney in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Plates are stained with H and E stains and viewed with 100 objectives.
Fig. 13.
The histology of the heart in NaAsO2 induced toxicity. Group A (Control), Group B (NaAsO2), Group C (Kolaviron 100 mg/kg) and Group D (Kolaviron 200 mg/kg). Plates are stained with H and E stains and viewed with 100 objectives.
5. Discussion
The incidence of cardiovascular and renal dysfunction associated with environmental pollutants is becoming increasingly worrisome and a source of global concern.45, 46, 47 Associated with these environmental threats are clinical complications including hypertension, stroke, obesity, immune suppression, respiratory failure, cognitive disorders, developmental abnormality in children with resultant negative impact on the ecosystem health.48, 49 Previous studies have recorded positive correlation between high levels of blood cadmium, mercury, lead and metabolic syndrome.50, 51
Several studies have reported generation of reactive oxygen/nitrogen species (ROS/RNS) from exposure to environmental pollutants with resultant oxidative stress which have played a significant role in the mechanism of toxicity associated with heavy metals.6, 52 Decrease in antioxidant defence system and with concomitant increase in ROS/RNS has become a biochemical yardstick in environmental pollutants-catalyzed oxidative stress reactions and chronic inflammation in vivo and in vitro.53, 54, 55
Results from the present study indicated that administration of NaAsO2 precipitated significant increase in markers of oxidative stress and depletion of enzymic and non-enzymic antioxidant status. Both cardiac and renal MDA levels and H2O2 generation increased whereas GPx, SOD, GST and CAT activities reduced significantly in NaAsO2 only treated rats. Our study confirmed previous reports on the negative impact of environment pollutants on markers of antioxidants defense system and oxidative stress.56, 57, 58 However, KV pre-treatment modulated, reversed and reduced markers of oxidative stress together with apparent improvement in the antioxidant defense system.
The activity of serum XO was assessed in this study. The administration of NaAsO2 increased the activity of serum XO which was indicative hyperuriceamia. However, accumulating evidences have established positive correlation and remarkable association between hyperuriceamia and oxidative stress, chronic heart failure, coronary artery, cerebrovascular disease, diabetes mellitus, metabolic syndrome and cardio-renal damage.59, 60, 61 Furthermore, hyperuriceamia has been shown to increase free radicals generation that will ultimately damage cardiomyocytes and vascular endothelium together with disrupted myocardial contractility, arterial stiffness, endothelial dysfunction and vasoconstriction.62 Increase in the activity of XO is directly proportional to uric acid production. Therefore, Riegersperger et al.,63 reported that excessive uric acid might contribute significantly to the development of atherosclerosis, endothelial dysfunction, renovascular hypertension, and cardiovascular disease. It is worth to note that the increased activity of serum XO was reversed in the present study. In line with study, KV might be another novel antihyperuricemic agent and thereby open another therapeutic window as far as drug discovery, development and innovation in the field of science is concerned.
From the present study, the activity of serum Myeloperoxidase (MPO) activity increased significantly and this might due to NaAsO2 intoxication. However, treatment of KV at the dose of 100 and 200 mg/kg decreased the serum MPO activity. This, therefore, might be indicative of anti-inflammatory and cardio-protective effect of KV. MPO has been found to be abundantly expressed in neutrophils and to a lesser extent in monocytes and some tissue macrophages.64 In fact, recent report documented that macrophages are now used as an imaging biomarker in inflammatory cardiovascular diseases.65 Several studies have suggested mechanistic links between myeloperoxidase, atherosclerosis, endothelial dysfunction, inflammation and are both acute and chronic manifestations of cardiovascular disease.66, 67 Together, KV could therefore open a new therapeutic window for the treatment of atherosclerosis and other cardiovascular diseases.
Endothelial dysfunction and decreased NO levels have been found to have a positive correlation between oxidative stress and hypertension.68, 69 Endothelial dysfunction has been reported to increase oxidative stress and reduce NO bioavalability.70, 71 Our data showed that NaAsO2 intoxication reduced NO bioavailability in the serum, cardiac and renal tissues. However, KV pre-treatment prior to NaAsO2 intoxication significantly improved NO bioavailability. Further, we hypothesized that inhibition of SOD activity might also contribute to the development of hypertension through excessive generation of superoxide anion radicals which can mop-up NO leading to reduce NO bioavailability. Alternatively, peroxynitrite (ONOO−) that is formed from the combination of NO and superoxide anion radical might also inhibit the endothelial nitric oxide synthase which is the key enzyme that produces NO from the endothelium for the maintenance of vascular tone and blood pressure. Similarly, KV might increase endothelial NO production, lower the systemic blood pressure, vasoconstriction, glomerular hypertension and arterial stiffness. Hence, KV could be a useful anti-hypertensive agent in the future.
The activities of serum ALT, AST and ALP were observed to have increased significantly in NaAsO2 intoxicated rats. This is suggestive that apart from the cardio-renal dysfunction, hepatic injury could also be accompanied side-by-side in NaAsO2 intoxication. Although, the increase in the activities of these enzymes along with other specific markers of cardiac damage have been reported in isoproterenol (ISO)-induced myocardial infarction (MI) in rats.72 Present study showed that in KV pre-treated rats, the increased activities of ALT, AST and ALP were restored compared to the control and the NaAsO2 intoxicated rats.
The electrocardiogram (ECG) revealed increase heart rate, QRS together with prolonged QT and QTc intervals in NaAsO2 intoxicated rats which is suggestive of negative influence of NaAsO2 on cardiovascular system. Epidemiological studies have shown that chronic arsenic poisoning through ingestion of arsenic-contaminated water is associated with various cardiovascular diseases in dose-response relationship.73 QT interval prolongation has been extensively documented in arsenic poisoning.74, 75, 76 The QT interval prolongation might be associated with increase in intracellular calcium overload as previously reported.77 Hence, we propose that KV pre-treatment might have a modulatory effect on intracellular calcium current.
Our results indicated that rats intoxicated with NaAsO2 had higher expressions of C-reactive protein (CRP), cardiac troponin I (CTnI) in the kidney and heart tissues, respectively. The CRP is a marker of inflammation whereas CTnI is a diagnostic marker of cardiac damage. Previous in vitro study on cardiac differentiation of rat myoblast H9c2 cell exposed to NaAsO2 reported enhanced expression of cardiac troponin T (cTnT), the appearance of multinucleated cells, and cell cycle arrest at G0/G1 phase.78 Also, higher expressions of high sensitive C-reactive protein (hs-CRP) has also been reported in experimental animal models exposed to NaAsO2 for 20 consecutive weeks with a resultant induction of arteriosclerosis.79
The inflammatory reactions induced by NaAsO2 intoxication in the kidney and heart was attenuated in KV pre-treated rats. Increase inflammation and decrease in glomerular filtration rate (eGFR) following NaAsO2 intoxication has also been reported80, 81 and this is in line with the present study. The mechanism of arsenic acid-induced arteriosclerosis might be through induction of inflammation and oxidative stress. The anti-inflammatory, cardioprotecive and nephroprotective effect of KV was therefore demonstrated through the inhibition of cardiac and renal CTnI and CRP, respectively. The BCL2 is a pro-survival and ant-apoptotic protein.82 The involvement of BCL2 in cell survival, differentiation, autophagy and apoptosis cannot be undermined. However, KV pre-treated rats increased the expressions of BCL2 in the renal tissues, and this was indicative of nephron-protective effect of KV on NaAsO2 intoxication renal damage. Our findings could therefore be supported from the work of Adil et al.83 who reported the elevation of kidney marker injury 1 (KIM-1) and caspase 3 mRNA expression in rats exposed to NaAsO2.
The histology of the kidney of rats administered NaAsO2 alone showed cellular infiltration of renal insterstitium and this was supported with increase in kidney weight of rats in the same group. The observed increase in the kidney weight might also be related to the inflammatory response elicited by NaAsO2 as indicated with higher expressions of CRP. Furthermore, inflammation of the epicardium and pericardium together with increase heart weight was also observed in rats administrated NaAsO2 without KV pre-treatment. In the KV pre-treated rats, the increase in kidney and heart weights combined with the pathology on the heart and kidney was restored.
6. Conclusion
In conclusion, KV may offer a new therapeutic regimen for the treatment of cardio-renal dysfunction associated with arteriosclerosis, hypertension and other cardiovascular dysfunctions. The administration of KV might also useful for the improvement of NO bioavailability which is necessary for the maintenance of vascular tone, blood pressure, improvement of coronary blood flow, vasodilation and prevention of arterial stiffness and vasoconstriction. Furthermore, the anti-inflammatory and cardio-renal protective effect of KV against NaAsO2− induced inflammation and cardio-renal damage could be developed as novel drug for the amelioration of inflammation associated with cardiac and renal damage.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Manna P., Sinha M., Sil P.C. Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin Pharmacol Toxicol. 2007;101(5):333–338. doi: 10.1111/j.1742-7843.2007.00132.x. [DOI] [PubMed] [Google Scholar]
- 2.Bustaffa E., Minichilli F., Andreassi M.G. Studies on markers of exposure and early effect in areas with arsenic pollution: methods and results of the project SEpiAs. Epidemiological surveillance in areas with environmental pollution by natural or anthropogenic arsenic. Epidemiol Prev. 2014;238(3–4 suppl 1):27–94. [PubMed] [Google Scholar]
- 3.Cubadda F., D'Amato M., Mancini F.R. Assessing human exposure to inorganic arsenic in high-arsenic areas of Latium: a biomonitoring study integrated with indicators of dietary intake. Ann Ig. 2015;27(1):39–51. doi: 10.7416/ai.2015.2021. [DOI] [PubMed] [Google Scholar]
- 4.Bustaffa E., Bianchi F. Studies on markers of exposure and early effect in areas with arsenic pollution: methods and results of the project SEpiAs. Epidemiological studies on population exposed to low-to-moderate arsenic concentration in drinking water. Epidemiol Prev. 2014;38(3–4 suppl 1):14–24. [PubMed] [Google Scholar]
- 5.Hirano S., Kobayashi Y., Cui X., Kanno S., Hayakawa T., Shraim A. The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol Appl Pharmacol. 2004;198(3):458–467. doi: 10.1016/j.taap.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Jomova K., Jenisova Z., Feszterova M. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 7.Hughes M.F. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh J., Das J., Manna P., Sil P.C. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol. 2009;240(1):73–87. doi: 10.1016/j.taap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Fu S., Wu J., Li Y. Urinary arsenic metabolism in a Western Chinese population exposed to high-dose inorganic arsenic in drinking water: influence of ethnicity and genetic polymorphisms. Toxicol Appl Pharmacol. 2014;274(1):117–123. doi: 10.1016/j.taap.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Das A.K., Bag S., Sahu R. Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem Toxicol. 2010;48(1):326–335. doi: 10.1016/j.fct.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Manna P, Sinha M, Sil PC. Arsenic-induced oxidative myocardial injury: protective role of arjunolic acid. Arch Toxicol. 82(3):137–149. [DOI] [PubMed]
- 12.Das A.K., Sahu R., Dua T.K. Arsenic-induced myocardial injury: protective role of Corchorus olitorius leaves. Food Chem Toxicol. 2010;48(5):1210–1217. doi: 10.1016/j.fct.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Liang S.X., Lu Y.F., Miao J.W., Wu Q., Shi J.S. Realgar and realgar-containing Liu-Shen-Wan are less acutely toxic than arsenite and arsenate. J Ethnopharmacol. 2011;134(1):26–31. doi: 10.1016/j.jep.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 14.Kordas K., Queirolo E.I., Mañay N. Low-level arsenic exposure: nutritional and dietary predictors in first-grade Uruguayan children. Environ Res. 2011;147:16–23. doi: 10.1016/j.envres.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adaramoye O.A., Lawal S.O. Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J Basic Clin Physiol Pharmacol. 2015;26(1):65–72. doi: 10.1515/jbcpp-2013-0139. [DOI] [PubMed] [Google Scholar]
- 16.Awogbindin I.O., Olaleye D.O., Farombi E.O. Kolaviron improves morbidity and suppresses mortality by mitigating oxido-inflammation in BALB/c mice infected with influenza virus. Viral Immunol. 2015;28(7):367–377. doi: 10.1089/vim.2015.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olajide O.J., Enaibe B.U., Bankole O.O., Akinola O.B., Laoye B.J., Ogundele O.M. Kolaviron was protective against sodium azide (NaN3) induced oxidative stress in the prefrontal cortex. Metab Brain Dis. 2016;31(1):25–35. doi: 10.1007/s11011-015-9674-0. [DOI] [PubMed] [Google Scholar]
- 18.Akinmoladun A.C., Akinrinola B.L., Olaleye M.T., Farombi E.O. Kolaviron, a Garcinia kola biflavonoid complex, protects against ischemia/reperfusion injury: pertinent mechanistic insights from biochemical and physical evaluations in rat brain. Neurochem Res. 2015;40(4):777–787. doi: 10.1007/s11064-015-1527-z. [DOI] [PubMed] [Google Scholar]
- 19.Adedara I.A., Daramola Y.M., Dagunduro J.O., Aiyegbusi M.A., Farombi E.O. Renoprotection of Kolaviron against benzo (A) pyrene-induced renal toxicity in rats. Ren Fail. 2015;37(3):497–504. doi: 10.3109/0886022X.2015.1006085. [DOI] [PubMed] [Google Scholar]
- 20.Akinrinde A.S., Olowu E., Oyagbemi A.A., Omobowale O.T. Gastrointestinal protective efficacy of Kolaviron (a bi-flavonoid from Garcinia kola) following a single administration of sodium arsenite in rats: biochemical and histopathological studies. Pharm Res. 2015;7(3):268–276. doi: 10.4103/0974-8490.157978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owoeye O., Adedara I.A., Adeyemo O.A., Bakare O.S., Egun C., Farombi E.O. Modulatory role of kolaviron in phenytoin-induced hepatic and testicular dysfunctions in Wistar rats. J Diet Suppl. 2015;12(1):105–117. doi: 10.3109/19390211.2014.952862. [DOI] [PubMed] [Google Scholar]
- 22.Ayepola O.R., Cerf M.E., Brooks N.L., Oguntibeju O.O. Kolaviron, a biflavonoid complex of Garcinia kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes-induced nephrotoxic rats. Phytomed. 2014;21(14):1785–1793. doi: 10.1016/j.phymed.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Owoeye O., Adedara I.A., Bakare O.S., Adeyemo O.A., Egun C., Farombi E.O. Kolaviron and vitamin E ameliorate hematotoxicity and oxidative stress in brains of prepubertal rats treated with an anticonvulsant phenytoin. Toxicol Mech Methods. 2014;24(5):353–361. doi: 10.3109/15376516.2014.913752. [DOI] [PubMed] [Google Scholar]
- 24.Abarikwu S.O. Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim Biochim Biophys Acta. 2014;1840(7):2373–2381. doi: 10.1016/j.bbagen.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Adaramoye O.A., Arisekola M. Kolaviron, a biflavonoid complex from Garcinia kola seeds, ameliorates ethanol-induced reproductive toxicity in male Wistar rats. Niger J Physiol Sci. 2013;28(1):9–15. [PubMed] [Google Scholar]
- 26.Abarikwu S.O. Anti-inflammatory effects of Kolaviron modulate the expressions of inflammatory marker genes, inhibit transcription factors ERK1/2, p-JNK, NF-κB, and activate Akt expressions in the 93RS2 sertoli cell lines. Mol Cell Biochem. 2015;401(1–2):197–208. doi: 10.1007/s11010-014-2307-9. [DOI] [PubMed] [Google Scholar]
- 27.Oluwatosin A., Tolulope A., Ayokulehin K. Antimalarial potential of Kolaviron, a biflavonoid from Garcinia kola seeds, against Plasmodium berghei infection in Swiss albino mice. Asian Pac J Trop Med. 2014;7(2):97–104. doi: 10.1016/S1995-7645(14)60003-1. [DOI] [PubMed] [Google Scholar]
- 28.Adaramoye O.A., Lawal S.O. Effect of Kolaviron, a biflavonoid complex from Garcinia kola seeds, on the antioxidant, hormonal and spermatogenic indices of diabetic male rats. Andrologia. 2014;46(8):878–886. doi: 10.1111/and.12160. [DOI] [PubMed] [Google Scholar]
- 29.Iwu M.M., Igboko O.A., Onwuchekwa U., Okunji C.O. Evaluation of the anti-hepatotoxicity of the biflavonoids of Garcinia kola seeds. J Ethnopharmacol. 1987;21:127–142. doi: 10.1016/0378-8741(87)90123-1. [DOI] [PubMed] [Google Scholar]
- 30.PHS (PUBLIC HEALTH SERVICE) US Department of Health and Humane services; Washington, DC: 1996. Public Health Service Policy on Humane Care and the Use of Laboratory Animals; pp. 99–158. [Google Scholar]
- 31.Shinha K.A. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 32.Misra H.P., Fridovich I. The role of superoxide anoin in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;217:3170–3175. [PubMed] [Google Scholar]
- 33.Oyagbemi A.A., Omobowale T.O., Akinrinde A.S., Saba A.B., Ogunpolu B.S., Daramola O. Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol. 2015;30:1235–1243. doi: 10.1002/tox.21994. [DOI] [PubMed] [Google Scholar]
- 34.Omobowale T.O., Oyagbemi A.A., Akinrinde A.S. Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environ Toxicol Pharmacol. 2014;37:1202–1211. doi: 10.1016/j.etap.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Jollow D.J., Mitchell J.R., Zampaglione N., Gillette J.R. JB Lippinocott; 1994. Bromobenzene-induced Liver Necrosis; Protective Role of GSH & Evidence for 3, 4 Bromobenzene Oxide as the Hepatotoxic Metabolite; pp. 108–144. [DOI] [PubMed] [Google Scholar]
- 36.Buetler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 37.Habig W.H., Pabst M.J., Jacoby W.B. Glutathione-S-transferase activity: the enzymic step in mercapturic acid formation. J Biol Chem. 1974;249:130–139. [PubMed] [Google Scholar]
- 38.Varshney R., Kale R.K. Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58(5):733–743. doi: 10.1080/09553009014552121. [DOI] [PubMed] [Google Scholar]
- 39.Wolff S.F. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 40.Olaleye S.B., Adaramoye O.A., Erigbali P.P., Adeniyi O.S. Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol-exposed rats. World J Gastroenterol. 2007;13:5121–5126. doi: 10.3748/wjg.v13.i38.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia Y., Zweier J.L. Measurement of myeloperoxidase in leukocyte-containing tissues. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245(1):93–96. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- 42.Akaike T., Ando M., Oda T. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest. 1990;85:739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gornal A.G., Bardawill J.C., David M.M. Determination of serum proteins by means of Biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 44.Drury R.A., Wallington E.A., Cancerson R. Carlton's Histopathological Techniques. 4th ed. Oxford University Press; Oxford/London/New York: 1976. [Google Scholar]
- 45.Kurt O.K., Zhang J., Pinkerton K.E. Pulmonary health effects of air pollution. Curr Opin Pulm Med. 2016;22(2):138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J., Xu X., Hylkema M.N. Early-life exposure to widespread environmental toxicants and health risk: a focus on the immune and respiratory systems. Ann Glob Health. 2016;82(1):119–131. doi: 10.1016/j.aogh.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Nadal M., Marquès M., Mari M., Domingo J.L. Climate change and environmental concentrations of POPs: a review. Environ Res. 2015;143(Pt A):177–185. doi: 10.1016/j.envres.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Rahman M., Sohel N., Yunus M. A prospective cohort study of stroke mortality and arsenic in drinking water in Bangladeshi adults. BMC Public Health. 2014;14:174. doi: 10.1186/1471-2458-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tutic A., Novakovic S., Lutovac M., Biocanin R., Ketin S., Omerovic N. The heavy metals in agrosystems and impact on health and quality of life. J Med Sci. 2015;3(2):345–355. doi: 10.3889/oamjms.2015.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon S.S. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine. 2014;46(2):263–271. doi: 10.1007/s12020-013-0061-5. [DOI] [PubMed] [Google Scholar]
- 51.Lee B.K., Kim Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. Am J Ind Med. 2013;56(6):682–692. doi: 10.1002/ajim.22107. [DOI] [PubMed] [Google Scholar]
- 52.Xia X., Hua C., Xue S. Response of selenium-dependent glutathione peroxidase in the freshwater bivalve Anodonta woodiana exposed to 2,4-dichlorophenol,2,4,6-trichlorophenol and pentachlorophenol. Fish Shellfish Immunol. 2016;55:499–509. doi: 10.1016/j.fsi.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Chai T., Cui F., Mu X. Stereoselective induction by 2,2′,3,4′,6-pentachlorobiphenyl in adult zebrafish (Danio rerio): implication of chirality in oxidative stress and bioaccumulation. Environ Pollut. 2016;215:66–76. doi: 10.1016/j.envpol.2016.04.075. [DOI] [PubMed] [Google Scholar]
- 54.Dzul-Caamal R., Hernández-López A., Gonzalez-Jáuregui M., Padilla S.E., Girón-Pérez M.I., Vega-López A. Usefulness of oxidative stress biomarkers evaluated in the snout scraping, serum and peripheral blood cells of Crocodylus moreletii from Southeast Campeche for assessment of the toxic impact of PAHs, metals and total phenols. Comp Biochem Physiol A Mol Integr Physiol. 2016 May 6 doi: 10.1016/j.cbpa.2016.05.006. S1095-6433(16) 30105-2. [DOI] [PubMed] [Google Scholar]
- 55.Pelclova D., Zdimal V., Fenclova Z. Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occup Environ Med. 2016;73(2):110–118. doi: 10.1136/oemed-2015-103161. [DOI] [PubMed] [Google Scholar]
- 56.Liu G.D., Sheng Z., Wang Y.F., Han Y.L., Zhou Y., Zhu J.Q. Glutathione peroxidase 1 expression, malondialdehyde levels and histological alterations in the liver of Acrossocheilus fasciatus exposed to cadmium chloride. Gene. 2016;578(2):210–218. doi: 10.1016/j.gene.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 57.Wilson J., Berntsen H.F., Zimmer K.E. Effects of defined mixtures of persistent organic pollutants (POPs) on multiple cellular responses in the human hepatocarcinoma cell line, HepG2, use high content analysis screening. Toxicol Appl Pharmacol. 2016;294:21–31. doi: 10.1016/j.taap.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Tan H.Y., Wang N. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16(11):26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuwabara M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse (Basel) 2016;3(3–4):242–252. doi: 10.1159/000443769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai H., Lu S., Tang X. Combined association of serum uric acid and metabolic syndrome with chronic kidney disease in hypertensive patients. Kidney Blood Press Res. 2016;41(4):413–423. doi: 10.1159/000443443. [DOI] [PubMed] [Google Scholar]
- 61.Wang M., Zhao J., Zhang N., Chen J. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomed Pharmacother. 2016;83:975–988. doi: 10.1016/j.biopha.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Maharani N., Kuwabara M., Hisatome I. Hyperuricemia and atrial fibrillation. Int Heart J. 2016;57(4):395–399. doi: 10.1536/ihj.16-192. [DOI] [PubMed] [Google Scholar]
- 63.Riegersperger M., Covic A., Goldsmith D. Allopurinol, uric acid, and oxidative stress in cardiorenal disease. Int Urol Nephrol. 2011;43(2):441–449. doi: 10.1007/s11255-011-9929-6. [DOI] [PubMed] [Google Scholar]
- 64.Pattison D.I., Davies M.J., Hawkins C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic Res. 2012;46(8):975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 65.Ali M., Pulli B., Chen J.W. Molecular imaging of macrophage enzyme activity in cardiac inflammation. Curr Cardiovasc Imaging Rep. 2014;7(4):9258. doi: 10.1007/s12410-014-9258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sadowski M., Ząbczyk M., Undas A. Coronary thrombus composition: links with inflammation, platelet and endothelial markers. Atherosclerosis. 2014;237(2):555–561. doi: 10.1016/j.atherosclerosis.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 67.Talib J., Cook N., Pattison D., Davies M. Disruption of the iron-sulfur cluster of aconitase by myeloperoxidase-derived oxidants. Free Radic Biol Med. 2014;75(l):S27–S28. doi: 10.1016/j.freeradbiomed.2014.10.752. [DOI] [PubMed] [Google Scholar]
- 68.Korsager L.M., Matchkov V.V. Hypertension and physical exercise: the role of oxidative stress. Med Kaunas. 2016;52(1):19–27. doi: 10.1016/j.medici.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Islam M.Z., Van Dao C., Shiraishi M., Miyamoto A. Methylmercury affects cerebrovascular reactivity to angiotensin II and acetylcholine via Rho-kinase and nitric oxide pathways in mice. Life Sci. 2016;147:30–38. doi: 10.1016/j.lfs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 70.Molina M.N., Ferder L., Manucha W. Emerging role of nitric oxide and heat shock proteins in insulin resistance. Curr Hypertens Rep. 2016;18(1) doi: 10.1007/s11906-015-0615-4. 1. [DOI] [PubMed] [Google Scholar]
- 71.Kao C.K., Morton J.S., Quon A.L., Reyes L.M., Lopez-Jaramillo P., Davidge S.T. Mechanism of vascular dysfunction due to circulating factors in women with pre-eclampsia. Clin Sci (Lond) 2016;130(7):539–549. doi: 10.1042/CS20150678. [DOI] [PubMed] [Google Scholar]
- 72.Geng Z.H., Huang L., Song M.B., Song Y.M. Protective effect of a polysaccharide from Salvia miltiorrhiza on isoproterenol (ISO)-induced myocardial injury in rats. Carbohydr Polym. 2015;132:638–642. doi: 10.1016/j.carbpol.2015.06.086. [DOI] [PubMed] [Google Scholar]
- 73.Wang C.H., Hsiao C.K., Chen C.L. A review of the epidemiologic literature on the role of environmental arsenic exposure and cardiovascular diseases. Toxicol Appl Pharmacol. 2007;222(3):315–326. doi: 10.1016/j.taap.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 74.Chen X., Shan H., Zhao J. L-type calcium current (ICa,L) and inward rectifier potassium current (IK1) are involved in QT prolongation induced by arsenic trioxide in rat. Cell Physiol Biochem. 2010;26(6):967–974. doi: 10.1159/000324005. [DOI] [PubMed] [Google Scholar]
- 75.Liao Y.T., Li W.F., Chen C.J. Synergistic effect of polymorphisms of paraoxonase gene cluster and arsenic exposure on electrocardiogram abnormality. Toxicol Appl Pharmacol. 2009;239(2):178–183. doi: 10.1016/j.taap.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 76.Zhao X.Y., Li G.Y., Liu Y. Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J Pharmacol. 2008;154(1):105–113. doi: 10.1038/bjp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan Y., Wang C., Zhang Y. Genistein ameliorates adverse cardiac effects induced by arsenic trioxide through preventing cardiomyocytes apoptosis. Cell Physiol Biochem. 2013;31(1):80–91. doi: 10.1159/000343351. [DOI] [PubMed] [Google Scholar]
- 78.Sumi D., Abe K., Himeno S. Arsenite retards the cardiac differentiation of rat cardiac myoblast H9c2 cells. Biochem Biophys Res Commun. 2013;436(2):175–179. doi: 10.1016/j.bbrc.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 79.Cheng T.J., Chuu J.J., Chang C.Y., Tsai W.C., Chen K.J., Guo H.R. Atherosclerosis induced by arsenic in drinking water in rats through altering lipid metabolism. Toxicol Appl Pharmacol. 2011;256(2):146–153. doi: 10.1016/j.taap.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Peters B.A., Hall M.N., Liu X. Renal function is associated with indicators of arsenic methylation capacity in Bangladeshi adults. Environ Res. 2015;143(Pt A):123–130. doi: 10.1016/j.envres.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kesavan M., Sarath T.S., Kannan K. Atorvastatin restores arsenic-induced vascular dysfunction in rats: modulation of nitric oxide signaling and inflammatory mediators. Toxicol Appl Pharmacol. 2014;280(1):107–116. doi: 10.1016/j.taap.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Chipuk J.E., Moldoveanu T., Llambi F., Parsons M.J., Green D.R. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adil M., Kandhare A.D., Visnagri A., Bodhankar S.L. Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α. Ren Fail. 2015;37(8):1396–1407. doi: 10.3109/0886022X.2015.1074462. [DOI] [PubMed] [Google Scholar]