Abstract
Background
This is the first review to analyze literature identifying risk factors for a multidrug-resistant urinary tract infection (MDR UTI). Risk factors for other infections involving multidrug-resistant organisms have been evaluated in other reviews, but they do not assess urinary tract infections. The purpose of this study is to collect currently published data to determine the most commonly and consistently identified risk factors for UTIs.
Material and methods
For this study, 3 independent researchers searched PubMed, Embase, and Cochrane database from 1966 to February 2016 for articles identifying risk factors for MDR UTI.
Results
A total of 25 studies including 31,284 patients with positive cultures provide evidence for 12 possible risk factors for MDR UTI . The most commonly identified risk factor was previous antibiotic usage as evidenced in 16 of the 20 studies that evaluated this possible risk factor. The time range utilized to define previous antibiotic usage ranged from 2 days to 365 days. Other risk factors with the strongest supporting data were urinary catheterization, previous hospitalization, and nursing home residence.
Conclusion
We identified 12 different possible risk factors for a MDR UTI, however several risk factors have minimal or conflicting evidence. The definitions of the risk factors varied widely among the studies, and should be standardized for future studies.
Keywords: Cystitis, Microbial drug resistance, Pyelonephritis, Risk factors, Systematic review, Urinary tract infection
1. Introduction
Urinary tract infections (UTIs) are the most frequently reported infections and drive antibiotic use around the world (Allen et al., 1999, Anthony, 2002). UTIs are the fourth most common type of healthcare-associated infection (Magill et al., 2014). Multidrug-resistant organisms (MDRO) are predominantly bacteria, that are resistant to one or more classes of antimicrobial agents. Sulfamethoxazole-trimethoprim resistance has been shown throughout the world for E. coli and has led to expanded use of fluoroquinolones and cephalosporins. Gram negative extended-spectrum beta lactamase producing enterobacteriaceae are an increasing concern in regards to antibiotic resistance and their potential cause of serious infections which are difficult to treat (Shaikh et al., 2015). Throughout the world there is increasing antimicrobial drug resistance, therefore it is important to identify factors that place patients at increased risk for a multidrug-resistant infection, so that broad spectrum antibiotics can be reserved for use in these patients. Limiting broad spectrum empiric antibiotics to patients with proven risk factors can help slow the prevalence of resistance to these antibiotics. The concern that our study addresses is how to define and identify the patients who are at increased risk of infection by these multidrug-resistant organisms in regards to a UTI.
This is the first review to analyze the literature identifying risk factors for a multidrug-resistant (MDR) urinary tract infections (UTIs). Risk factors for other infections involving multidrug-resistant organisms have been evaluated in other reviews, but these studies do not assess infections of the urinary tract system. The objective of our study is to identify and appraise the current literature to determine what are the possible risk factors for developing a MDR UTI’s and which risk factors have the strongest supporting data.
2. Material and methods
2.1. Data sources and search strategy
A literature search was completed independently by three authors using PubMed, Embase, and Cochrane databases. Search was conducted from January 1966 up to February 31st 2016. The following keywords were used as search terms: ([drug AND resistance AND multiple] OR [multidrug AND resistance]) AND ([urinary AND tract AND infection] OR pyelonephritis OR cystitis) AND (risk AND factors). Reference lists of included articles were also reviewed for eligible studies. We categorized the risk factors evaluated in the studies into 3 categories: Probable risk factor, possible risk factor, and unlikely risk factor or further research needed Table 2). Due to the likeliness of variable definitions of multidrug resistance those studies that follow the 2011 international consensus panel’s expert proposal definition for acquired resistance were also assessed (Magiorakos et al., 2012).
Table 2.
Study characteristics in determining risk factor supporting evidence.
| Probable risk factors requirements | Possible risk factor | Unlikely risk factor or more research needed |
|---|---|---|
| Number of studies ≥5 | Number of studies ≥ 5 | Number of studies ≤5 |
| Number of patients ≥1500 | Number of patients ≥2000 | Number of patients ≤2000 |
| Percent of patients in studies identified as positive for risk factor ≥70% | Percent of patients in studies identified as positive for risk factor ≥60% OR | Percent of patients in studies identified as positive for risk factor <60% |
| Percent of studies identified as positive for risk factor ≥70% | Percent of studies identified as positive for risk factor ≥60% | Percent of studies identified as positive for risk factor <60% |
2.2. Study selection
Studies were considered eligible for inclusion if the study identified and reported any risk factors associated or not associated with MDR UTIs in patients with positive cultures. Studies were eligible for inclusion only if published in English. Studies were limited to those reporting on human adult or pediatric patients. Titles and abstracts were reviewed for identification of risk factors for MDR UTIs. Articles deemed relevant were reviewed in full to determine inclusion in our analysis. All articles were evaluated for inclusion by three authors and a consensus was achieved whenever there was a disagreement on inclusion. The primary outcome assessed was the association of different risk factors with MDR UTIs.
2.3. Data extraction and quality assessment
The three reviewers independently extracted data from all eligible studies and agreed on any discrepancies by consensus. The extracted data for each study when available was placed into an Excel spreadsheet and included the country, study type, year of publication, number of patients, type of UTI (pyelonephritis vs. cystitis, complicated vs. uncomplicated), organism cultured, study setting (community, inpatient hospital, emergency department), drugs of focus in study, and all risk factors reviewed for association. No uniform use of a specified definition was utilized. Studies were included regardless of how the information that was collected was defined.
Studies included for analysis were rated using the Newcastle-Ottawa Quality Assessment Scale (NOS) (O’Connell, 2002). The NOS contains eight items, categorized into three dimensions including selection, comparability, and outcome. The NOS ranges between zero and nine. Each study was reviewed independently by two of the authors and assessed for quality using the NOS. Authors discussed any discrepancies in quality assessment and came to a consensus with the assistance of a third reviewer.
Descriptive statistics were used to quantitatively describe features of the studies when analyzed collectively. Studies were chronologically assessed by the age of the study to determine relevance and/or changes in MDR UTI risk factors.
3. Results
The review identified 25 studies including 31,284 patients with positive cultures that identify possible risk factors for multidrug-resistant UTI (Colodner et al., 2004, Ena et al., 1995, Eshetie et al., 2015, Faine et al., 2015, Gangcuangco et al., 2015, Gould et al., 2010, Gupta et al., 2011, Hertz et al., 2016, Ho et al., 2007, Hooton et al., 2010, Ikram et al., 2015, Jadoon et al., 2015, Johnson et al., 2008, Kalil et al., 2016, Kang et al., 2015, Khawcharoenporn et al., 2013, Killgore et al., 2004, Klevens et al., 2002, Lee et al., 2010, Magill et al., 2014, Magiorakos et al., 2012, Metlay et al., 2003, O’Connell, 2002, Osthoff et al., 2015, Shaikh et al., 2015). There has been an increasing trend in the number of articles published regarding risk factors for developing an MDR UTI in recent years. Individual study characteristics are described in Table 1. There were 13 retrospective studies, 11 prospective studies, and 1 study with both retrospective and prospective components. 14 studies took place in the inpatient setting, 7 in the community setting, and 4 had mixed settings. Study sizes ranged from 66 to 21,414 and 23 of the 25 studies had less than 1000 participants. The percent of positive cultures in the studies included that identified E. coli as the causative pathogen ranged from 29.2 to 100%. The studies were good to moderate based on the scoring from the NOS. All the studies scored a 7 or 8 out of 9 on the quality assessment with the exception of one study from Spain with 105 patients with positive cultures scoring a 5.
Table 1.
Background information of studies included in the review.
| Study characteristics |
Patient characteristics |
|||||
|---|---|---|---|---|---|---|
| Lead author, year of publication | Country | Prospective (P), retrospective (R), or both (B) | Definition of resistanceb | E. coli (%) | Number of patients with positive urine cultures | Female (%) |
| Allen, 1999 | Canada | P | Other | 100 | 548 | 65.6 |
| Arslan, 2005 | Turkey | P | Fluoroquinolone | 84.1 | 611 | 85.8 |
| Brown, 2002 | USA | R | TMP-SMX | 100 | 601 | 100 |
| Burman, 2003 | USA | B | TMP-SMX | 97.5 | 832 | 95.1 |
| Colgan, 2004 | USA | P | TMP-SMX | 83.5 | 103 | 100 |
| Colodner, 2004 | Israel | P | Other | 71.7 | 311 | 77.2 |
| Ena, 1995a | Spain | R | Fluoroquinolone | 6.4 | 105 | 58.1 |
| Eshetie, 2015 | Ethiopia | P | ≥2 classes | 61.2 | 183 | 63.8 |
| Faine, 2015 | USA | R | Other | 33.3 | 360 | 83.6 |
| Guangcuangco, 2015 | Philippines | P | TMP-SMX | 76.2 | 229 | 100 |
| Hertz, 2015 | Denmark | R | Other | 100 | 442 | 83.6 |
| Ho, 2010 | Hong Kong | P | ≥3 classes | 77 | 352 | 100 |
| Ikram, 2015 | New Zealand | R | ≥3 classes | 100 | 156 | 60.3 |
| Jadoon, 2015 | Pakistan | P | Fluoroquinolone | 100 | 66 | 75.3 |
| Johnson, 2008 | USA | R | Fluoroquinolone | 100 | 123 | 82.9 |
| Kang, 2015 | South Korea | R | Other | 29.2 | 1929 | 26.9 |
| Khawcharoenporn, 2013 | USA | R | Other | 72 | 431 | 81.4 |
| Killgore, 2004 | USA | R | Fluoroquinolone | 100 | 120 | 85 |
| Lee, 2010 | South Korea | P | Other | 100 | 225 | 100 |
| Metlay, 2003 | USA | R | TMP-SMX | 62 | 393 | N/A |
| Osthoff, 2015 | Australia | R | ESBL+ ≥3 classes | 72.5 | 200 | 74.5 |
| Seung, 2014 | South Korea | P | Other | 31.5 | 413 | 39.1 |
| Talan, 2008 | USA | P | TMP-SMX | 88 | 689 | 90 |
| Toner, 2015 | UK | R | ≥3 classes | 84.6 | 21,414 | 77 |
| Wright, 1999 | USA | R | TMP-SMX | 85 | 448 | 83.7 |
≥2/3 classes Resistance to ≥1 drug in a minimum of 2/3 different antibiotic classes.
Fluoroquinolone: Only required resistance to Ciprofloxacin or ≥1 drug in the fluoroquinolone class.
Only study with a Newcastle-Ottawa Quality Assessment Scale score of <7–8.
TMP-SMX: Only required resistance to sulfamethoxazole/trimethoprim.
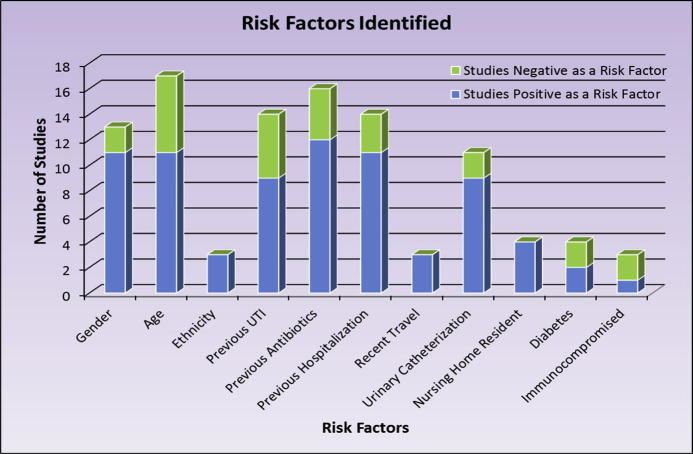
Some risk factors have been assessed much more often and much more consistently than other risk factors Fig. 1). Table 3 shows the risk factors stratified based on amount and consistency of supporting literature. The most commonly identified risk factor was previous antibiotic usage as seen in 16 of the 20 studies that evaluated this possible risk factor. The concern with the risk factor identification in the studies is that the time range utilized in studies to define previous antibiotic usage ranged from 2 days to 365 days. The other 11 risk factors identified in at least 2 different studies included: minority ethnicity, recent travel, nursing home residence, urinary catheterization, previous hospitalization, age, previous UTI’s, both male and female gender, immunocompromised patients, and diabetes.
Fig. 1.
Quantity of studies that assessed risk factor and found the risk factor to be related or not related to the chance of a UTI being from a MDR organism.
Table 3.
Risk factors stratified based on amount and consistency of supporting literature.
| Number of studies assessing for risk factor (n) | Number of patients assessed for risk factor (n) | % of Patients in studies positively identifying risk factor (%) | % of Studies positively identifying risk factor (%) | |
|---|---|---|---|---|
| Probable risk factors | ||||
| Urinary Catheter | 14 | 27,401 | 95.1 | 81.8 |
| Previous Hospitalization | 14 | 6353 | 84.8 | 72.7 |
| Previous Antibiotics | 20 | 6943 | 76.9 | 75 |
| Nursing Home Resident | 5 | 1959 | 100 | 100 |
| Possible risk factors | ||||
| Age | 19 | 29,626 | 87.6 | 64.7 |
| Previous UTI | 15 | 4526 | 59.1 | 64.3 |
| Male Gender | 19 | 27,701 | 92.7 | 61.5 |
| Unlikely risk factor or more research needed | ||||
| Diabetes | 6 | 1574 | 58.1 | 50 |
| Recent Travel | 3 | 1135 | 100 | 100 |
| Ethnicity | 3 | 1624 | 100 | 100 |
| Immunocompromised | 3 | 1255 | 28.6 | 33 |
| Female Gender | 19 | 27,701 | 2.7 | 23.1 |
Three of the risk factors are shown to be positive in all the studies in which they were assessed: nursing home residence (5/5), recent travel (3/3), and minority ethnicity (3/3). The three studies that assessed ethnicity were based out of the United States and assessed the ethnic minority of Hispanics in 2 studies and Asians in 1 study. 17 of the 24 studies that reported the proportion of each gender had 75% or more of the study population as females. Three studies evaluated immunosuppression as a risk factor, but all had different definitions (Faine et al., 2015, Ho et al., 2007, Kalil et al., 2016). One study assessed patients with human immunodeficiency virus, another evaluated patients taking immunosuppressive agents, and the third considered patients actively receiving chemotherapy and use of systemic corticosteroids (>10 mg prednisone-equivalent daily) or biological agents. 20 out of the 25 studies included were single center studies, and the 5 multicenter studies ranged from 11 to 54 centers. Fig. 2 shows the data on the percentage of patients and the percentage of studies that positively identified each risk factor. These results are shown together as whether the data is broken down by reproducibility (number of studies) or by study size (number of patients) the results appear fairly similar.
Fig. 2.

Proportion of studies and patients that positively identify the risk factor for a MDR UTI.
Only 3 studies that utilized the 2011 international consensus panel’s expert proposal for the definition of acquired resistance (resistance of 1 antimicrobial from 3 different classes) were identified. These three studies did include the majority of the culture positive patients included in our review 21,922 of 31,284 (70%). Patients in these 3 studies were 77.3% female and 84.4% of isolated pathogens were E. coli. Risk factors identified in these studies with proper definition of multidrug resistance alone are shown in Table 4. Urinary catheterization, hospitalization in Previous 12 months, UTI in previous 12 months, previous antibiotics, nursing home resident, both genders, diabetes mellitus, and older age were evaluated as risk factors. Older age was the only risk factor for MDR UTI that was identified by all three studies. 2 of the 3 studies assessed and agreed that urinary catheterization and previous antibiotics were a risk factor. All other risk factors had only 1 of the 3 studies evaluating it or there was a disagreement between studies.
Table 4.
Risk factors assessment analysis of studies using resistance definition from 2011 international consensus panel’s expert proposal for interim standard definitions for acquired resistance (resistance to 3 or more different drug classes with 1 or more antimicrobials in each class).
| Number of studies positive for risk factor/number of studies assessing risk factor (n/n) | Number of patients in studies positive for risk factor/number of patients assessed for risk factor (n/n) | |
|---|---|---|
| Urinary Catheter | 2/2 | 21,570/21,570 |
| Hospitalization in Previous 12 months | 0/1 | 0/156 |
| UTI in Previous 12 months | 1/2 | 156/508 |
| Previous Antibiotics | 2/2 | 508/508 |
| Nursing Home Resident | 1/1 | 156/156 |
| Male Gender | 1/1 | 21,414/21,414 |
| Female Gender | 1/1 | 156/156 |
| Diabetes Mellitus | 1/1 | 156/156 |
| Older Age (>51 or >85 or “increasing age”) | 3/3 | 21,922/21,922 |
4. Discussion
The present review of studies identifying MDR UTI risk factors has brought several key points to light. The foremost being the need of standardization of definitions for a risk factor so that the data can be uniformly collected and translated into clinical practice recommendations. Secondly, this study shows that the most probable risk factors for developing a MDR UTI are urinary catheterization, previous hospitalization, previous antibiotics, and residence in a nursing home. This review also helps identify risk factors that require further research to determine if they should be an influencing factor when deciding empiric therapy for a UTI.
The American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) released a joint guideline on the Management of Adults with Hospital-acquired, Ventilator-associated Pneumonia in 2016 (Kalil et al., 2016). This guideline provides much more detail on what should be considered as a risk factor for multidrug resistance. Although many risk factors have been assessed, the guideline lists the following as risk factors for multidrug-resistant pathogens as the causative pathogen for the hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP): intravenous antimicrobial use within 90 days, septic shock at time of VAP, acute respiratory distress syndrome (ARDS), current hospitalization of 5 days or more, and acute renal replacement therapy prior to VAP onset. Guidelines such as these with clear definitions of what should be considered a risk factor are useful tools in helping healthcare personnel decide when broader empiric therapy should be utilized for patients. Many of the risk factors listed in these guidelines are similar to the risk factors identified in our study, however due to a different anatomical site some differences can also be seen. As an example, it is unlikely that ARDS correlates with a MDR UTI as it does with a MDR pneumonia.
The Centers for Disease Control and Prevention developed guidelines published in 2009 for the prevention of catheter-associated urinary tract infections (CAUTI) (Gould et al., 2010). These guidelines provide recommendations to help minimize the occurrence of CAUTIs. Less total UTIs will lead to less MDR UTIs. General care measures outlined in the guidelines such as minimizing urinary catheter use and duration as well as aseptic insertion by trained personnel should be followed. 11 out of 14 of the studies that assessed urinary catheterization as a risk factor provided evidence that urinary catheters not only increase the risk of developing a UTI, but increase the risk of that UTI being Multidrug-resistant.
The IDSAs guidelines in conjunction with the European Society for Microbiology and Infectious Diseases on the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women released in 2010 is not as detailed on what may be considered a risk factor in dealing with genitourinary infections. These guidelines list 2 risk factors for sulfamethoxazole-trimethoprim resistant UTIs: the usage of sulfamethoxazole-trimethoprim within the last 3–6 months, and travel outside the United States in the previous 3–6 months based on the results of 2 studies for each of these 2 risk factor (Hooton et al., 2010). Also the IDSA’s guidelines on the diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults only discusses risk factors for developing a UTI and does not address risk factors for developing a UTI that is multidrug-resistant (Gupta et al., 2011). The lack of comprehensive evaluation of risk factors in these genitourinary tract related may be clarified by the results of our study.
The negative results identified in the study also provide some useful information as well. Despite having three studies (n = 752) that show female gender as a risk factor for developing an MDR UTI, there are 10 studies (n = 26,501) that provide evidence that female gender is not a risk factor for developing a MDR UTI.
Many of the evidence-based data on risk factors have been collected from retrospective observational studies, which cannot distinguish causation from noncausal association. The quality assessment of the studies showed very consistent results, this may provide supporting information to minimize the possibility that the data is skewed by several large studies of varying quality. The frequency of specific MDR pathogens causing a UTI may vary by hospital, patient population, exposure to antibiotics, type of ICU patient, and changes over time, emphasizing the need for timely, local surveillance data. When local studies are conducted standardized, common definitions should be utilized so the data can be better generalized to other populations.
Our study shows a recent increase in the number of studies reporting risk factors for multidrug-resistant urinary tract infections. Only the United States and South Korea have published more than 1 study reporting risk factors for MDR UTI in their countries. The biggest study was a retrospective cohort done in the United Kingdom with 21,414 patients. This study alone accounts for 68.5% of the patients with positive cultures included in our study (Toner et al., 2015).
A major concern identified through our study was the lack of standardization of definitions. For example, when looking at previous antibiotic exposure, the definition varied from any time within their life for one study, to within the last 48 hours for another study. The most common time window considered was within the past 3 months, utilized by only 25% of the studies that assessed this risk factor. 75% of the studies and 70.4% of the patients in these studies used other variable time frames. The other aspect lacking a consistent definition was that of immunosuppression. If evaluating immunosuppression as a risk factor studies should consider account for various forms of immunosuppression which may have more severe immunosuppression such as active hematologic malignancy, transplantation, immunosuppressive therapy, chemotherapy, or radiotherapy or a more moderate to mild immunosuppression such as chronic systemic steroid therapy (prednisone ≥25 mg/day), active solid malignancy, splenectomy, or autoimmune disease.
Four of the risk factors need to have clear definitions developed: Age and previous hospitalization, antibiotic usage, and urinary tract infection. Ages used varied greatly among the 19 studies that assessed age as a risk factor. 10 of the 19 studies used age thresholds of greater than or equal to 50–65 years. 8 of the 10 studies including 4380 patients that used age range threshold of greater than or equal to anywhere between 50–65 supported that advanced age is a risk factor for developing a MDR UTI. Although the ATS pneumonia guidelines consider antibiotics within the previous 3 months, only 5 of the 20 studies that assessed previous antibiotics as a risk factor MDR UTI used 3 months in the definition of previous antibiotics (Kalil et al., 2016). For previous hospitalization, the ATS guidelines again use 3 months or 90 days as part of their definition. Studies included in our literature review used various durations again with 5 studies using the previous 12 months and 3 studies used 3 months and 1 month each. Lastly, Previous UTI had a much more uniform definition of time frame evaluated utilized. Of the 15 studies, 11 considered previous UTI’s in the past 12 months. Future studies should consider using this as their time frame to support and be uniform with the current literature.
The definition of multidrug resistance varied greatly as well. Only three of the studies followed the 2011 international consensus panel’s expert proposal for interim standard definitions for acquired resistance (Gupta et al., 2011). MDR is defined as resistance to greater than or equal to 3 classes and where resistance to a class is defined as greater than or equal to 1 resistant agent within that class. Although only 11 of the studies were published after the expert panel consensus was published.
Similar to other reviews, several limitations in present review should be of concern. Firstly, only studies published in English were included. Secondly, definitions used throughout the studies varied greatly limiting the ability to make clear recommendations and implementing data from large ranges into clinical practice. As an example, the definition of previous antibiotic usage ranged from 2 to 365 days. Previous antibiotic usage is very likely a risk factor, but it is unknown where within this range the cutoff should be (i.e. 1 month or 3 months). Thirdly, this review is based on published articles, and publication bias may affect the results. Also, this review showed that there is an increasing trend in the number of publications regarding risk factors for a MDR UTI. This supports that the information gained from this review may quickly become outdated and another review may be required in the future. Lastly, the study has significant geographical difference that significantly decrease the generalizability of the study as resistance patterns and antibiotic usage rates vary based on geographic location.
5. Conclusion
Clear, universal definitions need to be used utilized in future studies when identifying risk factors for multidrug-resistant urinary tract infections. The risk factors with the most supporting data include previous hospitalization, previous antibiotic usage, urinary catheterization, and residence in a nursing home facility.
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Disclosures
All involved authors have approved this article and agree to this final draft.
Acknowledgements
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Allen U.D., MacDonald N., Fuite L., Chan F., Stephens D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ. 1999;160(10):1436–1440. [PMC free article] [PubMed] [Google Scholar]
- Anthony, J.S., 2002. Infections of the urinary tract. Campbell's Urology, eighth ed. pp. 515–602.
- Arslan H., Azap O.K., Ergonul O., Timurkaynak F. Risk factors for ciprofloxacin resistance among Escherichia coli strains isolated from community-acquired urinary tract infections in Turkey. J. Antimicrob. Chemother. 2005;56:914–918. doi: 10.1093/jac/dki344. [DOI] [PubMed] [Google Scholar]
- Brown P.D., Freeman A., Foxman B. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin. Infect. Dis. 2002;34:1061–1066. doi: 10.1086/339491. [DOI] [PubMed] [Google Scholar]
- Burman W.J., Breese P.E., Murray B.E., Singh K.V., Batal H.A., MacKenzie T.D. Conventional and molecular epidemiology of trimethoprim-sulfamethoxazole resistance among urinary Escherichia coli isolates. Am. J. Med. 2003;115:358–364. doi: 10.1016/s0002-9343(03)00372-3. [DOI] [PubMed] [Google Scholar]
- Colgan R., Johnson J.R., Kuskowski M., Gupta K. Risk factors for trimethoprim-sulfamethoxazole resistance in patients with acute uncomplicated cystitis. Antimicrob. Agents Chemother. 2008;52:846–851. doi: 10.1128/AAC.01200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodner R., Rock W., Chazan B., Keller N., Guy N., Sakran W. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur. J. Clin. Microbiol. Infect. Dis. Official Publ. Eur. Soc. Clin. Microbiol. 2004;23(3):163–167. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- Ena J., Amador C., Martínez C., Ortiz de la Tabla V. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin-resistant Escherichia coli. J. Urol. 1995;153:117–120. doi: 10.1097/00005392-199501000-00040. [DOI] [PubMed] [Google Scholar]
- Eshetie S., Unakal C., Gelaw A., Ayelign B., Endris M., Moges F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob. Resist. Infect. Control. 2015;17(4):12. doi: 10.1186/s13756-015-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faine B.A., Harland K.K., Porter B., Liang S.Y., Mohr N. A clinical decision rule identifies risk factors associated with antimicrobial-resistant urinary pathogens in the emergency departmenta retrospective validation study. Ann. Pharmacother. 2015;49(6):649–655. doi: 10.1177/1060028015578259. [DOI] [PubMed] [Google Scholar]
- Gangcuangco L.M., Alejandria M., Henson K.E., Alfaraz L., Ata R.M., Lopez M. Prevalence and risk factors for trimethoprim-sulfamethoxazole-resistant Escherichia coli among women with acute uncomplicated urinary tract infection in a developing country. Int. J. Infect. Dis. 2015;34:55–60. doi: 10.1016/j.ijid.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Gould C.V., Umscheid C.A., Agarwal R.K., Kuntz G., Pegues D.A. Guideline for prevention of catheter-associated urinary tract infections. Infect. Control Hospital Epidemiol. 2010;31:319–326. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- Gupta K., Hooton M.H., Naber K.G., Wullt B., Colgan R., Miller L.G. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women a 2010 update by the infectious diseases society of America and the european society for microbiology and infectious diseases. Clin. Infect. Dis. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- Hertz, F.B., Schønning, K., Rasmussen, S.C., Littauer, P., Knudsen, J.D., Løbner-Olesen, A., et al. 2016. Epidemiological factors associated with ESBL- and non ESBL-producing E. coli causing urinary tract infection in general practice. Infect. Dis. (Lond). 48(3) 241–245. [DOI] [PubMed]
- Ho P.L., Wong R.C., Yip K.S., Loke S.L., Leung M.S., Mak G.C. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women emerging multidrug resistance phenotypes. Diagn. Microbiol. Infect. Dis. 2007;59:439–445. doi: 10.1016/j.diagmicrobio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Hooton T.M., Bradley S.F., Cardenas D.D., Colgan R., Geerlings S.E., Rice J.C. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults 2009 international clinical practice guidelines from the infectious diseases society of America. Clin. Infect. Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- Ikram R., Psutka R., Carter A., Priest P. An outbreak of multidrug resistant Escherichia coli urinary tract infection in an elderly population a case-control study of risk factors. BMC Infect. Dis. 2015;9:224. doi: 10.1186/s12879-015-0974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoon R.J., Jalal-ud-Din M., Khan S.A. E. coli resistance to ciprofloxacin and common associated factors. J. Colloids Phys. Surg. Pak. 2015;25(11):824–827. [PubMed] [Google Scholar]
- Johnson L., Sabel A., Burman W.J., Everhart R.M., Rome M., MacKenzie T.D. Emergence of fluoroquinolone resistance in outpatient urinary Escherichia coli isolates. Am. J. Med. 2008;121:876–884. doi: 10.1016/j.amjmed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Kalil A.C., Metersky M.L., Klompas M., Muscedere J., Sweeney D.A., Palmer L.B. Management of adults with hospital-acquired and ventilator-associated pneumonia 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 2016;63:e61. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.S., Lee B.S., Lee H.J., Hwang S.W., Han Z.A. Prevalence of and risk factors for multidrug-resistant bacteria in urine cultures of spinal cord injury patients. Ann. Rehabil. Med. 2015;39(5):686–695. doi: 10.5535/arm.2015.39.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawcharoenporn, T., Vasoo, S., Singh, K., 2013. Urinary tract infections due to multidrug-resistant enterobacteriaceae prevalence and risk factors in a chicago emergency department. Emergency Medicine International, vol. 2013, Article ID 258517. [DOI] [PMC free article] [PubMed]
- Killgore K.M., March K.L., Guglielmo B.J. Risk factors for community-acquired ciprofloxacin-resistant Escherichia coli urinary tract infection. Ann. Pharmacother. 2004;38(7–8):1148–1152. doi: 10.1345/aph.1D622. [DOI] [PubMed] [Google Scholar]
- Klevens R.M., Edwards, J.R., Richards, Jr. C.L., Horan, T.C., Gaynes, R.P., Pollock, D.A., et al., 2002. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, Public Health Rep. 2007 Mar-Apr 122(2). pp. 160–166. [DOI] [PMC free article] [PubMed]
- Lee G., Cho Y.H., Shim B.S., Lee S.D. Risk factors for antimicrobial resistance among the Escherichia coli strains isolated from Korean patients with acute uncomplicated cystitis a prospective and nationwide study. J. Korean Med. Sci. 2010;25:1205–1209. doi: 10.3346/jkms.2010.25.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A. Multistate point-prevalence survey of health care-associated infections, 2011. New England J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Metlay J.P., Strom B.L., Asch D.A. Prior antimicrobial drug exposure a risk factor for trimethoprim-sulfamethoxazole-resistant urinary tract infections. J. Antimicrob. Chemother. 2003;51(4):963–970. doi: 10.1093/jac/dkg146. Epub 2003 Feb 25. [DOI] [PubMed] [Google Scholar]
- O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Appl. Eng. Agric. 2002;18:585–590. [Google Scholar]
- Osthoff, M., Mcguinness, S.L., Wagen, A.Z., Eisen, D.P., 2015. Urinary tract infections due to extended-spectrum beta lactamase-producing gram-negative bacteria identification of risk factors and outcome predictors in an Australian tertiary-referral hospital. Int. J. Infect. Dis. pii S1201-9712(15)00067-3. [DOI] [PubMed]
- Shaikh S., Fatima J., Shakil S., Rizvi S.M.D., Kamal M.A. Antibiotic resistance and extended spectrum beta-lactamasestypes, epidemiology and treatment. Saudi J. Biol. Sci. 2015;22(1):90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talan D.A., Krishnadasan A., Abrahamian F.M., Stamm W.E., Moran G.J. Group EMINS. Prevalence and risk factor analysis of trimethoprim-sulfamethoxazole- and fluoroquinolone-resistant Escherichia coli infection among emergency department patients with pyelonephritis. Clin. Infect. Dis. 2008;47:1150–1158. doi: 10.1086/592250. [DOI] [PubMed] [Google Scholar]
- Toner L., Papa N., Aliyu S.H., Dev H., Lawrentschuk N., Al-Hayek S. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospital urinary tract infections incidence and antibiotic susceptibility profile over 9 years. World J. Urol. 2015 doi: 10.1007/s00345-015-1718-x. [DOI] [PubMed] [Google Scholar]
- Wright S.W., Wrenn K.D., Haynes M.L. Trimethoprim-sulfamethoxazole resistance among urinary coliform isolates. J. Gen. Int. Med. 1999;14(10):606–609. doi: 10.1046/j.1525-1497.1999.10128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.B. Prevalence and risk factors for highly resistant microorganisms in urinary isolates from newly admitted patients in the National Rehabilitation Center, Korea. J. Rehabil. Med. 2014;46(8):814–818. doi: 10.2340/16501977-1835. [DOI] [PubMed] [Google Scholar]