Abstract
Periodontitis is characterized by inflammation of the periodontium and leads to loss of teeth if untreated. Although a number of surgical and pharmacological options are available for the management of periodontitis, it still affects a large proportion of population. Recently, metformin (MF), an oral hypoglycemic, has been used to treat periodontitis. The aim of this review is to systematically evaluate the efficacy of MF in the treatment of periodontitis. An electronic search was carried out using the keywords ‘metformin’, ‘periodontal’ and ‘periodontitis’ via the PubMed/Medline, ISI Web of Science and Google Scholar databases for relevant articles published from 1949 to 2016. The addressed focused question was: ‘Is metformin effective in reducing bone loss in periodontitis? Critical review and meta-analysis were conducted of the results obtained in the selected studies. Following the removal of the duplicate results, the primary search resulted in 17 articles and seven articles were excluded based on title and abstract. Hence, 10 articles were read completely for eligibility. After exclusion of four irrelevant studies, six articles were included. The topical application of MF resulted in improved histological, clinical and radiographic outcomes. Additionally, results from the meta-analysis indicated that application of metformin improved the clinical and radiographic outcomes of scaling and root-planing, but at the same time heterogeneity was evident among the results. However, because of a lack of histological and bacterial studies, in addition to short follow-up periods and risk of bias, the long-term efficacy of MF in the treatment of bony defects is not yet ascertained. Further studies are needed to envisage the long-term efficacy of MF in the management of periodontitis.
Keywords: Metformin, Periodontitis, Systematic review, Periodontal bone loss, Treatment of periodontitis
1. Introduction
Periodontitis, the inflammation of the periodontium, affects 40–90% of the global population (Pihlstrom et al., 2005). In the United States of America, it has been estimated that 46% of the adult population is affected by periodontitis (Eke et al., 2012). Periodontitis is characterized by the progressive destruction of cementum, periodontal ligament and alveolar bone and subsequently, loss of teeth (Socransky and Haffajee, 2005, Newman et al., 2012). A number of factors contribute to periodontitis. Local factors such as poor oral hygiene and use of tobacco are found to be implicated in periodontal disease (Newman et al., 2012) and tobacco (Winn, 2001, Fahad et al., 2015). Systemic conditions such as malnutrition (Najeeb et al., 2016a), diabetes, cardiovascular disease, infections (Newman et al., 2012) and pregnancy (Naseem et al., 2016, Trivedi et al., 2015) may also lead to manifestation of periodontitis. Lack of oral hygiene leads in the formation of dental plaque (Al-Otaibi et al., 2003, Niazi et al., 2016), a biofilm containing bacteria, lymphocytes, neutrophils, leuokocytes and macrophages. Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Bacteroides forsythus, Prevotella intermedia, Campylobacter rectus, Eubacterium nodatum, Streptococcus intermedius and Treponema denticola are the major bacteria that have been implicated in periodontitis (Socransky and Haffajee, 2005, Socransky et al., 1998, Haffajee et al., 1983). The presence of bacteria leads to an exaggerated immune response which leads to destruction of periodontal tissues. This destruction of tissues triggers a release of cytokines from immune cells which promote inflammation. Secretion of these proinflammatory cytokines, for example IL-1β, is linked to the production of collagenase and prostaglandin E which play a part in bone destruction (Richards and Rutherford, 1988). Management of chronic periodontitis mainly involves the removal of the causative factors and to repair, regenerate and maintain periodontal tissues. Scaling and root planing (SRP) is mainly used to remove plaque and calculus (Cugini et al., 2000). In SRP, debridement of supra- and subgingival plaque and calculus is carried out by ultrasonic and hand instruments, followed by smoothing of the exposed root surfaces. In some instances, SRP may be augmented by other treatments such as guided tissue regeneration, bone grafting and flap-procedures. Following SRP, antibiotics may be prescribed to treat the infection but may cause adverse effects (Slots, 2002). In addition, guided tissue regeneration aims to regenerate bone by means of placing barrier membranes along with bone grafts and substitutes (Sheikh et al., 2015, Bottino et al., 2012). If the cause is systemic, measures should be taken to improve the overall health. Recently a number of agents such as melatonin (Najeeb et al., 2016b, Arabacı et al., 2015), platelet rich fibrin (Najeeb et al., 2017, Agarwal et al., 2016, Pradeep et al., 2015) and metformin (Pradeep et al., 2015, Pradeep et al., 2016) have been explored for the cure of periodontitis.
Metformin (1, 1-dimethylbiguanide) HCl (MF) is a second-generation biguanide, derived from French lilac (Galega officinalis), used to manage type 2 diabetes mellitus (Witters, 2001). As an orally administered anti-hyperglycemic drug, it decreases blood glucose levels by inhibiting gluconeogesis (production of glucose) in the liver (UK Prospective Diabetes Study (UKPDS) Group, 1998). It has been suggested that MF inhibits intracellular binding of calcium at the mitochondria in the hepatocytes to decrease gluconeogenesis (Shaw et al., 2005). Moreover, MF is the drug of choice to treat type 2 diabetes mellitus in obese patients (Giugliano et al., 1993). Studies have also shown favorable effect of MF on bone formation. There are two mechanisms of action that have been suggested for the osteogenic effect of MF: increased proliferation of osteoblasts and reduction of osteoclast activity. Studies indicate that after MF is taken up by osteoblasts, their proliferation is incrased (Bak et al., 2010, Cortizo et al., 2006). In another study was observed that exposure to MF led to a decrease of osteoclast and bone formation. MF down-regulates the production of receptor activator of nuclear factor kappa B ligand (RANKL) and up-regulates the production of osteoprotegerin (OPG) from osteoblasts (Liu et al., 2012). This decreased RANKL/OPG ratio in turn decreases the osteoclast activity, thereby inducing bone formation and inhibiting bone resorption. More recently, MF has been used as an adjunct to surgical and non-surgical periodontal therapy to treat chronic periodontitis (Pradeep et al., 2015, Rao et al., 2013). Moreover, MF appears to augment the effect of platelet-rich fibrin in treating intrabony periodontal defects (Pradeep et al., 2015). To the authors’ best knowledge, there are no published systematic review studying the efficacy of MF therapy for treating periodontitis and related conditions. Hence, the aim of this review is to systematically evaluate the efficacy of MF in the treatment of periodontitis.
2. Methods
2.1. Focused question
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009), a focused question was constructed according to the Participants, Interventions, Control, Outcomes (PICO) principle (Boudin et al., 2010). The addressed focused question was: ‘Is metformin (MF) effective in reducing bone loss in periodontitis?’
2.2. Criteria for selection of studies
The inclusion criteria for this review were: (1) original studies published in the English language, (2) animal studies, (3) clinical studies and (4) Intervention: topical or systemic MF to treat periodontitis. Following types of studies were excluded: (1) historic reviews, (2) letters to the editor, (3) case series and reports and (4) cell culture studies.
2.3. Search methodology
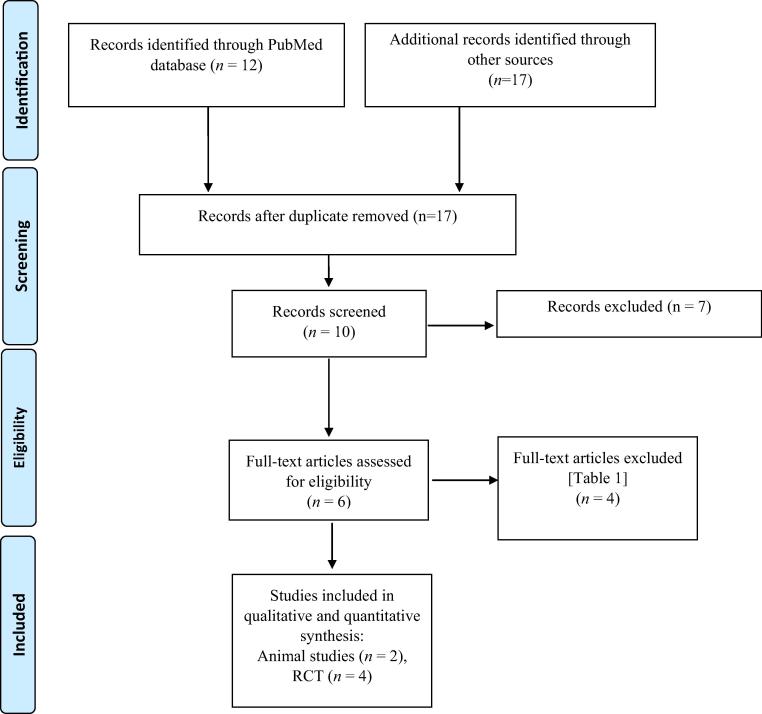
An electronic search was carried out using the keywords ‘metformin’, ‘periodontal’ and ‘periodontitis’ via the PubMed/Medline, ISI Web of Science and Google Scholar databases for relevant articles published from 1949 up to and including March 2016. The titles and abstracts of the articles found were read independently by two authors (S. N. and Z. K.). Both the authors checked for agreement via discussion. The references in the potentially relevant articles were read by both the authors to find additional studies which may not have been identified in the primary search following which the authors once again checked agreement via discussion. The search methodology is illustrated in the PRISMA flow diagram in Fig. 1.
Fig. 1.
Selection process of studies (PRISMA flow diagram) used in this review.
2.4. Quality assessment and meta-analysis
Each study was assessed for quality using the CONSORT principle by the two aforementioned reviewers (S. N. and Z. K.) (Moher et al., 2009). Additionally, meta-analysis was conducted using R and meta package (http://CRAN.R-project.org/package = meta; http://www.R-project.org). Due to differences in concentration of Metformin or control group used in the studies, only 3 RCTs were included in the meta-analysis.
3. Results
3.1. Search results
Following the removal of the duplicate search results, the primary search resulted in 17 articles in total. Seven articles were excluded based on title and abstract. Hence, remaining ten articles were read completely for eligibility. After exclusion of another four irrelevant studies (Han and You, 2010, Skamagas et al., 2008, González et al., 2012, Karageorgiou et al., 2014) as shown in Table 1, six studies (Pradeep et al., 2015, Pradeep et al., 2016, Bak et al., 2010, Liu et al., 2012, Rao et al., 2013, Pradeep et al., 2013) were included in this review.
Table 1.
List and reasoning of studies excluded from this review.
| Study (authors and year) | Reason for exclusion |
|---|---|
| Skamagas et al. (2008) | Review |
| Han and You (2010) | Chinese language |
| González et al. (2012) | Review |
| Karageorgiou et al. (2014) | Case report |
Two studies were animal studies (Bak et al., 2010, Liu et al., 2012), and remaining fours studies were human trials (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013) as listed in Table 2, Table 3, Table 4.
Table 2.
Characteristic features of animal studies included in this review.
| Study (authors& year) | Study design | Type of periodontal lesion(s) | Methods | Intervention |
Number of subject (n) | Follow-up | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Control | Test | |||||||
| Bak et al. (2010) | In vivo prospective | Periodontitis induced by ligature | Histological analysis, micro-CT analysis, cell assays | Saline | MF (IP, 10 mg/kg body weight) | 10 rats | 10 days | Significantly higher bone volume and less inflammation with MF. No effect of MF on osteoclasts and adipocytes. Higher number of osteoblasts with MF |
| Liu et al. (2012) | In vivo prospective | Periapical lesions inducted by pulpal exposure | Histological analysis, enzyme histo-chemistry, immunofluorescence labeling, immunohist-ochemistry, cell counting | Saline | MF (IM, 40 mg/kg body weight) | 40 rats | 28 days | RANKL/OPG ratio lower in test group. Significantly less osteoclasts seen in MF group |
CT, computer tomography; IM, intramuscular injection; IP, intraperitoneal injection; RANKL, receptor activator of nuclear factor kappa-B ligand; OPG, osteoprotegerin
Table 3.
General characteristics of the selected studies.
| Study, author and year | Study design | Number of patients | Number of smokers | Number of females | Number of IBDs included (n) | Age, mean & range (years) | Intervention and number of IBDs analysed |
Follow-up(weeks) | Main outcome at follow up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Test(n) | Control(n) | |||||||||
| Pradeep et al. (2013) | RCT | 41 | 0 | 21 | 118 | 37.2 (30–50) |
0.5% MF (26), 1% MF (27), 1.5% MF (29) | SRP + 0% MF (26) | 6 months | Clinical and radiographic parameters were significantly better in test groups than control. 1% MF provided highest improvement |
| Rao et al. (2013) | RCT | 50 | 50 | 0 | 71 | 34.6 (30–50) | 1% MF (36) | SRP + 0% MF (35) | 6 months | Clinical parameters and radiographic were significantly better in test groups than control |
| Pradeep et al. (2016) | RCT | 65 | 0 | 38 | 65 | 32.4 (25–50) | 1% MF (33) | SRP + 0% MF (32) | 6 months | Clinical parameters and radiographic were significantly better in test groups than control |
| Pradeep et al. (2015) | RCT | 120 | 0 | 68 | 120 | 41 (30–50) | OFD + PRF (30), OFD + 1% MF (30), OFD + PRF + 1%MF (30) | OFD (30) | 9 months | Clinical parameters and radiographic were significantly better in test groups than control. OFD + PRF+1% MF provided highest improvement |
IBDs, intrabony defects; MF, metformin gel; OFD, open-flap debridement; PRF, platelet-rich fibrin; RCT, randomized control trial; SRP, scaling and root planing.
Table 4.
Mean changes and clinical parameters reported by selected studies.
| Study (Authors and year) | PD (mm) Mean ± SD | RAL/CAL (mm) Mean ± SD | GML (mm) Mean ± SD | IBD depth (mm)Mean ± SD | IBD fill (%)Mean ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pradeep et al. (2013) | 0%MF + SRP | 1.77 ± 0.77 | 0%MF + SRP | 1.33 ± 0.66 | N/A | 0%MF + SRP | 1.19 ± 0.38 | 0%MF + SRP | 3.35 ± 8.36 | |
| 0.5%MF + SRP | 2.96 ± 0.93 | 0.5%MF + SRP | 2.23 ± 0.73 | N/A | 0.5%MF + SRP | 1.03 ± 0.20 | 0.5%MF + SRP | 21.11 ± 3.81 | ||
| 1%MF + SRP | 4.0 ± 1.05 | 1%MF + SRP | 3.83 ± 0.95 | N/A | 1%MF + SRP | 1.57 ± 0.57 | 1%MF + SRP | 31.89 ± 10.26 | ||
| 1.5%MF + SRP | 3.8 ± 1.03 | 1.5%MF + SRP | 3.6 ± 0.81 | N/A | 1.5%MF + SRP | 1.38 ± 0.69 | 1.5%MF + SRP | 26.8 ± 5.52 | ||
| Rao et al. (2013) | 0%MF + SRP | 0.87 ± 0.94 | 0%MF + SRP | 1.47 ± 0.78 | N/A | 0%MF + SRP | 0.22 ± 0.38 | 0%MF + SRP | 3.75 ± 8.06 | |
| 1%MF + SRP | 3.17 ± 0.75 | 1%MF + SRP | 3.27 ± 0.79 | N/A | 1%MF + SRP | 1.32 ± 0.4 | 1%MF + SRP | 26.17 ± 0.66 | ||
| Pradeep et al. (2016) | 0%MF + SRP | 2.0 ± 1.01 | 0%MF + SRP | 1.43 ± 0.97 | N/A | 0%MF + SRP | 0.23 ± 0.12 | 0%MF + SRP | 4.79 ± 2.3 | |
| 1%MF + SRP | 3.96 ± 0.66 | 1%MF + SRP | 4.06 ± 0.86 | N/A | 1%MF + SRP | 1.35 ± 0.34 | 1%MF + SRP | 26.17 ± 0.66 | ||
| Pradeep et al. (2015) | OFD | 3.00 ± 0.18 | OFD | 2.96 ± 0.18 | OFD | - 0.06 ± 0.04 | OFD | 0.49 ± 0.27 | OFD | 9.14 ± 0.004 |
| OFD + PRP | 4.00 ± 0.18 | OFD + PRP | 4.03 ± 0.18 | OFD + PRP | 0.27 ± 0.07 | OFD + PRP | 2.53 ± 0.30 | OFD + PRP | 48 ± 0.029 | |
| OFD + 1%MF | 3.93 ± 0.25 | OFD + 1%MF | 3.83 ± 0.25 | OFD + 1%MF | 0.27 ± 0.05 | OFD + 1%MF | 2.56 ± 0.28 | OFD + 1%MF | 48.69 ± 0.026 | |
| OFD + 1%MF + PRF | 4.90 ± 0.30 | OFD + 1%MF + PRF | 4.90 ± 0.30 | OFD + 1%MF + PRF | 0.33 ± 0.07 | OFD + 1%MF + PRF | 2.77 ± 0.3 | OFD + 1%MF + PRF | 52.65 ± 0.031 | |
CAL, clinical attachment level; GML, gingival marginal level; IBD, intrabony defect; MF, metformin gel; OFD, open flap debridement; PD, probing depth; SD, standard deviation; SRP, scaling and root planing.
3.2. Animal studies
Both the animal studies were in vivo prospective studies (Bak et al., 2010, Liu et al., 2012). The number of animals used as test subjects ranged from 10 to 40 (Bak et al., 2010, Liu et al., 2012). In one study, periapical periodontitis was induced by intentionally perforating the pulpal floor of the teeth (Liu et al., 2012) and in the other study, chronic periodontitis was induced by means of ligatures (Bak et al., 2010). MF was injected intraperitoneally (IP) to the periapical region in doses of 10 mg/kg body weight in one study (Bak et al., 2010) while it was administered to the animals via intramuscular (IM) injections in doses of 40 mg/kg body weight in the other study (Liu et al., 2012). The follow-up period ranged from 10 to 28 days (Bak et al., 2010, Liu et al., 2012). Saline was used as control in both the studies (Bak et al., 2010, Liu et al., 2012). The general characteristics of the animal studies are provided in Table 2.
3.3. Human studies
All human studies were randomized control trials (RCTs) (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). Number of patients included the studies ranged from 41 to 120 in which the number of female subjects ranged from 0 to 68 (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). Only one study included smokers which were 50 in number (Rao et al., 2013). The age of the patients ranged from 25 to 50 years (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). In all studies, MF gel was used in combination with other treatment modalities (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). Concentration of MF ranged from 0.5 to 1.5% (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). MF gel was injected into periodontal defects combination with scaling and root planing in 3 studies (SRP + MF) (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013) and was applied in combination with open-flap debridement (OFD) in one study (Pradeep et al., 2015). In one study, 1% MF was used in combination with platelet-rich fibrin (PRF) and OFD (1%MF + PRP + OFD) as intervention (Pradeep et al., 2015). Also, PRF was used as an adjunct to OFD in one study as intervention (OFD + PRF) (Pradeep et al., 2015). As controls, SRP combined with placebo gel (SRP + 0% MF) was used in 3 studies (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013) while in one study OFD was used (Pradeep et al., 2015). The follow-up period was 6 months in 3 studies (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013), while in one study the patients were followed-up for 9 months (Pradeep et al., 2015). A summary of the general characteristics of RCTs is provided in Table 3.
3.4. Assessment of parameters
In both animal studies, histological analysis, cell number and enzyme assays were used as primary parameters assessed (Bak et al., 2010, Liu et al., 2012). In one study, micro-CT analysis was also used (Bak et al., 2010). Mean changes in probing depth (PD), relative or clinical attachment level (RAL/CAL), intrabony defect depth (IBD depth) and intrabony defect fill (IBD fill) were measured by all human studies (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). Only one study measured mean changes in gingival marginal levels (GML) in addition to PD, RAL/CAL, IBD depth and IBD fill (Pradeep et al., 2015). Plaque index (PI) and modified sulcular bleeding index (mSBI) measured as a parameter of oral hygiene by all clinical studies (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). One study measured the level of MF in the gingival crevicular fluid (GCF), following treatment with 0.5%, 1% and 1.5% MF along with SRP, for a period of 28 days (Pradeep et al., 2013).
3.5. Main outcomes of the selected studies
Results from all 6 studies show that using MF is effective in reducing bone loss and improving outcomes of periodontal treatment (Pradeep et al., 2015, Pradeep et al., 2016, Bak et al., 2010, Liu et al., 2012, Rao et al., 2013, Pradeep et al., 2013). In both animal studies, IP and IM injections of MF led to more favorable results when compared to saline controls (Bak et al., 2010, Liu et al., 2012). IP injections of MF in doses of 10 mg/kg body weight resulted in a significantly higher number of osteoblasts and reduced bone loss but did not have significant effect on the number of adipocytes and osteoclasts (Bak et al., 2010). On the other hand, IM injections of MF in doses of 40 mg/kg body weight resulted in lower RANKL/OPG ratios and reduced number of osteoclasts in addition to diminished bone loss (Liu et al., 2012). In clinical studies, 1%MF + SRP and 1%MF + OFD resulted in better clinical and radiographic outcomes than SRP and OFD alone (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). 1%MF + SRP resulted in better outcomes and higher MF levels in GCF than 0.5%MF + SRP and 1.5%MF + SRP (Pradeep et al., 2013). Additionally, OFD + 1%MF + PRF resulted in significantly higher improvement in parameters than PRF + OFD, 1%MF + OFD and OFD (Pradeep et al., 2015). After 6 month follow-up, the highest IBD fill was demonstrated by 1%MF + SRP (26.1–31.89%) (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013) and the lowest was shown with 0%MF + SRP (4.79–3.35%) (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). After 9 month follow-up, the highest IBD fill was exhibited with OFD + 1%MF + PRF (52.65%) and the lowest IBD fill was seen with OFD (9.14%) (Pradeep et al., 2015). In three studies, when compared to SRP alone, application of MF along with SRP led to higher improvement in pocket depth (PD). The improvement in PD ranged from 2.96 mm to 4,0 mm (Pradeep et al., 2015, Rao et al., 2013, Pradeep et al., 2013). Additionally, addition of MF application to OFD also resulted in improved PD when compared to OFD alone in one study (Pradeep et al., 2015). The highest improvement in PD was exhibited by application of 1%MF and PRF along with OFD (Pradeep et al., 2015). In all studies, application of MF to surgical or non surgical periodontal therapy resulted in improvement IBD depth and IBD fill. The highest improvement in IBD depth and IBD fill was observed to be 2.77 mm and 52.6% respectively following application of 1%MF and PRF with OFD (Pradeep et al., 2015). The mean changes in clinical parameters are summarized in Table 4.
3.6. Meta-analysis
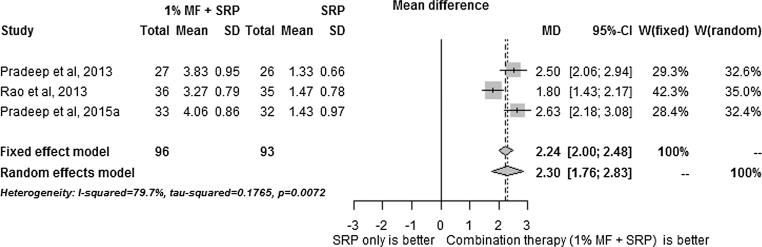
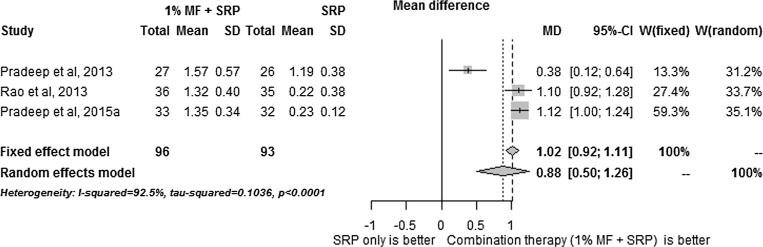
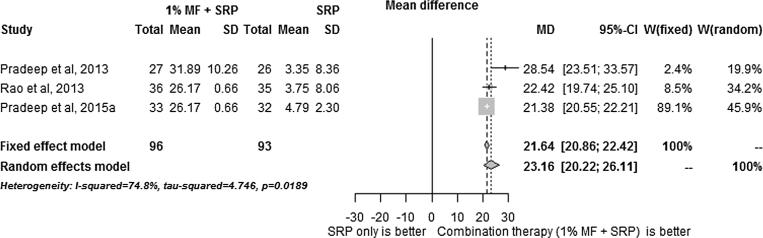
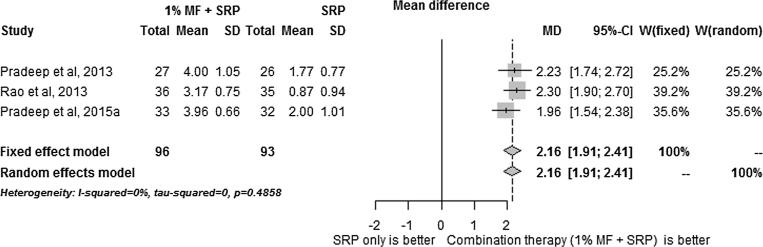
The results of the meta-analysis results are summarized in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5. SRP + 1% MF resulted in significantly higher improvements in all assessed clinical parameters. However, the I2 statistic values for CAL/RAL, IBD fill and IBD were higher than 50, indicating heterogeneity among the results (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5).
Fig. 2.
Meta-analysis reporting changes in clinical/relative attachment levels.
Fig. 3.
Meta-analysis reporting changes in intra-bony defect fill.
Fig. 4.
Meta-analysis reporting changes in intra-bony defect depth.
Fig. 5.
Meta-analysis reporting changes in pocket depth.
On the other hand, since meta-analysis of PD values demonstrated I2 statistic of less than 50, 1% MF application following SRP was found to have the highest impact on PD compared to other clinical parameters.
4. Discussion
In all of the six studies analyzed in this review, results showed that administration of MF, in either topical or systemic form, reduced bone resorption (Pradeep et al., 2015, Pradeep et al., 2016, Bak et al., 2010, Liu et al., 2012, Rao et al., 2013, Pradeep et al., 2013). However, only one animal study investigated the systemic administration of MF in animals (Liu et al., 2012) and none of the clinical trials investigated the effect of systemic administration of MF on periodontal bone loss in humans. Nevertheless, in 100% of the RCTs, local intrabony administration of MF following SRP resulted in better clinical and radiographic parameters compared to SRP + 0% MF (Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). In one study, OFD + 1%MF + PRF resulted in significantly better outcomes than OFD, PRF + OFD and 1% MF + OFD, indicating that MF augments the regenerative effects of PRF (Pradeep et al., 2015).
The effect of MF on osteoblasts in vitro was revealed previously (Cortizo et al., 2006, Kanazawa et al., 2008). In a study by Cortizo et al., exposing osteoblast-like cell lines to MF increased proliferation of the cells as well as higher alkaline phosphatase activity and an increased collagen-II turn over were observed (Cortizo et al., 2006). Moreover, MF induced formation of mineralized bone tissue for up to 3 weeks. In a study by Kanazawa et al., osteoblast-like cells cultured with MF exhibited increased proliferation by the activation of AMP-activated protein kinase (AMPK) (Kanazawa et al., 2008). Additionally, the cells increased expressions of endothelial nitric oxide synthase (eNOS) and bone morphogenetic protein-2 (BMP-2) were detected. eNOS has a vital role in maintaining and controlling bone turnover (Chambliss and Shaul, 2002, Armour et al., 2001). BMP-2 is also known to increase osteoblast differentiation and bone formation (Govender et al., 2010). Hence, results obtained in in vitro studies indicate that MF has an osteogenic effect which is triggered by the increased proliferation of osteoblasts.
The animal studies showed that MF has, both, a stimulatory effect on osteoblasts and an inhibitory effect on osteoclasts (Bak et al., 2010, Liu et al., 2012). In the 10-day study by Bak et al. using periodontitis model, IP injections of MF stimulated the proliferation of osteoblasts with little effect on osteoclasts and adipocytes (Bak et al., 2010). These results are similar to the increased osteoblast proliferation observed in the aforementioned in vitro studies (Cortizo et al., 2006, Kanazawa et al., 2008). However, in the 28-day study by Liu et al., MF had negligible effect on osteoblasts but decreased RANKL/OPG ratios and, hence, inhibited proliferation of osteoclasts (Liu et al., 2012). Hence, to date, the mode of action of MF is unclear and more long-term studies are needed to better-understand the mechanism of osteogenic effect of the drug.
MF has been known to prevent fractures in diabetic patients, indicating its osteogenic effect similar to that observed in animal models and in vitro studies (Rejnmark, 2008, Vestergaard et al., 2005). Hence, it is not surprising that intrabony injections of MF in a gel carrier augments the effect of surgical and non-surgical periodontal therapy and leads to increased IBD fill and reduced IBD as well as significant improvements in RAL/CAL, PD and GML (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). According to results obtained in the study by Pradeep et al., 1% MF is the optimal concentration in the management of periodontitis (Pradeep et al., 2013). It has also been observed that MF and PRF are more effective in improving outcomes when placed in intrabony defects following OFD than PFD, PRF or MF alone, suggesting that MF may augment the effect of PRF (Pradeep et al., 2015). PRF is autologous plasma derivative containing fibrin and growth factors which has been previously used to restore intrabony defects due to its regenerative and space maintaining effects (Pradeep et al., 2012). Combining MF with currently available GTR materials and scaffolds may provide additional effective options to manage periodontitis. However, more research is warranted to explore this hypothesis.
It is known that smoking has an adverse effect on periodontal health (Fiorini et al., 2014). Based on the results obtained in the study by Rao et al., MF may prove to be a potent drug for improving outcomes following SRP in smokers (Rao et al., 2013). Because smoking has a negative effect on the outcomes of periodontal regenerative therapy as well (Patel et al., 2012), the effect of MF on the outcomes of implant therapy, GTR and prosthodontic therapy in smokers needs to be investigated.
As shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, metaanalysis carried on the human studies in this reviewed indicates that MF increases the efficacy of surgical and non-surgical periodontal therapy. However, the I2 analysis showed that there was heterogenicity among the results obtained in studies. The RCTs included in this study have additional; limitations. The patients were followed-up for more than 9 months (Pradeep et al., 2015, Pradeep et al., 2016, Rao et al., 2013, Pradeep et al., 2013). Therefore, the long-term efficacy of MF in improving the outcomes of periodontal disease is unknown. Furthermore, none of studies included histological analysis or bacterial studies which are crucial for accurate assessment of attachment loss (Grossi et al., 1995). Hence, it is unknown what effect MF has on human periodontal tissues at the microscopic level and on the bacterial flora of the periodontal pockets. Moreover, only one study included smokers (Rao et al., 2013) and none of the studies included diabetic patients. Hence, studies focusing on the long-term outcome of MF on smokers and diabetic patients need to be conducted.
5. Conclusion
Metformin is an effective medicament in improving the outcomes of surgical and non-surgical periodontal therapy. However, owing to the lack of histological and bacterial studies and short follow-up periods of reported studies, there is risk of bias in the RCTs and long-term efficacy of metformin in the treatment of intra bony defects is not yet ascertained. Further research is required to envisage long-term efficacy of metformin for chronic periodontitis management.
Acknowledgments
Acknowledgements
None.
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal A., Gupta N.D., Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontol. Scand. 2016;74:36–43. doi: 10.3109/00016357.2015.1035672. [DOI] [PubMed] [Google Scholar]
- Al-Otaibi M., Al-Harthy M., Soder B., Gustafsson A., Angmar-Mansson B. Comparative effect of chewing sticks and toothbrushing on plaque removal and gingival health. Oral Health. Prev. Dent. 2003;1:301–307. [PubMed] [Google Scholar]
- Arabacı T., Kermen E., Özkanlar S., Köse O., Kara A., Kızıldağ A., Duman Ş.B., Ibişoğlu E. Therapeutic effects of melatonin on alveolar bone resorption after experimental periodontitis in rats: a biochemical and immunohistochemical study. J. Periodontol. 2015;86:874–881. doi: 10.1902/jop.2015.140599. [DOI] [PubMed] [Google Scholar]
- Armour K.E., Armour K.J., Gallagher M.E., Gödecke A., Helfrich M.H., Reid D.M., Ralston S.H. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase** This study was supported by grants from the arthritis research campaign (UK) and the medical research council (UK) Endocrinology. 2001;142:760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- Bak E.J., Park H.G., Kim M., Kim S.W., Kim S., Choi S., Cha J., Yoo Y. The effect of metformin on alveolar bone in ligature-induced periodontitis in rats: a pilot study. J. Periodontol. 2010;81:412–419. doi: 10.1902/jop.2009.090414. [DOI] [PubMed] [Google Scholar]
- Bottino M.C., Thomas V., Schmidt G., Vohra Y.K., Chu T.G., Kowolik M.J., Janowski G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration – a materials perspective. Dental Mater. 2012;28:703–721. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Boudin F., Nie J., Bartlett J.C., Grad R., Pluye P., Dawes M. Combining classifiers for robust PICO element detection. BMC Med. Inform. Decision Making. 2010;10:1. doi: 10.1186/1472-6947-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss K.L., Shaul P.W. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002;23:665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Cortizo A.M., Sedlinsky C., McCarthy A.D., Blanco A., Schurman L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur. J. Pharmacol. 2006;536:38–46. doi: 10.1016/j.ejphar.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Cugini M., Haffajee A., Smith C., Kent R., Socransky S. The effect of scaling and root planing on the clinical a microbiological parameters of periodontal diseases: 12-month results. J. Clin. Periodontol. 2000;27:30–36. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- Eke P.I., Dye B.A., Wei L., Thornton-Evans G., Genco R.J. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Fahad K., Aziz A., Shahab S., Zafar M. Laboratorial and clinical impacts of tobacco on periodontal health: a systematic review. Int. Dental J. Student’s Res. 2015;3:72–78. [Google Scholar]
- Fiorini T., Musskopf M.L., Oppermann R.V., Susin C. Is there a positive effect of smoking cessation on periodontal health? A systematic review. J. Periodontol. 2014;85:83–91. doi: 10.1902/jop.2013.130047. [DOI] [PubMed] [Google Scholar]
- Giugliano D., De Rosa N., Di Maro G., Marfella R., Acampora R., Buoninconti R., D'Onofrio F. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care. 1993;16:1387–1390. doi: 10.2337/diacare.16.10.1387. [DOI] [PubMed] [Google Scholar]
- González O.A., Tobia C., Ebersole J.L., Novak M.J. Caloric restriction and chronic inflammatory diseases. Oral Dis. 2012;18:16–31. doi: 10.1111/j.1601-0825.2011.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender S., Csimma C., Genant H. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures. Orthopedic Trauma Direct. 2010;8:31–37. [Google Scholar]
- Grossi S.G., Genco R.J., Machtet E.E., Ho A.W., Koch G., Dunford R., Zambon J.J., Hausmann E. Assessment of risk for periodontal disease. II. risk indicators for alveolar bone loss*. J. Periodontol. 1995;66:23–29. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- Haffajee A.D., Socransky S.S., Goodson J.M. Clinical parameters as predictors of destructive periodontal disease activity. J. Clin. Periodontol. 1983;10:257–265. doi: 10.1111/j.1600-051x.1983.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Han B., You L. Effect of initial periodontal therapy on diabetic patients with chronic periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2010;45:282–286. [PubMed] [Google Scholar]
- Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem. Biophys. Res. Commun. 2008;375:414–419. doi: 10.1016/j.bbrc.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Karageorgiou I., Chandler C., Whyte M.B. Silent diabetes mellitus, periodontitis and a new case of thalamic abscess. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhang C., Hu Y., Peng B. Protective effect of metformin on periapical lesions in rats by decreasing the ratio of receptor activator of nuclear factor kappa B ligand/osteoprotegerin. J. Endod. 2012;38:943–947. doi: 10.1016/j.joen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- Najeeb S., Khurshid Z., Agwan M.A.S., Ansari S.A., Zafar M.S., Matinlinna J.P. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: a systematic review of clinical studies. Tissue Eng. Regene. Med. 2017;14:735–742. doi: 10.1007/s13770-017-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najeeb S., Zafar M.S., Khurshid Z., Zohaib S., Almas K. The role of nutrition in periodontal health: an update. Nutrients. 2016;8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najeeb S., Khurshid Z., Zohaib S., Zafar M.S. Therapeutic potential of melatonin in oral medicine and periodontology. Kaohsiung J. Med. Sci. 2016;32:391–396. doi: 10.1016/j.kjms.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem M., Khurshid Z., Khan H.A., Niazi F., Zohaib S., Zafar M.S. Oral health challenges in pregnant women: recommendations for dental care professionals. Saudi J. Dental Res. 2016;7:138–146. [Google Scholar]
- Newman, Michael G., Takei, Henry H., Klokkevold, Perry R., Carranza,Fermín A., 2012. “Carranza's clinical periodontology”.
- Niazi F., Naseem M., Khurshid Z., Zafar M.S., Almas K. Role of Salvadora persica chewing stick (miswak): a natural toothbrush for holistic oral health. Eur. J. Dentist. 2016;10:301–308. doi: 10.4103/1305-7456.178297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.A., Wilson R.F., Palmer R.M. The effect of smoking on periodontal bone regeneration: a systematic review and meta-analysis. J. Periodontol. 2012;83:143–155. doi: 10.1902/jop.2011.110130. [DOI] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Pradeep A., Nagpal K., Karvekar S., Patnaik K., Naik S.B., Guruprasad C. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2015;86:729–737. doi: 10.1902/jop.2015.140646. [DOI] [PubMed] [Google Scholar]
- Pradeep A., Rao N.S., Agarwal E., Bajaj P., Kumari M., Naik S.B. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2012;83:1499–1507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- Pradeep A., Rao N.S., Naik S.B., Kumari M. Efficacy of varying concentrations of subgingivally delivered metformin in the treatment of chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2013;84:212–220. doi: 10.1902/jop.2012.120025. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Patnaik K., Nagpal K., Karvekar S., Ramamurthy B.L., Naik S.B., Suke D., Singh P., Raju A. Efficacy of locally-delivered 1% metformin gel in the treatment of intrabony defects in patients with chronic periodontitis: a randomized, controlled clinical trial. J Invest Clin Dentist. 2016;7:239–245. doi: 10.1111/jicd.12150. [DOI] [PubMed] [Google Scholar]
- Rao N.S., Pradeep A., Kumari M., Naik S.B. Locally delivered 1% metformin gel in the treatment of smokers with chronic periodontitis: a randomized controlled clinical trial. J. Periodontol. 2013;84:1165–1171. doi: 10.1902/jop.2012.120298. [DOI] [PubMed] [Google Scholar]
- Rejnmark L. Bone effects of glitazones and other anti-diabetic drugs. Current Drug Safe. 2008;3:194–198. doi: 10.2174/157488608785699478. [DOI] [PubMed] [Google Scholar]
- Richards D., Rutherford R.B. The effects of interleukin 1 on collagenolytic activity and prostaglandin-E secretion by human periodontal-ligament and gingival fibroblast. Arch. Oral Biol. 1988;33:237–243. doi: 10.1016/0003-9969(88)90184-7. [DOI] [PubMed] [Google Scholar]
- Shaw R.J., Lamia K.A., Vasquez D., Koo S.H., Bardeesy N., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh Z., Najeeb S., Khurshid Z., Verma V., Rashid H., Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials. 2015;8:5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamagas M., Breen T., LeRoith D. Update on diabetes mellitus: prevention, treatment, and association with oral diseases. Oral Dis. 2008;14:105–114. doi: 10.1111/j.1601-0825.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Selection of antimicrobial agents in periodontal therapy. J. Periodont. Res. 2002;37:389–398. doi: 10.1034/j.1600-0765.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- Socransky S.S., Haffajee A.D., Cugini M.A., Smith C., Kent R.L. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Socransky, S.S. and Haffajee, A.D., 2005. Periodontal microbial ecology. Periodontol. 2000, 38, 135–187. [DOI] [PubMed]
- Trivedi S., Lal N., Singhal R. Periodontal diseases and pregnancy. J. Orofacial Sci. 2015;7:67. [Google Scholar]
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Vestergaard P., Rejnmark L., Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- Winn D.M. Tobacco use and oral disease. J. Dent. Educ. 2001;65:306–312. [PubMed] [Google Scholar]
- Witters L.A. The blooming of the French lilac. J. Clin. Invest. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]