Abstract
The aim of the present study was to investigate the potential effect of thymoquinone (TQ) on the metabolic activity of four major drug metabolizing enzymes in human liver microsomes, namely cytochrome P450 (CYP) 1A2, CYP2C9, CYP2D6 and CYP3A4. The inhibition of CYP enzymatic activities by TQ was evaluated by incubating typical substrates (phenacetin for CYP1A2, tolbutamide for CYP2C9, dextromethorphan for CYP2D6, and testosterone for CYP3A4) with human liver microsomes and NADPH in the absence or presence of TQ (1, 10 and 100 µM). The respective metabolite of the substrate that was formed was measured by HPLC. Results of the presented study presented that the metabolic activities of all the investigated CYP enzymes, viz. CYP1A2, CYP2C9, CYP2D6 and CYP3A4, were inhibited by TQ. At 1 µM TQ, CYP2C9 enzyme activity was maximally inhibited by 46.35%, followed by CYP2D6 (20.26%) > CYP1A2 (13.52%) > CYP3A4 (12.82%). However, at 10 µM TQ, CYP2C9 enzyme activity was maximally inhibited by 69.69%, followed by CYP3A4 (23.59%) > CYP1A2 (23.51%) > CYP2D6 (11.42%). At 100 µM TQ, CYP1A2 enzyme activity was maximally inhibited by 81.92%, followed by CYP3A4 (79.24%) > CYP2C9 (69.22%) > CYP2D6 (28.18%). The IC50 (mean ± SE) values for CYP1A2, CYP2C9, CYP2D6 and CYP3A4 inhibition were 26.5 ± 2.9 µM, 0.5 ± 0.4 µM, >500 µM and 25.2 ± 3.1 µM, respectively. These findings suggest that there is a high probability of drug interactions resulting from the co-administration of TQ or herbs containing TQ with drugs that are metabolized by the CYP enzymes, particularly CYP2C9.
Keywords: Thymoquinone, Cytochrome P450, Metabolism, Human liver microsomes
1. Introduction
Thymoquinone [TQ, (Fig. 1)], an oxygenated monoterpene (Grosso et al. 2009), is the key active constituent of volatile oil of Nigella sativa L. also known as black seed belongs to family of Ranunculaceae (Salomi et al. 1991). It is conventionally utilized as flavoring agent and medicine in several societies including Indian subcontinents and Arabian countries. Traditional system of medicines have been encouraging to utilizing black seed oil for the cure of many human ailments such as headache, bronchial asthma, eczema, dysentery, gastrointestinal problems, obesity, and hypertension. Modern clinical studies have also reported the anti-inflammatory and antioxidant activity of N. sativa extracts administered orally (Entok et al., 2014, Hadi et al., 2016, Kanter et al., 2003).
Fig. 1.
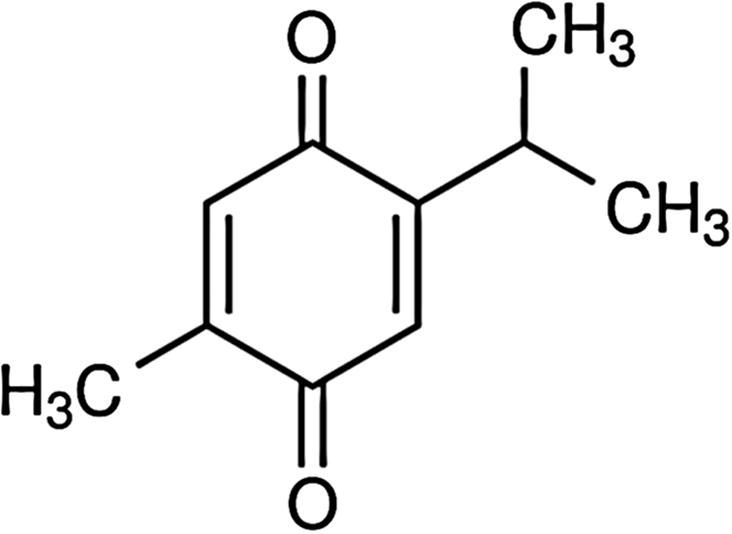
Chemical structure of TQ.
Previously, it was demonstrated that N. sativa seed extracts possesses inhibitory activity against hepatic cytochrome P450 (CYP)3A4 and CYP2D6 both in vitro and in vivo (Al-Jenoobi et al. 2010).
In another in vivo study, administration of N. sativa for one week resulted in a significant reduction in gene expression, as well as metabolic activity, of CYP2D (Al-Jenoobi et al. 2014). A similar study, using tolbutamide as a substrate, showed that its oil extract had considerable inhibitory effects against CYP2C11 (Korashy et al. 2015). However, when N. sativa was co-administered with cyclosporine in an animal model, the oral bioavailability of cyclosporine was significantly reduced, suggesting the possibility that N. sativa induced intestinal P-glycoprotein and/or hepatic CYP3A activity (Al-Jenoobi et al. 2013). Another study presented that the dietary dose of TQ substantially modulate the activity of hepatic xenobiotic metabolizing enzyme in rabbits (Elbarbry et al. 2012).
Several valuable properties of TQ, such as hepatoprotective (Al-Suhaimi, 2012, Hassan et al., 2012), chemopreventive (Aikemu et al. 2013), antibacterial (Chaieb et al. 2011) and anti-inflammatory (Entok et al. 2014) activities have been reported in literature and the mechanism of action of TQ has been attributed to, at least partly, to its capability to clean free radicals and/or constrain lipid peroxidation (Ragheb et al. 2011). Although the community usage of N. sativa oil is increasing, the effect of oral consumption of TQ, the major constituent of the oil, on drug metabolizing enzymes has not been investigated yet. Considering the above-mentioned literature, there is potential to investigate the effect of TQ on the metabolic activity of major drug metabolizing enzymes in pooled human liver microsomes (HLM).
2. Materials and methods
2.1. Materials
Thymoquinone (99%) and HPLC-grade acetonitrile were purchased from “Winlab laboratory and chemicals (Leicestershire, UK)”. HLM (“BD UltraPool” HLM 150, protein concentration: 20 mg/mL) were obtained from “BD Biosciences, Bedford, MA, USA”). “Reduced β-nicotinamide adenine dinucleotide 2′-phosphate (NADPH) tetrasodium salt hydrate” was procured from “Chem-Implex Int’l Inc., Wood Dale, IL, USA”. “Tolbutamide, testosterone and 6β-hydroxytestosterone” were purchased from “Sigma–Aldrich, MO, USA”. 4-hydroxytolbutamide was procured from “Cayman Chemical Company, MI, USA”. Dextromethorphan hydrobromide, dextrorphan-D-tartrate and phenacetin were purchased from “ICN Biomedicals, Inc. Eschwege, Germany”. Caffeine was purchased from “Alfa Aesar, Ward Hill, MA, USA”. Potassium dihydrogen phosphate was received from “Fisher Scientific, Leicestershire, UK”.
2.2. Microsomal incubation conditions for CYP1A2
For incubation, phenacetin (10.0 µL of a 5 mM stock, final concentration (FC) = 100 µM) was added to the eppendorf tubes, and then 10 µL from a TQ stock prepared in methanol (for 1 µM FC-10 µL from 50 µM stock, for 10 µM -10 µL from 500 µM stock, and for 100 µM -10 µL from 5000 µM stock) was added and samples were keep aside for approximately 10 min to allows organic solvent to evaporate. Then HLM (FC = 0.5 mg protein/mL) and “phosphate buffer (0.1 M, pH 7.4)” was added, mixed, and placed in water shaker bath maintained at 37 °C for 5 min. The reaction was started by the addition of 25 µL of 20 mM NADPH (FC = 1.0 mM) to produce a final volume of 0.5 mL, and the mixture was incubated for a further 30 min. The reaction was stopped by 70% perchloric acid (10 μL) with effective shaking for 2.0 min (Eagling et al., 1998, Kobayashi et al., 1998). Caffeine (25 µL) was added as internal standard (IS) in each eppendorf tubes from a stock solution of 10 µM and tubes were centrifuged at 12,000 rpm for 10 min and supernatant was withdrawn and injected to HPLC for analysis.
2.2.1. Analysis of acetaminophen in human liver microsomes
Acetaminophen in HLM was analyzed using an HPLC method (Kobayashi et al. 1998). A “Nucleodur C18 column (4.6 × 250 mm, 5 µm; Macherey-Nagel, Duren, Germany)” was used for detection of the metabolite. Acetonitrile: phosphate buffer (50 mM) in ratio of 15:85 was used as mobile phase which pumped at the flow rate of 1.3 mL/minute. Phenacetin, acetaminophen and caffeine stocks were prepared in methanol. The calibration curve of acetaminophen from 1.0 µM to 15 µM concentration was prepared and drug was detected at 245 nm.
2.3. Microsomal incubation conditions for CYP2C9
For incubation, tolbutamide (10.0 µL of 7.5 mM, FC = 0.15 mM) was added to the eppendorf tubes. Then 10 µL from the TQ stock prepared in methanol (for 0.01 µM FC-10 µL from 0.5 µm stock, for 0.05 µM FC-10 µL from 2.5 µm stock, and for 0.1 µM FC-10 µL from 5 µm stock, for 1 µM TQ FC- 10 µL from 50 µM stock, for 10 µM TQ -10 µL from 500 µM stock, and for 100 µM TQ -10 µL from 5000 µM stock) was added and samples were keep aside for approximately 10 min to allows organic solvent to evaporate. Further, phosphate buffer 0.1 M, pH 7.4 (405 µL) and HLM (FC = 0.25 mg/ml) was added, mixed well and placed in water shaker bath pre-warmed at 37 °C for 5.0 min. Reaction was started by adding 25 µL of 20 mM NADPH (FC = 1.0 mM) to produce a final volume of 0.5 mL, and incubated for 30 min. The reaction was stopped by adding cold methanol (250 µL) with vigorous shaking (Al-Jenoobi, 2010, Korashy et al., 2015). Nitrazepam (25 µL) as IS was added into each tubes from a stock of 1.0 µg/mL. All eppendorf tubes were centrifuged at 12,000 rpm for 10 min. The supernatant was pipette out and injected into an HPLC machine for analysis.
2.3.1. Analysis of 4-hydroxytolbutamide in human liver microsomes
For the analysis of 4-hydroxytolbutamide in HLM, “Symmetry C18 column (4.6 × 150 mm, 5 μm; Waters, Milford, MA, USA)” was used. Mixture of acetonitrile and potassium dihydrogen orthophosphate buffered (0.02 M, pH 3.4) in ratio of 25:75 was employed as mobile phase which was pumped at flow rate of 1.5 mL/minute. Stock solutions of substrate, and metabolite were prepared in acetonitrile and methanol respectively. The calibration curve of tolbutamide metabolite was prepared in the range of 0.05– 5.0 µM and the detection of metabolite was carried out at a wavelength of 230 nm (Al-Jenoobi, 2010, Korashy et al., 2015).
2.4. Microsomal incubation conditions for CYP2D6
Dextromethorphan solution (10 μL, FC = 25 μM) from a stock solution of 1.25 mM, prepared in methanol was added into clean eppendorf tubes. Then, 10 µL from TQ stock prepared in methanol (for 1 µM -10 µL from 50 µM stock, for 10 µm -10 µL from 500 µM stock, and for 100 µm -10 µL from 5000 µM stock) was added and samples were keep aside for approximately 10 min to allows organic solvent to evaporate, before the transfer of HLM (FC = 0.5 mg protein/mL) and “phosphate buffer (0.1 M, pH 7.4)”. The mixture was mixed well, and placed in pre-warmed water shaker bath at 37 °C for 5 min. The incubation reaction was started by the transfer of 25 µL of 20 mM NADPH (FC = 1 mM) and 10 µL of 0.3 M magnesium chloride (FC = 6 mM) in a final volume of 0.5 mL, and incubated in water bath for 30 min at 37 °C. The reaction was stopped by adding 10 μL of 70% perchloric acid, with vortexing for 2 min, the final mixture was centrifuged for 10 min at 12,000 rpm and the supernatant was pipetted out and transfer to HPLC machine for analysis of dextrorphan-D-tartrate (Al-Jenoobi et al., 2010, Al-Jenoobi et al., 2014).
2.4.1. Determination of Dextrorphan-D-tartrate in human liver microsomes
Analysis of dextrorphan-D-tartrate in HLM was carried out using HPLC (Bendriss et al. 2001). For analysis, a “Nucleodur C18 column (4.6 × 250 mm, 5 µm; Macherey-Nagel, Duren, Germany)” was used. The mobile phase comprised of acetonitrile: HPLC-grade water [containing glacial acetic acid (1.5%) and trimethylamine (0.1%), pH adjusted to 3] in ratio of 25:75 v/v. The flow rate of mobile phase was maintained at 1 mL/minute. The calibration curve of dextrorphan-D-tartrate was prepared in the concentration range of 0.5 to 5.0 µM and detection of dextrorphan-D-tartrate was carried out using fluorescence detector set at λEX = 280 nm and λEM = 330 nm.
2.5. Microsomal incubation conditions for CYP3A4
For incubation, testosterone (10.0 µL of 2.5 mM, FC = 50 µM) were added to eppendorf tubes. Then, 10 µL from TQ stock prepared in methanol (for 1 µM -10 µL from 50 µM stock, for 10 µM -10 µL from 500 µM stock, and for 100 µM -10 µL from 5000 µM stock) was added and samples were keep aside for approximately 10 min to allows organic solvent to evaporate, before the addition of HLM (FC = 0.25 mg protein/mL) and “phosphate buffer (0.1 M, pH 7.4)”, mixed well, and placed in water shaker bath pre-warmed at 37 °C for 5.0 min. The incubation reaction was started by adding 25 µL of 20 mM NADPH (FC = 1.0 mM) to produce a final volume of 0.5 mL, and incubated the mixture for 30 min at 37 °C. After 30 min, the incubation reaction was stopped by transferring 250 µL cold methanol with well mixing for 2.0 min (Baati et al., 2012, Wang and Yeung, 2011). Phenacetin (7.5 µL) as IS was transfer to each eppendorf tubes from a stock solution of 10 µg/mL (Borek-Dohalska et al. 2008) and the resulting mixture was centrifuged for 10 min at 12,000 rpm, and supernatant was pipette out and transferred to HPLC for analysis.
2.5.1. Analysis of 6-β-hydroxytestosterone in human liver microsomes
The analysis of 6-β-hydroxytestosterone in HLM was done using a “Nucleodur C18 column (4.6 × 250 mm, 5 µm; Macherey-Nagel, Duren, Germany)” by HPLC. Stock solutions of testosterone, and 6-β-hydroxytestosterone were prepared in methanol. The calibration curve of testosterone metabolite in the concentration range of 1–25 µM was prepared. The 70% methanol in water was used as mobile phase which pumped at the flow rate of 0.5 mL/minute. The detection of testosterone metabolite was carried out at the wavelength of 242 nm (Pan et al. 2012).
2.6. Data analysis
The determination of the IC50 values were done using “GraphPad Prism 6 (GraphPad, Software Inc., San Diego, CA, USA)”. “ANOVA followed by Dunnett’s test was analyzed”; “P value <.05 were considered as significant”.
3. Results and discussion
Presently, natural medications are receiving immense attention and the requirement for herbal products has been raising from year by year (Al-Ghamdi et al., 2017, Mohammad et al., 2015). Herbal medications are famous and are frequently utilized by patients in combination with several recommended remedies for the treatment of many ailments (Rotblatt, 2002). It is well known that the metabolism of a drug can be varied by another co-administered drug or foreign chemical and this could lead to drug interactions which can often be have clinical importance.
The reported modulation of CYP enzymes by various ancient medicines have led to the acceptance that conventional remedies can have adverse effects (Meng and Liu, 2014). However, this is conflicting to the general views in nations where there is a flowing practice of herbal treatment. Further, it has been reported that natural medicines and supplements have differentially modified the stimulation of CYP in humans and experimental models (Fugh-Berman and Ernst, 2001).
The most of the severe events of drug interactions are as a consequence of the intervention of the metabolic clearance of one active by one more concurrently taken natural product, food or drug (Delgoda and Westlake, 2004). Suppression of drug-metabolizing proteins could also contribute to upsurge plasma levels of drugs taken concurrently, prolonging their pharmacological effects, and enhancing the incidence of drug-induced toxicity and severe adverse effects (Angela and Kuerzel, 2013).
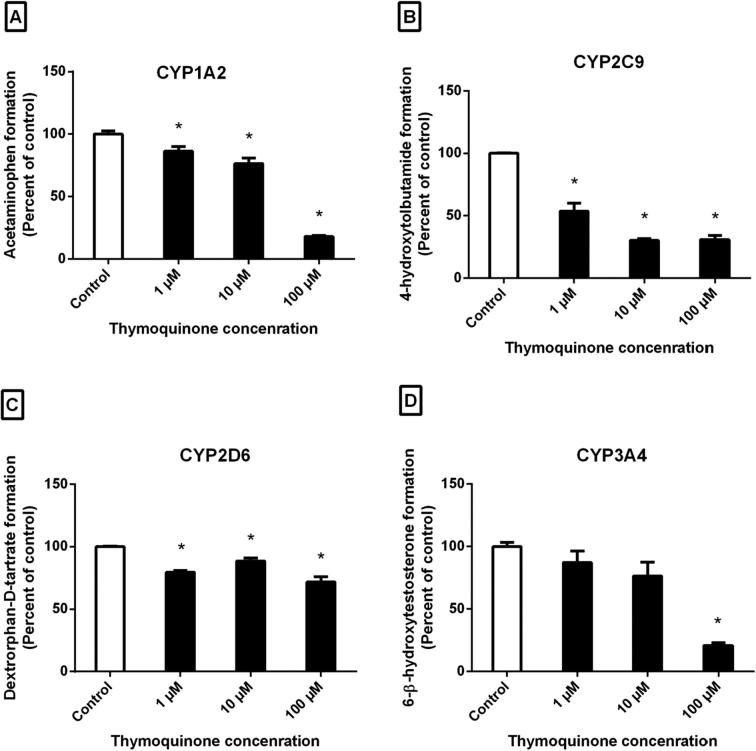
TQ is the main component of N. sativa herb, traditionally used in the Middle East and Southeast Asian countries (Sharma et al. 2009). Its medicinal use is still increasing but data about interactions of TQ-CYP enzymes are limited. In present study, the TQ effect on CYP1A2 enzyme activity was assessed by incubating phenacetin (1, 10 and 100 µM) in pooled HLM and the effect of TQ was evaluated based on the CYP1A2-mediated formation of acetaminophen from phenacetin in HLM. It was observed that TQ significantly inhibits CYP1A2 activity in a concentration-dependent manner, with a maximum inhibition (82%) observed at the highest concentration of TQ (100 µM) (Fig. 2A). TQ inhibited CYP1A2 activity by 14% (P < .05) and 24% (P < .01) at a concentration of 1 µM and 10 µM respectively (Fig. 2A). The rough IC50 value (3 concentrations) of TQ (mean ± standard error) was 26.5 ± 2.9 µM (Table 1).
Fig. 2.
Effect of TQ concentration on the formation of (A) acetaminophen (B) 4-hydroxytolbutamide (C) dextrorphan-D-tartrate and (D) 6-β-hydroxytestosterone in pooled HLM (n = 3, mean ± SEM), *p < .05 compared to control.
Table 1.
Rough IC50 values for inhibition of enzyme-selective substrates metabolites formation by TQ (n = 3, best-fit IC50 values ± SE). All resulting values had r2 value for goodness of fit of at least 0.9.
| Enzyme | IC50 (µM) |
|---|---|
| CYP1A2 | 26.5 ± 2.9 |
| CYP3A4 | 25.2 ± 3.1 |
| CYP2C9 | 0.5 ± 0.4 |
| CYP2D6 | >500 |
In CYP2C9 enzyme microsomes incubation study, the effect of TQ on CYP2C9 enzyme activity was assessed based on the CYP2C9-mediated formation of 4-hydroxytolbutamide from tolbutamide in HLM. TQ at 3 different concentrations (1, 10 and 100 µM) was incubated with tolbutamide. At concentration of 1 µM of TQ, statistically significant (P < .01) inhibition (46%) was observed. Additionally, TQ significantly (P < .01) inhibit CYP2C9 activity around 70% at concentrations of 10 and 100 µM (Fig. 2B). The rough IC50 value (3 concentrations) of TQ (mean ± standard error) was 0.5 ± 0.4 µM (Table 1). Since the IC50 value was low indicating a strong inhibition, we selected CYP2C9 for more investigation. A wide range of six TQ concentrations (0.01–100 µM) were used to assess the precise IC50 value and the precise IC50 value was 0.9 ± 0.3 µM (Table 2; Fig. 3).
Table 2.
Precise IC50 value for TQ showing strong inhibition of 4-hydroxytolbutamide formation.
| Enzyme | IC50 (µM) |
|---|---|
| CYP2C9 (Tolbutamide) | 0.9 ± 0.3 |
Tolbutamide and pooled HLM were incubated with six different concentrations of TQ (n = 3, best-fit IC50 values ± SE). The r2 values for goodness of fit were >0.9.
Fig. 3.
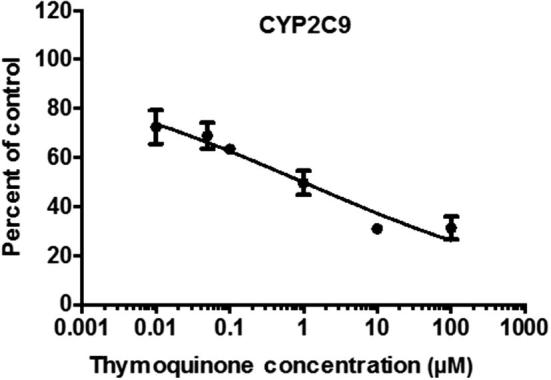
Inhibition of 4-hydroxytolbutamide formation by TQ in HLM. Data analysis was performed by nonlinear regressions (n = 3, mean ± SE).
The effect of TQ on CYP2D6 enzyme activity was assessed using HLM. TQ at 3 different concentrations (1, 10 and 100 µM) was incubated with dextromethorphan and the assay measured the formation of dextrorphan-D-tartrate from dextromethorphan in pooled HLM. The results were expressed as a percentage of the control activity. TQ significantly (P < .05) inhibited the formation of dextrorphan-D-tartrate, however, the inhibition was not potent. The maximum inhibition (∼28%) was observed at 100 µM of TQ, as shown in Fig. 2C. TQ also inhibited CYP2D6 activity by 20% and 11% at 1 µM and 10 µM, respectively, which was statistically significant (P < .05) (Fig. 2C). The rough IC50 value (3 concentrations) of TQ (mean ± standard error) was >500 µM (Table 1).
The inhibitory effect of TQ on the metabolic activity of CYP3A4 was studied. The HLM were incubated with testosterone, and the CYP3A4-mediated formation of 6-β-hydroxytestosterone from testosterone was measured by HPLC. It was observed that TQ inhibited CYP3A4 activity in a concentration-dependent manner. Its maximum inhibition (79%, P < .01) was observed at the highest concentration (100 µM) (Fig. 2D). TQ also inhibited CYP3A4 at 1 µM (Fig. 2D). However, this inhibition was not statistically significant. Similarly, at moderate TQ concentration (10 µM) a non-significant (P > .05) inhibition (∼24%) was observed (Fig. 2D). The rough IC50 value (3 concentrations) of TQ (mean ± standard error) was 25.2 ± 3.1 µM (Table 1).
These findings suggest that there is a high probability of drug interactions appeared from the co-administration of TQ or herbs containing TQ with drugs that are metabolized by these main CYP enzymes. Inhibition or induction of these CYP enzymes may result into rise or fall plasma levels of drugs taken concurrently, altering pharmacological actions, and raising the incidence of drug–induced toxicity. Therefore, the inhibitory effect of TQ on the activity of four major CYP enzymes supports the hypothesis that exposure to herbs or their chemical constituents can contribute to an unwanted pharmacological consequences of clinically used drugs. This is significant for the safety and efficacy of pharmacotherapy, particularly in cases of synthetic drugs with low therapeutic indexes.
4. Conclusion
The current work brings the indication that TQ, the primary chemical ingredient of the volatile oil of N. sativa, significantly inhibits the metabolic activity of four major drug metabolizing enzymes in HLM– CYP1A2, CYP2C9, and CYP3A4. This suggests that TQ may have the potential to modify the metabolic activities of these enzymes in humans. From a practical viewpoint, care should be taken while TQ or an herb with TQ as the main active constituent is concurrently taken with prescribed drugs that are mainly metabolized by CYP1A2, CYP2C9, and CYP3A4 enzymes, specifically drugs with narrow therapeutic ranges.
Conflict of interest
None
Acknowledgment
The “authors extend their appreciation to the deanship of scientific research and the research center, college of pharmacy, King Saud University” for funding this research”.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aikemu A., Xiaerfuding X., Shiwenhui C., Abudureyimu M., Maimaitiyiming D. Immunomodulatory and anti-tumor effects of Nigella glandulifera freyn and sint seeds on ehrlich ascites carcinoma in mouse model. Pharmacogn. Mag. 2013;9:187–191. doi: 10.4103/0973-1296.113258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi S., Aldossari K., Al-Zahrani J., Al-Shaalan F., Al-Sharif S., Al-Khurayji H., Al-Swayeh A. Prevalence, knowledge and attitudes toward herbal medication use by Saudi women in the central region during pregnancy, during labor and after delivery. BMC Complement. Altern. Med. 2017;17:196. doi: 10.1186/s12906-017-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jenoobi F.I. Effects of some commonly used Saudi folk herbal medications on the metabolic activity of CYP2C9 in human liver microsomes. Saudi. Pharm. J. 2010;18:167–171. doi: 10.1016/j.jsps.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jenoobi F.I., Al-Thukair A.A., Abbas F.A., Ansari M.J., Alkharfy K.M., Al-Mohizea A.M., Al-Suwayeh S.A., Jamil S. Effect of black seed on dextromethorphan O- and N-demethylation in human liver microsomes and healthy human subjects. Drug Metab. Lett. 2010;4:51–55. doi: 10.2174/187231210790980435. [DOI] [PubMed] [Google Scholar]
- Al-Jenoobi F.I., Al-Suwayeh S.A., Muzaffar I., Alam M.A., Al-Kharfy K.M., Korashy H.M., Al-Mohizea A.M., Ahad A., Raish M. Effects of Nigella sativa and Lepidium sativum on cyclosporine pharmacokinetics. Biomed. Res. Int. 2013;2013:953520. doi: 10.1155/2013/953520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jenoobi F.I., Korashy H.M., Ahad A., Raish M., Al-Mohizea A.M., Alam M.A., Al-Suwayeh S.A., Alkharfy K.M. Potential inhibitory effect of herbal medicines on rat hepatic cytochrome P450 2D gene expression and metabolic activity. Pharmazie. 2014;69:799–803. [PubMed] [Google Scholar]
- Al-Suhaimi E.A. Hepatoprotective and immunological functions of Nigella sativa seed oil against hypervitaminosis A in adult male rats. Int. J. Vitam. Nutr. Res. 2012;82:288–297. doi: 10.1024/0300-9831/a000121. [DOI] [PubMed] [Google Scholar]
- Angela D., Kuerzel G.U. Springer; Berlin Heidelberg: 2013. Drug-Drug Interaction: Enzyme Inhibition. Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; pp. 989–1004. [Google Scholar]
- Baati T., Horcajada P., Gref R., Couvreur P., Serre C. In vitro determination of the CYP 3A4 activity in rat hepatic microsomes by liquid-phase extraction and HPLC-photodiode array detection. J. Pharmacol. Toxicol. Methods. 2012;66:29–34. doi: 10.1016/j.vascn.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Bendriss E.K., Markoglou N., Wainer I.W. High-performance liquid chromatography assay for simultaneous determination of dextromethorphan and its main metabolites in urine and in microsomal preparations. J. Chromatogr. B Biomed. Sci. Appl. 2001;754:209–215. doi: 10.1016/s0378-4347(00)00609-5. [DOI] [PubMed] [Google Scholar]
- Borek-Dohalska L., Hodek P., Hudecek J., Stiborova M. Experimental approaches to evaluate activities of cytochromes P450 3A. Interdiscip. Toxicol. 2008;1:155–159. doi: 10.2478/v10102-010-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb K., Kouidhi B., Jrah H., Mahdouani K., Bakhrouf A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement Altern. Med. 2011;11:29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoda R., Westlake A.C. Herbal interactions involving cytochrome p450 enzymes: a mini review. Toxicol. Rev. 2004;23:239–249. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- Eagling V.A., Tjia J.F., Back D.J. Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br. J. Clin. Pharmacol. 1998;45:107–114. doi: 10.1046/j.1365-2125.1998.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbry F., Ragheb A., Marfleet T., Shoker A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand White rabbits. Phytother. Res. 2012;26:1726–1730. doi: 10.1002/ptr.4628. [DOI] [PubMed] [Google Scholar]
- Entok E., Ustuner M.C., Ozbayer C., Tekin N., Akyuz F., Yangi B., Kurt H., Degirmenci I., Gunes H.V. Anti-inflammatuar and anti-oxidative effects of Nigella sativa L.: 18FDG-PET imaging of inflammation. Mol. Biol. Rep. 2014;41:2827–2834. doi: 10.1007/s11033-014-3137-2. [DOI] [PubMed] [Google Scholar]
- Fugh-Berman A., Ernst E. Herb-drug interactions: review and assessment of report reliability. Br. J. Clin. Pharmacol. 2001;52:587–595. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso C., Figueiredo A.C., Burillo J., Mainar A.M., Urieta J.S., Barroso J.G., Coelho J.A., Palavra A.M. Enrichment of the thymoquinone content in volatile oil from Satureja montana using supercritical fluid extraction. J. Sep. Sci. 2009;32:328–334. doi: 10.1002/jssc.200800490. [DOI] [PubMed] [Google Scholar]
- Hadi V., Kheirouri S., Alizadeh M., Khabbazi A., Hosseini H. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J. Phytomed. 2016;6:34–43. [PMC free article] [PubMed] [Google Scholar]
- Hassan A.S., Ahmed J.H., Al-Haroon S.S. A study of the effect of Nigella sativa (Black seeds) in isoniazid (INH)-induced hepatotoxicity in rabbits. Indian J. Pharmacol. 2012;44:678–682. doi: 10.4103/0253-7613.103239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter M., Meral I., Dede S., Gunduz H., Cemek M., Ozbek H., Uygan I. Effects of Nigella sativa L. and Urtica dioica L. on lipid peroxidation, antioxidant enzyme systems and some liver enzymes in CCl4-treated rats. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003;50:264–268. doi: 10.1046/j.1439-0442.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Nakajima M., Chiba K., Yamamoto T., Tani M., Ishizaki T., Kuroiwa Y. Inhibitory effects of antiarrhythmic drugs on phenacetin O-deethylation catalysed by human CYP1A2. Br. J. Clin. Pharmacol. 1998;45:361–368. doi: 10.1046/j.1365-2125.1998.t01-1-00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korashy H.M., Al-Jenoobi F.I., Raish M., Ahad A., Al-Mohizea A.M., Alam M.A., Alkharfy K.M., Al-Suwayeh S.A. Impact of Herbal Medicines like Nigella sativa, Trigonella foenum-graecum, and Ferula asafoetida, on Cytochrome P450 2C11 Gene Expression in Rat Liver. Drug Res. (Stuttg) 2015;65:366–372. doi: 10.1055/s-0034-1384604. [DOI] [PubMed] [Google Scholar]
- Meng Q., Liu K. Pharmacokinetic interactions between herbal medicines and prescribed drugs: focus on drug metabolic enzymes and transporters. Curr. Drug Metab. 2014;15:791–807. doi: 10.2174/1389200216666150223152348. [DOI] [PubMed] [Google Scholar]
- Mohammad Y., Al-Ahmari A., Al-Dashash F., Al-Hussain F., Al-Masnour F., Masoud A., Jradi H. Pattern of traditional medicine use by adult Saudi patients with neurological disorders. BMC Complement Altern. Med. 2015;15:102. doi: 10.1186/s12906-015-0623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Mak J.W., Ong C.E. Development and validation of HPLC methods for the determination of CYP2D6 and CYP3A4 activities. Curr. Pharm. Anal. 2012;8:219–224. [Google Scholar]
- Ragheb A., Attia A., Elbarbry F., Prasad K., Shoker A. Attenuated combined action of cyclosporine a and hyperlipidemia on atherogenesis in rabbits by thymoquinone. Evid Based Complement Alternat. Med. 2011;2011:620319. doi: 10.1093/ecam/nep225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblatt M.Z.I. Hanley & Belfus Inc; Philadelphia: 2002. Evidence-Based Herbal Medicine. [Google Scholar]
- Salomi M.J., Nair S.C., Panikkar K.R. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr. Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- Sharma N.K., Ahirwar D., Jhade D., Gupta S. Medicinal and Phamacological Potential of Nigella sativa: A Review. Ethnobotan. Rev. 2009;13 946-455. [Google Scholar]
- Wang X., Yeung J.H. Effects of Salvia miltiorrhiza extract on the liver CYP3A activity in humans and rats. Phytother. Res. 2011;25:1653–1659. doi: 10.1002/ptr.3472. [DOI] [PubMed] [Google Scholar]