Abstract
This study assessed the wound healing potential and antimicrobial activity of henna, pomegranate and myrrh extract formulations and their blend in excision, and dead space wound models in rats in comparison to a marketed ointment (gentamycin). The natural extracts were used in ointment formulations alone or in a combination of three extracts at a total concentration of 15% w/w in medications. The percent of wound contraction in case of henna, myrrh, pomegranate, the blend and gentamycin (10 mg/kg) were 85.90–98.5%, 88.35–99.52%, 93.55–100%, 97.30–100%, and 90.25–100% from days 16 to 20, respectively. The blended formulation showed the highest increase in the percent of wound contraction and decrease in the epithelisation period compared to other formulations and showed comparable results to the standard ointment. The histological studies of excision biopsy at day 24 showed healed skin structures with normal epithelisation, the restoration of adnexa and fibrosis within the dermis in all of the formulation- and gentamycin-treated groups while the control group lagged behind in the formation of the amount of ground substance in the granulation tissue. The formulations showed antimicrobial activity against Candida, Staphylococcus aureus, mucous membrane infections and E. coli topical infections. The study proved the wound healing potential and antimicrobial activity of the herbal extract.
Keywords: Wound healing, Henna, Pomegranate, Myrrh, Hydrophilic ointment, Herbal extract
1. Introduction
A wound is a break formed in the normal continuity of the skin, thereby forming a disruption in its cellular and anatomic structures and affecting its functionality. Wounds may be classified by several methods; their location, aetiology, presenting symptoms or type of injury, wound depth and tissue loss or clinical appearance of the wound (Flanagan, 1994). Wound healing or wound repair is a complicated process in which the tissue repairs itself after being injured. It involves three overlapping phases, namely inflammation, cellular proliferation and remodelling, which are orchestrated in a controlled manner to resume their normal function (Clark, 1993, Sahl and Clever, 1994, Singer and Clark, 1999).
Many efforts have been made to explore new agents that can enhance healing and allow for the speedy recovery of injuries while saving patients from amputation, similar complications and additional problems. Medicinal plants were shown to play an important role in curing skin disorders like cuts and burns (Kokane et al., 2009). Nevertheless, the selection of plants based on their activity is critical and requires care in order to determine the value of the plant (Shivhare et al., 2010).
Traditional medicinal plants are often used to obtain preparations beneficial for wound healing purposes comprising a wide area of different skin-related diseases (Maver et al., 2015) such as Lawsonia inermis (Salih et al., 2017) and Myrrh (Walsh et al., 2010). Herbs such as henna, pomegranate and myrrh have been widely used in traditional systems of medicines due to their antiseptic and anti-inflammatory properties (Li et al., 2006, Shen et al., 2012).
Pomegranate is one of the important fruits stated in the Holy Qur’an. Punica granatum belongs to the family of Punicaceae, and is more commonly known as pomegranate, granats, grenade and punica apple (Voravuthikunchai et al., 2005). Pomegranate extract is becoming popular in the Western world for the treatment and prevention of arthritis and other inflammatory diseases (Ahmed et al., 2005). Also, of the peel, pulp and seed, pomegranate peel had the highest antioxidant activity (Guo et al., 2003). In folk medicine of many cultures, pomegranates have been used extensively (Longtin, 2003). Large amounts of polyphenols are found in pomegranate peel, for example ellagic tannins, ellagic acid and gallic acid. It has been utilised in the preparation of food recipes, cosmetics, tinctures and therapeutic formulae (Ben Nasr et al., 1996). The effectiveness of the dried fruit peel in the treatment of respiratory and urinary tract infections and diarrhoea has been reported (Li et al., 2006). It has also been reported to have anti-fertility effects (Umadevi et al., 2013) cytotoxic activity (Ampasavate et al., 2010) hepatoprotective activity (Ashoush et al., 2013) and hypoglycemic activity (Hontecillas et al., 2009). The ethanolic extract of pomegranate peel has an ameliorative effect against chlorpyrifos-ethyl-induced oxidative stress in rats (Mahgoub and Nashwah, 2009). It also has a potent nephroprotective action and suppresses ferric nitrilotriacetate-induced renal oxidative damage in rats (Mahgoub and Ali, 2010).
Myrrh is the stem resinous exudates that belong to various Commiphora species depending on their growing area. It is an oleo-gum resin that form emulsion upon mixing with water. Published researches showed different medicinal effects for myrrh. The medical uses including fasciolicidal effect, and for the treatment of schistosomiasis (El Ashry et al., 2003, Massoud et al., 2001). Myrrh, either as a whole or some of its components, appears to have therapeutic effectiveness in the treatment of gynaecological cancer diseases (Su et al., 2011), while proving effective as an aesthetic, antibacterial, antifungal and anti-hyperglycaemic agent (Abdallah et al., 2009). Other medicinal uses include anti-ulcer, antipyretic, analgesic, antioxidant and anti-inflammatory effects (Shen et al., 2012). Myrrh has been employed successfully in treatment of wounds and ulcers (Walsh et al., 2010) and is extremely useful topically to facilitate drying and provide wound cleansing (Nomicos, 2007). Myrrh contains about 2–8% essential oil, 40–60% water-soluble gum and 23–40% alcohol-soluble resins. The essential oil is rich in furanosesquiterpenoids, with about 20 compounds having been isolated (Zhu et al., 2003).
Henna, Laurus nobilis Linn., (Lauraceae) is a strong evergreen tree which breeds in the wild or can be cultivated. It has been reported to have carminative, astringent, diaphoretic, diuretic, digestive, emetic and stomachic properties (Nayak et al., 2006), antirheumatic and antineuralgic properties (Marc et al., 2008). Its oil can be used in liniments for sprains and bruising (Guru Prasad, 2011). Owing to its high content of lauric acid, it provides an insecticidal effect against moths. It has also an activity against ringworm infection (Badoni Semwal et al., 2014) and intestinal amoebiasis (Venkata Subbaiah and Savithramma, 2012). The oil extracted from the leaves contains compounds like linalool (monoterpenoids), which is the major compound (50%), whereas p-cymene, α-pinene, limonene and β-pinene are present at levels of around 5–10% each. Also, phenylpropanoids may be found in trace amounts (Nayak et al., 2006).
In light of these traditional uses of plants, cited activities and observations, the present study was performed to assess the synergistic wound healing potential of the aforementioned herb extract formulation blend in excision and dead space wound models in rats when applied topically in comparison to a marketed ointment. Surveying the literature revealed that the synergistic wound healing activity of such blend had not been previously examined. In addition, the study also aimed to evaluate the antibacterial and antifungal activities of the three extracts.
2. Materials and methods
2.1. Materials
Henna, pomegranate and myrrh extracts were obtained from Kuber Impex Limited (New Palasia, India). Yellow soft paraffin, mineral oil and yellow beeswax were purchased from Loba Chemie (Mumbai, India).
2.2. Methods
2.2.1. Ointment preparation
Ointments of henna extract, pomegranate extract, myrrh extract and the three blended extracts were prepared according to the following formula:
| Yellow Soft Paraffin | 52% |
| Bees wax | 3% |
| Liquid paraffin | 25% |
| Natural Extract | 15% |
Weighed amounts of yellow soft paraffin, yellow beeswax, and liquid paraffin were added to a 250 mL beaker. Beaker contents were heated over a water bath to 70 °C and then removed from the heat source. When the temperature of beaker contents lowered to 50 °C, the weighed amounts of natural extract were added to the beaker content and mixed vigorously for 5 min using a hand mixer. The natural extracts used here were 15% henna extract, 15% pomegranate extract, 15% myrrh extract or a blend of 5% of each of the three extracts.
2.2.2. Models for wound healing activity
2.2.2.1. Excision wound model
The excision wound study included the use of male Wistar rats (200–250 g). Animals were chosen and divided into six groups of five animals each. Anaesthetic ether was used to anaesthetise the rats, which were then depilated before wounding at the predetermined site. An excision wound was perpetrated by removing away approximately 500 mm2 full thickness of the assigned area on the anterior-dorsal side of each rat (Dash et al., 2001). The treatment of animals involved the external application of various formulations at a concentration of 10 mg/kg body weight. The assigned groups of animals including group I, group II, group III, group IV, group V and group VI were treated with methanolic extract of myrrh (M) paste (w/w), pomegranate (P) paste (w/w), henna (H) paste (w/w), blend (B) paste (w/w), base ointment alone (negative control group) and gentamycin ointment (positive control group), respectively. All of the formulations were applied to corresponding groups twice daily for 24 days, beginning from the day of wounding. The wound contraction as well as the wound closure time were taken as the criteria for evaluation of the wound healing property. Every three days, the wound area was measured by applying a transparent piece of paper over the wound and outlining it; then, the area of this measurement was calculated using a graph sheet (Werner et al., 1994). The wound contraction was described as the % of contraction. The time for wound closure was noted when total healing occurred. The protocol of the study for animal experiments was approved by the Institutional Animals Ethical Committee of the department.
2.2.3. Histopathological studies
The skin specimens were collected in 10% buffered formalin from rats of the six groups. Then, 5 μm thick sections were sliced and stained with haematoxylin and eosin. The light microscope was used to evaluate the sliced sections in terms of collagen formation, fibroblast proliferation, keratinisation, and epithelisation.
2.2.4. In vitro antimicrobial activity
The cup plate agar diffusion method (BSAC) was used to evaluate the antimicrobial activity of four hydrophilic ointments containing henna extract, myrrh extract, pomegranate extract and a blend of the three extracts.
Mueller-Hinton agar medium (Merck®, Darmstadt, Germany) was autoclaved after being prepared according to the manufacturer’s instructions. After autoclaving, the molten agar medium was allowed to cool down to 40–45 °C. Twenty-five mL of the agar medium was poured onto sterile Petri dishes (90 mm in diameter) to provide a depth of 4 ± 0.5 mm. The agar was left to solidify. The following standard strains were used to evaluate the antibacterial and antifungal activities of the topical formulations: Escherichia coli ATCC 25218, Pseudomonas aeruginosa ATCC 15442, Staphylococcus aureus ATCC 29213, Methicillin-Resistant S. aureus ATCC 29213, Bacillus subtilis ATCC 10400, and Candida albicans ATCC 1023. The standard strains were sub-cultured on 5% sheep blood agar medium. Three to five well-isolated colonies of the standard microorganism were suspended in 5 mL of sterile saline and vortexed well until a uniform suspension was obtained. The turbidity of the suspension was measured at 625 nm using a UV spectrophotometer (LKB® Ultrospec, Madison, WI, USA) to give an absorbance of 0.08–0.13 and an inoculum level of 1 × 108 CFU/ml. The suspension was diluted to 1:100 in sterile normal saline to obtain 1 × 106 CFU/ml for all standard strains, except S. aureus, which was diluted to 1:10. A sterile swab of cotton was immersed in the suspension and streaked over the surface of the agar plates. The inoculated plates were then left to dry at room temperature. The dried inoculated plates were used for the agar well-diffusion assay. A sterile cork borer was used to make four wells per plate by perforating holes on the inoculated plate. Each well was 7 mm in diameter, and the cut pieces of the agar were removed using a sterile needle. Then, 100 µg of each formulation was placed into each well. A 100 µg of gentamycin ointment (Garamycin® ointment) and standard discs (imipenem disc containing 10 μg and ketoconazole disc containing 10 μg) were used as positive controls. The inoculated agar plates were kept at 37 °C for 18 h. The detected diameters of the inhibition zones were measured using a ruler to the nearest millimetre. The experiment was carried out in duplicate and the mean diameter was taken.
3. Results
3.1. Wound healing activity
3.1.1. Excision model
The % of wound contraction ranged from 17.45% to 69.91% in the period from 4 to 12 days and from 85.19% to 97.96% in the period from 16 to 20 days in the control (ointment base) group of rats, whereas whole epithelisation and healing were shown on day 24. The shedding of eschar lasted for an average of 12.4 days without leaving any residual raw wound in the control rats. The % of wound contraction in rats treated externally with henna (10 mg/kg) ranged from 19.64% to 72.78% in the period from 4 to 12 days and from 85.90% to 98.50% in the period from 16 to 20 days, respectively. The % rate of wound contraction in rats treated externally with myrrh (10 mg/kg) ranged from 37.92% to 75.31% in the period from 4 to 12 days and from 88.35% to 99.52% in the period from 16 to 20 days, respectively. The % rate of wound contraction in rats treated externally with pomegranate (10 mg/kg) ranged from 41.80% to 79.45% in the period from 4 to 12 days and from 93.55% to 100% in the period from 16 to 20 days, respectively. The % rate of wound contraction in rats treated externally with the blend (10 mg/kg) ranged from 49.33% to 88.66% in the period from 4 to 12 days and from 97.30% to 100% in the period from 16 to 20 days, respectively. The gentamycin-treated rats showed increased wound contraction from 61.03% to 90.25% in the period from 4 to 12 days and from 90.25% to 100% in the period from 16 to 20 days, respectively. Meanwhile, the mean epithelialisation time decreased from 12.4 days in controls, to 12.2 for henna, 11.8 for myrrh, 11.4 for pomegranate, and 9.4 for the blend, while standard gentamycin showed the lowest time of 9.2 days. Among the prepared herbal formulations, the blended formulation showed the highest increase in % of wound contraction and decrease in epithelisation period in comparison with controls, and showed comparable results to the standard ointment (Table 1).
Table 1.
The measurements of wound areas over a period of 24 days showing the percent of contraction among different formulations.
| External treatment (10 mg/kg, twice daily) | Wound area in mm2/rat (% contraction) |
Epithelization period (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 day | 4th day | 8th day | 12th day | 16th day | 20th day | 24th day | ||
| Control (ointment base) | 545.35 ± 6.35 | 450.16 ± 7.36 | 325.32 ± 5.48 | 164.08 ± 8.18 | 80.74 ± 5.18 | 11.12 ± 0.63 | 2.4 ± 0.14 | 12.4 |
| 0 | 17.45 ± 1.51 | 40.34 ± 1.12 | 69.91 ± 1.67 | 85.19 ± 1.06 | 97.96 ± 0.13 | 99.55 ± 0.03 | ||
| Henna ointment | 546.82 ± 7.78 | 439.42 ± 6.79 | 301.28 ± 4.94 | 148.82 ± 4.81 | 77.09 ± 3.18 | 8 ± 0.73 | 1.04 ± 0.3 | 12.2 |
| 0 | 19.64 ± 1.24 | 44.90 ± 0.90 | 72.78 ± 0.88 | 85.90 ± 0.58 | 98.53 ± 0.13 | 99.80 ± 0.02 | ||
| Myrrh ointment | 545.02 ± 6.10 | 338.32 ± 3.93 | 282.36 ± 7.25 | 134.54 ± 3.70 | 63.45 ± 3.09 | 2.6 ± 0.36 | 0 | 11.8 |
| 0 | 37.92 ± 0.72 | 48.19 ± 1.33 | 75.31 ± 0.67 | 88.35 ± 5.6 | 99.52 ± 0.07 | 100 | ||
| Pomegranate ointment | 547.7 ± 6.70 | 318.52 ± 4.06 | 255.98 ± 2.28 | 112.54 ± 2.98 | 35.28 ± 3.17 | 0 | 0 | 11.4 |
| 0 | 41.8 ± 0.74 | 53.26 ± 0.41 | 79.45 ± 0.54 | 93.55 ± 0.58 | 100 | 100 | ||
| Blend ointment | 551.86 ± 9.62 | 279.62 ± 5.54 | 140.32 ± 8.1 | 62.54 ± 2.98 | 14.88 ± 3.31 | 0 | 0 | 9.8 |
| 0 | 49.33 ± 1 | 74.57 ± 1.46 | 88.66 ± 0.54 | 97.30 ± 0.6 | 100 | 100 | ||
| Gentamycin ointment | 552.06 ± 7.87 | 215.12 ± 4.05 | 118.24 ± 2.39 | 53.8 ± 3.73 | 0 | 0 | 0 | 9.4 |
| 0 | 61.03 ± 0.73 | 78.58 ± 0.43 | 90.25 ± 0.67 | 100 | 100 | 100 | ||
3.2. Histopathological studies
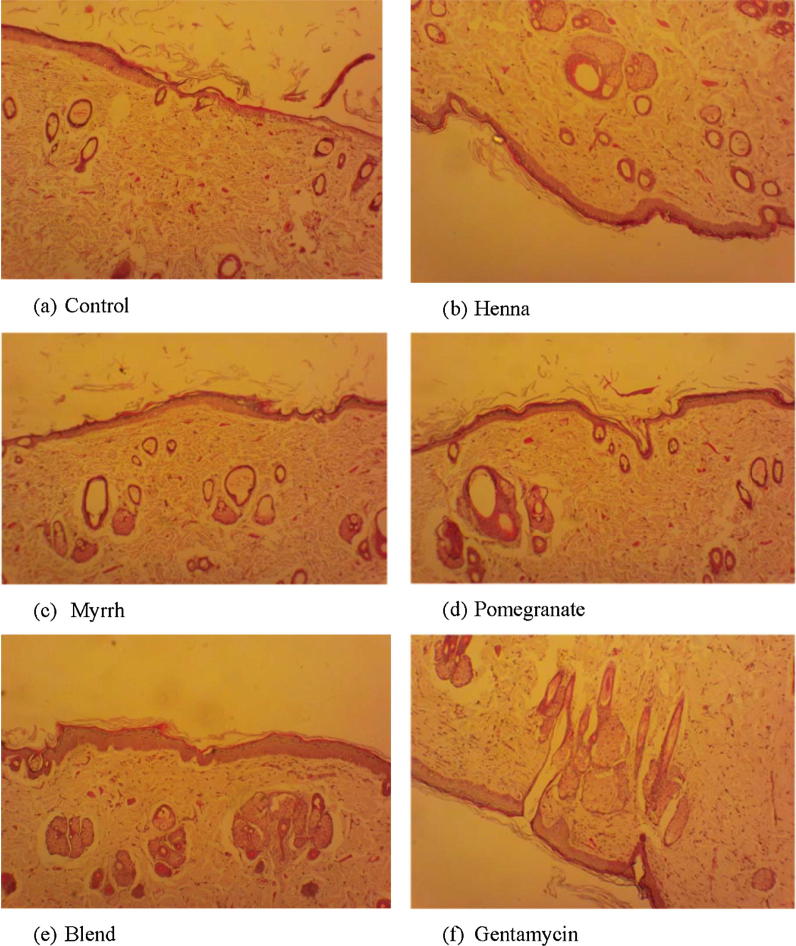
Histology of excision biopsy at day 24 demonstrated healed skin structures of skin wounds with normal epithelisation, the restoration of adnexa and fibrosis within the dermis in all of the formulations and gentamycin-treated groups. The control group lagged behind the treated groups with respect to the formation of the amount of ground substance in the granulation tissue, as observed in tissue sections (Fig. 1a–f). The histology of the deeper structure within the granulation tissue of the control group demonstrated mononuclear inflammatory cells with scattered fibroblasts (minimal fibrosis), and few proliferating vasculature in granulation tissue indicating low healing properties, whereas the granulation tissue of the groups treated with either the herbal formulations or gentamycin showed an abundance of eosinophilic collagen tissue and neovascularisation, with few inflammatory cells indicative of healing by fibrosis. The order of healing can be arranged as follows: control < henna < myrrh < pomegranate < blend = gentamycin. These results were similar to that which was observed for wound contraction and rate of epithelisation.
Fig. 1.
Histopathology of skin at day 24 stained with H&E (100×), showing: (a) Skin of control rat showing ulceration and edema, early epithelisation and granulation tissue and abundance of mononuclear inflammatory cells, (b) & (c) Henna & Myrrh treated rats, respectively, showing large amount of granulation tissue, small number of mononuclear inflammatory cells, and restoration of adnexa and extensive fibrosis, (d), (e) & (f) Pomegranate, blend and gentamycin treated rats, respectively, showing healed skin structures with well formed, near to normal epidermis, restoration of adnexa, and extensive fibrosis and collagen tissue within the dermis.
3.3. Microbiological results
The results of the in vitro evaluation of antibacterial and antifungal activity of five of the tested formulations by cup plate diffusion method are shown in Table 2. The results revealed that all of the tested formulations have excellent antibacterial activities against Gram-negative bacteria, such as E. coli ATCC25218, Gram-positive bacteria such as S. aureus ATCC 29213, MRSA ATCC29213, and B. subtilis ATCC10400. No formulations showed any activity against Gram-negative P. aeruginosa ATCC15442. On the other hand, all formulations showed amazing antifungal activities against C. albicans ATCC 10231.
Table 2.
Evaluation of antimicrobial activities of four hydrophilic ointments containing henna extract, myrrh extract, pomegranate extract and blend of the three extracts respectively by Cup plate diffusion method.
| Formulation | Cup plate diffusion method (mm) |
|||||
|---|---|---|---|---|---|---|
| Gram-negative bacteria |
Gram-positive bacteria |
Yeast |
||||
| E. coli ATCC25218 | P. aeruginosa ATCC15442 | S. aureus ATCC 29213 | MRSA ATCC33591 | B. subtilis ATCC10400 | C. albicans ATCC 10231 | |
| Control (ointment base) | NIL | NIL | NIL | NIL | NIL | NIL |
| Henna ointment | 20 | NZa | 26 | 25 | 20 | 30 |
| Myrrh ointment | 19 | NZ | 23 | 22 | 25 | 30 |
| Pomegranate ointment | 21 | NZ | 20 | 20 | 21 | 29 |
| Blend ointment | 18 | NZ | 25 | 25 | 25 | 30 |
| Garamycin ointment | 29 | 25 | 27 | 26 | 28 | NDb |
| Imipenem 10 µg/disc | 30 | 25 | 35 | 30 | 30 | ND |
| Ketokonazole 25 µg/disc | ND | ND | ND | ND | ND | 35 |
NZ means no inhibition zone.
ND means not determined.
4. Discussion
A wound is a kind of skin injury in which a tear, cut or puncture occurs in the skin (an open wound). Pathologically, it reflects a sharp damaging injury to the dermis of the skin (Thomas and Wysocki, 1990) or it may be epidermal wounds (Moll et al., 1998). The main processes that are involved in wound healing are epithelisation, contraction, and connective tissue deposition. Healing processes are governed by the biosynthesis and deposition of new collagens at the site of the wound (Agarwal et al., 2009). The process of wound healing involves the migration of endothelial cells, leading to the neovascularisation of connective tissues which synthesise extracellular matrices including collagens, and keratinocytes leading to re-epithelialisation of the wounded tissue (Clark, 1993). Herbs such as henna, pomegranate and myrrh were widely used in the traditional system of medicines for their antiseptic and anti-inflammatory properties. Therefore, the present study was performed as an in-depth investigation of the synergistic wound healing activities of the aforementioned herb extract formulation blend in excision and dead space wound models in rats when applied topically on wounds in comparison to a marketed gentamycin ointment (Garamycin®, Schering-Plough). In the excision wound model, the blend formulation showed faster healing properties compared with the control group. Further excision biopsy of the skin wound at day 10 showed healed skin structures with normal epithelisation, restoration of adnexa and fibrosis within the dermis in both the blend formulation- and gentamycin-treated groups, while the control group lags behind the treated groups in the amount of ground substance formed in the granulation tissue. The faster wound contraction by the blend formulation may be due to the stimulation of cytokine, an inflammatory α-chemokine by flavonoids, terpines and gallic acid (Moyer et al., 2002). Phytochemical constituents of the herbal extract henna contain monoterpenoides, which have anti-inflammatory and antimicrobial properties (Chmit et al., 2014, Tabanca et al., 2013). Pomegranate contains polyphenols such as ellagic tannins, ellagic acid and gallic acid, with known antimicrobial and anti- inflammatory properties (Nayak et al., 2013) whereas myrrh contains furanosesquiterpenes, β-sitosterol and alcohol-soluble resins with potent antiseptic, antioxidant and anti-inflammatory properties (Walsh et al., 2010). In the excision wound model, the results showed the extent of wound healing by various formulations in the following order: Blend > Pomegranate > Myrrh > Henna.
Antimicrobial studies were performed to evaluate the antibacterial and antifungal activities of the hydrophilic ointments of henna extract, myrrh extract, pomegranate extract and the blend of the three extracts. These topical pharmaceutical formulations can be used to cure Candida, Staphylococcus aureus, mucus membrane infections and E. coli topical infections. The advantage of the tested formulations is demonstrated with respect to their activity against both bacteria and Candida.
5. Conclusion
The present investigation involving excision wound models, which included the observation of different physical, histological, and antimicrobial activities, indicated the wound healing activity of herbal extracts formulations. The healing effects seemed to be due to promoting faster collagen deposition, the formation of other connective tissue constituents, and antibacterial activity.
Acknowledgments
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research and Research Center, College of Pharmacy at King Saud University for supporting this research.
Conflict of interest
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdallah E.M., Khalid A.S., Ibrahim N. Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA) Sci. Res. Essays. 2009;4:351–356. [Google Scholar]
- Agarwal P.K., Singh A., Gaurav K., Goel S., Khanna H.D., Goel R.K. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian J. Exp. Biol. 2009;47(1):32–40. [PubMed] [Google Scholar]
- Ahmed S., Wang N., Hafeez B.B., Cheruvu V.K., Haqqi T.M. Punica granatum L. extract inhibits IL-1β–Induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-κB in human chondrocytes in vitro. J Nutr. 2005;135(9):2096–2102. doi: 10.1093/jn/135.9.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampasavate C., Okonogi S., Anuchapreeda S. Cytotoxicity of extracts from fruit plants against leukemic cell lines. Afr. J. Pharm. Pharmacol. 2010;4:13–21. [Google Scholar]
- Ashoush I.S., El-Batawy O.I., El-Shourbagy G.A. Antioxidant activity and hepatoprotective effect of pomegranate peel and whey powders in rats. Ann. Agric. Sci. 2013;58(1):27–32. [Google Scholar]
- Badoni Semwal R., Semwal D.K., Combrinck S., Cartwright-Jones C., Viljoen A. Lawsonia inermis L. (henna): ethnobotanical, phytochemical and pharmacological aspects. J. Ethnopharmacol. 2014;155(1):80–103. doi: 10.1016/j.jep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Ben Nasr C., Ayed N., Metche M. Quantitative determination of the polyphenolic content of pomegranate peel. Z. Lebensm. Unters. Forsch. 1996;203(4):374–378. doi: 10.1007/BF01231077. [DOI] [PubMed] [Google Scholar]
- BSAC. Methods for Antimicrobial Susceptibility Testing – Version 14. Available online: <http://bsac.org.uk/wp-content/uploads/2012/02/BSAC-Susceptibility-testing-version-14.pdf> (accessed on 9 April 2015).
- Chmit M., Kanaan H., Habib J., Abbass M., McHeik A., Chokr A. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pac. J. Trop. Med. 2014;7S1:S546–S552. doi: 10.1016/S1995-7645(14)60288-1. [DOI] [PubMed] [Google Scholar]
- Clark R.A. Regulation of fibroplasia in cutaneous wound repair. Am. J. Med. Sci. 1993;306(1):42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Dash G.K., Suresh P., Ganapathy S. Studies on hypoglycaemic and wound healing activities of Lantana camara Linn. J. Nat. Rem. 2001;1:105–110. [Google Scholar]
- El Ashry E.S., Rashed N., Salama O.M., Saleh A. Components, therapeutic value and uses of myrrh. Die Pharmazie. 2003;58(3):163–168. [PubMed] [Google Scholar]
- Flanagan M. Wound care. Assessment criteria. Nursing Times. 1994;90(35):76–88. [PubMed] [Google Scholar]
- Guo C., Yang J., Wei J., Li Y., Xu J., Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003;23(12):1719–1726. [Google Scholar]
- Guru Prasad, B.R., 2011. Assessment of Ethno-medicinal Plants from Chamundi Hill, Mysore, pp. 5200–5202.
- Hontecillas R., O'Shea M., Einerhand A., Diguardo M., Bassaganya-Riera J. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 2009;28(2):184–195. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- Kokane D.D., More R.Y., Kale M.B., Nehete M.N., Mehendale P.C., Gadgoli C.H. Evaluation of wound healing activity of root of Mimosa pudica. J. Ethnopharmacol. 2009;124(2):311–315. doi: 10.1016/j.jep.2009.04.038. [DOI] [PubMed] [Google Scholar]
- Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96(2):254–260. [Google Scholar]
- Longtin R. The pomegranate: nature's power fruit? J. Natl. Cancer Inst. 2003;95(5):346–348. doi: 10.1093/jnci/95.5.346. [DOI] [PubMed] [Google Scholar]
- Mahgoub M.A., Ali S.E. Protective effect of pomegranate peel ethanol extract against ferric nitrilotriacetate induced renal oxidative damage in rats. J. Cell. Mol. Biol. 2010;7:35–43. [Google Scholar]
- Mahgoub M.A., Nashwah I.Z. Assessment the ameliorative effect of pomegranate and rutin on chlorpyrifos-ethyl-Induced oxidative stress in rats. Nat. Sci. 2009;7:49–61. [Google Scholar]
- Marc E.B., Nelly A., Annick D.-D., Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008;120(3):315–334. doi: 10.1016/j.jep.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Massoud A., El Sisi S., Salama O., Massoud A. Preliminary study of therapeutic efficacy of a new fasciolicidal drug derived from Commiphora molmol (myrrh) Am. J. Trop. Med. Hyg. 2001;65(2):96–99. doi: 10.4269/ajtmh.2001.65.96. [DOI] [PubMed] [Google Scholar]
- Maver T., Maver U., Stana Kleinschek K., Smrke D.M., Kreft S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015;54(7):740–751. doi: 10.1111/ijd.12766. [DOI] [PubMed] [Google Scholar]
- Moll I., Houdek P., Schmidt H., Moll R. Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes1. J. Invest. Dermatol. 1998;111(2):251–258. doi: 10.1046/j.1523-1747.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Moyer K.E., Saggers G.C., Allison G.M., Mackay D.R., Ehrlich H.P. Effects of interleukin-8 on granulation tissue maturation. J. Cell. Physiol. 2002;193(2):173–179. doi: 10.1002/jcp.10160. [DOI] [PubMed] [Google Scholar]
- Nayak S., Nalabothu P., Sandiford S., Bhogadi V., Adogwa A. Evaluation of wound healing activity of Allamanda cathartica L. and Laurus nobilis L. extracts on rats. BMC Complem. Altern. Med. 2006;6:12–18. doi: 10.1186/1472-6882-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S.B., Rodrigues V., Maharaj S., Bhogadi V.S. Wound healing activity of the fruit skin of Punica granatum. J. Med. Food. 2013;16(9):857–861. doi: 10.1089/jmf.2012.0229. [DOI] [PubMed] [Google Scholar]
- Nomicos E.Y. Myrrh: medical marvel or myth of the Magi? Holistic Nurs. Pract. 2007;21(6):308–323. doi: 10.1097/01.HNP.0000298616.32846.34. [DOI] [PubMed] [Google Scholar]
- Salih M.A., Kakamad F., Salih R.A., Hussein D.A., Hassan H.M., Maikel T., Abdul Aziz J., Aube H. Effect of Lawsonia inermis (Henna) on wound healing in sprague-dawley rats: a pilot study. Wound Med. 2017;18:41–42. [Google Scholar]
- Sahl W.J., Jr., Clever H. Cutaneous scars: Part I. Int. J. Dermatol. 1994;33(10):681–691. doi: 10.1111/j.1365-4362.1994.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Shen T., Li G.-H., Wang X.-N., Lou H.-X. The genus Commiphora: a review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2012;142(2):319–330. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Shivhare Y., Singour P.K., Patil U.K., Pawar R.S. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb (fruits) in rats. J. Ethnopharmacol. 2010;127(3):614–619. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Singer A.J., Clark R.A. Cutaneous wound healing. N. Engl. J. Med. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Su S., Wang T., Chen T., Duan J., Yu L., Tang Y. Cytotoxicity activity of extracts and compounds from Commiphora myrrha resin against human gynecologic cancer cells. J. Med. Plants Res. 2011;5:1382–1389. [Google Scholar]
- Tabanca N., Avonto C., Wang M., Parcher J.F., Ali A., Demirci B., Raman V., Khan I.A. Comparative investigation of Umbellularia californica and Laurus nobilis leaf essential oils and identification of constituents active against Aedes aegypti. J. Agric. Food Chem. 2013;61(50):12283–12291. doi: 10.1021/jf4052682. [DOI] [PubMed] [Google Scholar]
- Thomas A.C., Wysocki A.B. The healing wound: a comparison of three clinically useful methods of measurement. Decubitus. 1990;3(1) 18–20, 24–15. [PubMed] [Google Scholar]
- Umadevi M., Sampath Kumar P.K., Bhowmik D., Duraivel S. Medicinal plants with potential antifertility activity. J. Med. Plants Stud. 2013;1:26–33. [Google Scholar]
- Venkata Subbaiah K.P., Savithramma N. Bio-prospecting and documentation of traditional medicinal plants used to treat itching, psoriasis and wounds by ethnic groups of Kurnool District, Andhra Pradesh, India. Int. J. Pharm. Pharm. Sci. 2012;5:127–131. [Google Scholar]
- Voravuthikunchai S.P., Sririrak T., Limsuwan S., Supawita T., Iida T., Honda T. Inhibitory effects of active compounds from Punica granatum pericarp on verocytotoxin production by enterohemorrhagic Escherichia coli O157: H7. J. Health Sci. 2005;51(5):590–596. [Google Scholar]
- Walsh M.E., Reis D., Jones T. Integrating complementary and alternative medicine: use of myrrh in wound management. J. Vasc. Nurs. 2010;28(3):102. doi: 10.1016/j.jvn.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Werner S., Breeden M., Hubner G., Greenhalgh D.G., Longaker M.T. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J. Invest. Dermatol. 1994;103(4):469–473. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- Zhu N., Sheng S., Sang S., Rosen R.T., Ho C.-T. Isolation and characterization of several aromatic sesquiterpenes from Commiphora myrrha. Flavour Fragrance J. 2003;18(4):282–285. [Google Scholar]