Abstract
Guiera senegalensis J.F. Gmel is a broad-spectrum African folk- medicinal plant, having activities against fowlpox and herpes viruses. Very recently, we have shown the anti-hepatitis B vius (HBV) potential of G. senegalensis leaves extract (GSLE). Here, we report the antioxidative and hepatoprotective efficacy of GSLE, including HPTLC quantification of four biomarkers of known antioxidative and antiviral activities. In cultured liver cells (HuH7) GSLE attenuated DCFH-induced oxidative stress and cytotoxicity. This was supported by in vitro DPPH radical-scavenging and β-carotene-linoleic acid bleaching assays that showed strong antioxidant activity of GSLE. Further, two simple and sensitive HPTLC methods (I and II) were developed and validated to quantify β-amyrin, β- sitosterol, lupeol, ursolic acid in GSLE. While HPTLC-I (hexane: ethylacetate; 75:25; v/v) enabled quantification of β-amyrin (Rf = 0.39; 20.64 μg/mg) and β-sitosterol (Rf = 0.25; 18.56 μg/mg), HPTLC-II (chloroform: methanol; 97:3; v/v) allowed estimation of lupeol (Rf = 0.47; 6.72 μg/mg) and ursolic acid (Rf = 0.23; 5.81 μg/mg) in GSLE. Taken together, the identified biomarkers strongly supported the antioxidant and anti-HBV potential of GSLE, suggesting its activity via abating the oxidative stress. To our knowledge, this is the first report on HPTLC analysis of these biomarkers in G. senegalensis that could be adopted for standardization and quality-control of herbal-formulations.
Keywords: Guiera senegalensis, HPTLC, β-Amyrin, β-Sitosterol, Lupeol, Ursolic acid, Antioxidant, Antiviral, Anti-HBV
1. Introduction
Oxidative stress-induced cellular injury is caused by the imbalance between the oxidant and antioxidant molecules or by overabundance of reactive oxygen species (ROS), produced by endogenous or exogenous sources (Opara et al., 2006). The accumulating excess of ROS can damage lipids, proteins or nucleic acids, and inhibit the normal growth and function of the cells (Ames et al., 1993). Several in vitro and in vitro studies have suggested the association of oxidative stress and damages with different forms of liver diseases (Ha et al., 2010). The hepatitis B virus (HBV) infection results in acute and chronic liver diseases, such as hepatitis B, cirrhosis and hepatocellular carcinoma (HCC). Evidences have shown that HBV can induce oxidative stress in vitro and in vivo including chronic hepatitis B patients (Severi et al., 2006, Niu et al., 2009, Bolukbas et al., 2005). Notably, the oncogenic ‘X’ gene of HBV (HBx) is trans-activated by ROS, and plays a crucial role in the development of HCC. Moreover, the polyunsaturated fatty acids residues of phospholipids of cell membranes and intracellular organelles are highly reactive to ROS that lead to lipid peroxidation (LPO), and produce cytotoxic malondialdehyde and hydroxynonenal (Djordjevic, 2004). It is reported that the total peroxide level, LPO and oxidative DNA damage are significantly higher in hepatitis B patients (Shaban et al., 2014).
Currently, the use of medicinal plants is massively increasing due to fewer or insignificant side effects as well as its low-cost. The African medicinal plant, Guiera senegalensis J.F. Gmel, commonly known as ‘Cure all’ is a popular folk medicine in the treatment of several types of metabolic and infectious diseases (Suleiman, 2015, Bosisio et al., 1997, Somboro et al., 2011). The dried leaves preparations of G. senegalensis is used to treat cough, sexual, gastrointestinal, respiratory and skin diseases, including their use as acaricidal, antimalarial, and antimicrobial, antioxidant, anti-inflammatory and gastroprotective agents (Osman et al., 2014, Bouchet et al., 1998, Sombié et al., 2011, Akuodor et al., 2013). Importantly, the galls of the plant are reported to have in vitro antiviral efficacies against fowlpox virus (FPV) (Lamien et al., 2005) and herpes simplex virus (HSV) (Silva et al., 1997). Very recently, we have shown the in vitro anti-HBV efficacy of G. senegalensis leaves (Arbab et al., 2017).
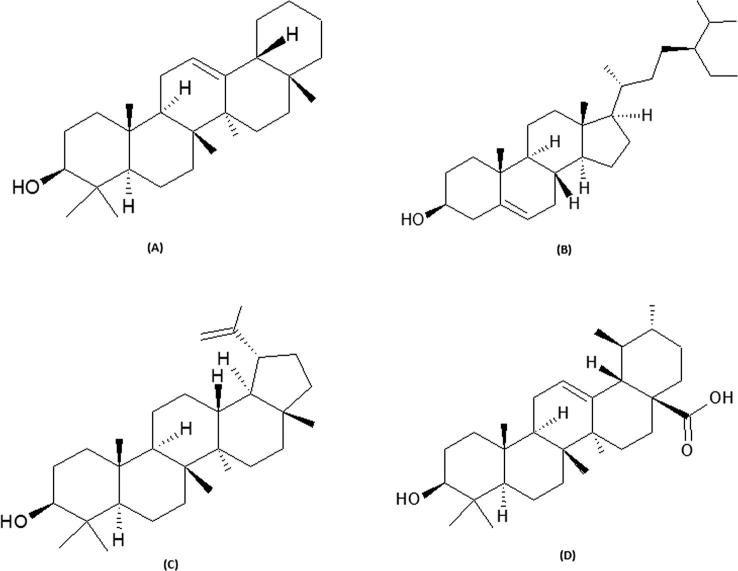
It is known that the pharmacological activity of a herbal-formulation is attributed to bioactive constituents, and their amount can differ considerably depending on the plant’s part used, its geographical origin and the season of harvest. Therefore, development of sensitive methods for quantitative analysis of active biomarker(s) in a claimed plant extract or marketed formulation is fundamental for ensuring its therapeutic quality. Therefore, owing to its low-cost, high-throughput and minimum sample clean-up properties, the high-performance thin-layer chromatography (HPTLC) has become a convenient analytical method. The phytochemical analysis of G. senegalensis has identified various bioactive flavonoids, alkaloids, tannins and a naphthyl butenone (Ficarra et al., 1997; Bouchet et al., 1996). However, natural triterpenes (the structurally diverse group of pentacyclic triterpenoids) and phytosterols of known antioxidant and antiviral activities are hitherto, not explored in G. senegalensis. The present study was therefore, designed to evaluate the antioxidative and hepatoprotective property of anti-HBV active G. senegalensis leaves ethanol-extract (GSLE) and, to quantify biomarkers (β-amyrin, β-sitosterol, lupeol, ursolic acid; Fig. 1) by validated HPTLC methods.
Fig. 1.
Chemical structures of antioxidant biomarkers analyzed in the present study. (A) β-amyrin, (B) β-sitosterol, (C) lupeol, and (D) ursolic acid.
2. Experimental
2.1. Plant material and extract preparation
Fresh and clean leaves of G. senegalensis (Family: Combretaceae) were collected from Kordofan region, and authenticated (voucher specimen no. 798) at the Forestry Research Center (FRC), Khartoum, Sudan. A further verification was done by a taxonomist at the herbarium of College of Pharmacy, King Saud University, Saudi Arabia. The leaves were washed and dried at room temperature for a week and powdered (50 g) using mortar-pestle. The extraction was done with 500 mL of 70% ethanol (Merck) for 24 h with intermittent shaking, and repeated twice with fresh solvent. The extracts were pooled, passed through Whatmann filter paper, and dried under reduced pressure using rotary evaporator (R-210, BUCHI).
2.2. Antioxidant activity assays of GSLE
2.2.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The antioxidant activity was tested by DPPH radical scavenging ability of GSLE in a 96-well microplate as described elsewhere (Bouchet et al., 1998, Wong et al., 2014a, Wong et al., 2014b). Briefly, triplicates of 100 μL of GSLE (0.0, 31.25, 62.5, 125, 250 and 500 μg/mL) was mixed with 50 μL of 0.2 mM DPPH (Sigma, USA) in a 96-well flat-bottom microplate (Becton-Dickinson Labware, USA). Rutin, an antioxidant natural flavonoid was used as positive control. Following a 30 min incubation at 25 °C in dark, the absorbance (λ= 517 nm) was recorded using microplate spectrophotometer (BioRad, USA). The data was analyzed for radical scavenging activity of SGEE, using the following equation:
2.2.2. β-Carotene-linoleic acid bleaching assay
The antioxidant activity of GSLE was based on peroxidation of β-carotene and linoleic acid in a 96-well microplate as described elsewhere (Wong et al., 2014a, Wong et al., 2014b). Briefly, 0.5 mL β-carotene (500 μg/mL, in chloroform) was mixed with a solution of 12.5 μg of linoleic acid and 100 mg of Tween-40 followed by chloroform evaporation at 43 °C using speed vacuum concentrator (Savant, Thermo Electron Co.). The mixture was immediately diluted to 25 mL with distilled water and shaken vigorously for 2–3 min to form an emulsion. A 150 μL aliquot of the emulsion was added to wells of a 96-well plate containing 50 μL of GSLE or rutin (500 μg/mL), including a negative solvent control The plate was incubated at 50 °C for 2 h, and absorbance (λ = 470 nm) was recorded at 30 min intervals. The antioxidant activity of GSLE or controls was expressed as% inhibition of LPO using the formula:
2.3. In vitro hepatoprotective assessment of GSLE
The in vitro hepatoprotective activity of GSLE was tested on human hepatoma cell line, HuH7 cells, using MTT cell proliferation assay kits (Tervigen, UAS). HuH7 were grown in RPMI-1640 medium, supplemented with 10% heat-inactivated bovine serum (Gibco, UAS), 1x penicillin-streptomycin, and 1x sodium pyruvate streptomycin (HyClone Laboratories, USA) at 37 °C with 5% CO2. 2,7-Dichlorofluorescein (DCFH; Sigma, USA) was used to induce in vitro chemical cytotoxicity at pre-determined dose (IC50: 100 μg/mL for Huh7). Briefly, cells were seeded (0.5 × 105 cells/well, in triplicate) in a 96-well flat-bottom plate (Becton-Dickinson Labware, USA) and grown over night. Stock of GSLE was prepared in DMSO (100 mg/mL), followed by its dilution with culture media to different doses (250.0, 125.0, 62.5, 31.25, and 0.0 μg/mL). The final concentration of DMSO never exceeded > 0.1%, and therefore, the prepared doses showed non-cytotoxicity, including the highest one. The culture monolayer were replenished with fresh media containing DCFH (100 μg/mL) plus a dose of GSLE, including untreated as well as DCFH only-treated controls. The treated cells were incubated for 48 h at 37 °C followed by MTT assay as per the manufacturer’s guidelines. The absorbance (OD; λ = 570 nm) was recorded (BioTek, ELx800), and non-linear regression analysis was performed in Excel software to determine the cell survival.
The treated cells were also visually monitored under an inverted microscope (Optica) for the morphological alternations under the experimental conditions.
2.4. Solvents, standards and stock preparation
The commercial standards: rutin, β-carotene, β-amyrin, β-sitosterol, lupeol, ursolic acid and linoleic acid were procured (Sigma Aldrich, USA). The AR grade organic solvents (hexane, ethyl acetate, chloroform and methanol) and p-anisaldehyde were purchased (BDH, UK). HPLC grade methanol (Merck, Germany) was used for the preparation of standard stock solutions (1000 μg/ml) and their serial dilutions (β-amyrin and β-sitosterol: 10–100 μg/ml; lupeol and ursolic acid: 10–120 μg/mL).
2.5. Apparatus and instruments
For the application of different tracks of standards and GSLE, glass-backed silica gel 60F254 HPTLC plates (Merck, Germany) were used. CAMAG automatic TLC sampler-4 was used for the application of the standards and GSLE, band-wise to the plates and developed in automatic development chamber (ADC2) (CAMAG, Muttenz, Switzerland). The developed HPTLC Plates were documented by CAMAG TLC Reprostar3 and scanned in CATS 4 (CAMAG).
2.6. Instrumentation and conditions
The HPTLC analyses of β-amyrin and β-sitosterol (Method-I), and lupeol and ursolic acid (Method-II) in GSLE were carried out on 10 × 10 cm precoated HPTLC plates where the band size of each track was 6 mm wide and 7.3 mm apart. All the solvent dilutions (10 μL, each) were applied by micro syringe attached with the applicator on the HPTLC plate to furnish the linearity range of 100–1000 ng/band of β-amyrin and β-sitosterol, and 100–1200 ng/band of lupeol and ursolic acid. The rate of application of all samples on the HPTLC plates was 160 nL/s. The plates were developed in pre-saturated 20 × 10 cm twin-trough glass chamber at room temperature (25 ± 2 °C) and humidity (60 ± 5%). The solvents used were hexane and ethyl acetate (7.5:2.5, v/v) for Method-I, and chloroform and methanol (97:3, v/v) for Method-II. The developed plate was dried, derivatized with p-anisaldehyde, re-dried and quantified at UVmax 540 nm (Method-I) and at 630 nm (Method-II). Since, all marker compounds were soluble in methanol, the extraction of GSLE was carried out in methanol.
2.7. Method validation
Validations of the two developed HPTLC methods (Method-I and -II) were carried out as per the International Conference on Harmonization guidelines for linearity range, precision, recovery as accuracy, robustness, including limit of detection (LOD) and limit of quantification (LOQ) (ICH guideline, 2005). Recovery as accuracy studies involved the addition of a known amount of the analyte to the given sample and to determine how much percentage of analyte was added to the samples. In the present study, for β-amyrin, β-sitosterol, lupeol and ursolic acid known amounts i.e. 50%, 100% and 150% of each standard (200 ng) were added and, the percentage of these added amount were estimated to find out the recovery of the spiked standards. The precision (intra- and inter-day) of the two developed methods were evaluated by performing replicate analysis (n = 6) at low (400 ng/band), medium (600 ng/band), and high (800 ng/band), concentrations of the four biomarkers. The precision was recorded as standard deviation (SD) and% standard deviation of the response (% RSD) of each calibration level. The robustness of the HPTLC methods was performed to analyze their capacity to remain unaffected by small, but deliberate variations in mobile phase composition and volume, used for saturation and saturation-time in developing chamber that indicates reliability of the methods.
The robustness study was performed in replicate analysis (n = 6) for the four biomarkers at 300 ng/band concentration. The results were evaluated in terms of standard SD, %RSD and SEM of peak area. In method-I, the mobile phases were prepared with hexane and ethyl acetate (7.5:2.5, v/v) in different proportions (7.3:2.7 and 7.7:2.3, v/v) for the analysis of β-amyrin and β-sitosterol. In method-II, the different mobile phases (95:5 and 98:2, v/v) were prepared from chloroform: methanol (97:3, v/v) for the analysis of lupeol and ursolic acid. In addition to the slight variations in the mobile phases for robustness study, the mobile phase volume used for saturation was also varied to 18 mL to 22 mL from 20 mL. In addition, the saturation-time was also varied from 10 min to 30 min from 20 min. The calculation of LOD and LOQ were based on the RSD and slope (S) of the calibration curve, using the formulae: LOD = 3.3 (SD/S); LOQ = 10 (SD/S).
2.8. Statistical analysis
Results were expressed as mean ± SEM. Total variation present in a set of data was estimated by one-way analysis of variance (ANOVA) followed by Dunnet’s-test. P < 0.01 was considered significant.
3. Results
3.1. Free-radical scavenging and antioxidant activity of GSLE
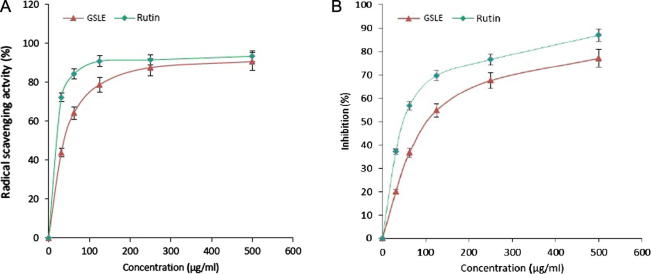
Two in vitro assays were employed to determine the antioxidative potential of GSLE. In the DPPH radical scavenging assay, GSLE (IC50: 82.71 μg/mL) showed dose-dependent activity (Fig. 2A). At concentrations of 31.25, 61.5, 125, 250 and 500 μg/ml, the radical scavenge activity were about 43.74%, 64.25%, 78.72%, 87.5%, and 90.6%, respectively. Interestingly, the radical scavenging activity of the extract at 500 μg/ml was comparable to that of rutin (93.3%). In the β-carotene-linolenic acid assay, GSLE (IC50: 128.7 μg/mL) also inhibited LPO in a dose-dependent manner (Fig. 2B), confirming its strong antioxidative potential.
Fig. 2.
In vitro antioxidant assays of G. senegalensis (leaves) ethanol-extract (GSLE). (A) DPPH radical scavenging method showing antioxidative activity, (B) β-carotene-linoleic acid bleaching method showing inhibition of lipid peroxidation by different concentrations (31.25–500 µg/mL) of GSLE compared to the standard antioxidant (rutin).
3.2. In vitro hepatoprotective potential of GSLE
In the HuH7 cell culture mode, DCFH (IC50: 100 μg/mL), induced severe hepatotoxicity as reflected by altered cell morphology compared to untreated control, observed under microscope. Interestingly, the DCFH-treated cells when supplemented with GSLE (125 and 250 μg/mL), showed normal morphology (data not shown). This was in accordance with the MTT assay that demonstrated the protection of DCFH-toxicated HuH7 cells to about 74% and 86% by 125 and 250 μg/mL doses of GSLE, respectively (Fig. 3). In line with our in vitro data, our GSLE showed significant hepatoprotective activity via attenuation of DCFH-induced oxidative stress and cytotoxicity.
Fig. 3.
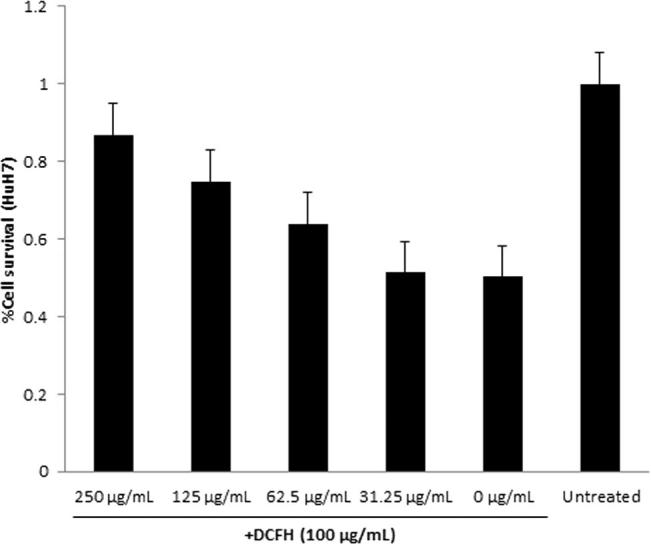
MTT cell proliferation assay, showing in vitro hepatoprotective effect of GSLE on cultured HuH7 cells. Values (Y-axis): means of three determinations.
3.3. Method development
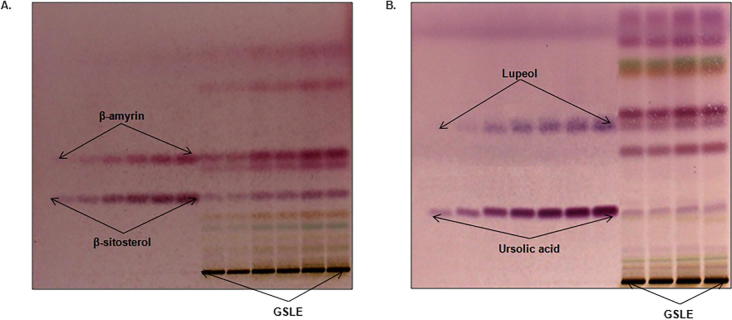
The selection of best mobile phase for the analysis of different biomarkers in Method-I and Method-II were carried out by running several TLC plates with different solvent combinations. The final solvent combination was chosen on the basis of its accurate reproducibility, separation efficiency of maximum phytoconstituents as well as different markers in simultaneous analysis. The mobile phase that fulfilled these criteria for Method-I was hexane and ethyl acetate (7.5:2.5, v/v) (Fig. 4A) whereas for Method-II it was chloroform and methanol (97:3, v/v) (Fig. 4B). The optimized saturation time and volume of mobile phase were observed as 20 min and 20 m, respectively. The developed HPTLC plates were then derivatized by spraying p-anisaldehyde reagent and heated to give compact spots of markers as well as different phytoconstituents present in GSLE.
Fig. 4.
Pictograms of TLC plates for G. senegalensis (leaves) ethanol-extract (GSLE) derivatized with p-anisaldehyde in day light. (A) Method-I (β-amyrin and β-sitosterol): mobile phase- hexane: ethylacetate (7.5:2.5; v/v), (B) Method-II (lupeol and ursolic acid): mobile phase- chloroform: methanol (97:3; v/v).
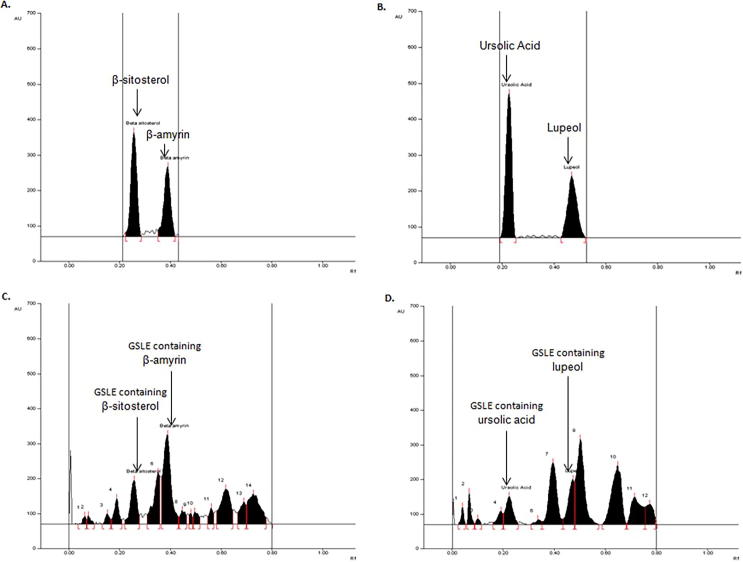
In Method-I, the densitometric analysis was carried out at 540 nm in the absorbance mode that furnished compact, sharp, symmetrical and high resolution bands of β-amyrin and β-sitosterol at Rf 0.39 ± 0.004 and 0.25 ± 0.005, respectively (Fig. 5A). In Method-II, the analysis was carried out at 630 nm that produced compact, sharp and high resolution band of lupeol and ursolic acid at Rf 0.47 ± 0.004 and 0.23 ± 0.005, respectively (Fig. 5B). Thus, the developed HPTLC methods were found to be quite selective with good baseline resolution.
Fig. 5.
Chromatograms of antioxidant biomarkers analysis in GSLE. (A) Chromatogram of β-sitosterol (Rf = 0.25) and β-amyrin (Rf = 0.39) scanned at λmax = 540 nm; mobile phase (Method I) - hexane: ethylacetate (7.5: 2.5; v/v). (B) Chromatogram of ursolic acid (Rf = 0.23) and lupeol (Rf = 0.47) scanned at λmax = 630 nm; mobile phase (Method II) – chloroform: methanol (97:3; v/v). (C) Chromatogram of GSLE (β-sitosterol, spot 5, Rf = 0.25; β-amyrin, spot 7, Rf = 0.39) scanned at λmax = 540 nm; mobile phase (Method I) – hexane: ethylacetate (7.5: 2.5; v/v). (D) Chromatogram of GSLE (ursolic acid, spot 5, Rf = 0.23; lupeol, spot 8, Rf = 0.47) scanned at λmax = 630 nm; mobile phase (Method II) - chloroform: methanol (97:3; v/v).
3.4. Method validation
Linearity of compounds β-amyrin, β-sitosterol, lupeol and ursolic acid were validated by using the linear regression equation (Y) and correlation coefficient (r2). The six-point calibration curve for β-amyrin and β-sitosterol was found to be linear in the range of 100–1000 ng/band while for lupeol and ursolic acid it was in the range of 100–1200 ng/band. The respective values of Y and r2 for the biomarkers were β-amyrin (8.727x + 306.63 and 0.9975 ± 0.0002), β-sitosterol (8.843x + 1249.27 and 0.9983 ± 0.0005), lupeol (3.892x + 324.92 and 0.9971 ± 0.0005) and ursolic (9.952x + 146.74 and 0.9987 ± 0.0004). Further analysis revealed a good linearity response for the developed methods (Table 1). The mean value (±SD) of the slope and intercept were found to be 8.727 ± 0.042 and 306.63 ± 11.53 for β-amyrin, 8.843 ± 0.049 and 1249.27 ± 20.955 for β-sitosterol, 3.892 ± 0.061 and 324.92 ± 13.259 for lupeol, and 9.952 ± 0.089 and 146.74 ± 11.432 for ursolic acid, respectively.
Table 1.
Rf, Linear regression data for the calibration curve of β-amyrin, β-sitosterol, lupeol and ursolic acid (n = 6).
| Parameters | β-Amyrin | β-Sitosterol | Lupeol | Ursolic Acid |
|---|---|---|---|---|
| Linearity range (ng/spot) | 100–1000 | 100–1000 | 100–1200 | 100–1200 |
| Regression equation | Y = 8.727x + 306.63 | Y = 8.843x + 1249.27 | Y = 3.892x + 324.92 | Y = 9.952x + 146.74 |
| Correlation (r2) coefficient | 0.9975 ± 0.0002 | 0.9983 ± 0.0005 | 0.9971 ± 0.0005 | 0.9987 ± 0.0004 |
| Slope ± SD | 8.727 ± 0.042 | 8.843 ± 0.049 | 3.892 ± 0.061 | 9.952 ± 0.089 |
| Intercept ± SD | 306.63 ± 11.53 | 1249.27 ± 20.955 | 324.92 ± 13.259 | 146.74 ± 11.432 |
| Standard error of slope | 0.017 | 0.020 | 0.025 | 0.036 |
| Standard error of intercept | 4.709 | 8.553 | 5.412 | 4.666 |
| Rf | 0.39 ± 0.004 | 0.25 ± 0.005 | 0.47 ± 0.004 | 0.23 ± 0.005 |
| LOD (ng) | 16.24 | 18.56 | 52.24 | 29.57 |
| LOQ (ng) | 49.21 | 56.26 | 158.32 | 89.61 |
The recoveries as accuracy study for the developed methods were recorded for β-amyrin, β-sitosterol, lupeol and ursolic acid (Table 2). The recovery was 99.23–100.14% for β-amyrin, 99.26–100.18% for β-sitosterol, 99.58–100.23% for lupeol and 99.03–100.78% for ursolic acid. The %RSD was 0.338–0.396 for β-amyrin, 0.608–0.703 for β-sitosterol, 0.270–0.331 for lupeol, and 0.204–0.184 for ursolic acid, respectively. The intra-/inter-day precision (n = 6) for β-amyrin and β-sitosterol in Method-I and those for lupeol and ursolic acid in Method-II (Table 3) were recorded. In Method-I, the respective intra-/inter-day %RSD for β-amyrin and β-sitosterol were 0.205–0.235%/0.190–0.232% and 0.355–0.375%/0.337–0.355%, respectively which demonstrated the good precision of this method. Likewise, in Method-II, the respective intra-/inter-day %RSD for lupeol and ursolic acid were 0.238–0.262%/0.229–0.249% and 0.131–0.147%/0.127–0.140% that also demonstrated the good precision of this method.
Table 2.
Recovery as accuracy studies of the proposed method of β-amyrin, β-sitosterol, lupeol and ursolic acid (n = 6).
| Stand. added to analyte (%) | Theor. conc. of stand. (ng/μL) | Method I |
Method II |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Amyrin |
β-Sitosterol |
Lupeol |
Ursolic acid |
||||||||||
| Conc. found (ng/μL) ± SD | % RSD | % Recovery | Conc. found (ng/μL) ± SD | % RSD | % Recovery | Conc. found (ng/μL) ± SD | % RSD | % Recovery | Conc. found (ng/μL) ± SD | % RSD | % Recovery | ||
| 0 | 200 | 199.91 ± 0.69 | 0.347 | 99.95 | 199.94 ± 1.21 | 0.608 | 99.97 | 199.83 ± 0.65 | 0.326 | 99.91 | 201.57 ± 0.41 | 0.204 | 100.78 |
| 50 | 300 | 298.94 ± 1.01 | 0.338 | 99.65 | 300.55 ± 1.96 | 0.652 | 100.18 | 300.69 ± 0.81 | 0.270 | 100.23 | 297.09 ± 0.52 | 0.176 | 99.03 |
| 100 | 400 | 400.56 ± 1.42 | 0.356 | 100.14 | 398.34 ± 2.71 | 0.682 | 99.58 | 398.31 ± 1.16 | 0.290 | 99.58 | 401.25 ± 0.71 | 0.178 | 100.31 |
| 150 | 500 | 496.13 ± 1.96 | 0.396 | 99.23 | 496.32 ± 3.49 | 0.703 | 99.26 | 499.04 ± 1.65 | 0.331 | 99.80 | 500.56 ± 0.92 | 0.184 | 100.11 |
Stand.: Standards; Theor.: Theoretical; Conc.: Concentration.
Table 3.
Precision of the proposed HPTLC Method-I & II (n = 6).
| Concentration of standard added (ng/spot) | β-Amyrin (Method I) |
β-Sitosterol (Method I) |
||||||
|---|---|---|---|---|---|---|---|---|
| Intra-day precision |
Inter-day precision |
Intra-day precision |
Inter-day precision |
|||||
| Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | |
| 400 | 399.87 ± 0.81 | 0.205 | 398.72 ± 0.76 | 0.190 | 400.55 ± 1.42 | 0.355 | 399.42 ± 1.39 | 0.348 |
| 600 | 598.71 ± 1.33 | 0.222 | 597.56 ± 1.29 | 0.216 | 597.02 ± 2.16 | 0.362 | 595.89 ± 2.01 | 0.337 |
| 800 | 797.29 ± 1.87 | 0.235 | 793.86 ± 1.84 | 0.232 | 796.75 ± 2.99 | 0.375 | 793.36 ± 2.82 | 0.355 |
| Concentration of standard added (ng/spot) | Lupeol (Method II) | Ursolic acid (Method II) | ||||||
| Intra-day precision | Inter-day precision | Intra-day precision | Inter-day precision | |||||
| Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | Average Conc. found ± SD | %RSD | |
| 400 | 399.51 ± 0.95 | 0.238 | 396.94 ± 0.91 | 0.229 | 399.71 ± 0.52 | 0.131 | 397.19 ± 0.50 | 0.127 |
| 600 | 600.69 ± 1.51 | 0.251 | 598.12 ± 1.40 | 0.234 | 598.79 ± 0.81 | 0.136 | 595.77 ± 0.78 | 0.131 |
| 800 | 798.12 ± 2.09 | 0.262 | 795.55 ± 1.98 | 0.249 | 794.83 ± 1.17 | 0.147 | 791.81 ± 1.11 | 0.140 |
The data for the robustness studies for β-amyrin and β-sitosterol (Table 4) and for lupeol and ursolic acid (Table 5) were documented. The low value of SD and %RSD obtained after introducing small deliberate changes indicated that the robustness of the two methods. The respective LOD/LOQ for β-amyrin, β-sitosterol, lupeol and ursolic acid were 16.24/49.21, 18.56/56.26, 52.24/158.32 and 29.57/89.61 ng/band (Table 1). This observation further indicated that the two HPTLC methods had good sensitivity for the quantification of the four compounds in GSLE.
Table 4.
Robustness of the proposed HPTLC Method I at 300 ng/band (n = 6).
| Optimization condition | β-Amyrin |
β-Sitosterol |
||
|---|---|---|---|---|
| SD | %RSD | SD | %RSD | |
| Mobile phase composition; (hexane: ethyl acetate) | ||||
| (7.5:2.5) | 1.526 | 0.383 | 2.662 | 0.669 |
| (7.3:2.7) | 1.589 | 0.399 | 2.581 | 0.650 |
| (7.7:2.3) | 1.685 | 0.425 | 2.515 | 0.630 |
| Mobile phase volume (for saturation) | ||||
| (18 mL) | 1.506 | 0.378 | 2.619 | 0.662 |
| (20 mL) | 1.529 | 0.385 | 2.513 | 0.637 |
| (22 mL) | 1.585 | 0.400 | 2.573 | 0.649 |
| Duration of saturation | ||||
| (10 min) | 1.496 | 0.376 | 2.589 | 0.653 |
| (20 min) | 1.517 | 0.383 | 2.574 | 0.650 |
| (30 min) | 1.581 | 0.396 | 2.549 | 0.641 |
Table 5.
Robustness of the proposed HPTLC Method II at 300 ng/band (n = 6).
| Optimization condition | Lupeol |
Ursolic acid |
||
|---|---|---|---|---|
| SD | %RSD | SD | %RSD | |
| Mobile phase composition; (chloroform: methanol) | ||||
| (97:3) | 0.824 | 0.276 | 0.612 | 0.207 |
| (95:5) | 0.832 | 0.278 | 0.724 | 0.246 |
| (98:2) | 0.845 | 0.282 | 0.916 | 0.309 |
| Mobile phase volume (for saturation) | ||||
| (18 mL) | 0.829 | 0.280 | 0.912 | 0.309 |
| (20 mL) | 0.851 | 0.287 | 0.924 | 0.314 |
| (22 mL) | 0.812 | 0.273 | 0.856 | 0.290 |
| Duration of saturation | ||||
| (10 min) | 0.831 | 0.279 | 0.812 | 0.277 |
| (20 min) | 0.862 | 0.289 | 0.844 | 0.289 |
| (30 min) | 0.875 | 0.294 | 0.866 | 0.294 |
3.5. Application of the validated HPTLC methods in the quantitative analysis of β-amyrin, β-sitosterol, lupeol and ursolic acid in GSLE
Further applicability of the developed and validated HPTLC methods were tested for the quantitative analysis of β-amyrin and β-sitosterol (Fig. 5C) and lupeol and ursolic acid (Fig. 5D) in GSLE. With these methods, the contents of β-amyrin, β-sitosterol, lupeol and ursolic acid were estimated to be 20.64 μg/mg, 16.35 μg/mg, 6.73 μg/mg, and 5.81 μg/mg of the dry weight of GSLE. To the best of our knowledge, this is the first report on simple, accurate and rapid HPTLC methods developed and validated for the simultaneous quantification of β-amyrin, β-sitosterol, lupeol and ursolic acid in G. senegalensis.
4. Discussion
G. senegalensis J.F. Gmel is a very popular African folk-medicinal plant with antioxidative, gastroprotective, anti-microbial and antiviral potential (Suleiman, 2015, Bosisio et al., 1997, Somboro et al., 2011). DCFH is commonly used to evaluate experimental oxidative stress caused by free-radicals or ROS through the principle of oxidation of DCFH to the fluorescent DCF (Rota et al., 1999). Notably, DCFH is also highly cytotoxic to cultured human cell lines, including hepatoma cells (Al-Yahya et al., 2013, Arbab et al., 2016). In the present study, MTT assay has demonstrated the hepatoprotective activity of GSLE via attenuation of DCFH-induced oxidative stress and cytotoxicity in cultured hepatoma cells, HuH7. Further, our DPPH radical-scavenging and β-carotene-linoleic acid bleaching assays showed dose-dependent antioxidant efficacy of GSLE. Very recently, we have demonstrated a robust anti-HBV activity of G. senegalensis (Arbab et al., 2017) in accordance with its previously reported antiviral property against FPV and HSV (Lamien et al., 2005, Silva et al., 1997). The antiviral activities by natural or herbal products are suggested via three mechanisms: firstly, direct inhibition; secondly, enhancing host immunity; and thirdly, abating inflammation and protecting cells from oxidative stress or damages. The indirect antiviral efficacy of Ampelopsis silica root extract against HSV (Chen and Yang, 1999) and duck hepatitis B virus (DHBV) (Chen et al., 2000) was thus, attributed to its anti-inflammatory and antioxidative activity (Chen et al., 2005). In line with this, the in vitro and in vitro antioxidative, hepatoprotective and anti-HBV potential of Acacia mellifera leaves extract has also been demonstrated, recently (Arbab et al., 2015).
The phytochemical analysis of G. senegalensis has revealed presence of various bioactive flavonoids (eg., catechin, myricitrin, rutin and quercetin), alkaloids (e.g., harman and eleagnine), a naphthyl butenone (guieranone A) and tannins (eg., epicatechin, epigallocatechin gallate and galloylquinic acid (Ficarra et al., 1997, Silva and Gomes, 2003, Bouchet et al., 1998). Of the identified tannins, radical scavenging and antioxidant activities of galloylquinic acid have been studied. However, to our knowledge, the triterpenes of known therapeutic potentials including antioxidant and antiviral activities are not reported in G. senegalensis. Natural triterpenes are structurally diverse group of triterpenoids, having linear and, tetra or pentacyclic carbon skeleton. Bioactive triterpenes are isolated from a variety of plants with antimicrobial, anticancer, hepatoprotective, anti-inflammatory, and antioxidative salutations (Pavlova et al., 2003, Woldemichael et al., 2003, Aiken and Chen, 2005, Fernandez, 2011, Sultana and Saify, 2012). Moreover, the pentacyclic triterpenes are shown to have antiviral properties against HSV (Heidary et al., 2014, Joycharat et al., 2008, Tanaka et al., 2004) and HIV (Cichewicz and Kouzi, 2004), the genetically close viruses to HBV. Notably, β-amyrin (oleanane-type pentacyclic triterpenoid) is shown to have antiviral efficacies against influenza A and HSV (Rao et al., 1974). In our analysis, the high quantity of β-amyrin (20.64 μg/mg) in antioxidant GSLE strongly supports its anti-HBV activity, probably by attenuating the cellular oxidative mechanism.
Ursolic acid (ursane-type pentacyclic triterpenoid), also known as urson, prunol, micromerol or malol is one of the most promising therapeutic natural compound. It has shown in vitro and in vitro hepatoprotective activity against ethanol-toxicity by elevating levels of antioxidant molecules and serum protein while decreasing the total bilirubin and LPO markers (Saraswat et al., 2000, Saravanan et al., 2006). Also, ursolic acid treatment is shown to ameliorate paracetamol- and carbon tetrachloride-induced liver injury by increasing hepatocytes viability and improvement of serum markers (Shukla et al., 1992, Martin-Aragón, 2001). Interestingly, ursolic acid is also found to have potent antiviral activity against HSV, human immunodeficiency virus (HIV) and human hepatitis C virus (HCV) (Filho et al., 2010, Hattori et al., 2013, Ma et al., 1999, Quéré et al., 1996). Our identification of ursolic acid (5.81 μg/mg) in the anti-HBV active GSLE thus, endorses its antioxidative potential, and could correlate with its anti-HBV activity via abating cellular oxidative stress.
The triterpenoids, also known as phytosterols have wide spectrum of biological activities including, anti-inflammatory, hypocholesterolemic, and insulin-regulating potential (Kong et al., 2013, Yamamoto et al., 1991, Bouic et al., 1996). Of these, lupeol has in vivo and in vitro anti-inflammatory, anti-microbial, anti-protozoal, anti-proliferative, anti-invasive, anti-angiogenic and hypocholesterolemic efficacies (Ivorra et al., 1988). Lupeol is reported to decrease the ROS level and restore the antioxidant enzyme activities in mouse liver against chemical-induced oxidative stress (Siddique et al., 2011). Also, lupeol treatment is shown to induce growth inhibition and apoptosis in HCC cell line, SMMC7721 (Prasad et al., 2007). Though lupeol has shown weak antiviral activities in several studies, it has served as a lead drug for the generation of more effective compounds against Influenza A and HSV (Zhang et al., 2009). On the contrary, lupeol isolated from Strobilanthes cusia root has demonstrated a robust anti-HSV activity, in vitro (Flekhter et al., 2004). Notably, to the best of our knowledge, the antiviral activity of lupeol against hepatitis viruses has not been reported, so far. Nevertheless, lupeol (6.72 μg/mg) identified in the antioxidant GSLE, could have a direct or indirect role in the plant’s anti-HBV activity.
β-sitosterol, a phytosterol has been shown to exhibit in vitro and in vitro anti-HIV activity by immunomodulatory mechanism though stabilization of CD4+ T-lymphocyte counts, and a significant decrease in interleukin-6 level (Tanaka et al., 2004). Very recently, antiviral effect of β-sitosterol isolated from cottonseed oil against tobacco mosaic virus (TMV) is reported (Bouic, 1997). Interestingly, β-sitosterol has been also shown to attenuate in vitro chemical-induced hepatotoxicity (Arbab et al., 2016) and cardiotoxicity by enhancing mitochondrial glutathione redox mechanism (Zhao et al., 2015). In line with this, our identification of β-sitosterol (l6.72 μg/mg) as the second most abundant compound in GSLE strongly supports its in vitro antioxidative, hepatoprotective and anti-HBV activities, independently or interdependently.
5. Conclusion
The present study has demonstrated antioxidative and hepatoprotective potential of anti-HBV active GSLE. Further, HPTLC analysis of biomarkers (β-amyrin, β-sitosterol, lupeol and ursolic acid) of known antioxidant and antiviral activities, strongly supported the anti-HBV efficacy of GSLE via abating the cellular oxidative stress molecules. Our findings therefore, warrant for the therapeutic potential of G. senegalensis against hepatitis B associated chronic liver disease. Nevertheless, bioactivity guided fractionation and isolation of active principles from G. senegalensis leaves, and their detailed analysis would be necessary. To our knowledge, this is the first report on HPTLC analysis of triterpenes in G. senegalensis that could be further adopted for the standardization and quality-control of marketed herbal-formulations.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RG-1435-053.
Acknowledgments
Conflict of interest statement
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad K. Parvez, Email: mohkhalid@ksu.edu.sa.
Perwez Alam, Email: aperwez@ksu.edu.sa.
Ahmed H. Arbab, Email: arbabssn@gmail.com.
Mohammed S. Al-Dosari, Email: mdosari@ksu.edu.sa.
Tawfeq A. Alhowiriny, Email: talhowiriny@ksu.edu.sa.
Saleh I. Alqasoumi, Email: sqasoumi@ksu.edu.sa.
References
- Aiken C., Chen C.H. Betulinic acid derivatives as HIV-1 antivirals. Trends Mol. Med. 2005;11:31–36. doi: 10.1016/j.molmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Akuodor G.C., Essien A.D., David-Oku E., Chilaka K.C., Akpan J.L., Ezeokpo B., Ezeonwumelu J.O. Gastroprotective effect of the aqueous leaf extract of Guiera senegalensis in Albino rats. Asian Pac. J. Trop Med. 2013;6:771–775. doi: 10.1016/S1995-7645(13)60136-4. [DOI] [PubMed] [Google Scholar]
- Al-Yahya Y., Al-Dosari M.S., Al-Sohaibani M., AlSaid M., Mothana R., Parvez M.K., Rafatullah S. Attenuation of CCl4-induced oxidative stress and Hepato-nephrotoxicity by Saudi Sidr Honey in Rats. Evid. Based Complementary Altern. Med. 2013;2013:1–10. doi: 10.1155/2013/569037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A.H., Parvez M.K., Al-Dosari M.S., Al-Rehaily A.J. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp. Ther. Med. 2017;14:626–634. doi: 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A.H., Parvez M.K., Al-Dosari M.S., Al-Rehaily A.J., Ibrahim K.E., Alam P., AlSaid M.S., Rafatullah S. Therapeutic efficacy of ethanolic extract of Aerva Javanica aerial parts in the amelioration of CCl4-induced hepatotoxicty and oxidative damage in rats. Food Nutr. Res. 2016;60:30864–30873. doi: 10.3402/fnr.v60.30864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A.H., Parvez M.K., Al-Dosari M.S., Rafatullah S., Al-Sohaibani M., Zorag E.A., Al-Rehaily A.J., AlSaid M.S. Hepatoprotective and antiviral efficacy of Acacia mellifera leaves fractions against hepatitis B virus. BioMed. Res. Int. 2015;2015:929131. doi: 10.1155/2015/929131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolukbas C., Bolukbas F.F., Horoz M., Aslan M., Celik H., Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect. Dis. 2005;5:95–98. doi: 10.1186/1471-2334-5-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosisio E., Mascetti D., Verotta L., Zani F., Mazza P., Talbot P. Guiera senegalensis J.F. Gmelin (Combretaceae): Biological activities and chemical investigation. Phytomed. 1997;3:339–348. doi: 10.1016/S0944-7113(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Bouchet N., Barrier L., Fauconneau B. Radical scavenging activity and antioxidant properties of tannins from Guiera senegalensis (Combretaceae) Phytother. Res. 1998;12:159–162. [Google Scholar]
- Bouchet N., LeÂ, vesque J., Blond A., Bodo B., Pousset J.L. 1,3-di-O-galloylquinic acid from Guiera senegalensis. Phytochemistry. 1996;42:189–190. [Google Scholar]
- Bouic P.J.D., Etsebeth R.W., Liebenberg C.F. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- Bouic P.J.D. Immunomodulation in HIV/AIDS: The tygerberg/stellenbosch university experience. AIDS Bull. 1997;6:18–20. [Google Scholar]
- Chen K., Yang Z. The acting part anti-HSV-1 in infected cells of extract from Ampelopsis sinica roots. J. Chin. Drugg. 1999;2:225–226. [Google Scholar]
- Chen K., Li H., Chen Y., Zhang C. Inhibition of extracts of Ampelopsis sinica roots on DHB vs. Ag in sera of ducklings. Zhong Yao Cai. 2000;23:46–47. [PubMed] [Google Scholar]
- Chen K., Plumb G.W., Bennett R.N., Bao Y. Antioxidant activities of extracts from five anti-viral medicinal plants. J. Ethnopharmacol. 2005;96:201–205. doi: 10.1016/j.jep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004;24:90–114. doi: 10.1002/med.10053. [DOI] [PubMed] [Google Scholar]
- Djordjević V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- Fernandez M.A., de las Heras B., Garcia M.D., Saenz M.T., Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2011;53:1533–1539. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- Ficarra R., Ficarra P., Tommasini S., Carulli M., Melardi S., Di Bella M.R., Calabro M.L., De Pasquale R., Germano M.P., Sanogo R., Casuscelli F. Isolation and characterization of Guiera senegalensis J.F. Gmel. active principles. Boll Chim Farm. 1997;136:454–459. [PubMed] [Google Scholar]
- Filho J.R., de Sousa Falcão H., Batista L.M., Filho J.M., Piuvezam M.R. Effects of plant extract on HIV-1 protease. Curr. HIV Res. 2010;8:531–544. doi: 10.2174/157016210793499204. [DOI] [PubMed] [Google Scholar]
- Flekhter O.B., Boreko E.I., Nigmatullina L.P., Pavlova N.I., Medvedeva N.I., Nikolaeva S.N., Ashavina O.A., Savinova O.V., Baltina L.A., Galin F.Z., Tolstikov G.A. Synthesis and antiviral activity of lupane triterpenoids and their derivatives. Pharm. Chem. J. 2004;38:355–358. [Google Scholar]
- Hattori M., Ma C.M., Wei Y., Salah El Dine R., Sato N. Survey of anti-HIV and anti-HCV compounds from natural sources. Can. Chem. Tran. 2013;1:116–140. [Google Scholar]
- Ha H.L., Shin H.J., Feitelson M.A., Yu D.Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010;16:6035–6043. doi: 10.3748/wjg.v16.i48.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary N.M., Laszczyk-Lauer M.N., Reichling J., Schnitzler P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine. 2014;21:1273–1280. doi: 10.1016/j.phymed.2014.06.007. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Text and Methodology Q2 (R1), Comp. Guideline Methodol. incorporated in November 2005 by the ICH Steering Committee, IFPMA, Geneva.
- Ivorra M.D., D’Ocon M.P., Paya M., Villar A. Antihyperglycemic and insulin releasing effects of beta-sitosterol 3-B-D-glucoside and its aglycone beta-sitosterol. Arch. Int. Pharmacodyn. Therapy. 1988;296:224–231. [PubMed] [Google Scholar]
- Joycharat N., Greger H., Hofer O., Saifah E. Flavaglines and triterpenoids from the leaves of Aglaia forbesii. Phytochemistry. 2008;69:206–211. doi: 10.1016/j.phytochem.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Kong L., Li S., Liao Q., Zhang Y., Sun R., Zhu X., Zhang Q., Wang J., Wu X., Fang X. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013;98:44–53. doi: 10.1016/j.antiviral.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Lamien C.E., Meda A., Mans J., Romito M., Nacoulma O.G., Viljoen G.J. Inhibition of fowlpox virus by an aqueous acetone extract from galls of Guiera senegalensis J. F. Gmel (Combretaceae) J. Ethnopharmacol. 2005;96:249–253. doi: 10.1016/j.jep.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ma C.M., Nakamura N., Miyashiro H., Hattori M., Shimotohno K. Inhibitory effect of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem. Pharm. Bull. 1999;47:141–145. doi: 10.1248/cpb.47.141. [DOI] [PubMed] [Google Scholar]
- Martin-Aragón S., de las Heras B., Sanchez-Reus M.I., Benedi J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001;53:199–206. doi: 10.1078/0940-2993-00185. [DOI] [PubMed] [Google Scholar]
- Niu D., Zhang J., Ren Y., Feng H., Chen W.N. HBx genotype D represses GSTP1 expression and increases the oxidative level and apoptosis in HepG2 cells. Mol. Oncol. 2009;3:67–76. doi: 10.1016/j.molonc.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opara E.C. Oxidative stress. Dis Mon. 2006;2:183–198. doi: 10.1016/j.disamonth.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Osman I.M., Mohammed A.S., Abdalla A.B. Acaricidal properties of two extracts from Guiera senegalensis J.F. Gmel. (Combrataceae) against Hyalomma anatolicum (Acari: Ixodidae) Vet. Parasitol. 2014;199:201–205. doi: 10.1016/j.vetpar.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Pavlova N.I., Savinova O.V., Nikolaeva S.N., Boreko E.I., Flekhter O.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia. 2003;74:489–492. doi: 10.1016/s0367-326x(03)00123-0. [DOI] [PubMed] [Google Scholar]
- Prasad S., Kalra N., Shukla Y. Hepatoprotectic effects of lupeol and mango pulp extract on carcinogen induced alteration in Swiss albino mice. Mol. Nutr. Food Res. 2007;51:352–359. doi: 10.1002/mnfr.200600113. [DOI] [PubMed] [Google Scholar]
- Quéré L., Wenger T., Schramm H.J. Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem. Biophys. Res. Commun. 1996;227:484–488. doi: 10.1006/bbrc.1996.1533. [DOI] [PubMed] [Google Scholar]
- Rao G.S., Sinsheimer J.E., Cochran K.W. Antiviral activity of triterpenoid saponins containing acylated β-amyrinaglycones. J. Pharm. Sci. 1974;63:471–473. doi: 10.1002/jps.2600630341. [DOI] [PubMed] [Google Scholar]
- Rota C., Chignell C.F., Mason R.P. Evidence for free radical formation during the oxidation of 2'-7'-dichlorofluorescin to the fluorescent dye 2'-7'- dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic. Biol. Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Saraswat B., Visen P.K.S., Agarwal D.P. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother Res. 2000;14:163–166. doi: 10.1002/(sici)1099-1573(200005)14:3<163::aid-ptr588>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Saravanan R., Pugalendi V., Pugalendi K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78:713–718. doi: 10.1016/j.lfs.2005.05.060. [DOI] [PubMed] [Google Scholar]
- Severi T., Ying C., Vermeesch J.R., Cassiman D., Cnops L., Verslype C., Fevery J., Arckens L., Neyts J., van Pelt J.F. Hepatitis B virus replication causes oxidative stress in HepAD38 liver cells. Mol. Cell Biochem. 2006;290:79–85. doi: 10.1007/s11010-006-9167-x. [DOI] [PubMed] [Google Scholar]
- Shukla B., Visen P.K.S., Patnaik G.K., Tripathi S.C., Srimal R.C., Dayal R., Dobhal P.C. Hepatoprotective activity in the rat of ursolic acid isolated from Eucalyptus hybrid. Phytother. Res. 1992;6:74–79. [Google Scholar]
- Siddique H.R., Saleem M. Beneficial health effects of lupeol triterpene: a review of preclinical studies. Life Sci. 2011;88:285–293. doi: 10.1016/j.lfs.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Silva O., Barbosa S., Diniz A., Valdeira M.L., Gomes E. Plant extracts antiviral activity against Herpes simplex virus type 1 and African swine fever virus. Int. J. Pharmacogn. 1997;35:12–16. [Google Scholar]
- Silva O., Gomes E.T. Guieranone A, a naphthyl butenone from the leaves of Guiera senegalensis with antifungal activity. J. Nat. Prod. 2003;66:447–449. doi: 10.1021/np0204904. [DOI] [PubMed] [Google Scholar]
- Shaban N.Z., Salem H.H., Elsadany M.A., Ali B.A., Hassona E.H., Mogahed F.A.K. Alterations in lipid peroxidation and antioxidants in patients with different stages of Hepatitis B Virus infection in Egypt. Life Sci. 2014;11:960–967. [Google Scholar]
- Somboro A.A., Patel K., Diallo D., Sidibe L., Chalchat J.C., Figueredo G., Ducki S., Troin Y., Chalard P. An ethnobotanical and phytochemical study of the African medicinal plant Guiera senegalensis J. F. Gmel. J. Med. Plants Res. 2011;5:1639–1651. [Google Scholar]
- Sombié P.A.E.D., Hilou A., Mounier C., Coulibaly A.Y., Kiendrebeogo M., Millogo J.F., Nacoulma O.G. Antioxidant and anti-inflammatory activities from galls of Guiera senegalensis J.F. Gmel. (Combretaceae) Res. J. Med. Plants. 2011;5:448–461. [Google Scholar]
- Suleiman M.H. An ethnobotanical survey of medicinal plants used by communities of Northern Kordofan region, Sudan. J. Ethnopharmacol. 2015;176:232–242. doi: 10.1016/j.jep.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Sultana N., Saify Z.S. Naturally occurring and synthetic agents as potential anti-inflammatory and immunomodulants. Antiinflamm Antiallergy Agents Med Chem. 2012;11:3–19. doi: 10.2174/187152312803476264. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ikeda T., Kaku M., Zhu X.H., Okawa M., Yokomizo K., Uyeda M., Nohara T.A. new lignan glycoside and phenylethanoid glycosides from Strobilanthes cusia BREMEK. Chem. Pharm. Bull. 2004;52:1242–1245. doi: 10.1248/cpb.52.1242. [DOI] [PubMed] [Google Scholar]
- Woldemichael G.M., Singh M.P., Maiese W.M., Timmermann B.N. Constituents of antibacterial extract of Caesalpinia paraguariensis BURK Z. Naturforsch C. 2003;58:70–75. doi: 10.1515/znc-2003-1-213. [DOI] [PubMed] [Google Scholar]
- Wong H.S., Chen J.H., Leong P.K., Leung H.Y., Chan W.M., Ko K.M. β-sitosterol protects against carbon tetrachloride hepatotoxicity but not gentamicin nephrotoxicity in rats via the induction of mitochondrial glutathione redox cycling. Molecules. 2014;19:17649–17662. doi: 10.3390/molecules191117649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.S., Chen N., Leong P.K., Ko K.M. β-Sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: protection against oxidant injury in H9c2 cells and rat hearts. Phytother. Res. 2014;28:999–1006. doi: 10.1002/ptr.5087. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Matsui T., Sugiyama K., Yokota M., Nukagomi K., Nakazawa H. Anti-inflammatory active constituents of Aloe arborescens Miller. Agric. Biol. Chem. 1991;55:1627–1629. [Google Scholar]
- Zhang L., Zhang Y., Zhang L., Yang X., Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;27:163–170. doi: 10.1080/07357900802210745. [DOI] [PubMed] [Google Scholar]
- Zhao L., Feng C., Hou C., Hu L., Wang Q., Wu Y. First discovery of acetone extract from cottonseed oil sludge as a novel antiviral agent against plant viruses. PLoS One. 2015;10:e0117496. doi: 10.1371/journal.pone.0117496. [DOI] [PMC free article] [PubMed] [Google Scholar]