Abstract
Two novel polysaccharides, Plumula nelumbinis (P. nelumbinis) polysaccharide I (LNP I) and P. nelumbinis polysaccharide II (LNP II), were extracted and purified from P. nelumbinis, and a sulfated polysaccharide, P. nelumbinis polysaccharide III (LNP III), with a substitution degree of 0.62 was prepared from LNPI. The structures of the LNPs were preliminarily characterized using high performance size exclusion chromatography (HPSEC), gas chromatography-mass spectrometry (GC–MS), Fourier transformed infrared spectrometry (FT-IR), and nuclear magnetic resonance (NMR) spectrometry. In addition, evaluation of the antioxidant activity of the LNPs showed that they could significantly increase the proliferation of RAW264.7 macrophages (P < 0.05) and improve the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) based on cell model of H2O2-induced oxidative damage. This suggested that these LNPs may be used as potential antioxidants.
Keywords: Plumula nelumbinis, Polysaccharides, Sulfated modification, Antioxidant activity
1. Introduction
Plumula nelumbinis (P. nelumbinis) (called “Lian Zi Xin” in Chinese) is the embryo of the seed of Nelumbo nucifera Gaertn. P. nelumbinis, is not only used for making tea in China, but has also been widely used as a traditional Chinese medicine for hundreds of years for the treatment of various diseases, especially mood disorders, thirsty, spermatorrhea, and bleeding (Jiangsu New Medical College, 1986). Modern pharmacological studies have revealed that the P. nelumbinis extract ameliorates loss of pancreatic islets, improves serum lipid profiles (Liao and Lin, 2013a), exerts cytoprotective effect against oxidative stress (Xie et al., 2013), possesses anti-inflammatory (Liao and Lin, 2013b), suppresses angiotens in II–induced fractalkine production (Cao et al., 2014), inhibits hepatic fibrosis (Liu et al., 2015), and shows anti-arrhythmic activity (Wu and Xiao, 2006). A variety of chemical constituents with different structural features have been reported from the extracts of P. nelumbinis, such as alkaloids (Lin et al., 2013, Lin et al., 2014, Itoh et al., 2011), flavonoids (Shao et al., 2016), phytosteroids (Lou et al., 1995), and polysaccharides (Liao et al., 2011, Liao and Lin, 2012, Liao and Lin, 2013a, Liao and Lin, 2013b).
Oxidation, induced by oxygen radicals, occurs in all organisms, and contributes to the development of various diseases, including cancer, rheumatoid arthritis, and atherosclerosis, as well as in degenerative processes like aging (Finkel and Holbrook (2000)). Reactive oxygen species (ROS), which includes various free radicals, are produced during cellular metabolism and play a crucial role in oxidation, cell signaling, apoptosis, gene expression, and ion transportation (Vajragupta et al., 2004). The superoxide anion radical (•O2−), hydroxyl radical (•OH), and hydrogen peroxide (H2O2) are the main ROS, and their over-production can damage DNA, RNA, proteins and lipids, resulting in an increased risk for cardiovascular disease, cancer, autism, and other diseases (Lü et al., 2010). Therefore, antioxidation play an important role in protection from ROS damage and may help in maintaining adequate antioxidant status in the organism. It has reported that polysaccharide is a potential antioxidant (Zhang et al., 2013, Varoni et al., 2017, Huang et al., 2016, Liu et al., 2018).
Currently, polysaccharides have drawn increased attention from researchers and consumers, due to their relatively low toxicity and potent biological activity, such as anti-tumor, antioxidative, antidiabetic, and anti-hyperlipidemia effects. Several polysaccharides have been isolated and characterized from P. nelumbinis (Liao et al., 2011, Liao and Lin, 2012, Liao and Lin, 2013a, Liao and Lin, 2013b). Moreover, activities of these polysaccharides have been evaluated for amelioration of the loss of pancreatic islets and improvement of serum lipid profiles in non-obese diabetic mice, anti-inflammatory activity via decrease in Toll-like receptor-2 and -4 expression in mouse primary splenocytes, and decrease in the secretion ratios of pro-/anti-inflammatory (IL-6/IL-10) cytokinesin LPS-stimulated RAW264.7 macrophages (Liao et al., 2011, Liao and Lin, 2012, Liao and Lin, 2013a, Liao and Lin, 2013b). However, the monosaccharide composition of polysaccharides isolated from P. nelumbinis in this study were different from the polysaccharides reported in literature (Liao et al., 2011, Liao and Lin, 2012, Liao and Lin, 2013a, Liao and Lin, 2013b). In addition, the antioxidative activity of the P. nelumbinis polysaccharides, especially the sulfated polysaccharide, has not been evaluated.
In this study, we performed preliminary profiling of the chemical characteristics of polysaccharides I and II (LNP I and LNP II, respectively) isolated from P. nelumbinis, and prepared a sulfated polysaccharide (LNP III). Furthermore, the antioxidant activities of LNP I, LNP II, and LNP III were assayed in vitro.
2. Materials and methods
2.1. Materials and chemicals
Standard monosaccharides and dextran standards were obtained from Pharmacia Co., Ltd. (Uppsala, Sweden). N,O-bis(trimesilyl)trifluoroacetamide (BSTFA) and pyridine were purchased from Sigma-Aldrich (USA). All other reagents were of analytical grade unless otherwise stated. The RAW264.7 macrophages were obtained from the cell center of Xiangya Hospital, Central South University, China. DEAE-52 cellulose and Sephadex G-200 were purchased from Amersham Pharmacia Co. (Sweden). The dried P. nelumbinis was purchased in August 2013 from the culture field in Xiangtan, Hunan Province, China. Plant identity was verified by Professor Rong Zeng (Central South University, Changsha, China). A voucher specimen was deposited at the author’s laboratory (ID: 2013001).
2.2. Extraction and purification
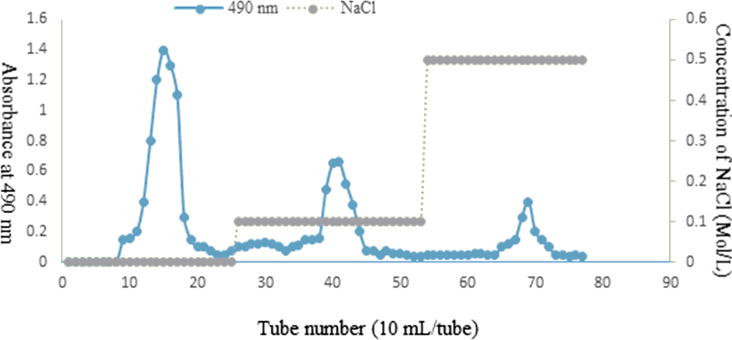
The dried P. nelumbinis (510 g) was extracted in 3.5 L distilled water at 100 °C three times for 2 h. After centrifugation, the supernatant was collected and concentrated in a rotary evaporator under reduced pressure. The concentrated extract was precipitated with 80% ethanol (final concentration) for 24 h and further deproteinised using the combined method of Sevage and Papain. After removing the solvent (CHCl3 and n-butanol) in a rotary evaporator under reduced pressure, the deproteinised extract was precipitated using 80% ethanol (final concentration) for 24 h. The crude polysaccharide (2.5 g) obtained after lyophilization was precipitated and washed twice with acetone and anhydrous ethanol. Then, the crude polysaccharide (1.0 g) was loaded onto a DEAE-52 cellulose chromatography column (2.6 cm × 40 cm). The column was sequentially eluted using distilled water and gradient eluted with 0.1 M and 0.5 M sodium chloride, and 10 mL eluted fractions were collected. The small fractions combined into three fractions according to the sugar content, as determined by the phenol–sulfuric acid method (Fig. 1). Furthermore, two major fractions were purified using a Sephadex-G200 column (2.0 cm × 60 cm), eluted with distilled water, and sequentially discolored by activated carbon, H2O2, and macroreticular resin D315. The two polysaccharides, LNP I (280.8 mg) and LNP II (200.4 mg), were obtained after dialysis (molecular weight cut off 3000 Da) and were lyophilized (Fig. 1).
Fig. 1.
Elution curve of crude P. nelumbinis polysaccharide on DEAE-52 cellulose chromatography column.
2.3. Preparation of sulfated derivatives
The sulfation agent and anhydrous pyridine (30 mL) were prepared by dropping HClSO3 (10 mL) dropwise for 1 h under agitation in a 100 mL three-necked flask maintained under cooling conditions in an ice water bath. LNP I (150.0 mg) was added in anhydrous formamide (20.0 mL) at room temperature with stirring to form a suspension. Then, the suspension was added into the sulfation agent with stirring for 2 h at 70 °C. After reaction, the mixture was cooled down to room temperature and neutralized with 2.5 mol/L NaOH solution. Then, the mixture was precipitated using 80% ethanol (final concentration) for 24 h. The precipitate was collected and washed with anhydrous ethanol, dissolved in distilled water, and dialyzed against distilled water for 72 h. The sulfated LNP I derivative (LNP III) was obtained by concentrating under reduced pressure at 60 °C.
The sulfur contents of LNP III were determined using a reported method (Li et al., 2014). A calibration curve was constructed using potassium sulfate as a standard. The linear regression equation of the standard curve was as follows:
Where Y represents the concentration (μg/mL) of sulfur, and X represents the UV absorbance at 360 nm.
The degrees of substitution (DS) was calculated from the sulfur content according to the equation DS = (1.62 × S%) ÷ (32 − 1.02 × S%) (Xu et al., 2016).
2.4. Determination of carbohydrate, sulfuric radical, protein and uronic acid content
The carbohydrate content in crude LNP and its purified fractions were determined using phenol–sulfuric acid method (Dubois et al., 1956) with glucose as the standard. The sulfate radical content was determined according to a reported method (Li et al., 2014) using K2SO4 as the standard. The protein content was determined using Bradford method (Bradford, 1976) with bovine serum albumin as the standard. The uronic acid content was determined per carbazole–sulfuric acid method (Xu et al., 2016) using D-glucuronic acid as the standard.
2.5. Determination of the molecular weight of LNP I and LNP II
The molecular weights of LNP I and LNP II were determined using high performance size exclusion chromatography on Agilent 1200 system (Agilent Technologies, CA, USA) equipped with a Shodex Ohpak SB-804HQ gel column (30 cm × 8.0 mm, Thermo Fisher Scientific., Massachusetts, USA) and a refractive index detector. The column was eluted with 0.1 M sodium chloride at 35 °C using a flow rate of 0.5 mL/min. Dextrans of different molecular weights (D0, D1, D2, D3, D4, D5, D6, D7, D8, and D2000) were used to establish a standard curve. Each dextran standard solution was prepared by dissolving 10.0 mg sample in 1.0 mL 0.1 M sodium chloride. The solutions of LNP I and LNP II were prepared in the same way. Then, dextran standard solutions were separately injected into the column and a linear regression standard curve of log molecular weight versus HPSEC retention time was calculated. The linear regression equation of the standard curve was as follows:
Where Mw represents the molecular weight, and tR represents retention time. Afterwards, LNP I and LNP II solutions were injected into the column and the molecular weight of LNP I and LNP II were determined according to the above standard curve.
2.6. Analysis of the monosaccharide composition of LNP I and LNP II
Polysaccharides of LNP I (1.0 mg) and LNP II (1.0 mg) were completely hydrolyzed with 200 μL trifluoroacetic acid (2.0 mol/L) at 110 °C for 4 h. Excessive acid was removed by co-blowing with 200 L methanol to dryness in N2 thrice. Briefly, the derivatives of hydrolysate were derivatized using the BSTFA method (Hsu et al., 2007) and the aldononitrile acetate method (Liang and Balser, 2010, Lv et al., 2014). Next, the monosaccharide composition of LNP I and LNP II were determined using gas chromatography-mass spectrometry analysis on an Agilent 7890A-5975C instrument with a HP-5MS column (30 m × 0.25 mm × 0.25 µm) and a NIST08 mass spectral library. The temperature was programmed as follows: 120 °C for 3 min, then rise to 200 °C at 10 °C/min, and finally rise from 200 °C to 240 °C at 5 °C/min.
2.7. Spectroscopic analysis
2.7.1. UV absorption peak detection
LNP I, LNP II, and LNP III were dissolved in distilled water to a final concentration of 5% and were analyzed on ultraviolet–visible (UV–vis) Agilent Cary 60 spectrophotometer. The UV absorption spectrum of the sample was recorded in the wavelength range of 200–800 nm.
2.7.2. FT-IR spectrometric analysis
Three milligrams of each sample (LNP I, LNP II, and LNP III) was mixed with 100 mg KBr powder, thoroughly ground, and compressed in a mold for FT-IR measurement at the frequency range 4,000–400 cm−1. IR spectra were recorded on a Bruker Alpha-T spectrometer (Bruker, Rheinstetten, Germany)
2.7.3. NMR spectrometric analysis
LNP I, LNP II, and LNP III (3.0 mg) were dissolved in 0.6 mL 99.9% D2O respectively. Spectra were recorded using Bruker AV-500 spectrometer (Bruker Co., Germany) with 500 MHz frequency for 1H NMR. The spectra were observed at 25 °C from 120 scans. Data processing was performed using standard Bruker Topspin-NMR software.
2.8. Cell culture
The mouse macrophage cell line RAW264.7 was incubated at 37 °C in a humidified atmosphere with 5% CO2 and 95% air. Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin and 100 U/mL penicillin (Life Technologies). Cells seeded on 96-well culture plates at 1.0 × 104 cells/well before polysaccharide treatment.
2.9. In vitro antioxidant activity assays
2.9.1. Proliferation assays for RAW264.7 macrophages
The antioxidative activities of the samples (LNP I, LNP II, LNP III and vitamin C) were evaluated against H2O2-induced oxidative stress in RAW264.7 cells using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Tang et al., 1999). Briefly, RAW264.7 cells (1 × 104 cells/well) were seeded on 96-well culture plates and incubated for 24 h in the presence of different concentrations (0.125, 0.5, 2.0 μg/mL) of these samples, followed by treatment with 0.658 mM H2O2 for 12 h (Jin et al., 2014). After renewal of the media, 20 μL MTT (5.0 mg/mL) was added to each well incubated for 4 h. One hundred and fifty microliters dimethyl sulfoxide (DMSO) was later added into each well to solubilize the formazan crystals. Absorbance was measured using a microplate reader (Bio-Tek Elx-800, USA) at 490 nm. The inhibition of cell growth was calculated using the following formula: Inhibition (%) = (1 − OD/ODm) × 100, where OD and ODm indicated the absorbance of treated and model groups, respectively.
2.9.2. Measurement of the SOD and GSH-Px activity
The RAW264.7 cells were cultured and treated with samples and H2O2 as mentioned above. After collection and washing with PBS, the treated cells were disrupted using ultrasonic waves on ice and centrifuged at 1000 r/min for 10 min. The supernatant was used to measure the SOD and GSH-Px activities using SOD and GSH-Px kits (Nanjing Jiancheng Institute, China) according to the manufacturer’ instructions.
2.10. Statistical analysis
Values are expressed as means ± standard deviation (SD) of three replicated measurements. Data was analyzed statistically using one way analysis of variance (ANOVA) and Student’s t-test of SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). P-values < 0.05 were considered statistically significant.
3. Result analysis and discussion
3.1. LNP I and LNP II were isolated and purified from P. nelumbinis
Approximately 4.4% crude polysaccharide was extracted and isolated from P. nelumbinis after precipitation, deproteinisation, and lyophilization. The protein concentration in the crude polysaccharide analyzed using the Bradford method (Bradford, 1976) was lower than 0.1%, which was further confirmed by UV–vis spectrometry. The results of preliminary chemical analysis showed that the neutral sugars and uronic acid contents in crude polysaccharide were 79.10% and 10.82%, respectively. The three fractions polysaccharide fractions, LNP-1, LNP-2, and LNP-3 isolated from the crude polysaccharide by DEAE-52 cellulose column chromatography, were sequentially eluted using deionized water, 0.1 M NaCl, and 0.5 M NaCl solution (Fig. 1). Then, two major fractions, LNP-1 and LNP-2, were further purified using a Sephadex-G200 column, decolored, dialyzed and lyophilized to obtain two dry polysaccharide white powders, LNP I and LNP II, respectively.
3.2. Molecular weight and chemical composition of LNP I, LNP II, and the sulfur contents of LNP III
The molecular weights of LNP I and LNP II were determined to be 11.75 × 104 Da and 41.19 × 104 Da by HPSEC on Agilent 1200 system. After completed acid hydrolyzed of LNP I and LNP II, the hydrolysates were derivatized using the BSTFA method and the aldononitrile acetate method, respectively. The result of BSTFA method showed that LNP I and LNP II contained few ketoses. In addition, analysis of the monosaccharide composition by the two methods showed that LNP I consisted of 17.4% mannose, 6.6% arabinose, 45.7% xylose, and 30.3% galactose, whereas LNP II consisted of 12.7% mannose, 9.5% arabinose, 50.2% xylose, and 27.6% galactose. LNP III was prepared successfully, and the sulfur content of LNP III determined to 90.85 μg/mL by a reported method (Li et al., 2014). The DS of LNP III was calculated to be 0.62.
3.3. Spectroscopic analysis of LNPs
3.3.1. UV–vis spectra analysis of LNPs
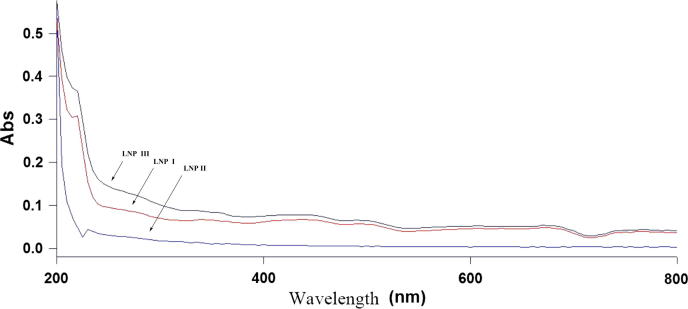
The purity of the polysaccharide was confirmed by UV–vis spectrophotometry as shown in Fig. 2. The absence of peaks between 260 and 280 nm indicated that the LNP I and LNP II are pure or free of nucleic acid and proteins, which confirm the results mentioned above.
Fig. 2.
UV spectra of the LNPs.
3.3.2. FT-IR spectra analysis of LNPs
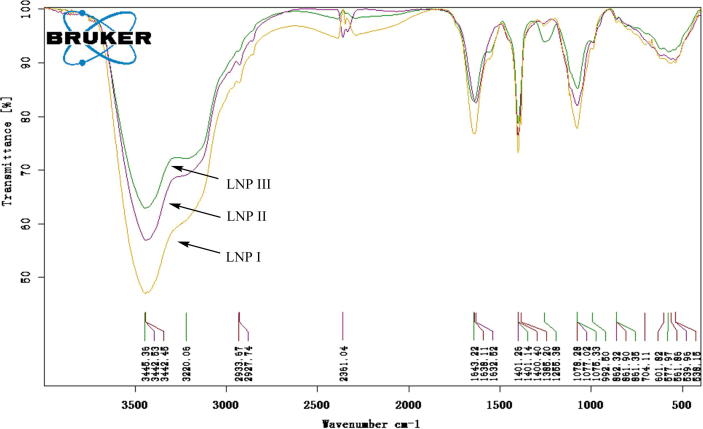
FT-IR spectra of LNPs were recorded in the region of 4,000–400 cm−1. As shown in Fig. 3, the spectra of LNPs showed similar characteristic absorption peaks apart from a peak at 1225 cm−1 in LNP III, and displayed typical absorption peaks for polysaccharides. The spectra of the three polysaccharides showed a strong and broad absorption peak at around 3442 cm−1 for O—H stretching vibrations (Xu et al., 2012) and a weak peak at approximately 2930 cm−1 for C—H stretching vibrations (Xu et al., 2012). The absorption peaks in all spectra at approximately 1638 cm−1 were caused by the bending mode of bound water, and the absorption peaks at approximately 1400 cm−1 was the C—O stretching vibration (Chen et al., 2016). Moreover, the weak small absorption band at approximately 860 cm−1 was attributed to be characteristic of α-glycosidic linkages in the polysaccharide chains (Chen et al., 2016). In addition, the peak appeared at approximately 1077 cm−1 was the result of the glycosidic linkage stretch vibration of C—O—C bond (Sellimi et al., 2014). In addition, a weak peak at 1225 cm−1 in the LNP III spectrum (Fig. 3), which is more intense than the peak observed in LNP I spectrum, was assigned to asymmetric O S O stretching vibration of sulfate esters (Xu et al., 2012, Sellimi et al., 2014), indicating the success of the sulfation reaction.
Fig. 3.
FT-IR spectra of the LNPs.
3.3.3. NMR spectra analysis of the LNPs
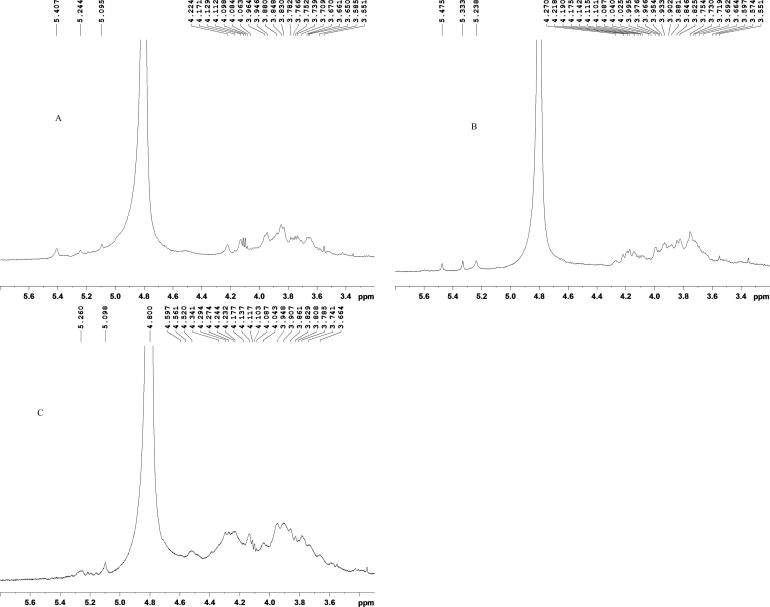
As shown in Fig. 4, a set of wide and complex signals between δH 3.2 and 5.3 ppm was characteristic of typical polysaccharide signals (Wang et al., 2017). Based on literature, the signal peaks between 3.20 and 4.50 ppm were identified to be CH2—O and CH—O groups on sugar rings (Ktari et al., 2017). Therefore, the signal peaks between 3.35 and 4.40 ppm were assigned to protons of CH2—O and CH—O groups on sugar rings. Apart from the signals might be overlapped with the intense signal of the solvent signal peak, signals between 5.00 and 5.50 suggested that the three polysaccharides contained α-configuration of saccharide residues (Wang et al., 2017) which corroborated the results of the FT-IR spectra.
Fig. 4.
1H NMR spectra of the LNPs (A: LNP I, B: LNP II, C: LNP III).
3.4. In vitro antioxidant activity analysis
3.4.1. Effects of LNPs on the proliferation ability of H2O2-treated RAW264.7 cells
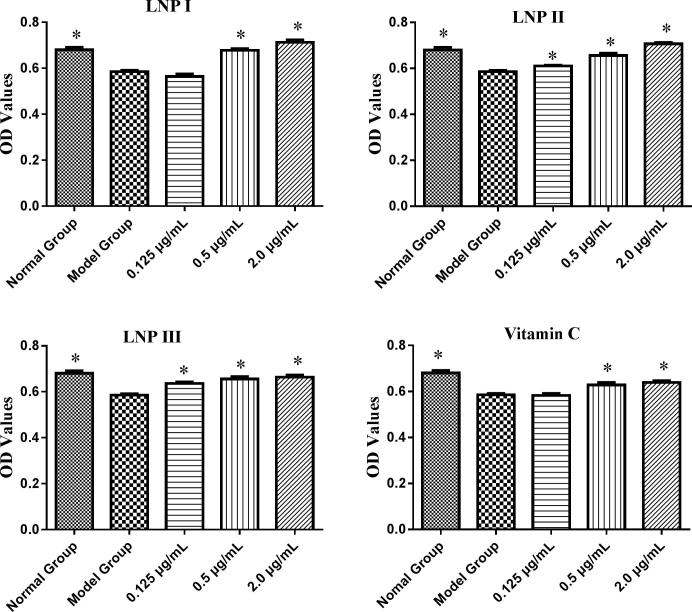
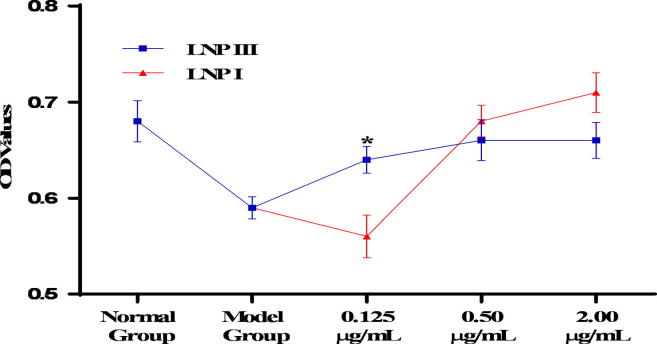
Antioxidants could prevent RAW264.7 cells death through the suppression of H2O2-induced ROS formation. In this study, the antioxidant activity of LNPs was evaluated by assessing the proliferation of RAW264.7 cells induced H2O2 after treated with different concentrations (0.125, 0.5, 2.0 μg/mL) of LNP I, LNP II, and LNP III (Jin et al., 2014). Compared to the model group (treatment with 0.658 mM H2O2 for 12 h alone), normal control group showed a significant increasing proliferation level (P < 0.05), and middle dose (0.5 μg/mL) and high dose (2.0 μg/mL) group of LNP I, LNP II, and LNP III had a significant increasing proliferation of RAW264.7 cells (P < 0.05) (Fig. 5). Moreover, the proliferation of the LNP I, LNP II, and LNP III treatment groups were similar to that of vitamin C control group at the concentration of 0.50 μg/mL and 2.0 μg/mL concentration. However, compared to the LNP I group, the LNP III (sulfated LNP I) group did not show any significant increase in proliferation except for at the concentration of 0.125 μg/mL (Fig. 6).
Fig. 5.
The proliferation ability of cells treated with LNPs on H2O2-treated RAW264.7 cells at different concentrations (0.125, 0.5, 2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the model control.
Fig. 6.
The proliferation ability of cells treated with LNPⅠand LNP III on H2O2-treated RAW264.7 cells at different concentrations (0.125、0.5、2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the LNPⅠgroup.
3.4.2. Effects of the LNPs on the antioxidant enzyme activity of H2O2-treated RAW264.7 cells
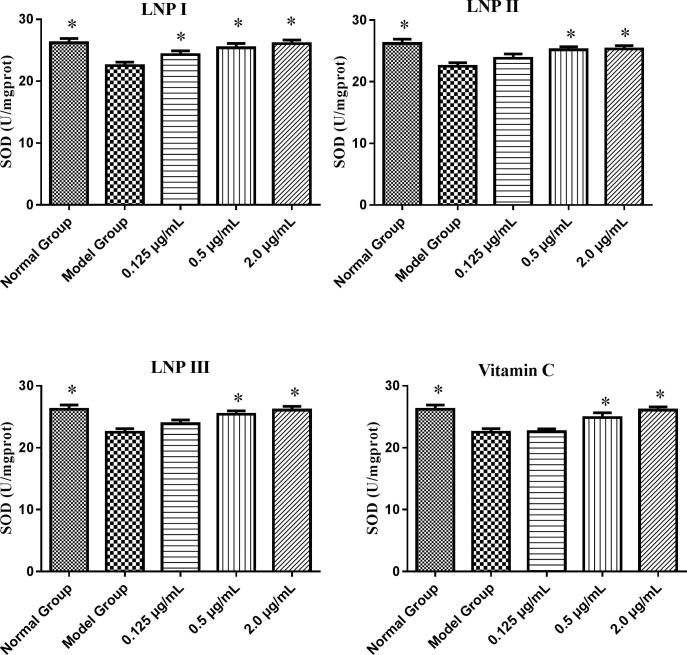
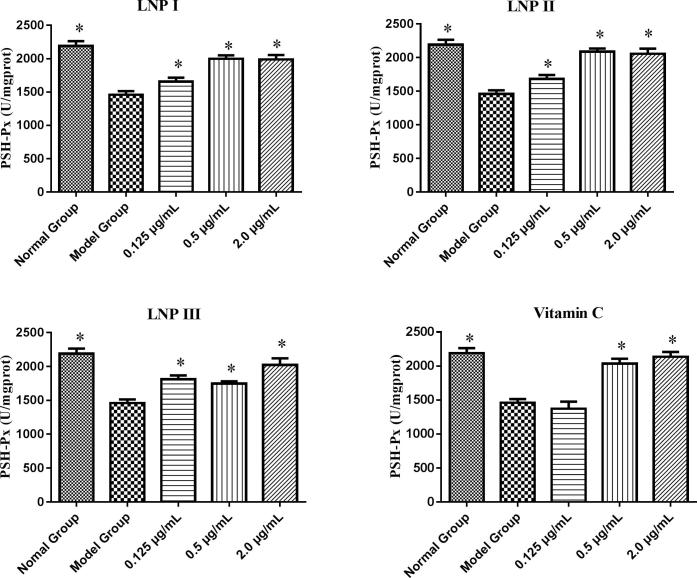
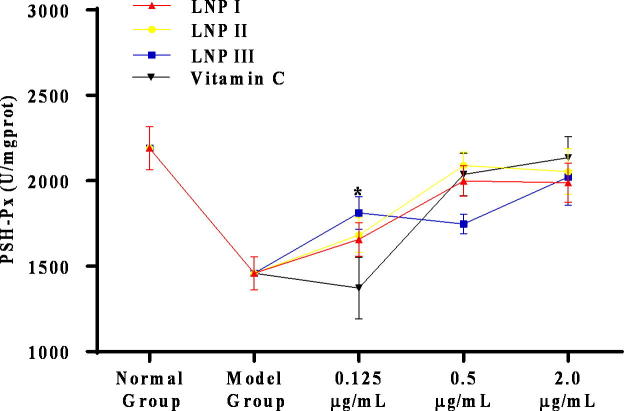
SOD and GSH-Px are two important antioxidant enzymes that prevent cellular damage from the superoxide anion and hydrogen peroxide. Our results indicated that 0.5 μg/mL and 2.0 μg/mL of LNP I, LNP II, and LNP III each significantly increased the concentrations of SOD and GSH-Px compared to that in the model group (P < 0.05) (Fig. 7, Fig. 8). In addition, there were no statistically (P > 0.05) significant increase in the level of the two antioxidant enzymes in these experimental groups (LNP I, LNP II, and LNP III) compared to the vitamin C control group (Fig. 9, Fig. 10) except for the level of GSH-Px at a concentration of 0.125 μg/mL of LNP III group. Moreover, compared to the LNP I group, the LNP III (sulfated LNP I) group did not show any significant increase in the concentrations of SOD and GSH-Px.
Fig. 7.
The antioxidant enzyme of SOD treated with the LNPs on H2O2-treated RAW264.7 cells at different concentrations (0.125、0.5、2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the model control.
Fig. 8.
The antioxidant enzyme of GSH-Px treated with the LNPs on H2O2-treated RAW264.7 cells at different concentrations (0.125、0.5、2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the model control.
Fig. 9.
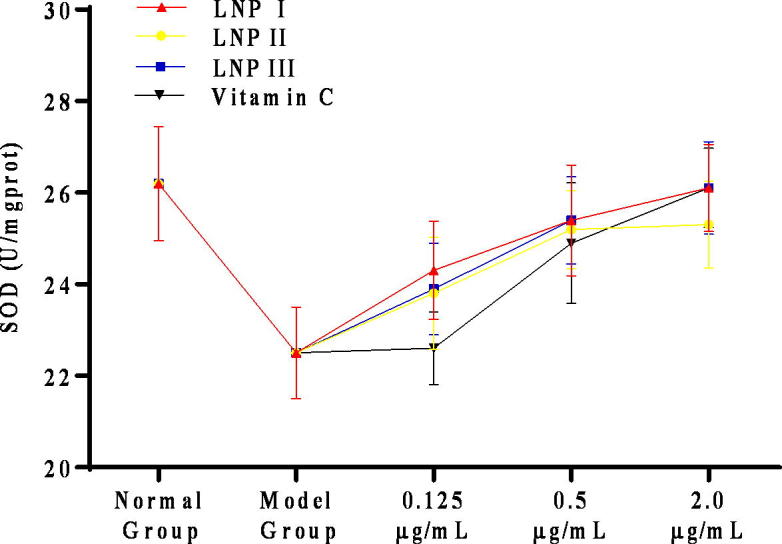
The antioxidant enzyme of SOD treated with LNPs on H2O2-treated RAW264.7 cells at different concentrations (0.125、0.5、2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the vitamin C group.
Fig. 10.
The antioxidant enzyme of GSH-Px treated with LNPs on H2O2-treated RAW264.7 cells at different concentrations (0.125、0.5、2.0 μg/mL). Data were presented as mean ± standard deviation: (a) *P < 0.05, differ significantly from the vitamin C group.
4. Conclusions
In this study, two natural polysaccharides (LNP I and LNP II) were isolated from P. nelumbinis and one sulfated polysaccharide (LNP III) was prepared. The structures of the LNPs were preliminarily characterized using HPSEC, GC–MS, FT-IR, and NMR spectrometry. Compared to these polysaccharides have been isolated from P. nelumbinis (Liao et al., 2011, Liao and Lin, 2012, Liao and Lin, 2013a, Liao and Lin, 2013b) and other polysaccharides have been reported in online database, the two novel polysaccharides (LNP I and LNP II) possessed exclusive monosaccharide composition and molecular weight. Moreover, analysis of the antioxidant activities of the three polysaccharides showed that they significantly (P < 0.05) increased the proliferation of H2O2-treated RAW264.7 macrophages, and enhanced the level of SOD and GSH-Px compared to that in the model group. Studies show that sulfation of polysaccharides improves antioxidant activity (Zhang et al., 2015, Chen et al., 2015). However, in this study, the sulfated polysaccharide (LNP III) did not show any significant improvement of antioxidant activity compared to the unsulfated polysaccharide (LNP I). Nonetheless, these results demonstrated that these polysaccharides possessed moderate antioxidant activity and might be responsible for the healthcare function of P. nelumbinis. Further studies on their structure and function are underway.
Acknowledgments
This work were supported by the National Natural Sciences Foundation of China (NNSFC) [grant number 81703384]; the Department of Science and Technology of Hunan Province of China [grant number 2014sk3014]; and the Hunan Provincial Natural Science Foundation of China [grant number 2017JJ3498].
Footnotes
Peer review under responsibility of King Saud University.
References
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cao Y., Zheng L., Liu S., Peng Z., Zhang S. Total flavonoids from Plumula nelumbinis suppress angiotens in II–induced fractalkine production by inhibiting the ROS/NF–κB pathway in human umbilical vein endothelial cells. Exp. Ther. Med. 2014;7:1187–1192. doi: 10.3892/etm.2014.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Wang Y., Nie S., Li C., Xie M. Sulfated modification of the polysaccharides from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2015;186:231–238. doi: 10.1016/j.foodchem.2014.10.032. [DOI] [PubMed] [Google Scholar]
- Chen T., Zhang M., Li J., Surhio M.M., Li B., Ye M. Structural characterization and hypoglycemic activity of Trichosanthes peel polysaccharide. LWT - Food Sci. Technol. 2016;70:55–62. [Google Scholar]
- Dubois M., Gilles K., Hamilton J., Rebers P., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Hsu C., Cheng C., Lee C., Ding W. Derivatization procedures and determination of levoglucosan and related monosaccharide anhydrides in atmospheric aerosols by gas chromatography-mass spectrometry. Talanta. 2007;72:199–205. doi: 10.1016/j.talanta.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Huang G., Chen X., Huang H. Chemical modifications and biological activities of polysaccharides. Curr. Drug Targets. 2016;17:1799–1803. doi: 10.2174/1389450117666160502151004. [DOI] [PubMed] [Google Scholar]
- Itoh A., Saitoh T., Tani K., Uchigaki M., Sugimoto Y., Yamada J., Nakajima H., Ohshiro H., Sun S., Tanahashi T. Bisbenzylisoquinoline alkaloids from Nelumbo nucifera. Chem. Pharm. Bull. 2011;59:947–951. doi: 10.1248/cpb.59.947. [DOI] [PubMed] [Google Scholar]
- Jiangsu New Medical College . Shanghai Science and Technology Publishing House; Shanghai: 1986. Dictionary of Traditional Chinese Medicine; p. 1806. [Google Scholar]
- Jin M., Wang Y., Huang M., Lu Z., Wang Y. Sulphation can enhance the antioxidant activity of polysaccharides produced by Enterobacter cloacae Z0206. Carbohyd. Polym. 2014;99:624–629. doi: 10.1016/j.carbpol.2013.08.072. [DOI] [PubMed] [Google Scholar]
- Ktari N., Feki A., Trabelsi I., Triki M., Maalej H., Slima S.B., Nasri M., Amara I.B., Salah R.B. Structure, functional and antioxidant properties in Tunisian beef sausage of a novel polysaccharide from Trigonella foenum-graecum seeds. Int. J. Biol. Macromol. 2017;98:169–181. doi: 10.1016/j.ijbiomac.2017.01.113. [DOI] [PubMed] [Google Scholar]
- Lou H., Yuan H., Ji M., Xu D. Sitosterol esters from embryo of the seed of Nelumbo nucifera. Acta Acad. Med. Shandong. 1995;33:346–348. [Google Scholar]
- Lü J., Lin P., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Guo S., Lin J. Characterisation of the chemical composition and in vitro anti-inflammation assessment of a novel lotus (Nelumbo nucifera Gaertn) plumule polysaccharide. Food Chem. 2011;125:930–935. [Google Scholar]
- Liao C., Lin J. Purification, partial characterization and anti-inflammatory characteristics of lotus (Nelumbo nucifera Gaertn) plumule polysaccharides. Food Chem. 2012;135:1818–1827. doi: 10.1016/j.foodchem.2012.06.063. [DOI] [PubMed] [Google Scholar]
- Liao C., Lin J. Lotus (Nelumbo nucifera Gaertn) plumule polysaccharide ameliorates pancreatic islets loss and serum lipid profiles in non-obese diabetic mice. Food Chem. Toxicol. 2013;58:416–422. doi: 10.1016/j.fct.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Liao C., Lin J. Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing Toll-like receptor-2 and -4 expressions using mouse primary splenocytes. J. Ethnopharmacol. 2013;147:164–173. doi: 10.1016/j.jep.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Lin Z., Wang H., Fu Q., An H., Liang Y., Zhang B., Hashi Y., Chen S. Simultaneous separation, identification and activity evaluation of three butyrylcholinesterase inhibitors from Plumula nelumbinis using on-line HPLC-UV coupled with ESI-IT-TOF-MS and BChE biochemical detection. Talanta. 2013;110:180–189. doi: 10.1016/j.talanta.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Lin Z., Yang R., Guan Z., Chen A., Li W. Ultra-performance LC separation and quadrupole time-of-flight MS identification of major alkaloids in Plumula nelumbinis. Phytochem. Anal. 2014;25:485–494. doi: 10.1002/pca.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xiao J., Zha X., Pan L., Asghar M., Luo J. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohyd. Polym. 2014;106:247–254. doi: 10.1016/j.carbpol.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Liang C., Balser T. Mass spectrometric characterization of amino sugar aldononitrile acetate derivatives used for isotope enrichment assessment of microbial residues. Soil Biol. Biochem. 2010;42:904–909. [Google Scholar]
- Lv L., Cheng Y., Zheng T., Li X., Zhai R. Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb. Carbohyd. Polym. 2014;99:765–777. doi: 10.1016/j.carbpol.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Liu Z., Yi J., Xiao X. Study on the effective mechanism of Plumula nelumbinis extract against hepatic fibrosis in rats. Chin. J. Clin. Pharmacol. 2015;31:1749–1753. [Google Scholar]
- Liu Q., Ge X., Chen L., Cheng D., Yun Z., Xu W., Shao R. Purification and analysis of the composition and antioxidant activity of polysaccharides from Helicteres angustifolia L. Int. J. Biol. Macromol. 2018;107:2262–2268. doi: 10.1016/j.ijbiomac.2017.10.095. [DOI] [PubMed] [Google Scholar]
- Shao J., Liu Y., Li X., Xv Q., Pei X., Yang S. Chemical constituents from Nelumbinis Plumula. Chin. Tradit. Herbal Drugs. 2016;47:1661–1664. [Google Scholar]
- Sellimi S., Kadri N., Barragan-Montero V., Laouer H., Hajji M., Nasria M. Fucans from a Tunisian brown seaweed Cystoseira barbata: structural characteristics and antioxidant activity. Int. J. Biol. Macromol. 2014;66:281–288. doi: 10.1016/j.ijbiomac.2014.02.041. [DOI] [PubMed] [Google Scholar]
- Tang W., Jin H., Luo D., Zhong S., Su Z., Peng X., Gao X. Comparison of MTT with SRB assays in vitro anticancer drug screening. Nat. Prod. Res. Dev. 1999;11:17–21. [Google Scholar]
- Vajragupta O., Boonchoong P., Berliner L.J. Manganese complexes of curcumin analogues: evaluation of hydroxyl radicals cavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radic. Res. 2004;38:303–314. doi: 10.1080/10715760310001643339. [DOI] [PubMed] [Google Scholar]
- Varoni M.V., Pasciu V., Gadau S.D., Baralla E. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ. Sci. Pollut. Res. 2017;24:2946–2955. doi: 10.1007/s11356-016-8050-x. [DOI] [PubMed] [Google Scholar]
- Wu Z., Xiao X. Anti-arrhythmic action of Plumula nelumbinis microcapsule. China Pharm. 2006;17:1770–1771. [Google Scholar]
- Wang L., Liu H., Qin G. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017;234:314–322. doi: 10.1016/j.foodchem.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhang Y., Zhang L., Shao S., Guo Z., Zheng B. Protective effects of alkaloid compounds from Nelumbinis Plumula on tert-Butyl hydroperoxide-induced oxidative stress. Molecules. 2013;18:10285–10300. doi: 10.3390/molecules180910285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Song S., Wei Y., Wang F., Zhao M., Guo J., Zhang J. Sulfated modification of the polysaccharide from Sphallerocar pusgracilis and its antioxidant activities. Int. J. Biol. Macromol. 2016;87:180–190. doi: 10.1016/j.ijbiomac.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Xu R., Ye H., Sun Y., Tu Y., Zeng X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis) Food Chem. Toxicol. 2012;50:2473–2480. doi: 10.1016/j.fct.2011.10.047. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang J., Nie S., Wang Y., Cui S., Xie M. Sulfated modification, characterization and property of a water-insoluble polysaccharide from Ganoderma atrum. Int. J. Biol. Macromol. 2015;79:248–255. doi: 10.1016/j.ijbiomac.2015.04.070. [DOI] [PubMed] [Google Scholar]
- Zhang L., Gu J., Chen Y., Zhang L. A study on four antioxidation effects of Lycium barbarum polysaccharides in vitro. Afr. J. Tradit. Complement Altern. 2013;10:494–498. doi: 10.4314/ajtcam.v10i6.18. [DOI] [PMC free article] [PubMed] [Google Scholar]