Abstract
Superficial fungal infections caused by Candida species are common skin diseases. Therefore, this study aimed to develop a new formulation containing oxiconazole nitrate, which is an azole group derivative for antifungal treatment, as a thermosensitive gel since there has been no literature study until now.
MIC value of the novel thermosensitive formulation against three Candida species was calculated and time-dependent antifungal activity analysis was performed. Viscosity, transition temperature Tsol-gel (°C) and gelation time of the thermosensitive gel formulation were also determined in the viscometer. The measurements performed on the tensilometer device were analyzed for adhesion hardness and elongation percentages of the formulation. In the FT-IR spectrometer, the spectrum of solution and gel state was compared between 650 and 4000 cm−1 and it was found that there is no difference between them.
It was found that the temperature is reversible on the formulation and did not cause any disruption of its components. Characterization parameters of the thermosensitive gel formulation containing oxiconazole nitrate and time-dependent activity against Candida species was observed to be the same as those of the solution containing only oxiconazole nitrate. MIC, MFC and time-dependent antifungal analysis did not show any particular difference between formulation and oxiconazole nitrate itself. Thermosensitive gel formulation containing oxiconazole nitrate was found to be effective on superficial fungal infections. We believe it is also appropriate for in vivo usage, but it is necessary to perform animal and human research. It is also needed to evaluate the formulation against other etiologic agents of superficial fungal infections.
Keywords: Oxiconazole nitrate, Thermosensitive gels, Poloxamer 407, Candida, Antifungal activity
1. Introduction
Superficial fungal infections are common diseases at all ages and for both sexes. There is a wide variation of infection type (pityriasis versicolor, etc.), and different pathogens may be observed as causative agents. Treatment options may change according to the severity of the disease, the physician’s choice and the medical status of the patient (Rezabek and Friedman, 1992).
More than 200 Candida species were identified and about 30 species of them cause human infections. The infection spectrum shows a wide variability from superficial to invasive infections. Candida albicans colonizes the skin, genital, and/or intestinal mucosa and is the most frequently isolated species from infections, but species distribution may vary worldwide. Although invasive Candida infections get all attention, skin and soft tissue infections have a wide space among Candida infections (Rezabek and Friedman, 1992, Leeyaphan et al., 2016, Del-Rosso and Kircik, 2013).
There are many options on treating superficial fungal infections, but unclearity on optimal treatment period, application type and dosage may result with low efficacy (Crawford and Hollis, 2007). Therefore, the studies have focused on the development of alternative dosage forms. Structural modifications on azoles showed high in vitro effects against C. albicans, whereas correlation on in vivo and in vitro effectiveness of azole derivates is questionable (Heeres et al., 2010). On the other hand, azoles are still in use and many studies showed high efficiency even in vivo (Denning and Hope, 2010).
The azole antifungals are the most widely used class in treatment of candidiasis. Especially for invasive candidiasis, there is an emergent situation on antifungal resistance to azoles. But, for superficial candidiasis, the data is very limited. Azole resistance is strongly related with administration route, and systemic application is more inclined to this condition. However, the situation against oxiconazole nitrate (ON) is a mystery. Although resistance mechanisms against ON might be similar to the others, virulence of Candida species causing skin infections is significantly different from ones causing invasive infections, which is related with antifungal resistance (Whaley et al., 2016, Kashem and Kaplan, 2016).
Oxiconazole active substance was selected from this group because it has low-medium molecular weight, high permeability, no deep irritation, high local bioavailability and good compatibility with polymers. Transdermally administered thermosensitive gels are new drug delivery systems that have been studied frequently since they have in situ activity depending on the temperature (Masteikova et al., 2003). The thermosensitive gels are superior to the conventional gels in terms of stability, ease of use for patients, improved local bioavailability, and efficacy at low doses, easy preparation and low production costs.
In this study we aimed to develop thermosensitive gel formulation (TGF) of ON showing antifungal activity, which explores gelling at body temperature when it is applied to the skin. After performing the characterization studies of the formulation, we tried to determine its effectiveness by comparing our findings with ON solution using time-dependent analysis.
2. Materials and methods
2.1. Materials
ON was kindly donated by the Deva Pharmaceutical Industry (Turkey) for the studies. Poloxamer 407 was purchased from Sigma-Aldrich Co (St Louis, MO, USA). All other chemicals were used as reagent grade without further purification.
2.2. Preparation of TGF
The TGF was prepared by “cold method” (Bilensoy et al., 2006). The poloxamer 407 solution was prepared at 4 °C and it was left to stand overnight for the loss of foam formation. ON was dissolved in a mixture of ethanol and polyethylene glycol 400 (Sigma-Aldrich Co, St Louis, MO, USA) and added to the poloxamer 407 solution slowly and by being stirred at 4 °C. A sufficient amount of distilled water was used in preparation of the solution. The details of all components of formulation are shown in Table 1. In the process of mixing the solutions, ice battery was used to keep the ambient temperature at 4 °C. The mixture was allowed to stand at 4 °C overnight to begin in vitro assays.
Table 1.
The components and concentrations of thermosensitive gel formulation.
| Contents | Concentration (%) |
|---|---|
| Oxiconazole nitrate | 1 (w/v) |
| Poloxamer 407 | 22 (w/v) |
| Ethanol | 18 (v/v) |
| PEG 400 | 9 (v/v) |
| Distilled water | q.s. |
2.3. The preparation of ON and TGF solutions
Stock solution of ON in dimethyl sulfoxide (DMSO) (Merck Millipore, MA, USA) in concentration of 12.8 mg/mL was prepared. Stock solutions of TGF in DMSO can also be prepared with the same last concentration of ON. 50 µL from each solutions were completed to 20 mL with Roswell Park Memorial Institute 1640 medium (Sigma-Aldrich Co, MO, USA) and 400-fold serial dilutions were applied to final concentration of 32 µg/mL. Roswell Park Memorial Institute medium, which is commonly referred to as RPMI medium or RPMI 1640, is a form of medium used in cell culture and tissue culture used to grow a variety of mammalian cell lines. RPMI 1640 uses a bicarbonate buffering system and differs from most of the mammalian cell culture media in its typical pH 8 formulation.
2.4. The characterization studies and rheological and mechanical properties of formulation
2.4.1. FT-IR spectrum of TGF
Spectra of all the compounds used in the formulation were obtained by scanning over a wide range of 650–4000 cm−1 using FT-IR spectrophotometer (Perkin Elmer, MA, USA). The peaks of the polymers used in the formulation were analyzed (Dibern et al., 2002). The solution state of the TGF at 4 °C was compared with the gel state at 35 °C by FT-IR spectrophotometer (Kim et al., 2010).
2.4.2. The determination of sol-gel transition temperature and time of TGF
The sol-gel transition temperature of TGF was determined using Haake Viscotester 7 Plus viscosimetry device (Thermo-Fisher Scientific, MA, USA) with R7 spindle at 0.1 rpm. The temperature was changed from 4 °C to 45 °C increasing by 0.5 °C per minute. The viscosity changes of the formulation were recorded. The time was calculated since the beginning of a significant increase in viscosity and the middle point of the region where the increase in viscosity happened due to the increase of the temperature was the basis of the calculation of the sol-gel transformation of the prepared TGF.
2.4.3. The viscosity, hardness and adhesion measurements of TGF
Haake Viscotester 7 Plus device was used in the rheological examination of the TGF. The study by Chang et al. (2002) was modified and the measurements were done by increasing 0.5 °C/min between 0.1 °C and 45 °C at 0.1 rpm R7 spindle and viscosities were determined. The study by Yong et al. (2002) was modified and the hardness and adhesion measurements of the TGF were performed on software-assisted H5KS Benchtop Tensilometer (Tinius Olsen, Redhill, UK). The measurements started at 35 °C after the completion of the sol-gel transition of the formulation. The hard silicone probe 20 mm in diameter was immersed into the gel 15 mm at rate of 10 mm/min to evaluate the hardness of the prepared TGF. Then, the adhesion and elasticity measurements were analyzed and recorded while the probe was leaving from the gel surface with the same parameters.
2.5. In vitro antifungal analysis
2.5.1. The preparation of Candida strains
The strains of Candida albicans ATCC 10231, Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22,019 were prepared to 0.5 MacFarland turbidity standard (1–5 × 106 CFU/mL) in 0.9% NaCl. These solutions were 1000-fold diluted to 1–5 × 103 CFU/mL.
2.5.2. Broth macrodilution
To get reliable data on in vitro antifungal efficiency of ON and TGF, an antifungal analysis was applied within a broth macrodilution method according to CLSI, 2008a, CLSI, 2008b standard guidelines. Due to the lack of information about ON in the guidelines, modified azole standards and methods were used. As a solvent, DMSO is a best choice except fluconazole, which also worked excellently for ON and TGF, as previously reported (Gebhart et al., 1984). Because of the evaluation of dilution range is not clear for oxiconazole, the widest range in CLSI guidelines (0.016–128 mg/L) with 14 serial dilutions and also positive and negative control tubes were applied. Following the addition of RPMI medium and Candida solutions, final concentrations were 100-fold diluted. The tubes were incubated in ambient atmosphere and 37 °C temperature for 24 h, and visual evaluation was done to detect minimum inhibitory concentration (MIC) levels (CLSI, 2008a, EUCAST, 2015). Furthermore, to evaluate the minimum fungicidal concentration (MFC), visual turbidity-detected tubes were additionally inoculated to standard methods agar (SMA) (Thermo-Fisher Scientific, MA, USA) quantitatively (1 μL, 10 μL), as applied previously by Cantón et al., (2003). For MFC, fungicidal rate of ≥98% is necessary and according to this data, tubes with a maximum of 20 colonies for 10 μL and 2 colonies for 1 μL inoculation were accepted as MFC (CLSI, 2009, Balouiri et al., 2016, Arendrup et al., 2010, Pfaller et al., 2008, EUCAST, 2008).
2.5.3. The time-dependent fungicidal activity
The method modified from CLSI M26-A guidelines was applied to compare the fungicidal activity alterations of ON and TGF in time (CLSI M26-A, 1999). 40 μL from each previously prepared stock solutions were added to 3960 μL RPMI media (100-fold diluted). Then, 2.225 mL from this solution were added into 29.775 mL RPMI media, which results final concentrations to 8.9 μg/mL. With the same methodology in broth macrodilution, serial dilutions were made at 8.9, 4.45, 2.225, 1.1125, 0.5563, 0.2781 and 0.1391 μg/mL final concentrations.
Solutions of Candida species ATCC 10,231 at 0.5 MacFarland turbidity standard (1–5 × 106 CFU/mL) was 10-fold serially diluted. After combining fungal and antifungal solutions, the concentrations of ON and TGF were finally at 8, 4, 2, 1, 0.5, 0.25 and 0.125 μg/mL.
At 0, 4, 8, 16 and 24th h, 1 μL and 10 μL from each tube were quantitatively inoculated to SMA and plates were incubated in ambient atmosphere at 35 °C temperature for 24 h. Colonies were counted and noted (Cantón et al., 2003, CLSI, 1999, Klepser et al., 1998, Clancy et al., 2006, Moore et al., 2001, Ernst et al., 2000).
3. Results and discussion
Although various studies of different dosage forms of imidazole derivatives are available, studies on ON are very limited (Kumar et al., 2014). Transdermally administered thermosensitive gels are new drug delivery systems that have been studied. Thermal sensitive carrier systems have a number of advantages due to their suitability for both lipophilic and hydrophilic active agents, the presence of low systemic side effects, the specificity of the application site and the possibility of controlled release. The thermosensitive gels which are solid when applied to body at the temperature of the skin surface have excellent thermal conversion and have an advantage of having a high bioadhesive activity (James et al., 2014, Cao et al., 2007, Akash et al., 2014). In this study, we aimed to develop a TGF of ON for antifungal activity.
3.1. Temperature of the sol-gel transition
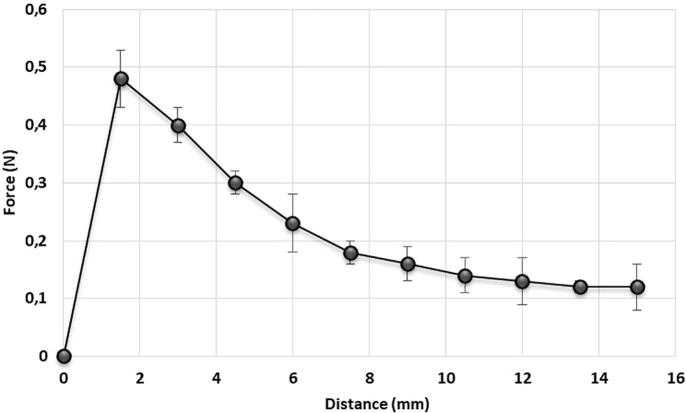
The temperature was gradually increased in the study of transition from liquid to gel form. The results are shown in Fig. 1. The gelling temperature is measured as a value close to the average surface temperature of the human skin (Ramanathan, 1964). The gelation temperature and gelation time are shown in the Table 2. It was observed that the use of ethanol and PEG 400 in the formulation caused the increased gelation temperature (Dumortier et al., 2006), while the use of poloxamer 407 at 22% would reduce the gelation temperature. By using the ratios of the formula components which is shown in Table 1, it is possible to obtain the optimum formula for body surface temperature by changing the ratios of ingredients of the formulation
Fig. 1.
Viscositiy and Tsol-gel measurements of TGF.
Table 2.
The gelation temperature and the gelation time of the formulation.
| Gelation temperature (°C) ± SD | Gelation time (sec) ± SD |
|---|---|
| 33.01 ± 0.025 | 343.100 ± 0.177 |
3.2. Viscosity, hardness and adhesion
The amount of gelling agent in a formulation is very crucial for its mechanical properties. The gel hardness with the association of gel adhesiveness indicates the gel applicability and this may be indicative to obtain the retention time in the application area. This is also directly related to the polymer concentration and is a very important parameter in wound healing (Rencber et al., 2017, Hurler et al., 2012). The temperature of the formulation was checked before starting the analysis of this study. The adhesion measurement of the TGF is shown in Fig. 2 and the hardness measurements are shown in Fig. 3. The viscosity changes obtained by gradually increasing the temperature are shown in the Fig. 1. The hardness and adhesion measurements were carried out after the gelation. The results are shown in Table 3. In the study of Jones et al. (2009), it was observed that the increase of the concentration of poloxamer 407 is directly proportional to increase of adhesion and hardness. This is compatible with our findings.
Fig. 2.
The adhesion measurements of TGF.
Fig. 3.
The hardness measurements of TGF.
Table 3.
Viscositiy of sol-gel, hardness and adhesion properties of the formulation (n = 3).
| Viscositiy 4 °C (pa·s) | Viscositiy 33 °C (pa·s) | Hardness (N) | Adhesion (N) |
|---|---|---|---|
| 30.94 ± 0.5 | 5726 ± 2.6 | 0.5001 ± 0.02 | 0.4855 ± 0.1 |
3.3. FT-IR spectroscopy
Individual FT-IR spectra of the compounds at the formula were determined. When the spectra of the components in the formulation were examined, it was found that some peaks are not detected due to the decrease in concentration with the presence of water in the formulation. However, it was found that the characteristic peaks of ON and poloxamer 407 were preserved (Dibern et al., 2002). FT-IR spectrums before and after gelation were superimposed to observe any difference for comparison. It was investigated whether there was any difference after gelling. As a result, it was observed that the spectra overlapped in great scale and there isn't any peak appeared. However, with the increase in temperature, it shows that there are slightly differences in 1413, 2917 and 3295 cm−1. This can be explained with the evaporation of the water after gelation. This result reveals the fact that the temperature does not cause any disruption to the ingredients in the formulation and the process of the consistent gelationis reversible. It is observed that FT-IR spectrometer analysis results are compatible with the study by Kim et al. (2010). The results are shown in Fig. 4.
Fig. 4.
A; FT-IR spectrum of oxiconazole nitrate, B; FT-IR spectrum of PEG 400, C; FT-IR spectrum of poloxamer 407, D; FT-IR spectrum of thermosensitive gel formulation (TGF) at 4 °C, E; the overlapped FT-IR spectrum of thermosensitive gel formulation (TGF) at 4 °C (solution) and 35° (gel).
3.4. In vitro antifungal analysis
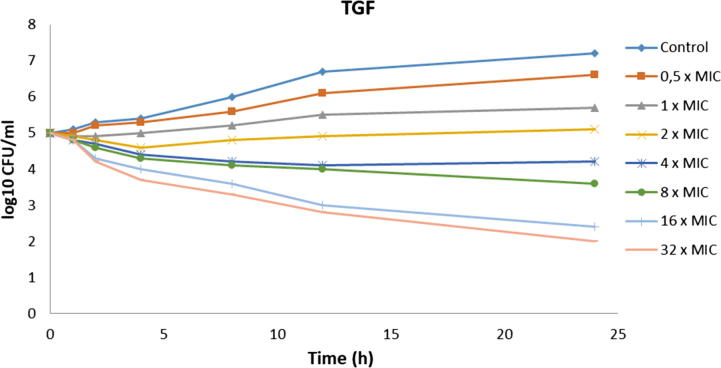
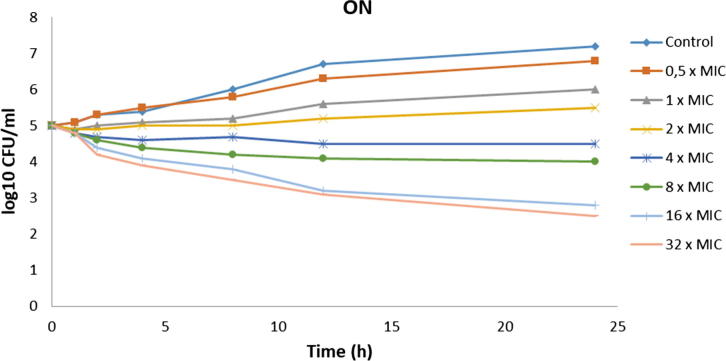
Broth macrodilution, a standardized method, is a necessity to get true MIC and MFC data and to evaluate exact susceptibilities to ON and TGF. Table 4 shows MIC and MFC levels of ON and TGF. According to broth macrodilution and serial quantitative inoculations, ON and TGF showed similar antifungal activity results. Although same MIC levels were observed, in vivo studies must be evaluated to verify this conclusion. MIC results do not always correlate with clinical outcome, and for this reason, MFC testing generally gives supportive data. Studies indicate that MFC has a promising potential with stated clinical correlation (Perkhofer et al., 2010). In our study, despite same MIC levels, a higher MFC level was detected for ON (128 μg/mL) than for TGF (64 μg/mL). This situation may be caused by ethanol which is used to solve ON, and it seems this is an advantage of TGF over purely tested ON on antifungal activity (McDonnell and Russell, 1999). Fig. 5, Fig. 6 show the time-dependent antifungal activities of TGF and ON. TGF did not lose their activity and was also correlated with the activity of ON.
Table 4.
MIC and MFC values of ON and TGF.
| MIC (µg/mL) |
MFC (µg/mL) |
|||
|---|---|---|---|---|
| ON | TGF | ON | TGF | |
| C. albicans | 0.25 | 0.25 | 64 | 64 |
| C. krusei | 0.125 | 0.125 | 128 | 64 |
| C. parapsilosis | 0.125 | 0.125 | 64 | 64 |
Fig. 5.
The time-dependent antifungal activity profile of TGF (C. albicans ATCC 10231).
Fig. 6.
The time-dependent antifungal activity profile of ON (C. albicans ATCC 10231).
Both CLSI, 2008a, CLSI, 2008b, EUCAST, 2015 guidelines recommend the usage of DMSO as a solvent in susceptibility testing of water-insoluble drugs. However, studies indicated that DMSO in low concentrations (<4%) may cause alterations (both as inducer or suppressive) on the growth of Candida species, which may affect susceptibility results. On the other hand, researchers claimed that there is an effect on yeast growth, but this effect may not be observed on MIC levels, which makes these alterations controversial (Hazen et al., 2013). To check DMSO effect on Candida growth, we used same concentrations of DMSO in each tested tube and tests were done in the same conditions. According to our study, this kind of effect can be disregarded and we do not think that this is a major limitation.
Potency takes a primary place in susceptibility tests. Both CLSI, 2008a, CLSI, 2008b, EUCAST, 2015 guidelines have a necessity of calculation with potency. Unfortunately, it was unable to reach potency of ON from the manufacturer guidelines and academic articles, so the effect of potency results was not given in this study. This limitation may cause a problem related with actual antifungal activity of ON such as the underestimation of MIC levels. Even if it was true, this would not affect the results of the comparison between TGF and ON.
Visual turbidity determination as in CLSI may show a difference with spectrophotometric measurement (EUCAST) (Fothergill, 2012), but researchers state that there is no statistically meaningful alterations between these two methods (Perkhofer et al., 2010, Espinel-Ingroff et al., 2005).
Another MIC evaluation is “epidemiological cut-off value (ECOFF)”, which is a very important parameter for non-wild type strains. These microorganisms may show acquired or mutational resistance to the antifungal agent that is observed phenotypically (EUCAST, 2017). Since this condition might cause treatment failures, it is crucial to test a novel drug for various types of strains, even of a single species, to define ECOFF values and its potential in treatment. As we have only studied wild-type strains, this can be a limitation. However, ON is not a novel drug and we have just studied it on different formulations of a known agent, so it is possible to comment that this limitation can be underestimated. To obtain reliable data on this ECOFF issue, various types of strains should be tested with all our formulations.
Another issue is “tissue-treated vs. non-tissue-treated” microdilution plates, which is commented in EUCAST (2015) worksheets. Although there is insufficient data to decide whether it is a problem or not (EUCAST, 2017), it should be noted that our study based on CLSI guidelines can be improved according to this notification.
4. Conclusion
By examining physicochemical properties of ON, it is observed that the solubility in water is very slightly, so it is difficult to develop a poloxamer-based TGF, which has good water solubility. We developed a TGF containing ON and carried out the characterization study. Characterization studies and time-dependent antifungal activity analysis showed that ON did not show any incompatibility with the adjuvants in the formulation and also retained its activity on C. albicans strain.
Commercial ON products have already proven to be efficient, but the data we found supports that TGF can be a good alternative to reach the same success. It is crucial to perform long-term treatment studies, especially for drug-resistant species. The obtained data are thought to be valuable, due to the lack of such studies in the literature. It is also necessary to expand the sample size and perform human researches to evaluate the applicability and patient comfort and to observe same success on patients. We believe that TGF of ON will bring a new approach to the treatment of candidiasis.
Acknowledgements
-
1.
This article project was financially supported by University of Health Sciences (formerly known as Gulhane Military Medical Academy) with the Gulhane Scientific Research Board decision; Date and Number: 16 Nov 2015; AR-2015/55.
-
2.
The data in this article was presented as academic thesis of the first author, Pharm. Alper ARSLAN, in University of Health Sciences, Gulhane Campus (formerly known as Gulhane Military Medical Academy), Department of Pharmaceutical Technology, Ankara, Turkey.
-
3.
We declare special thanks to Deva Pharmaceutical Industry (Istanbul, Turkey) for their support of providing oxiconazole nitrate and Prof. Sinasi Taner YILDIRAN, MD (University of Health Sciences, Department of Medical Microbiology, Gulhane Campus, Ankara, Turkey) for his precious guidance.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akash M.S.H., Rehman K., Chen S. Pluronic F127-based thermosensitive gels for delivery of therapeutic proteins and peptides. Polym. Rev. 2014;54(4):573–597. [Google Scholar]
- Arendrup M.C., Garcia-Effron G., Lass-Flörl C. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27–A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob. Agents. Chemother. 2010;54(1):426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilensoy E., Abdur-Rouf M., Vural I. Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: β-cyclodextrin complex. AAPS PharmSciTech. 2006;7(2):E54–E60. doi: 10.1208/pt070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantón E., Pemán J., Viudes A. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 2003;45(3):203–206. doi: 10.1016/s0732-8893(02)00525-4. [DOI] [PubMed] [Google Scholar]
- Cao Y., Zhang C., Shen W. Poly (N-isopropylacrylamide)–chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control Release. 2007;120(3):186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Chang J.Y., Oh Y.K., Choi H.G. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int. J. Pharm. 2002;241(1):155–163. doi: 10.1016/s0378-5173(02)00232-6. [DOI] [PubMed] [Google Scholar]
- Clancy C.J., Huang H., Cheng S. Characterizing the effects of caspofungin on Candida albicans, Candida parapsilosis and Candida glabrata isolates by simultaneous time-kill and postantifungal-effect experiments. Antimicrob. Agents. Chemother. 2006;50(7):2569–2572. doi: 10.1128/AAC.00291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI), 1999. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline, CLSI Document M26-A. Pennsylvania, USA.
- Clinical Laboratory Standards Institute (CLSI), 2008. Reference Methods for Broth Dilution Antifungal Susceptibility Testing for Yeasts, CLSI Document M27-A3, third ed. Pennsylvania, USA.
- Clinical Laboratory Standards Institute (CLSI), 2008. Reference Methods for Broth Dilution Antifungal Susceptibility Testing for Yeasts, CLSI Document M27-S3, third informational supplement. Pennsylvania, USA.
- Clinical Laboratory Standards Institute (CLSI), 2009. Method for Antifungal Disk Diffusion Susceptibility Testing for Yeasts: Approved Guideline, CLSI Document M44-A2, second ed. Pennsylvania, USA.
- Crawford, F., Hollis, S., 2007. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst. Rev. <https://doi.org/10.1002/14651858.CD001434.pub2>. [DOI] [PMC free article] [PubMed]
- Del-Rosso J.Q., Kircik L.H. Optimizing topical antifungal therapy for superficial cutaneous fungal infections: focus on topical naftifine for cutaneous dermatophytosis. J. Drugs. Dermatol. 2013;12(11Suppl):165–171. [PubMed] [Google Scholar]
- Denning D.W., Hope W.W. Therapy for fungal diseases: opportunities and priorities. Trends. Microbiol. 2010;18(5):195–204. doi: 10.1016/j.tim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Dibern H.W., Müller R.M., Wirbitzki E. Edition Canto Verlag; Aulendorf, Germany: 2002. UV and IR Spectra: Pharmaceutical Substances (UV and IR) and Pharmaceutical and Cosmetic Excipients (IR) [Google Scholar]
- Dumortier G., Grossiord J.L., Agnely F. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- Ernst E.J., Klepser M.E., Pfaller M.A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents. Chemother. 2000;44(4):1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Barchiesi F., Cuenca-Estrella M. International and multicenter comparison of EUCAST and CLSI M27–A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 2005;43(8):3884–3889. doi: 10.1128/JCM.43.8.3884-3889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST), 2008. EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia–forming moulds. Clin. Microbiol. Infect. 14(10), 982–984. [DOI] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2015. EUCAST Document E.DEF 7.3 - Method for the determination of broth dilution of antifungal agents for fermentative yeasts; revised December 2015. Copenhagen, Denmark.
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST), 2017. EUCAST Definitive Document E.DEF 7.3.1 - Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts; January 2017. Copenhagen, Denmark.
- Fothergill A.W. Antifungal susceptibility testing: clinical laboratory and standards institute (CLSI) methods. In: Hall G.S., editor. Interactions of Yeasts, Moulds, and Antifungal Agents. Springer Science-Business Media; Amsterdam: 2012. pp. 65–74. [Google Scholar]
- Gebhart R.J., Espinel-Ingroff A., Shadomy S. In vitro susceptibility studies with oxiconazole (Ro 13–8996) Chemotherapy. 1984;30(4):244–247. doi: 10.1159/000238275. [DOI] [PubMed] [Google Scholar]
- Hazen K.C. Influence of DMSO on antifungal activity during susceptibility testing in vitro. Diagn. Microbiol. Infect. Dis. 2013;75(1):60–63. doi: 10.1016/j.diagmicrobio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Heeres J., Meerpoel L., Lewi P. Conazoles. Molecules. 2010;15(6):4129–4188. doi: 10.3390/molecules15064129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurler J., Engesland A., Poorahmary-Kermany B., Škalko-Basnet N. Improved texture analysis for hydrogel characterization: gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012;125(1):180–188. [Google Scholar]
- James H.P., John R., Alex A. Smart polymers for the controlled delivery of drugs–a concise overview. Acta. Pharm. Sin. B. 2014;4(2):120–127. doi: 10.1016/j.apsb.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.S., Bruschi M.L., de Freitas O. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009;372(1):49–58. doi: 10.1016/j.ijpharm.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Kashem S.W., Kaplan D.H. Skin immunity to Candida albicans. Trends. Immunol. 2016;37(7):440–450. doi: 10.1016/j.it.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Nishimoto S.K., Bumgardner J.D. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31(14):4157–4166. doi: 10.1016/j.biomaterials.2010.01.139. [DOI] [PubMed] [Google Scholar]
- Klepser M.E., Ernst E.J., Lewis R.E. Influence of test conditions on antifungal time-kill curve results: proposal for standardized methods. Antimicrob. Agents. Chemother. 1998;42(5):1207–1212. doi: 10.1128/aac.42.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.J., Muralidharan S., Parasuraman S. Antifungal agents: new approach for novel delivery systems. J. Pharm. Sci. Res. 2014;6(5):229–235. [Google Scholar]
- Leeyaphan C., Bunyaratavej S., Foongladda S. Epidemiology, clinical characteristics, sites of infection and treatment outcomes of mucocutaneous candidiasis caused by non-albicans Species of Candida at a dermatologic clinic. J. Med. Assoc. Thai. 2016;99(4):406–411. [PubMed] [Google Scholar]
- Masteikova R., Chalupova Z., Sklubalova Z. Stimuli-sensitive hydrogels in controlled and sustained drug delivery. Medicina. 2003;39(2):19–24. [PubMed] [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.B., Walls C.M., Denning D.W. In vitro activities of Terbinafine against Aspergillus species in comparison with those of Itraconazole and Amphotericin B. Antimicrob. Agents. Chemother. 2001;45(6):1882–1885. doi: 10.1128/AAC.45.6.1882-1885.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkhofer S., Mrazek C., Hartl L. In vitro susceptibility testing in fungi: What is the role in clinical practice? Curr. Infect. Dis. Rep. 2010;12(6):401–408. doi: 10.1007/s11908-010-0134-z. [DOI] [PubMed] [Google Scholar]
- Pfaller M.A., Boyken L.B., Hollis R.J. Validation of 24-hour fluconazole MIC readings versus the CLSI 48-hour broth microdilution reference method: results from a global Candida antifungal surveillance program. J. Clin. Microbiol. 2008;46(11):3585–3590. doi: 10.1128/JCM.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan N.L. A new weighting system for mean surface temperature of the human body. J. Appl. Physiol. 1964;19(3):531–533. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- Rencber S., Karavana S.Y., Senyigit Z.A. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2017;22(4):551–561. doi: 10.3109/10837450.2016.1163385. [DOI] [PubMed] [Google Scholar]
- Rezabek G.H., Friedman A.D. Superficial fungal infections of the skin. Drugs. 1992;43(5):674–682. doi: 10.2165/00003495-199243050-00004. [DOI] [PubMed] [Google Scholar]
- Whaley S.G., Berkow E.L., Rybak J.M. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2016;7:2173. doi: 10.3389/fmicb.2016.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong C.S., Choi J.S., Quan Q.Z. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive force of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2002;226(1):195–205. doi: 10.1016/s0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]