Abstract
Objectives
We have encountered patients who developed large joint fluid collections with massive elevations in chromium (Cr) and cobalt (Co) concentrations following metal-on-metal (MoM) hip arthroplasties. In some cases, retrieval analysis determined that these ion concentrations could not be explained simply by the wear rates of the components. We hypothesized that these effects may be associated with aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL).
Patients and Methods
We examined the influence of the ALVAL grade on synovial fluid Co and Cr concentrations following adjustment for patient and device variables, including volumetric wear rates. Initially restricting the analysis to include only patients with one MoM hip resurfacing device, we performed multiple regression analyses of prospectively collected data. We then repeated the same statistical approach using results from a larger cohort with different MoM designs, including total hip arthroplasties.
Results
In the resurfacing cohort (n = 76), the statistical modelling indicated that the presence of severe ALVAL and a large fluid collection were associated with greater joint fluid Co concentrations after adjustment for volumetric wear rates (p = 0.005). These findings were replicated in the mixed implant group (n = 178), where the presence of severe ALVAL and a large fluid collection were significantly associated with greater fluid Co concentrations (p < 0.001).
Conclusion
The development of severe ALVAL is associated with elevations in metal ion concentrations far beyond those expected from the volumetric loss from the prosthetic surfaces. This finding may aid the understanding of the sequence of events leading to soft-tissue reactions following MoM hip arthroplasties.
Cite this article: D. J. Langton, R. P. Sidaginamale, T. J. Joyce, J. G. Bowsher, J. P. Holland, D. Deehan, A. V. F. Nargol, S. Natu. Aseptic lymphocyte-dominated vasculitis-associated lesions are related to changes in metal ion handling in the joint capsules of metal-on-metal hip arthroplasties. Bone Joint Res 2018;7:388–396. DOI: 10.1302/2046-3758.76.BJR-2018-0037.
Keywords: Hip, Arthroplasty, Metal-on-metal, Adverse reaction to metal debris, Taper, Chromium, Cobalt, Aseptic lymphocyte-dominated vasculitis-associated lesion
Article focus
In our clinical practice, we had frequently encountered patients with metal-on-metal (MoM) total hip arthroplasties (THAs) with greater concentrations of cobalt (Co) in their hip synovial fluid samples than patients with equivalent MoM hip resurfacing designs.
However, examination of explanted devices indicated significantly lower total wear rates in the THA group.
We hypothesized that the presence and severity of the aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) response in periprosthetic tissue may influence, or be influenced by, the rate of clearance of metal from the joint fluid.
Key messages
After adjustment for volumetric wear rates, severe ALVAL was associated with greater Co and chromium (Cr) concentrations in synovial fluid samples compared with samples taken from patients without severe ALVAL.
After adjustment for wear rates, the presence of a large fluid collection was also associated with greater concentrations of metal in synovial fluid, rather than a reduction as might be expected via the effects of dilution.
The findings indicate that ALVAL and fluid collections are associated with impaired clearance of metal debris from the joint capsule.
Strengths and limitations
The study describes novel findings in a large sample size involving patients with resurfacing and THAs.
While an association between ALVAL and changes in metal handling have been identified, cause or effect cannot be determined.
Introduction
For several years, a cohort of patients implanted with one type of metal-on-metal (MoM) hip arthroplasty, the Articular Surface Replacement (ASR) (DePuy Synthes, Warsaw, Indiana) have been followed up in our institution. The ASR was available in two forms, the ASR resurfacing and the ASR XL total hip arthroplasty (THA), though both of these implants have been withdrawn since 2010. However, we have continued to investigate the relationship between the wear of MoM prostheses, periprosthetic cellular responses and clinical outcomes.
Many patients have been encountered who developed large joint effusions containing massive concentrations of chromium (Cr) and cobalt (Co) metal ions.1 Yet, in some cases, most commonly ASR XL THA patients, retrieval analysis determined that the Cr and Co ion concentrations were higher than would be expected from the actual amount of material loss from the components. Furthermore, it was found that patients implanted with the ASR XL THA experienced an adverse reaction to metal debris (ARMD)-related failure at a rate double to that of patients implanted with ASR resurfacings.1 The reactions tended to be more severe in the THA patients yet, on average, they were exposed to significantly less volumetric wear than the resurfacing patients (Table I). Paradoxically, the median Co concentration in the synovial fluid samples of the THA patients was higher than that in the resurfacing patients. These observations prompted us to investigate the differences in synovial fluid biochemistry associated with metal debris generated from bearings, compared with that from the head taper.2
Table I.
Details of the Articular Surface Replacement (ASR) resurfacing and XL patients revised at our unit. The whole cohorts have been described in detail in previous publications1,3
| Resurfacings | ASR THAs | Significance | |
|---|---|---|---|
| Hips, n | 76 | 45 | |
| Age (yrs)* | 54 | 56 | 0.061† |
| Male:female | 33:43 | 14:29 | 0.239‡ |
| Total volumetric wear rate (mm3 per yr)* | 7.10 | 3.26 | 0.001† |
| Joint fluid Cr (µg/l)* | 1622 | 562 | 0.024† |
| Joint fluid Co (µg/l)* | 855 | 1192 | 0.812† |
| ALVAL grade 3 (severe), % | 17 | 26 | 0.290‡ |
| Soft-tissue injury grade 3 (severe), % | 9 | 35 | 0.001‡ |
median value
Mann-Whitney U test
Fisher’s exact test
THA, total hip arthroplasty; Cr, chromium; Co, cobalt; ALVAL, aseptic lymphocyte-dominated vasculitis-associated lesion
Contrary to our expectations though, we found that metal debris release from the taper junction was not associated with significantly greater Co concentrations in the joint when matched by dose to bearing debris.2 We speculated, therefore, that these variations in metal ion concentrations might be associated with changes in the cellular environment in the joint capsule, specifically the grade of aseptic lymphocyte-dominated associated lesion (ALVAL).4
Our primary hypothesis for this investigation, therefore, was that fluid samples taken from hip joints with higher grade ALVAL scores would contain greater Co concentrations than those taken from joints with lower grade ALVAL, after adjustment for wear rates. Our secondary hypothesis was that, like ALVAL, larger fluid collections would also be associated with greater Co concentrations than small fluid collections exposed to similar amounts of metal debris.
Patients and Methods
Since 2008, patients who experience failure of MoM hip prostheses at our centre routinely undergo pre-revision blood and hip joint synovial fluid Co and Cr ion measurements. Tissue samples excised at revision surgery are assessed by a pathologist (SN).5 Explanted prostheses are analyzed to determine volumetric material loss from the bearing and female taper surfaces.2 In order to exclude the contribution of taper junction debris, and variations in metallurgy between designs,2 we initially included only ASR hip resurfacings with recorded volumetric wear rates, corresponding joint fluid metal ion concentrations and documented ALVAL grades. Patients with incomplete data sets were excluded.
The study was part of an ethically approved project (IRAS reference 14119).
Histopathological tissue assessment
Samples were taken from between two and four sites surrounding the implant. Up to ten paraffin blocks were processed per site to ensure the viable tissue was well represented. Samples were also sent to the microbiology department to rule out sepsis. A single consultant histopathologist (SN), examined the slides independently of the clinical findings, blinded to the results of the wear or fluid metal ion analyses. The size of aggregated particle masses was recorded as has been described.2 In 2011, the significant correlation between the mean diameter of lymphocytic perivascular cuffs and the extent of synovial membrane necrosis was reported,6 along with the variation in cellular responses to MoM prostheses.5 The grading system used in this paper (the NTH grading system) is standard and used in clinical practice for all MoM cases. The ALVAL response was graded from 0 (absent) to 3 (severe) according to the integrity of the synovial membrane and the stage of lymphocytic infiltration (Figs 1 to 3, Table II and supplementary material).
Fig. 1.
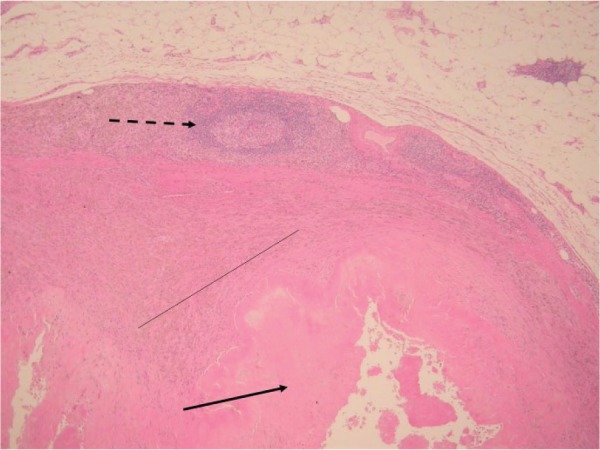
Histology showing grade 1 (mild) aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL). There is fibrin deposition on the surface membrane with minimal necrosis (unbroken arrow). Macrophages are present, with visible metal debris (thin, unbroken line). There are thin perivascular lymphocytic cuffs forming aggregates but not follicles (broken arrow).
Fig. 3.
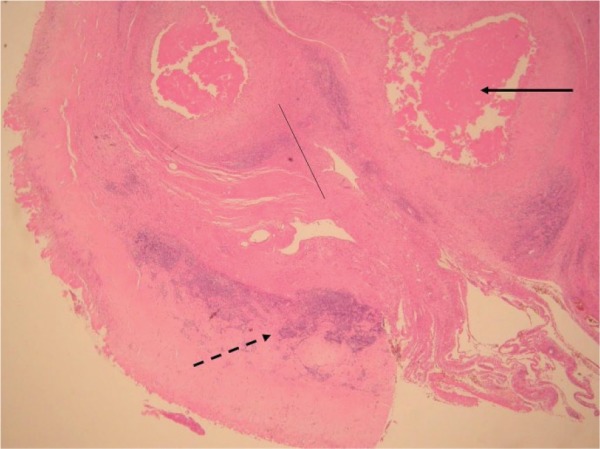
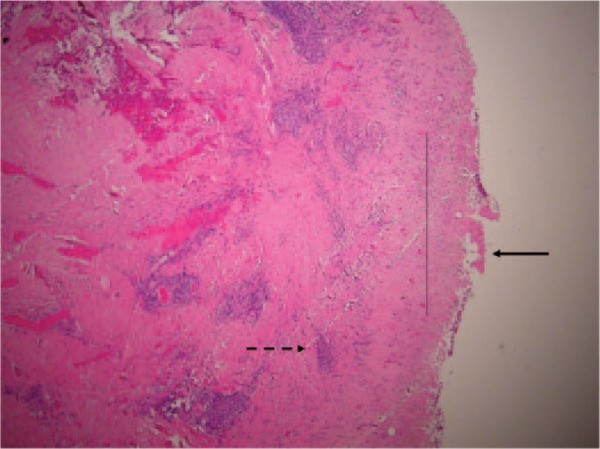
Histology showing grade 3 (severe) aseptic lymphocyte-dominated vasculitis-associated lesion. There are coalescing perivascular lymphocytic aggregates and development of lymphoid follicles (broken arrow). There is extensive surface necrosis (unbroken arrow) and sheets of macrophages containing metal debris (thin, unbroken line).
Table II.
NTH aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) grading system. The development of the grading system, interobserver reliability scores and comparison with other grading systems are described in the supplementary material
| Grade 0: ALVAL absent | Grade 1: Mild ALVAL | Grade 2: Moderate ALVAL | Grade 3: Severe ALVAL | |
|---|---|---|---|---|
| Vascular cuffs | None | Yes | Large perivascular lymphocytic aggregates | Large confluent lymphocytic aggregates |
| Synovial membrane | Intact | Grade 0 or 1 | Grade 2 or 3 | Grade 4 |
| Hyalinization | No | No | Can be present | Present |
| Germinal centres | No | No | Can be present | Present |
Fig. 2.
Histology showing grade 2 (moderate) aseptic lymphocyte-dominated vasculitis-associated lesion. There is fibrin deposition as well as necrosis of the surface membrane (unbroken arrow). Sheets of macrophages containing metal debris are visible (thin, unbroken line). Lymphoid aggregates are present but there is no follicle formation (broken arrow).
Synovial membrane
The extent of synovial surface necrosis was recorded as type 1 to 4, as described originally by Davies et al4 and later modified by us.5
Lymphocytic cuffs
The development of perivascular lymphocytic cuffs appears to be a dynamic process.5 Thin perivascular cuffs initially appear which increase in thickness as the recruitment of lymphocytes is further stimulated. These cuffs then either expand to develop into aggregates or coalesce into one another, forming larger aggregates. Once this process of lymphoid neogenesis is complete, pale germinal centres appear.
The differences between the ALVAL grading system used in the current paper and those used in previous publications7,8 are discussed in the supplementary material.
Wear analysis
Explanted prostheses were analyzed using a coordinate measuring machine (Legex 322; Mitutoyo Ltd, Halifax, United Kingdom) to calculate the total amount of material that had been removed from the components in vivo. This material loss can be expressed in volumetric terms as ‘total volumetric wear’ (in mm3) or the total value can be divided by the number of years in vivo to provide a mean ‘volumetric wear rate’ (expressed in mm3/year). The accuracy of such methods has been validated and is of the order of 0.5 mm3 per component for bearings and 0.2 mm3 for tapers.2,9 Throughout this paper, wear rates refer only to volumetric Co-Cr material loss. For resurfacings, ‘total volumetric wear rates’ refer to the bearing surface wear rates (combined head and acetabular component volumetric wear rates). For THAs, ‘total volumetric wear rates’ refer to the combined wear rates of the bearings and female taper surface.
Joint fluid Co and Cr concentrations
Prior to revision surgery, joint fluid was extracted under local anaesthetic to rule out infection and to analyze the Co and Cr concentrations. Metal ion concentrations were measured using inductively coupled plasma mass spectrometry (ICPMS) at the Royal Surrey Hospital. Samples did not undergo acid digestion prior to ICPMS analysis2 (supplementary material).
Fluid at revision surgery
The amount of fluid observed at revision was categorized as described previously,2 albeit this time using a binary approach. Findings of no abnormal fluid or ‘small’ collections of fluid were designated ‘small’. Joints with copious amounts of abnormal fluid (approximately > 20 mls) or those found to have pressurized fluid or fistulation of fluid were designated ‘large’.
Statistical analysis
Initially, the distribution of blood and joint Co and Cr concentrations, as well as volumetric wear rates from the bearing and taper surface, were examined using the Shapiro-Wilk test. All values were determined to be non-parametrically distributed (p < 0.001 for all tests) and were therefore log normalized. Forwards and backwards stepwise multiple regression models (including forwards and backwards stepwise approaches) were constructed to investigate whether any differences in the joint Co and Cr concentrations were brought about due to differences in the ALVAL grade. Statistical analysis was carried out using Minitab v17 software (Minitab Ltd, Coventry, United Kingdom). For completeness, and to rule in or out effects of dilution in larger patients, we included other continuous and categorical variables as shown in Table III. Our previous investigations showed these variables to have limited, if any, influence on the relationship between wear and ion concentrations.2
Table III.
Variables included in the regression models
| Predictors | Dependent variables |
|---|---|
| Patient factors | Joint fluid Co† |
| Joint fluid Cr† | |
| Age at primary | |
| Gender* | |
| Bilateral devices* | |
| Cellular environment: | |
| ALVAL grade* | |
| Agglomerated particle grade* | |
| Device factors: | |
| Total volumetric wear rate† | |
| Duration in vivo | |
| Dilutional effects: | |
| Acetabular component size | |
| Fluid grade* |
categorical variables
log normalized values used in the analysis
Co, cobalt; Cr, chromium; ALVAL, aseptic lymphocyte-dominated vasculitis-associated lesion
Fluid and ALVAL synergy
We noted in our previous investigations the positive correlation between higher grades of ALVAL and larger joint fluid collections at revision.6 In order to investigate a potential synergistic effect between ALVAL and fluid development, two groups of patients were selected from the data. The first consisted of patients with both severe ALVAL and large fluid collections. The second was composed of patients with no/mild/moderate ALVAL with small fluid collections. The regression models were repeated, this time directly comparing these two groups.
Mixed implant cohort
In the final part of this investigation, we took the data from all other MoM patients revised at our centre with documented explant analysis, ALVAL, fluid grades and joint fluid metal ion concentrations. Only implants with cobalt-chromium-molybdenum (CoCrMo) bearings were included and, if a THA construct, only those used with uncemented titanium femoral stems. Patients were excluded only if data sets were incomplete. We repeated the statistical tests to determine whether the same relationships between ALVAL, wear and joint Co concentrations identified in the ASR resurfacing cohort existed in this larger sample of patients. Cr concentrations were not included in these tests as previous investigations have shown that, with debris released from taper junctions, there is a tendency for Cr to adhere to the taper surface.2
The statistical models reported are those that best described the response variables where all the coefficients of the explanatory variables were significantly different from 0, with significance drawn at a p-value < 0.05. Standardized beta coefficients are reported as β with standard error as SE. The power of statistical models to explain variation in the dependent variables is reported as percentages throughout (these percentages are equivalent to R2 values).
We also conducted three small parallel studies which are described in the supplementary material. The first study aimed to quantify the changes in joint fluid metal ion concentrations following acid digestion of samples. The second was intended simply to characterize the nature of fluid effusions as having normal or abnormal albumin concentrations. The third was a basic theoretical study to demonstrate how the effect of changes in fluid volumes would be expected to affect joint fluid metal ion concentrations.
Results
A total of 76 explanted ASR devices were included in the first analysis. There were 178 explants in the mixed implant cohort. Patient demographic, clinical parameters and implant details are shown in Table IV.
Table IV.
Details of the patient cohorts included in the investigation of the influence of aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) on joint fluid metal ion concentrations
| ASR resurfacings | Mixed implant cohort | |
|---|---|---|
| Hips, n | 76 | 178 |
| Device type | ASR resurfacings | 36 mm MoM: 105 |
| ASR XL: 45 | ||
| BHR resurfacings: 26 | ||
| Durom resurfacings: 2 | ||
| Age (yrs)* | 54 (29 to 75) | 59 (22 to 84) |
| Male:female | 33:43 | 53:125 |
| Total volumetric wear rate (mm3 per yr)* | 7.10 (0.62 to 95.5) | 2.28 (0.23 to 69.1) |
| Duration in vivo (mths)* | 54 (8 to 108) | 61 (11 to 136) |
| Acetabular component size (mm)* | 54 (46 to 64) | 53 (44 to 64) |
| Joint fluid Cr (µg/l)* | 1622 (26 to 195 947) | 455 (13 to 133 120) |
| Joint fluid Co (µg/l)* | 855 (26 to51 487) | 605 (9 to 46 433) |
| ALVAL grade 3 (severe), % | 17 | 22 |
| Ratio of small:large fluid collection: | 20:56 | 49:129 |
median values
ASR, Articular Surface Replacement; MoM, metal-on-metal; BHR, Birmingham Hip Resurfacing; Cr, chromium; Co, cobalt
ALVAL and joint fluid Co concentrations
In the best-fitting regression model, total wear rate was the dominant variable (β = 0.669, SE = 0.08, p < 0.001). Joints with grade 3 (severe) ALVAL were significantly associated with greater Co concentrations than those with grade 0, 1 or 2 ALVAL (p = 0.05, p = 0.012, p = 0.005, respectively) (Fig. 4). Smaller fluid collections were significantly associated with lower Co concentrations (β = -0.240, SE = 0.083, p = 0.005). A longer duration in vivo was associated with greater Co concentrations (β = 0.180, SE = 0.085, p = 0.038) and there was a trend for larger joints to have lower Co concentrations (β = -0.157, SE = 0.084, p = 0.068). These variables accounted for approximately 62% of the variation in Co (p < 0.001). The equation for the best-fit model was: log joint Co = 3.824 - (0.031 × acetabular component size) + (0.007 × duration in vivo) + (0.891 × log wear) - (0.412 if ALVAL absent) - (0.453 if ALVAL mild) - (0.438 if ALVAL moderate) - (0.388 if small fluid collection).
Fig. 4.
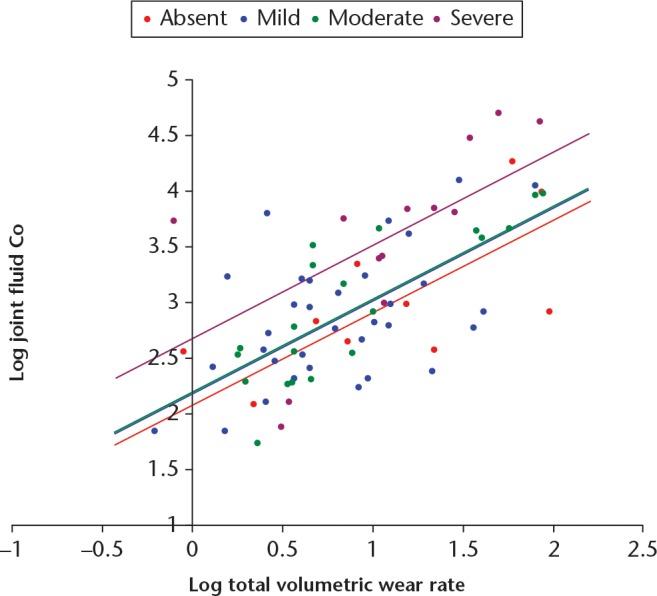
Articular Surface Replacement resurfacing patients. Best-fit regression lines for the relationship between logged values of fluid cobalt (Co) and volumetric wear, for each grade of aseptic lymphocyte-dominated vasculitis-associated lesion.
ALVAL and fluid synergy with Co
The influence of the combined effect of a large fluid collection and severe ALVAL on joint fluid Co can be seen in Figure 5. Total wear rate was again the dominant variable (β = 0.530, SE = 0.112, p < 0.001), however, the presence of a large fluid collection with severe ALVAL had a comparable influence (β = 0.502, SE = 0.112, p < 0.001). The equation of the best-fit regression model was: log joint Co = 2.721 + 0.816 × log total volumetric wear - (0.846 if small fluid collection and non-severe ALVAL). This model provided approximately 67% of the variation in the joint Co concentrations. Essentially, the effect of the presence of a large fluid collection and severe ALVAL was to increase the fluid Co concentrations as if the volumetric wear rate had increased by approximately 7 mm3 per year.
Fig. 5.
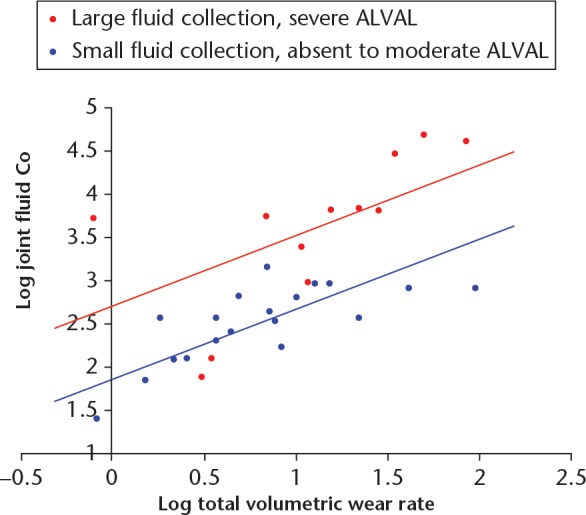
In this chart, the Articular Surface Replacement resurfacing patients have been placed into two groups: those with severe aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) and a large fluid collection (n = 12, red); and those with absent/mild/moderate ALVAL and a small fluid collection (n = 19, blue). The regression modelling demonstrated that patients with severe ALVAL and large fluid collections were found to have greater cobalt (Co) concentrations when adjusted for volumetric wear rates.
ALVAL and joint fluid Cr concentrations
In the best-fitting regression model, total wear rate was the dominant variable (β = 0.765, SE = 0.071, p < 0.001). Joints with small fluid collections were significantly associated with lower Cr concentrations than those with large fluid collections (β = -0.159, SE = 0.071, p = 0.030). Neither ALVAL, nor any of the other examined variables, was found to have significant influences, with wear rate and fluid providing approximately 63% of the variation in the fluid Cr concentration. The equation for the best-fit model was: log joint Cr = 2.303 + 1.192 × log wear rate (- 0.301 if small fluid collection).
ALVAL and fluid synergy with Cr
As was observed with Co, the presence of severe ALVAL and a large fluid collection was associated with significantly larger Cr concentrations, however, this effect was less powerful. The equation for the best-fit regression model was: log joint Cr = 2.489 + 1.098 × log wear rate - 0.440 × (absent to moderate ALVAL and small fluid collection) (Fig. 6). This model provided approximately 64% of the variation in Cr. Beta coefficients for these variables were 0.697 (SE = 0.117, p < 0.001) for volumetric wear rate and 0.255 (SE = 0.117, p = 0.038) for the presence of severe ALVAL with a large fluid collection.
Fig. 6.
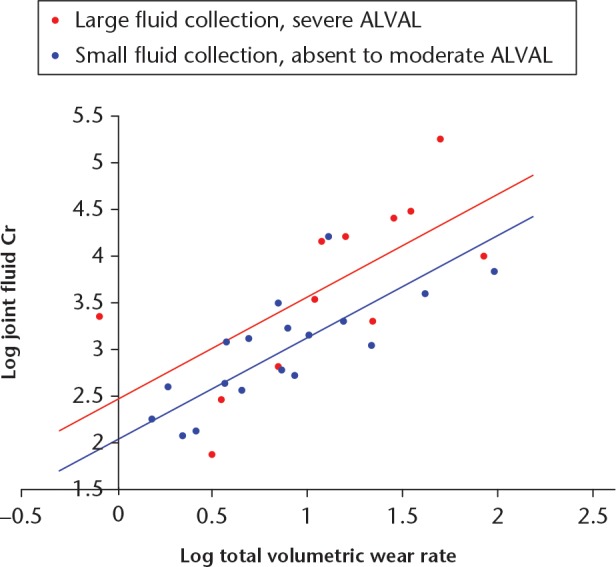
In this chart, the Articular Surface Replacement resurfacing patients have been placed into two groups: those with severe aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) and a large fluid collection (n = 12, red); and those with absent/mild/moderate ALVAL and a small fluid collection (n = 19, blue). The regression modelling demonstrated that patients with severe ALVAL and large fluid collections were found to have greater chromium (Cr) concentrations when adjusted for volumetric wear rates.
Mixed implant cohort
In the best-fitting regression model, wear rate was the dominant variable (β = 0.562, SE = 0.06, p < 0.001). Joints with grade 0 (absent) ALVAL were significantly associated with lower Co concentrations than those with grade 3 (severe) ALVAL (β = -0.210, SE = 0.078, p = 0.007), as were those with mild ALVAL (β = - 0.180, SE = 0.080, p = 0.020) (Fig. 7). In this cohort, the moderate ALVAL group were not found to have significantly lower joint Co concentrations than the severe group (β = 0.124, SE = 0.080, p = 0.121). There was no significant difference in Co concentrations between the patients with small and large fluid collections (p = 0.688).
Fig. 7.
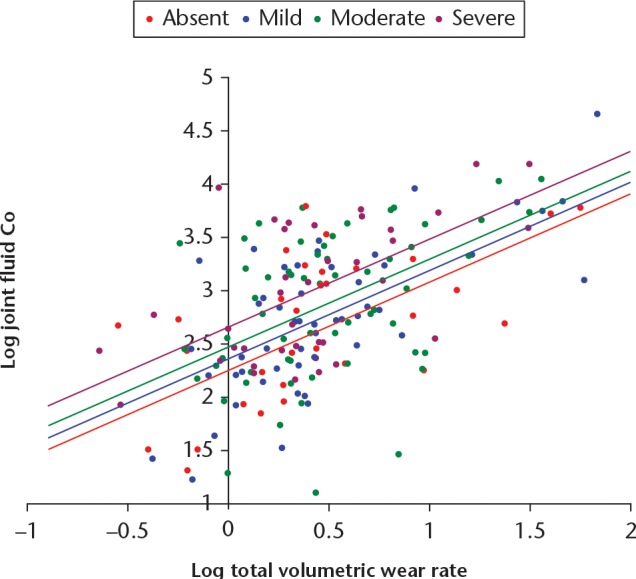
Mixed implant cohort. Best-fit regression lines for the relationship between logged values of fluid cobalt (Co) and volumetric wear, for each grade of aseptic lymphocyte-dominated vasculitis-associated lesion.
The combined influence of a large fluid collection and severe ALVAL on joint fluid Co can be seen in Figure 8. Wear rate was again the dominant variable (β = 0.516, SE = 0.094, p < 0.001). The presence of a large fluid collection with severe ALVAL had a weaker influence (β = 0.239, SE = 0.094, p < 0.001) than the resurfacing only group. The equation of the best-fit regression model was: log joint Co = 2.346 + 0.799 × log wear rate + (0.340 if large fluid collection and severe ALVAL).
Fig. 8.
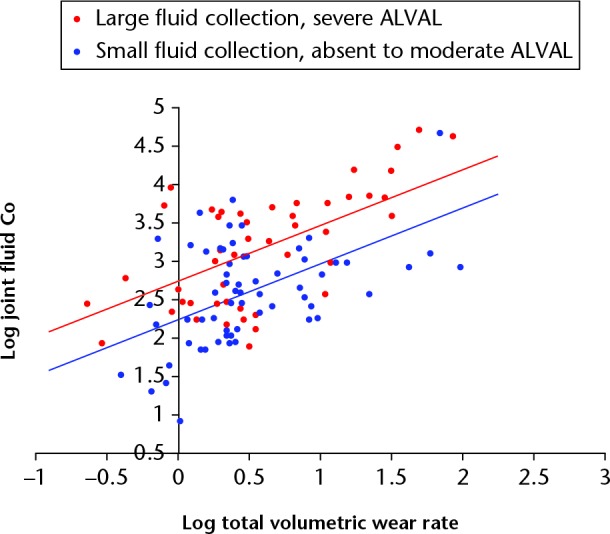
In this chart, the mixed implant cohort patients have been placed into two groups: those with severe aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL) and a large fluid collection (n = 34, red); and those with absent/mild/moderate ALVAL and a small fluid collection (n = 44, blue). The regression modelling demonstrated that patients with severe ALVAL and large fluid collections were found to have greater cobalt (Co) concentrations when adjusted for volumetric wear rates.
Discussion
We hypothesized that patients with ALVAL and large fluid effusions would have greater joint fluid metal ion concentrations when compared with patients with implants wearing at similar rates who had not developed ALVAL. Initially limiting our investigation to patients with one type of hip resurfacing, then expanding it to include different designs and modular devices, we have shown that the accumulation of Co ions in joint fluid is more pronounced than Cr.
‘Normal’ wear and synovial fluid ion concentrations
The standard CoCrMo alloy from which contemporary MoM prostheses are manufactured is composed, by weight, of approximately 65% Co and 30% Cr.2 Yet the most frequent biochemical picture encountered in patients with hip resurfacings is that of a synovial fluid rich in Cr.10 There can only be two explanations for this finding. The first is that there is a selective release of Cr from the prosthetic surfaces. However, as the volume loss can often be greater than 100 mm3,6 this explanation seems implausible. It is more likely, therefore, that there is a difference in the host’s capacity to clear the two different types of metal from the joint. Multiple hip simulator studies have indicated that larger particles generated from MoM bearing surfaces are composed predominantly of Cr, in the form of chromium dioxide.11,12 This has been substantiated by the examination of ex vivo fluid samples, analyzed pre- and post-acid digestion.13 Analysis of a hip fluid sample taken from a patient experiencing high wear of their MoM prosthesis, with a characteristic Cr-rich biochemistry (Cr = 15 200 µg/l; Co = 7,900 µg/l), found that the median (range) Co particle diameter was 39 nm (32 to 136), with the Cr particles being significantly larger.14 The cell layer of the synovial lining is discontinuous, with the intercellular space of the acetabular component filled with a complex interstitial matrix. This matrix has an estimated pore diameter of 30 nm to 118 nm and can act as a size-selective barrier.15 The size of particulate matter, therefore, affects the route and therefore, the rate, of its clearance from synovial fluid.16 Thus we speculate that the clearance of Cr occurs more slowly due to its lower solubility, greater average particle size and thus greater dependence on the lymphatic channels. We propose that this situation explains why MoM joint with minimal synovial membrane damage contain synovial fluid, which is typically rich in Cr, a finding which, we suggest, may be considered ‘normal’.
When Co concentrations rise in excess of the Cr concentration in joint fluid, it may be indicative of a disturbance in the normal function of the synovial membrane, a change in the composition or volume of the joint fluid, a change in the protein binding of the metal debris or a change in the rate, composition or morphology of the debris generated by the prosthesis.
Changes in particle size
Metal ion concentrations in this study were measured using fluid samples which had not undergone acid digestion prior to ICPMS. It is, therefore, conceivable that in situations where a greater proportion of metal debris was shed from the components in ionic form, the measured Cr and Co concentrations could be larger than those measured in a patient with a prosthesis wearing at the same rate but generating larger particles. Therefore, if ALVAL is stimulated by exposure to smaller particles, the relationships we have observed could be representative of the promotion of the cellular response rather than ALVAL itself causing the changes in biochemistry. However, acid digestion has a much greater effect on Cr than on Co,13 and, in the current study, severe ALVAL was associated primarily with elevations of Co rather than Cr.
Changes in the rate of metal debris generation
The wear rates we have quoted and used in the statistical analyses are mean annual wear rates, but it is possible that implants could experience rapid increases in wear prior to revision. This could occur, for example, when a wear patch extends to incorporate the edge of the bearing surface, or, with THAs, when micromotions at the taper interface become progressively greater in magnitude. From our data, we were not able to identify fluctuations in wear rates. This is an important area for future research as a rapid increase in metal debris release could in fact be the immune stimulus leading to the development of ALVAL.
Changes to synovial membrane function
As described above, the synovial membrane plays an important role in solute transit. Severe ALVAL, by definition, indicates severe disruption to the regulation of the blood joint barrier, with widespread necrosis of the synovial membrane. It seems only logical, therefore, that such severe destruction to this complex structure could result in the potential impairment of metal ion clearance.
Synovial fluid changes
One of the classical findings of ARMD is the development of large fluid collections in the joint which can frequently reach volumes many times larger than those reported in healthy hip joints (< 5 ml).17 In the patients in this study, the presence of ALVAL and the development of fluid collections appeared to be intimately linked. Of the 186 patients with ‘large’ joint effusions, only 19 (10%) had no features of ALVAL. Of the 139 patients with moderate/severe ALVAL, 115 (83%) had ‘large’ fluid collections. The composition of ARMD-related fluid collections has not, to our knowledge, been fully characterized. Our early findings (described in the supplementary material) demonstrate that these effusions, as with those found in other inflammatory conditions, contain excess concentrations of albumin, indicating a breakdown in the regulation of protein transport at the blood-joint barrier. For larger solutes such as albumin, the barrier between the blood and the joint resides mainly in the small pores of the endothelial layer of vessels which restrict protein exchange far more than the intimal matrix.18 Hallmark features of ALVAL include the development of perivascular lymphocytic aggregates and associated changes to the vessel walls. The changes in vessel wall permeability, combined with structural damage to the synovial membrane seen in advanced cases of ALVAL could, therefore, lead to changes in protein flux via leakage of protein through vessel walls or a reduction in the rate of fluid/albumin egress from the joint.
Changes in metal protein binding
The results presented in this paper show that the development of fluid collections leads to an increase in local metal ion concentrations rather than a decrease, as might be expected secondary to dilution (supplementary material). We have previously shown evidence that in aspirated hip fluid, Co and Cr ions are primarily bound to albumin and transferrin, respectively.14 In studies of drug delivery to the knee joint, it has been demonstrated that the binding of solutes to proteins in synovial fluid retards their transport out of the joint space.19 We believe, therefore, the most likely explanation for the accummulation of Co in the fluid samples is secondary to an increase in the proportion of Co which is protein bound. The clinical meaning of the effects of increases in fluid protein content, coupled with rising metal concentrations, is unclear. In theory, this environment could lead to the concentration of metalloproteins which may be recognized as ‘foreign’ by the host. The presence of metals other than Co and Cr, such as Mo, may also prove to be of greater significance in this respect than has previously been recognized.
In conclusion we have shown that the development of severe ALVAL is associated with elevations in metal ion concentrations far beyond those expected from volumetric loss from the prosthetic surfaces. It is also apparent that there is a complex interaction between ALVAL, joint fluid protein homeostasis and metal ion handling. We are currently unable to determine whether the changes in metal ion handling are caused by the presence of ALVAL or vice versa.
Footnotes
Author Contributions: D. J. Langton: Designed the study, Developed wear analysis techniques, Performed explant analysis, Statistical analysis, Produced the initial draft of the article.
R. P. Sidaginamale: Co-designed the study, Performed explant analysis, Collected and interpreted histopathological data, Involved in drafting and revision of the manuscript.
T. J. Joyce: Co-designed the study, Supervised wear analysis, Involved in drafting and revision of the manuscript.
J. G. Bowsher: Helped coordinate the study, Interpreted data, Helped draft and approve the final content of the manuscript.
J. P. Holland: Co-designed the study, Recruited patients, Provided explanted prostheses, Helped draft and approve the final content of the manuscript.
D. Deehan: Co-designed the study, Recruited patients, Provided explanted prostheses, Helped draft and approve the final content of the manuscript.
A. V. F. Nargol: Initiated the study, provided explanted prostheses, interpreted data, helped draft and approve the final manuscript
S. Natu: Co-designed the study, Provided histopathological assessment of retrieved tissues, Interpretation of this data, Drafting and approval of the final content of the manuscript.
Conflicts of Interest Statement: J. P. Holland, S. Natu, A. V. F. Nargol, T. J. Joyce and D. J. Langton are expert witnesses for plaintiffs in metal on metal litigation, however this is not related to this article. In addition, A. V. F. Nargol and D. J. Langton have a whistleblower case against Depuy which could result in financial reward, but is also not related to this article.
Follow us @BoneJointRes
Supplementary material
The development of the grading system, interobserver reliability scores and comparison with other grading systems.
Funding Statement
Funding has been received from the William Leech Charity and the Food and Drug Administration which is related to this article.
References
- 1. Langton DJ, Jameson SS, Joyce TJ, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg [Br] 2011;93-B:1011-1016. [DOI] [PubMed] [Google Scholar]
- 2. Sidaginamale RP, Joyce TJ, Bowsher JG, et al. The clinical implications of metal debris release from the taper junctions and bearing surfaces of metal-on-metal hip arthroplasty: joint fluid and blood metal ion concentrations. Bone Joint J 2016;98-B:925-933. [DOI] [PubMed] [Google Scholar]
- 3. Langton DJ, Jameson SS, Joyce TJ, et al. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg [Br] 2010;92-B:38-46. [DOI] [PubMed] [Google Scholar]
- 4. Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg [Am] 2005;87-A:18-27. [DOI] [PubMed] [Google Scholar]
- 5. Natu S, Sidaginamale RP, Gandhi J, Langton DJ, Nargol AV. Adverse reactions to metal debris: histopathological features of periprosthetic soft tissue reactions seen in association with failed metal on metal hip arthroplasties. J Clin Pathol 2012;65:409-418. [DOI] [PubMed] [Google Scholar]
- 6. Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg [Br] 2011;93-B:164-171. [DOI] [PubMed] [Google Scholar]
- 7. Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res 2010;468:2321-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grammatopoulos G, Pandit H, Kamali A, et al. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg [Am] 2013;95:e81. [DOI] [PubMed] [Google Scholar]
- 9. Langton D, Ahmed I, Avery P, et al. Investigation of Taper Failure in a Contemporary Metal-on-Metal Hip Arthroplasty System Through Examination of Unused and Explanted Prostheses. J Bone Joint Surg [Am] 2017;99:427-436. [DOI] [PubMed] [Google Scholar]
- 10. De Smet K, De Haan R, Calistri A, et al. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg [Am] 2008;90-A(Suppl 4):202-208. [DOI] [PubMed] [Google Scholar]
- 11. Bowsher JG, Hussain A, Williams PA, Shelton JC. Metal-on-metal hip simulator study of increased wear particle surface area due to ‘severe’ patient activity. Proc Inst Mech Eng H 2006;220:279-287. [DOI] [PubMed] [Google Scholar]
- 12. Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater 2018;106:986-996. [DOI] [PubMed] [Google Scholar]
- 13. Davda K, Lali FV, Sampson B, Skinner JA, Hart AJ. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg [Br] 2011;93-B:738-745. [DOI] [PubMed] [Google Scholar]
- 14. Loeschner K, Harrington CF, Kearney JL, Langton DJ, Larsen EH. Feasibility of asymmetric flow field-flow fractionation coupled to ICP-MS for the characterization of wear metal particles and metalloproteins in biofluids from hip replacement patients. Anal Bioanal Chem 2015;407:4541-4554. [DOI] [PubMed] [Google Scholar]
- 15. Sabaratnam S, Arunan V, Coleman PJ, Mason RM, Levick JR. Size selectivity of hyaluronan molecular sieving by extracellular matrix in rabbit synovial joints. J Physiol 2005;567:569-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simkin PA. Assessing biomarkers in synovial fluid: consider the kinetics of clearance. Osteoarthritis Cartilage 2013;21:7-9. [DOI] [PubMed] [Google Scholar]
- 17. Moss SG, Schweitzer ME, Jacobson JA, et al. Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology 1998;208:43-48. [DOI] [PubMed] [Google Scholar]
- 18. Levick JR, McDonald JN. Fluid movement across synovium in healthy joints: role of synovial fluid macromolecules. Ann Rheum Dis 1995;54:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owen SG, Francis HW, Roberts MS. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol 1994;38:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]


